Abstract
Introduction
Ovulation is similar to an inflammatory response and is associated with increased production of prostaglandins as well as local growth regulatory factors. However, the expression and function of innate immune cell-related genes in non-immune cells within the ovary has been reported recently and provides a novel and important regulatory system during ovulation.
Discussion
Several members of the Toll-like receptor (TLR) surveillance system are expressed in granulosa cells and cumulus cells. These receptors can be activated by pathogens as well as endogenous ligands leading to the induction and release of potent cytokines and chemokines from cumulus cells.
Conclusion
These inflammatory factors exert potent effects on cumulus cell-oocyte expansion, ovulation, transport and fertilization indicating that ovulation is a more complex immune-inflammatory process than previously recognized.
Keywords: Immune, Ovulation, Toll-like receptors, Cytokines, Ovary, Cumulus cells
Introduction
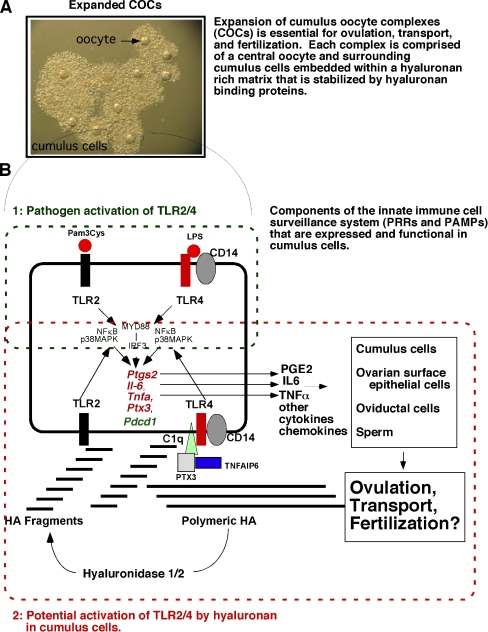
Ovulation is a luteinizing hormone (LH) surge induced process in which a mature oocyte (egg) and surrounding cumulus cells, known as the cumulus cell-oocyte complex (COC), are released from the surface of the ovary into the oviduct for transportation and fertilization [1–3]. During this process, the follicles become hyperemic and produce large amounts of prostaglandins (mainly PGE2). In addition, the cumulus cells synthesize a hyaluronan rich matrix that controls COC expansion, a process essential for ovulation to occur (Fig. 1A) [3]. These events are dependent on several LH-induced signaling cascades leading to the induction of the EGF-like factors (Areg, Ereg, Btc) [4–7]. These factors in turn regulate the induction of genes in cumulus cells. These include: prostaglandin synthase 2 (Ptgs2) and numerous matrix factors, e.g. hyaluronan synthase 2 (Has2), tumor necrosis stimulated gene 6 (Tnfaip6), pentraxin 3 (Ptx3), versican (Cgs2) [3, 4, 7]. Also critical for ovulation is the induction of the progesterone receptor (Pgr) [8, 9] and Pgr-regulated genes, such as Pparγ, Areg, Il6 [10] and specific proteases (Adamts1, Ctsl) [9]. In addition, ovulation is associated with the induced expression and function of the acute phase gene Cebpb [11]. More recent studies indicate that signaling cascades activated in response to the LH surge are more diverse and should be expanded to include additional gene categories such as those associated with the innate immune responses and the immune cell functions [12, 13]. Members of the innate immune systems, such as the Toll-like receptor (TLR) factors, are expressed/induced in granulosa/cumulus cells in mouse, bovine and human ovaries and therefore they may function to affect fertility [13–15]. Furthermore, cumulus cells express Pdcd1, a gene linked to autoimmune functions in mice and humans [12]. Thus, it is likely that the innate immune system plays a role in ovulation (Fig. 1A and B).
Fig. 1.
Schematic of TLR pathway activation and functions in cumulus cells during COC expansion, ovulation, transport and fertilization. A In response to the ovulatory surge of LH, cumulus cell-oocyte complexes (COCs) undergo expansion. This process requires the synthesis of a hyaluronan (HA)-rich matrix and factors that bind HA to stabilize the matrix. This process is critical for ovulation. B Cumulus cells express members of the Toll-like receptor (TLR) superfamily and can respond to specific ligands (1: pathogen-derived or 2: matrix-derived) leading to the induction of inflammation- and innate immune-related genes. These include: Ptgs2, Il6, Tnfaip6, Tnfa and Pdcd1. Prostaglandins (PGE2) synthesized by PTGS2, IL6, TNFa as well as other cytokines and chemokines are released from cumulus cells and can impact the function not only of the cumulus cells but also oviductal cells during transport and sperm during fertilization. The degradation of polymeric HA by hyaluronidases is presumed to lead to the generation of HA fragments that activate TLR2 and TLR4
The toll-like receptor family
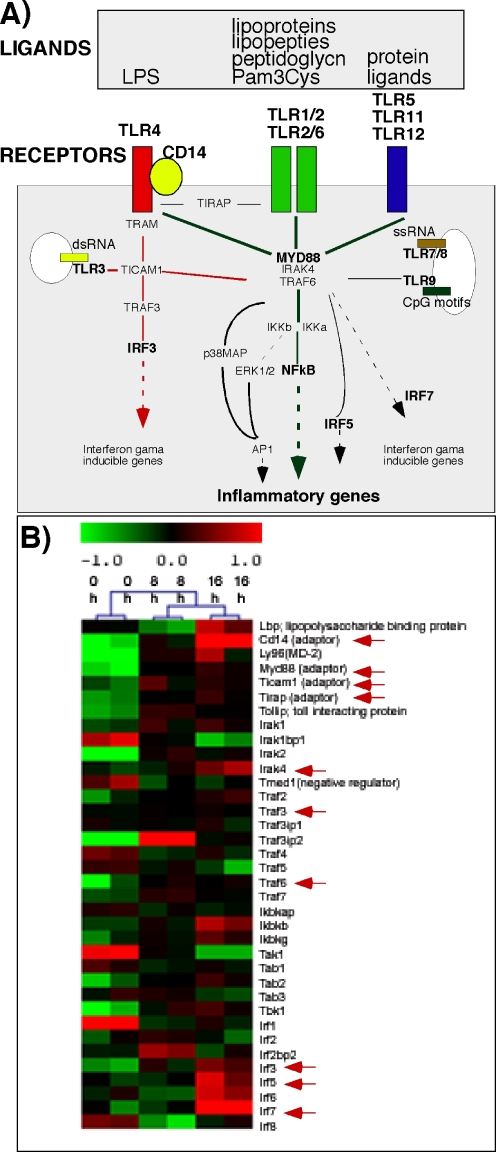
In mammals there are two kinds of immune responses, the innate response and the acquired immune response. While acquired immune responses operate later in an infection and are highly specific for the pathogen that induces them, the innate immune responses react immediately after exposure to pathogens and serve as the first line of host defense. The innate immune system depends on the pattern recognizing receptors (PRRs), e.g., components of the complement system and the Toll-like receptor family, that detect the pathogen-associated molecular patterns (PAMPs) and execute subsequent immune cell responses [16–18] (Fig. 2A).
Fig. 2.
Schematic of the Toll-like receptor superfamily signaling cascades, their ligands and putative functions. A The TLR surveillance system is comprised of a complex array of receptors, adaptors and downstream signaling molecules. There are 13 members of TLR family of membrane receptors (mouse) and many intracellular signaling components of which a few are shown (adapted from [17]). Whereas most TLRs are on the cell surface TLR3, TLR7/8 and TLR9 are on intracellular membranes and respond to dsRNA, ssRNA and CpG motifs, respectively. B The expression profile shows key TLR signaling pathway members detected in mouse ovarian COC samples collected at hCG 0, 8 and 16 h. The intensity values are log2 transformed and median centered across genes. Green color indicates relatively lower expression levels whereas red indicates relatively higher expression levels
The Toll-like receptors (TLRs) are a group of membrane-bound proteins characterized by a leucine-rich (LRR) repeat extracellular domain and a Toll/IL-1R (TIR) cytoplasmic domain [16]. TLRs can recognize a variety of PAMPs including lipoprotein, lipopolysaccharide, peptidoglycan, zymosan, bacterial flagella, CpG DNA, double strand and single strand RNAs (for reviews, see [16, 19]; Fig. 2A). To date, ten human TLRs and thirteen mouse TLRs have been identified (for reviews, see [16]). While each of them is activated by specific ligands, most of them mediate their actions via a MyD88-dependent intracellular pathway, leading to the activation of NFkB and MAPK pathways that regulate the induction of target genes. However, TLR3 is activated by dsRNA and employs a MyD88-independent pathways that involves Ticam1 and TLR4 can utilize both pathways [16, 17] (Fig. 2A).
Expression of TLRs in non-immune cells
TLRs seem to be derived from an ancient, evolutionarily conserved receptor recognition system that exists in both vertebrate and invertebrate species [20]. The expression of these receptors in immune cells is linked unequivocally to the detection of self from non-self [18]. However, an increasing number of reports indicate that TLRs are expressed and functional in non-immune, somatic cells such as adipose cells [22], mouse bone marrow derived mesenchymal stem cells (MSCs) [20] and human adipose tissue and bone marrow derived MSCs [23]. Several groups have also reported the expression of TLRs in ovarian tissues. In hen ovarian follicles, the theca cell layer expresses TLR-2, TLR-4, TLR-5 and TLR-7, whereas the granulosa cell layer expresses only TLR-4 and TLR-5 [24]. In the porcine ovary, TLR4 was detected [25]. TLR5 is expressed predominantly in human ovary, peripheral blood leukocytes, and prostate [26]. In mice, at least nine TLRs have been detected in the ovary of the three mouse strains (NIH, Balb/c and C57BL/6) [27]. Our lab has also reported the expression of TLRs 2, 4, 8 and 9 in mouse granulosa cells and cumulus cells [13]. Moreover, we have shown that TLR2 and TLR4 are functional because their ligands, Pam3Cys and LPS, respectively induce expression of known TLR2/TLR4 target genes, including Ptgs2, Il6 and Tnfa [13] (Fig. 1B). In addition to the TLRs, our microarray database on COC samples collected from preovulatory follicles at 0 h, 8 h and 16 h post-hCG showed that most of the key components of the complex TLR signaling pathway are present (Fig. 2B). These include the genes encoding adaptor factors (CD14, Ly96, Myd88, Ticam1, Tirap and Tollip), interleukin-1 receptor-associated kinases (Iraks), TNFα receptor associated factors (Trafs) and interferon regulatory factors (Irfs). Interestingly, many of them are differentially regulated between different time-points, strongly indicating the potential involvement of the TLR system in the ovulation process (Fig. 2B).
Function of TLRs in non-immune cells
In Drosophila Toll-like receptors were shown originally to regulate embryonic development and morphogenesis [28]. Recently the expression of the Toll-like receptor, 18-wheeler (18 w), was found to affect cell migration and egg morphology in Drosophila [29]. Although none of the single TLR knockout mice are infertile, there is increasing evidence indicating that TLRs are functional in the ovary and they could affect fertility. Therefore, there may be redundant functions of some receptors. Recent studies have shown that LPS is present in bovine follicles and is associated with reduced levels of estradiol and impaired fertility [14]. Because IL6 is one cytokine produced by COCs of ovulating follicles, its production may be related in part to TLR2/4 activation (Fig. 1B) [13]. Moreover, this cytokine and others may exert specific functions on cumulus cells and other cells [15]. Importantly, IL6 itself induces expression of many genes in cumulus cells and can stimulate COC expansion (our unpublished observations; [30]). IL6 and other cytokines may also impact ovarian surface epithelia cells at the time of ovulation to stimulate their proliferation and repair of the rupture site [31]. Once the COCs are in the oviduct, cytokines released from the cumulus cells may impact the function of oviductal cells. For example, COCs interact with the ciliated cells of the oviduct during transport and cluster in a region where the oviduct is “ballooned” and hence appears to be responding to the presence of the COCs. Although the extent to which factors from cumulus cells regulate these events is not entirely clear, transport and oviductal swelling are not observed in Ptx3 null mice in which the matrix disappears rapidly following ovulation [32, 33]. The Toll-like receptors may also be involved in fertilization [15]. Specifically, in in vitro fertilization assays, sperm induce cumulus cells to release specific cytokines and chemokines. Certain chemokines then bind chemokine receptors on sperm and enhance fertilization. These events are dramatically reduced in the presence of TLR2 and TLR4 blocking antibodies [15]. Thus, we propose that there is a regulatory loop between sperm and COCs during the fertilization process. TLRs have also been reported in uterine [34] and oviductal tissue and to impact pre-term labor [35]. TLR2/4 may also respond to factors in addition to sperm that enter the reproductive tract, including bacteria, at times of infection [35]. Based on these observations, chronic inflammatory conditions, such as endometriosis, that produce elevated levels of cytokines could impact the ovary and other reproductive tissues in many ways.
Non-pathogenic ligands that activate TLRs
Although LPS and Pam3Cys are bacterial ligands that activate TLR2/4 and initiate innate immune responses in immune cells [18], endogenous ligands generated at sites of tissue injury have also been identified. Thus, the local production of specific ligands can alert cells to alterations in their surrounding environment. One such ligand is hyaluronan (HA) that has size and cell specific effects (Fig. 1B) [19, 36]. In its polymeric form, HA appears to protect epithelial cells from apoptosis. In contrast, fragments of HA generated by endogenous hyaluronidases at sites of tissue injury or when added exogenously to cells exert size dependent effects that are remarkably diverse [36]. Thus HA fragments have been characterized as “an information-rich system” [36]. Because cumulus cells are embedded in a hyaluronan-rich matrix [12] it is tempting to speculate that polymeric HA as well as local production of HA fragments by hyaluronidases generate potent and diverse autocrine effects on cumulus cells (Fig. 1A&B). Although high molecular weight HA does not activate TLR2/4, fragments less than 230 kDA do exhibit stimulatory effects and those of 30 mer were reported to be the most effective [37]. In acute lung injury, very small HA fragments activate TLR2/4 and in the ovary we have shown that fragments of ∼150 kDA are functional [38]. In this regard, the ability of sperm to induce COC expression of potent cytokines and chemokines in in vitro fertilization procedures was mimicked by the presence of hyaluronan fragments or hyaluronidase and blocked by TLR2/4 neutralizing antibodies [15]. These results indicate that the release of hyaluronidase from sperm at the time of capacitation degrades the hyaluronan-rich matrix of the COCs leading to activation of cumulus cell TLR2/4 signaling events [15]. Other endogenous ligands that have been reported include HMB1, fatty acids, biglycan, defensin 2, nuclei acids, heme and Hspd1 [19, 36, 39]. Of note, HMB1 is present in COCs (our unpublished observations). Caution has been raised that the purity of all ligands has not been ensured. This is critical since any bacterial contaminant would be active.
Future direction or significance of the TLR system in reproduction
The potential role of TLRs in reproduction is just beginning to be recognized [13, 35]. Therefore, it will be important to analyze the reproductive phenotypes of mice that are null for various components of the TLR pathway. Importantly, these mice may reveal roles for components of this pathway in other non-immune related tissues. Most striking are the similarities between pre-adipocytes and granulosa/cumulus cells [13, 20, 21]. For example, each cell type expresses a battery of TLRs (TLR2, 4, 8 and 9), the adaptor factors CD14 and C1q, the transcription factor PPARγ and respond to LPS/Pam3Cys with increased production of Il6 mRNA. Moreover, cumulus cells express an adipocyte related adipokine, resistin, a marker of inflammatory conditions that can induce TLR2 (our unpublished observation). Even more provocative is the hypothesis that adipose-tissue mesenchymal stem cells as well as pre-adipocytes express TLRs and that “macrophages” may trans-differentiate from these cells [20, 21]. If this occurs in adipocyte cells, it is equally possible that granulosa cells and cumulus cells represent ovarian cells that can transdifferentiate, or are even derivatives of immune precursor stem cells. Would it not seem critical that the oocyte be “protected” by surveillance cells?
Whereas regulated synthesis and release of cytokines appears to impact events associated with ovulation, abnormal production of cytokines can lead to infertility. For example, LPS is present at high/detectible levels in follicular fluid of cattle with endometriosis compared to healthy cattle and follicles in the affected cattle exhibit reduced levels of estradiol and impaired development [14]. Systemic levels of IL6 are elevated in human patients with endometriosis and other chronic inflammatory related diseases and therefore may alter gene expression patterns in ovarian cells leading to infertility. Obesity may also increase chances of infertility because of altered TLR function in adipocytes that is associated with the release of immune cell-related factors, cytokines, FFA or other metabolic products, that impact the components of the reproductive system including the ovary [20]. Therefore, the role(s) of important cytokines and chemokines released from ovarian cells and other cells need to be more clearly defined in relation to inflammation and cancer.
Conclusion
Based on these observations and considerations, we propose that ovulation not only is similar to an inflammatory response but also involves the expression and function of molecules that exert potent roles in innate immune responses. In this way granulosa cells and more specifically cumulus cells appear to play immuno-protective-like functions for the ovulated oocyte. Not only do cumulus cells synthesize a HA rich matrix that serves as a physical protective barrier around the oocyte, these cells also have the potential to respond to external cues and release potent cytokines and chemokines. There remains much to be learned about the TLR pathway in the ovary and the ligands that activate these receptors and therefore novel information should emerge as more studies are done.
Acknowledgements
Supported in part by NIH-HD-16229 and HD-07495 (SCCPIR)(ZL, JSR) and Grant-in-Aid for Scientific Research (18688016) from the Japan Society for the Promotion of Science (JSPS) (MS)
References
- 1.Espey LL, Richards JS. Knobil and Neill’s physiology of reproduction. In: Neill JD, editor. Ovulation, 3rd ed. San Diego, CA: Academic; 2006. p. 425–74.
- 2.Russell DL, Robker RL. Molecular mechanisms of ovulation: co-ordination through the cumulus complex. Hum Reprod Updat. 2007;13:289–312. [DOI] [PubMed]
- 3.Richards JS. Genetics of ovulation. Semin Reprod Med. 2006;25:235–42. [DOI] [PubMed]
- 4.Conti M, Hsieh M, Park J-Y, Su Y-Q. Role of the EGF network in ovarian follicles. Mol Endocrinol. 2005;20:715–23. [DOI] [PubMed]
- 5.Park J-Y, Su Y-Q, Ariga M, Law E, Jin S-LC, Conti M. EGF-like growth factors as mediators of LH action in the ovulatory follicle. Science 2004;290:395–8. [DOI] [PubMed]
- 6.Hsieh M, Lee D, Panigone S, Horner K, Chen R, Theologis A, Lee D, Threadgill WD, Conti M. Luteinizing Hormone-dependent activation of the epidermal growth factor network is essential for ovulation. Mol Cell Biol. 2007;27:1914–1924. [DOI] [PMC free article] [PubMed]
- 7.Shimada M, Gonzalez-Robayna I, Hernandez-Gonzalez I, Richards JS. Paracrine and autocrine regulation of EGF-like factors in cumulus oocyte complexes and granulosa cells: key role for prostaglandin synthase 2 (Ptgs2) and progesterone receptor (Pgr). Mol Endocrinol. 2006;20:348–64. [DOI] [PubMed]
- 8.Lydon JP, DeMayo F, Funk CR, Mani SK, Hughes AR, Montgomery CA, et al. Mice lacking progesterone receptor exhibit reproductive abnormalities. Genes Dev. 1995;9:2266–78. [DOI] [PubMed]
- 9.Robker RL, Russell DL, Espey LL, Lydon JP, O’Malley BW, Richards JS. Progesterone-regulated genes in the ovulation process: ADAMTS-1 and cathepsin L proteases. Proc Natl Acad Sci USA. 2000;97:4689–94. [DOI] [PMC free article] [PubMed]
- 10.Kim J, Sato M, Li Q, Lydon JP, DeMayo FJ, Bagchi IC, et al. Peroxisome proliferator-activated receptor gamma is a target of progesterone receptor regulation in preovulatory follicles and controls ovulation in mice. Mol Cell Biol. 2008;28:1770–82. [DOI] [PMC free article] [PubMed]
- 11.Sterneck E, Tassarollo L, Johnson PF. An essential role for C/EBPb in female reproduction. Genes Dev. 1997;11:2153–62. [DOI] [PMC free article] [PubMed]
- 12.Hernandez-Gonzalez I, Gonzalez-Robayna IJ, Shimada M, Wayne CM, Ochsner SA, White L, et al. Gene expression profiles of cumulus cell oocyte complexes (COCs) during ovulation reveal cumulus cells express neuronal and immune-related genes: does this expand their role in the ovulation process. Mol Endocrinol. 2006;20:1300–21. [DOI] [PubMed]
- 13.Shimada M, Hernandez-Gonzalez I, Gonzalez-Robanya I, Richards JS. Induced expression of pattern recognition receptors (PRRs) in cumulus oocyte complexes (COCs): novel evidence for innate immune-like cells functions during ovulation. Mol Endocrinol. 2006;20:3228–39. [DOI] [PubMed]
- 14.Herath S, Williams EJ, Lilly ST, Gilbert RO, Dodson H, Bryant CE, et al. Ovarian follicular cells have innate immune capabilities that modulate their endocrine functions. Reproduction 2007;134:683–93. [DOI] [PMC free article] [PubMed]
- 15.Shimada M, Yanai Y, Okazaki T, Noma N, Kawashima I, Mori T, et al. Activation of TLR2 and TLR4 in cumulus cells of ovulated COCs stimulates production of cytokines/chemokines that induce sperm capacitation and enhance fertilization. Development. 2008; in revision. [DOI] [PubMed]
- 16.Akira S, Tadeka K. Toll-like receptor signalling. Nat Rev Immunol. 2004;4:499–511. [DOI] [PubMed]
- 17.Kawai T, Akira S. TLR signaling. Semin Immunol. 2007;19:24–32. [DOI] [PubMed]
- 18.Elward K, Gasque P. “Eat me” and “don’t eat me” signals govern the innate immune response and tissue repair in the CNS: emphasis on the critical role of the complement system. Mol Immunol. 2003;40:85–94. [DOI] [PubMed]
- 19.Jiang D, Liang J, Li Y, Noble PW. The role of Toll-like receptors in non-infectious lung injury. Cell Res. 2006;16:693–701. [DOI] [PubMed]
- 20.Pevsner-Fischer M, Morad V, Cohen-Sfady M, Rousso-Noori L, Zanin-Zhorov A, Cohen S, et al. Toll-like receptors and their ligands control mesenchymal stem cell functions. Blood 2008;109:1422–32. [DOI] [PubMed]
- 21.Schaffler A, Scholmerich J, Salzberger B. Adipose tissue as an immunological organ: Toll-like receptors, C1q/TNFs and CTRPs. Trends in Immunol., 2007;28:393–99. [DOI] [PubMed]
- 22.Bes-Houtmann S, Roche R, Hoareau L, Gonthier MP, Festy F, Caillens H, et al. Presence of functional TLR2 and TLR4 on human adipocytes. Histochem Cell Biol. 2007;127:1310137. [DOI] [PubMed]
- 23.Cho HH, Bae YC, Jung JS. Role of toll-like receptors on human adipose-derived stromal cells. Stem Cells. 2006;24:2744–52. [DOI] [PubMed]
- 24.Subedi K, Isobe N, Nishibori M, Yoshimura Y. Changes in the expression of Toll-like receptor mRNAs during follicular growth and in response to lipopolysaccharide in ovarian follicles of laying hens. J Reprod Dev. 2008;53:1227–35. [DOI] [PubMed]
- 25.Alvarez B. Molecular cloning, characterization and tissue expression of porcine Toll-like receptor 4. Dev Comp Immunol. 2006;30:345–55. [DOI] [PubMed]
- 26.Chaudhary PM, Ferguson C, Nguven V, Nguven O, Massa HF, Ebv M, et al. Cloning and characterization of two Toll/Interleukin-1 receptor-like genes TIL3 and Til4: evidence for a multi-gene family in humans. Blood 1998;91:4020–7. [PubMed]
- 27.Rodriguez-Martinez S, Cancino-Diaz ME, Jimenez-Zamudio L, Garcia-Latorre E, Cancino-Diaz JC. TLRs and NODs mRNA expression pattern in healthy mouse eye. Br J Ophthalmol. 2005;89:904–10. [DOI] [PMC free article] [PubMed]
- 28.Kambris Z, Hoffmann JA, Imler JL, Capovilla M. Tissue and stage specific expression of the Tolls in Drosophila embryos. Gene Expr Patterns. 2002;2:311–7. [DOI] [PubMed]
- 29.Kleve CD, Siler DA, Sved SK, Eldon ED. Expression of 18-wheeler in the follicle cell epithelium affects cell migration and egg morphology in Drosophila. Dev Dyn. 2006;235:1953–61. [DOI] [PubMed]
- 30.Liu Z, Richards JS. IL6: a potent regulator of mouse cumulus cell-oocyte complex expansion process. Biol Reprod. 2008; SSR Meeting Kona Hawaii, May 25–28.
- 31.Wong AST, Leung PCK. Role of endocrine and growth factors on the ovarian surface epithelium. J Obstet Gynaecol Res. 2007;33:3–16. [DOI] [PubMed]
- 32.Salustri A, Garlanda C, Hirsch E, DeAcetis M, Maccagno A, Bottazzi B, et al. PTX3 plays a key role in the organization of the cumulus oophorus extracellular matrix and in in vivo fertilization. Development 2004;131:1577–86. [DOI] [PubMed]
- 33.Varani S, Elvin JA, Yan C, DeMayo J, DeMayo FJ, Horton HF, et al. Knockout of pentraxin 3, a downstream target of growth differentiation factor-9, causes female subfertility. Mol Endocrinol. 2002;16:1154–67. [DOI] [PubMed]
- 34.Soboll G, Shen L, Wira CR. Expression of Toll-like receptors (TLR) and responsiveness to TLR agonists by polarized mouse uterine epithelial cells in culture. Biol Reprod. 2006;75:131–9. [DOI] [PubMed]
- 35.Girling JE, Hedger MP. Toll-like receptors in the gonads and reproductive tract: emerging roles in reproductive physiology and pathology. Immunol Cell Biol. 2007;85:481–9. [DOI] [PubMed]
- 36.Stern R, Asari AA, Sugahara KN. Hyaluronan fragments: an information-rich system. Eur J Cell Biol. 2006;85:699–715. [DOI] [PubMed]
- 37.Chang EJ, Kim HJ, Ha J, Kim HJ, Ryu J, Park KH, et al. Hyaluronan inhibits osteoclast differentiation via Toll-like receptor 4. J Cell Sci. 2007;120:166–76. [DOI] [PubMed]
- 38.Jiang D, Liang J, Fan J, Yu S, Chen S, Luo Y, et al. Regulation of lung injury and repair by Toll-like receptors and hyaluronan. Nat Med. 2005;11:1173–9. [DOI] [PubMed]
- 39.Richards JS, Liu Z, Shimada M. The role of immune like functions in ovulation. Trends Endocrinol Metab. 2008 (in press). [DOI] [PubMed]