Abstract
Purpose
To our knowledge, little is known about the effect of polarized and non-polarized uterine epithelial cells on cryopreserved embryo growth. This study was, therefore, set up to investigate the effect of these monolayers together with sequential culture media on vitrified-warmed mouse embryos in terms of blastocyst development, blastocyst quality, incidence of apoptosis and related genes expression.
Methods
Two cell vitrified-warmed mouse embryos were cultured in G-1™ver3 medium to the eight-cell stage when they were randomly assigned to three treatment groups of no co-culture (control), non-polarized and polarized mouse uterine epithelial monolayer co-culture. The culture medium was G-2™ver3 during the treatment phase. After 96 h on treatment, the significance of differences were evaluated by the one way analysis of variance for continuous data.
Results
In the polarized monolayer group, the hatched blastocyst formation and blastocyst quality improved significantly than other two groups (P < 0.05). Whereas the incidence of apoptosis and related gene expression such as Bax were higher in the blastocysts of control group (P < 0.05). The relative abundance of Bcl-2 mRNA to the β-tubulin was similar for all treatments.
Conclusion
Co-culture system involving polarized uterine epithelial cells and sequential culture media is a promising method for the improvement of vitrified-warmed mouse embryo development.
Keywords: Vitrification, Co-culture, Polarized uterine epithelial cells, Apoptosis, Mouse
Introduction
Since the first report of successful cryopreservation of mouse embryo [1], the cryopreservation of embryos from many mammalian species has been widely used in reproductive medicine, animal breeding and preservation of genetic variants. Despite many efforts, cryopreservation still causes drastic chromosomal, morphological and biochemical alterations including polyploidy formations [2], damages to membranes [3], cellular organelle dysfunctions [4, 5], cytoplasm disorganizations [6], and DNA fragmentation [7]. Such detrimental effects then may lead to the death of embryos by triggering the apoptotic cascade [7], which in turn may decrease developmental and implantational capabilities of embryos [8, 9].
Apoptosis is an endogenous cell degeneration mechanism necessary for normal development, but is more likely to occur during in vitro embryo culture and has received an increasing amount of attention because of its potential role in cellular response to suboptimal developmental conditions and stress (reviewed in [10]). As shown in previous studies, cryopreservation increases the number of apoptotic cells compared with that of fresh embryos [11]. It has been well known that the embryos can be severely altered or damaged by being cooled below physiological temperature, and DNA fragmentation is associated with cooling or cryo-sensivity [12]. In general, one central difficulty in successful cryopreservation lies in the production of high quality embryos without reducing in developmental competence. To resolve this problem, efforts are focused either to improve in cryopreservation methods or to in vitro culture conditions. The effect of improving in cryopreservation procedure on the rate of embryo development has been extensively studied [13–19]. Vitrification as a rapid protocol by increasing the cooling and warming rate and subsequence reducing in the formation of ice crystals and chilling injuries seems promising for solving some of the problems associated with cryopreservation of embryos. By improvements in the procedures and offspring born from embryos transferred following vitrification, it has been commonly used for mammalian embryos [20–22] and rarely used for human embryos [23]. Despite the fact that cryopreservation in general, and vitrification in particular, causes various types of injuries to embryos, attention needs to be paid to the improvement of subsequent culture conditions for embryos after cryopreservation as well improving cryopreservation protocols.
Several culture systems have been used for this purpose and most of them include somatic cells such as granolosa, oviduct, Vero and buffalo rat liver cells (BRL) as the co-culture support [24–26]. Fong et al. proposed the employment of a co-culture technique combined with sequential culture media and reported that Vero cells together with the sequential culture media for human embryos resulted in improved implantation rates [27]. Their results are confirmed by other investigations [28]. Recently, particular attention has been paid to the improved co-culture systems that could generate viable embryos by using polarized cells. Using techniques developed for cell polarization in vitro, it has been shown that polarized epithelial cells could improve in vitro embryo development [29]. The beneficial effects of in vitro cell polarization have been shown on uterine epithelial cells by analyzing the epithelial cell morphology and the secretion of proteins into the culture medium [30]. Based on these observations and our recent finding that co-culture with polarized and non-polarized uterine epithelial cells combined with sequential culture media enhances the in vitro development of the mouse embryos [31], it seems that improvement in the post warming culture conditions by using polarized and non-polarized uterine epithelial cells combined with sequential culture media can affect the embryo development and the quality of resulting blastocysts. The purpose of this study was to evaluate the developmental competence of vitrified-warmed mouse embryos in co-culture system using non-polarized or polarized uterine epithelial cells together with the sequential culture media with particular reference to apoptosis as a cause of the post-warm embryonic cell death.
Materials and methods
Epithelial cell co-culture
Uterine tissues were obtained from 4- to 6-week-old female NMRI mice following stimulation with standard hormonal treatment using an injection of 7.5 IU of pregnant mare serum gonadotropin (PMSG; Intervet, Holland), followed 48 h later by an injection of 7.5 IU of human chorionic gonadotropin (HCG; Organon, Holland). For each experiment, uterine horns from ten to 15 mice were removed (72 h after HCG administration) and placed into a petri dish containing sterile Hank’s balanced salt solution (HBSS; Gibco, USA). Having been washed with HBSS and having had their the remaining fatty and connective tissues trimmed off, the uteri were sliced longitudinally before being incubated in HBSS containing collagenase (type II, 0.25%; Sigma, USA) for 1 h at 37°C. Following incubation, the uterine tissues were carefully removed. The uterine epithelial cell preparation was enriched for epithelial cell clusters by sedimentation at unit gravity for 10–15 min. A series of gravity sedimentation steps was performed until few or no single cells were observed by microscopic examination. The epithelial cell clusters were seeded either on plastic dishes as non-polarized or on the apical compartment of a millicell culture plate insert with a membrane pore size of 0.4 μm (Millipore, USA) as a polarized culture. Prior to plating, pre-cooled filter inserts were coated with extra-cellular matrix extract (ECM gel; Sigma, USA), diluted 1:2 with a culture medium without any additions. The 0.1-ml ECM gel/insert was applied. The gelation process was completed after 1 h at 37°C. The epithelial cell clusters were seeded in a volume of 250 μl at a ratio of approximately three to four uterine horns per insert. The millicell inserts were placed in a 24-well tissue culture plate (Nunc, Denmark) containing 500 μl of the culture medium. The result was a dual-chambered system, which provided the medium with access to both sides of the membrane. The epithelial cell clusters were also cultured in the well of a 24-well tissue culture plate for non-polarized culture. The cultures were incubated at 37°C in 5% CO2 and 95% air atmosphere and reached confluence in 5–7 days. The medium was replaced every 2 days. The cultures were assessed under an inverted microscope (Nikon, Japan) and photographed for documentation during culture.
Evaluation of epithelial cells
Uterine epithelial cells were identified and visualized by cell-specific staining of cytoskeleton proteins. After 4 days in culture, the cells were washed with phosphate buffer saline (PBS) pH 7.4 for 10 min. The cells were fixed with 4% paraformaldehyde for 30 min before being permeabilized in PBS containing 0.5% Triton X-100 for 10 min. After 15 min of PBS washing, unspecific binding was blocked by 10% goat serum (Gibco, USA) in PBS for 1 h at room temperature. The cells were incubated for 1 h at 37°C with monoclonal antibody raised to cytokeratin (Chemicon, MAB1636) at a dilution of 1:10 in PBS containing 0.1% Triton X-100/0.1% bovine serum albumin (BSA; Sigma, USA). After having been briefly washed six times with PBS, the cells were incubated with fluorescein isothiocyanate (FITC)-labeled anti-mouse immunoglobulins (Sigma, USA) at a dilution of 1:00 in PBS containing 0.1% Triton X-100/0.1% BSA for 60 min at 37°C. The cells were once more washed briefly six times with PBS before being examined under an inverted fluorescence microscope (Nikon, TE 2000, Japan). The negative control was a monolayer undergoing the staining procedure without a primary antibody.
For transmission electron microscopy, the cultured epithelial cells were fixed with 2.5% glutaraldehyde in 0.1 M PBS buffer (pH 7.2–7.4) for 1 h at room temperature. Post-fixation was carried out in 1% osmium tetroxide in 0.1 M PBS buffer. The membranes supporting the ECM gel and cells were cut out with scalpels, and then the ECM gel and cells were embedded in Araldite (Sigma, USA). The detached monolayer from the plastic dishes was also embedded. For light microscopy, 1-μm-thick semi-thin sections were stained with 1% toluidine blue dye and photographed, while 50–70-nm-thick ultra-thin sections were stained with uranyl acetate and lead citrate for examination under an electron microscope (Zeiss, EM 900, Germany).
Preparation of pretreatment vitrification and dilution solution
Chemicals for pre-cooling, vitrification and dilution were obtained from Sigma Chemical Co.(St. Louis, MO). The solution for pretreatment, vitrification and dilution were prepared using T6 medium supplemented with 20% recombinant human albumin (rHA; Vitrolife, Sweden) as a holding medium. T6 medium composition was as follows: NaCl, 4,725; KCl, 110; NaH2PO4, 47; MgCl2, 47; CaCl2, 196; NaHCO3, 2,100; Na lactate, 2,610; Na pyrovate, 30; d-glucose, 1,000; penicillin G, 63 and streptomycin, 50 mg/l. The pretreatment solution (VS1) consisted of 1.5 mol/l ethylene glycol. The vitrification solution (VS2) consisted of 5.5 mol/l ethylene glycol and 1.0 mol/l sucrose. Whereas the solution for serial dilution contained 0.5, 0.25, 0.125 mol/l sucrose.
Embryo production
Six to 8-week-old female NMRI mice were superovulated by an injection of 7.5 IU of PMSG, followed 48 h later by an injection of 7.5 IU of HCG. They were subsequently caged individually with a 10-week-old male mouse; the presence of vaginal plug the following morning confirmed successful mating (day 1 of pregnancy). The plugged female mice were sacrificed by cervical dislocation on day 2 of pregnancy for recovery of two-cell embryos. The fallopian tubes were dissected and flushed with pre-warmed T6 medium.
Embryos vitrification and warming
A vitrification protocol was employed, based on the method originally designed for oocytes by Chen et al. using a closed system, called closed pulled straw (CPS) with a minor modifications [32]. The 0.25 ml straws used for the CPS system were obtained from I.V.M. L’Aigle, France. The end of a straw were heat-softened over a hot plate and pulled manually from near the cotton plug end, so that the inner diameter of tip was 0.8 mm with a wall thickness of 0.07 mm [33].
The vitrification procedure was carried out to use a loading temperature near to room temperature (25°C) and a two step loading process with cryoprotectant. Before vitrification embryos were placed in holding medium at room temperature for 5 min to cool down. The vitrification procedure was initiated in culture dishes (Nalge Nunc International, Rochester, NY, USA) prepared with three drop of 100 μl VS1, three drop of 100 μl VS2 and an additional 20 μl drop of VS2. The embryos were pretreated with VS1 for 5 min, after pre-equilibrating were transferred into VS2 and mixed for equlibration, and then transferred into the 20 μl drop of VS2. The tip of the pulled straw was loaded with 2 mm VS2 solution, 2 mm of air, and 2 mm of VS2 solution containing embryos, 2 mm of air, and 2 mm of VS2 solution. The straw was then placed into a prelabelled 5 ml cryovial (Nalge Nunc International, Rochester, NY, USA) held under liquid nitrogen. The total exposure to VS2 solution lasted for 1 min. After storage for a week, the straws were taken out of the liquid nitrogen. The cryoprotectants were removed by warming the embryos and diluting them using a three-step dilution with 500 μl of each dilution solutions. In brief, embryos were submerged in a solution of 0.5 mol/l sucrose. After 3 min, they were transferred into 0.25 mol/l sucrose for 3 min, and finally into 0.125 mol/l sucrose for 2 min. After warming, the recovered embryos were placed in 20 μl droplets of G-1™ver 3. (Vitrolife; Sweden) supplemented with 10% recombinant human albumin (rHA; Vitrolife, Sweden) under embryo tested mineral oil (Sigma; USA) and incubated at 37°C in 5% CO2/95% air atmosphere.
Survival assay
Survival of warmed embryos was determined based on visual examination of the integrity of the blastomere membrane and the normality of the cytoplasm one hour after warming. The validity of morphological classification was confirmed by vital staining with propodium iodide as already used by Cossarizza et al. [34]. To perform vital stain, samples of visually viable and dead embryos were mounted separately on microscopic slides and stained with propodium iodide (PI; Sigma, USA) at a final concentration of 20 μg/ml in PBS. The embryos were examined under a fluorescence microscope (Nikon, TE 2000, Japan). The remaining embryos were evaluated by morphological observation. Visually dead embryos were discarded, and the morphologically intact embryos were subject to culture.
Embryo co-culture
The survived embryos with normal morphology were then cultured in 20 μl droplets of G-1™ver 3. supplemented with 10% rHA under embryo tested mineral oil and incubated at 37°C in 5% CO2/95% air atmosphere. After 24 h in culture, the embryos in eight-cell stage were allocated into three in vitro culture treatments for further embryonic growth. The first treatment was embryo culture in 500 μl G-2™ver 3. (Vitrolife; Sweden) supplemented with 10% rHA in 24-well plastic dishes. The second treatment was embryo culture in 500 μl G-2™ver 3. with non-polarized uterine epithelial cell monolayer, which were only 60% confluent (3–4 days after seeding), so as to provide space for the growth of cells throughout the co-culture period. The same protocol was used for embryo co-culture with polarized uterine epithelial cell monolayer as the third treatment, the cells were cultured on an ECM-gel-coated filter insert except that the culture medium volume was 250 and 500 μl in the inner and outer chambers, respectively, and that the cells were used on day 7 after seeding so that they would reach cell polarity.
Embryo monitoring
The embryos were monitored daily under an inverted microscope, and the embryo developmental rate was recorded. At the end of culture period, the expanded blastocysts with a clear inner cell mass (ICM) were randomly selected by another observer and made ready for size measurement, differential staining, TUNEL labeling and RT-PCR analysis.
Blastocyst measurements
Blastocyst quantitative measurements of morphological features were performed according to the method described previously for human embryos [35]. They were taken with the use of the measurement program for the laser hatching system (Zilos-tk, Hamilton, USA). Blastocyst diameter (from outer zona to outer zona) was recorded, along with the longest length and widest perpendicular width (in micrometers). In addition, the same measurement for each ICM was calculated. A single size measurement for each blastocyst and ICM was calculated by using Factor Analysis. Blastocyst and ICM shapes were quantified by calculating a roundness index (RI; RI = length divided by width).
Blastocyst differential staining
Differential staining of the trophectoderm (TE) and ICM was performed according to the method described previously [36]. The zona-intact blastocysts were first incubated in 500 μl of 100 μg/ml propidium iodide (PI; Sigma, USA) with 1% triton X-100 in serum free T6 medium for up to 10 s or until trophectoderm visibly changed color to red and shrank slightly under a dissecting microscope. The blastocysts were then immediately transferred into 500 μl of 25 μg/ml bisbenzamide (Hoechst 33258; Sigma, USA) in 100% ethanol at 4°C overnight. Fixed and stained blastocysts were subsequently mounted in glycerol and observed under a microscope with an ultraviolet lamp (Nikon, Japan) and excitation filters (460 nm for blue and red fluorescence, and 560 nm for red only).
Blastocyst TUNEL and propidium iodide labeling
The TUNEL procedure was used to detect DNA fragmentation in combination with PI counterstaining in order to assess nuclear morphology. Nuclear DNA fragmentation in embryos was detected by the TUNEL method using an In Situ Cell Death Detection Kit (Roche Diagnostics Corporation, Hvidver, Denmark) in the same manner as described previously with some modifications [37]. The embryos were removed from the culture medium and washed four times in PBS (pH 7.4) containing 3 mg/ml polyvinylpyrolidone (PVP; Sigma, USA), the embryos being transferred from drop to drop. The zona pellucida-intact embryos were fixed in 4% paraformaldehyde in PBS (pH 7.4) for 30 min at room temperature, washed twice in PBS–PVP and permeabilized in 0.1% Triton X-100 for 15 min. The embryos were washed in PBS–PVP and incubated with 50 μl of TUNEL reaction mixture (containing fluorescein isothiocyanate-conjugated dUTP) and the enzyme terminal deoxynucleotidyl transferase (as prepared by the manufacturer) for 1 h at 37°C in the dark. The negative controls were incubated in the absence of terminal deoxynucleotidyl transferase. After that, the embryos were incubated with 50 μg/ml of PI for 30 min at room temperature. The embryos were washed four times in PBS–PVP to remove excess propidium iodide, and then they were mounted in glycerol onto a slide under a cover slip. Next, the embryos were observed under a fluorescent microscope (Nikon TE 2000, Japan). DNA fragmentation was assessed by the observation of a distinct TUNEL reaction of chromatin (bright yellow staining). Nuclear morphology was assessed on the basis of PI staining. The nuclei were classified according to four clear types of morphology: (1) healthy interphase nuclei with uniform PI staining and a clear outline; (2) mitosis, which included cells at the prophase, metaphase or anaphase stages with visible chromosomes counted as single nuclei; (3) fragmented nuclei, which included discrete clusters of membrane-bounded vesicles; and (4) condensed nuclei with intense PI staining, which were smaller than ‘healthy’ interphase nuclei. According to the above criteria, the nuclei displaying morphological characteristics of apoptosis (condensation and fragmentation) and biochemical characteristics of apoptosis (TUNEL reaction positive) were considered to be apoptotic nuclei. The apoptotic index of the embryos was calculated as the percentage of the apoptotic cells relative to the total number of the cells. The percentage of blastocysts having at least one apoptotic nucleus (apoptotic blastocysts) was calculated as the number of blastocysts with apoptotic nuclei relative to the total number of blastocysts.
RT-PCR analysis
The RT-PCR analysis was utilized to assess the presence of Bax and Bcl-2 mRNAs in early in vitro produced embryos at the blastocyst stage. The method was performed according to the RT-PCR for oocytes and embryos as previously described [38] with some modifications. For each group, 30 embryos were divided over three replicates before being analyzed. After zona pellucida removal with acid Tyrode’s (pH 2.3–2.4), the embryos were washed in sterile PBS and lysed in 5 μl lysis buffer [0.5% Nonidet P-40, 10 mM Tris (pH 8.0), 10 mM NaCl and 3 mM MgCl2] in an Ependorf tube on ice. If reverse transcription was not carried out immediately, the lysates were snap frozen in liquid nitrogen and stored at −70°C for a maximum of 2 weeks. Reverse transcription was performed using oligo (dt) primers and reverstranscriptase (k-1622; Fermentas). The lysate was made up to 20 μl with 2 μl of a 10 mM dNTP mix, 4 μl of 5× buffer reaction, 1 μl of ribonuclease inhibitor with and without (negative control) 1 μl (200 U/ml) Moloney murine leukemia virus (M-MLV) reverse transcriptase and 1 μl (0.5 μg/ml) oligo (dT) primer before incubation at 37°C for 1 h. The reaction was terminated by heating the tubes at 77°C for 10 min before they were immediately placed on ice. All the tubes were used for PCR or stored at −20°C until further use. Oligonucleotide primer pairs were used for the amplification of Bax and Bcl-2 mRNA, based on the mouse Bax and Bcl-2 cDNA sequence as described in Gene Bank accession numbers NM-177410 and NM-007527, respectively. Oligonucleotide primer pairs are summarized in Table 1. In all the experiments, β-tubulin was used as an internal control. After reverse transcription, 5 μl of reverse transcribed cDNA product was made up to 25 μl with 2.5 μl of 10 × PCR buffer, 0.5 μl of 10 mM dNTP mix, 0.75 μl of 50 mM MgCl2 solution, 0.5 μl Taq polymerase (Fermentas) and 1 μl of each primer. PCR conditions were denaturation at 93°C for 45 s, annealing at 55°C for 30 s, extension at 72°C for 45 s and a final extension of 10 min. Ten microlitres of each amplification product was separated on 1.5% (w/v) agarose gel, stained with 1 μg/ml ethidium bromide (Sigma, USA) and photographed under UV light. The intensity of each band was assessed by densitometry using an image analysis program (Total Lab; Version10, UK). The relative amount of each mRNA species was calculated by dividing the intensity of the bands by the intensity of the corresponding β-tubulin.
Table 1.
Primers designed for RT-PCR of apoptotic genes and β-tubulin in mouse embryos
| Gene | Primer sequences | RT-PCR product size (bp) |
|---|---|---|
| Bcl-2 | Forward: TAC CGT CGT GAC TTC GCA GAG | 240 |
| Reverse: GGT GTG CAG ATG CCG GTT CA | ||
| Bax | Forward: CGG CGA ATT GGA GAT GAA CTG | 160 |
| Reverse: GCA AAG TAG AAG AGG GCA ACC | ||
| β-tubulin | Forward: GGA ACA TAG CCG TAA ACT GC | 317 |
| Reverse: TCA CTG TGC CTG AAC TTA CC |
Experimental design
To investigate whether non-polarized or polarized uterine epithelial cells using sequential culture media are effective on in vitro development of vitrified-warmed mouse embryos, embryos were cultured as described above. The treatment in which embryos were cultured in media alone was the control, those co-cultured with non-polarized cells was designated as treatment 1 and those co-cultured with polarized cells was designated as treatment 2. Consequently, the three treatments are referred to as control (cultured in media alone), non-polarized and polarized co-culture. In each replication, a total of 20–25 embryos were randomly allocated to each treatment and treatments were repeated seven to nine times for the developmental rate evaluation. Three to four replications were also considered for blastocyst quality (blastocyst diameter and cell number), apoptosis, Bcl-2 and Bax gene expression analysis.
Statistical analysis
The developmental rates to the blastocyst stage of treated embryos were submitted to a one-way analysis of variance (ANOVA). Data were transformed by square root transformation. After testing for normality (Kolmogorov–Smirnov test) statistical analysis of embryo development was carried out on transformed data. When a significant level (P < 0.05) was observed, post hoc LSD test was used to compare treatments. The means of blastocyst and ICM diameter, blastocyst cell count, values for apoptotic blastocyst and apoptotic index and relative amount of Bcl-2 and Bax mRNAs after testing for normality were submitted to a one-way ANOVA. When a significant level (p < 0.05) was observed, post-hoc Tukey’s HDS test was used to compare pairwise means. All the analysis was carried out by using SPSS (software program, Version 13, SPSS.ink, Chicago, USA).
Results
Characteristics of epithelial cells
Comparisons of growth properties of uterine epithelial cells cultured on the ECM gel and plastic surface indicated that the epithelial cells cultured on ECM gel formed small cell aggregates that attached to the ECM gel readily 1 day after culture. They packed clearly together and formed tight complex three-dimensional structures that extended into different planes of the ECM gel and reached confluency 7 days after culture. The cells cultured on plastic adhered to the plastic surface and began to form a complete epithelioid monolayer. They had characteristic polygonal morphology similar to that observed in other cultured epithelial cells, and they reached confluency 5 days after culture. Most of the cultured uterine cells exhibited intense labeling when immunohistochemistry was used for epithelial cell markers in the cytoplasm.
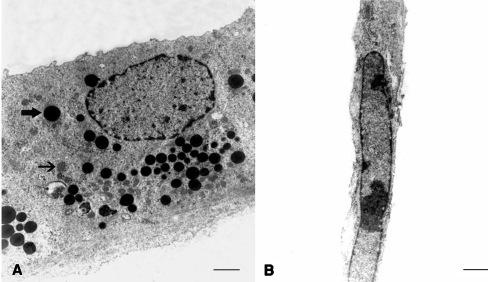
Ultrastructural analyses of cultures showed uterine epithelial cells cultured on ECM gel were well-polarized epithelial cells. The adjoining cells were connected by lateral junctions, and a basal-lamina-like structure was formed under the epithelia cells. Abundant electron dense and electron clear vacuoles were seen in the cytoplasm of the cells. Cellular organelles, such as mitochondria and prominent endoplasmic reticulum, were well presented (Fig. 1). The epithelial cells cultured on a plastic surface showed a few points of contact between the neighboring cells, with overlapping lateral cell borders. The cells contained a large nucleus with a few electron clear vesicles and mitochondria in the cytoplasm (Fig. 1).
Fig. 1.
Transmission electron micrograph of mouse uterine epithelial cells in vitro. A Polarized epithelial cells, culture on ECM gel; B non-polarized epithelial cells, culture on plastic surface; the accumulation of dense (bold arrow) and clear (thin arrow) cytoplasmic vacuoles in the polarized epithelial cells cultured on ECM gel. Bar: A 750 nm, B 1 μm
Embryo survival
One hour after warming 84% (154/183) of the embryos was judged by morphological examination to have survived the process of vitrification and warming. These results agree with the result obtained by membrane permeability assay showing 82% (152/183) viability rate.
Embryo development
The percentages of embryos cleaving to ten to 16-cell, morula and blastocyst stages in non-polarized and polarized cell co-culture as well as control are shown in Table 2. After 48 h of culture of all the embryos cultured as the control, 12.9% cleaved to the ten to 16-cell stage, 57.3% cleaved to the morula stage and 12.9% reached the blastocyst stage compared to 11.6%, 58.9% and 16.3%, and 9.6%, 65.5% and 22.1% in non-polarized and polarized cell co-culture, respectively (p < 0.05). The percentage of blastocysts formed was higher for the polarized co-culture treatment than for the control and non-polarized co-culture treatments. After 72 h of culture, the differences in the percentages of morulae were not significant. The percentage of non-hatching blastocysts was significantly lower in polarized cell co-culture compared to non-polarized cell co-culture and control (26.2% versus 48.8% and 43.5%% respectively; p < 0.05). However, blastocyst hatching was significantly higher in polarized cell co-culture compared to non-polarized cell co-culture and control (50.3% versus 19.4% and 11.7%, respectively; p < 0.05). After 96 h of culture, the percentage of hatched blastocysts was significantly higher in polarized cell co-culture compared to non-polarized cell co-culture and control (63.4% versus 42.7% and 39.5%, respectively; p < 0.05). At the end of the cultivation period, embryo degeneration was higher in control than non-polarized and polarized cell co-cultures (45.2%, 41.9%, and 29.0% respectively; p < 0.05).
Table 2.
Pre-implantation development of vitrified-warmed mouse embryos when cultured in sequential media alone or co-cultured with non-polarized or polarized mouse uterine epithelial cell monolayers
| 48 h | 72 h | 96 h | ||||||
|---|---|---|---|---|---|---|---|---|
| 10–16 cells | Morula | Blastocysts | Morula | Blastocysts | Hatching blastocysts | Hatched blastocysts | Degenerate embryos | |
| Control | 12.9a | 57.3a | 12.9a | 6.5a | 43.5a | 11.7a | 39.5a | 45.2a |
| Treatment 1 | 11.6a | 58.9a | 16.3a | 7.0a | 48.8a | 19.4a | 42.7a | 41.9a |
| Treatment 2 | 9.6a | 65.5b | 22.1b | 2.8a | 26.2b | 50.3b | 63.4b | 29.0b |
The culture time is the period from warming of two-cell stage vitrified embryos. Control: culture in sequential media alone (G-1™ ver3 followed by G-2™ ver3 media); treatment 1: co-culture in sequential media with non-polarized monolayer; treatment 2: co-culture in sequential media with polarized monolayer. The data are presented as the percentage and are the untransformed values but the statistical analyses were done on the transformed data. Values within columns with different superscripts are significantly different (ANOVA, P < 0.05).
Blastocyst diameter and cell count
In general, the mean diameter of blastocysts and ICMs as quantified by blastocyst and ICM single size were significantly higher in polarized and non-polarized cell co-cultures compared to control (3.1 ± 0.2, 2.8 ± 0.2 and 3.4 ± 0.3, 2.6 ± 0.3 versus 2.1 ± 0.3, 0.5 ± 0.1 respectively; p < 0.05). Blastocyst shape was similar for all treatments. However, the ICMs in control had slightly wider shape compared to non-polarized and polarized cell co-cultures as quantified by ICMs RI (2.6 ± 0.1 versus 2.1 ± 0.2 and 2.0 ± 0.1, respectively; P < 0.05; Table 3). Co-culture with polarized cells resulted in the production of blastocysts with significantly more cells compared to non-polarized cell co-culture and control (59 ± 2.8 versus 40.8 ± 2.7 and 34.2 ± 2.7 respectively; P < 0.05). The mean percentage of TE and ICM were similar for all treatments (Table 4).
Table 3.
Size of blastocyst and inner cell mass (ICM) in vitrified-warmed mouse embryos developed in sequential media alone or by co-culture with non-polarized or polarized mouse uterine epithelial cell monolayers
| Total blastocyst | Blastocyst diameter | ICM diameter | Blastocyst RI | ICM RI | |
|---|---|---|---|---|---|
| Control | 11 | 2.1 ± 0.3a | 0.5 ± 0.1a | 1.03 ± 0.07a | 2.6 ± 0.1a |
| Treatment 1 | 11 | 3.4 ± 0.3b | 2.6 ± 0.3b | 1.02 ± 0.03a | 2.1 ± 0.2b |
| Treatment 2 | 12 | 3.1 ± 0.2b | 2.8 ± 0.2b | 1.03 ± 0.09a | 2.0 ± 0.1b |
Control: culture in sequential media alone (G-1™ ver3 followed by G-2™ ver3 media); treatment 1: co-culture in sequential media with non-polarized monolayer; treatment 2: co-culture in sequential media with polarized monolayer. RI: roundness index = length divided by width. Data are means ± SEM. Values within columns with different superscripts are significantly different (ANOVA, P < 0.05).
Table 4.
Cell proliferation and differentiation in blastocysts drived from vitrified-warmed mouse embryos and developed in sequential media alone or by co-culture with non-polarized or polarized mouse uterine epithelial cell monolayers
| Total blastocyst | Total cell number | % ICM cell | % TE cell | |
|---|---|---|---|---|
| Control | 11 | 34.2 ± 2.7a | 15.1 ± 1.2a | 84.9 ± 1.2a |
| Treatment 1 | 11 | 40.8 ± 2.7a | 15.3 ± 0.5a | 84.7 ± 0.5a |
| Treatment 2 | 12 | 59 ± 2.8b | 16.1 ± 0.5a | 83.9 ± 0.5a |
Control: culture in sequential media alone (G-1™ ver3 followed by G-2™ ver3 media); treatment 1: co-culture in sequential media with non-polarized monolayer; treatment 2: co-culture in sequential media with polarized monolayer. Data are means ± SEM. Values within columns with different superscripts are significantly different (ANOVA, P < 0.05).
ICM inner cell mass, TE trophectoderm.
Apoptotic cell death in blastocysts
Cell death was seen in all treatments (Table 5). The proportion of blastocysts with apoptotic blastomere was higher for the control culture (100%) than for the non-polarized (76%) or polarized cell (75%) co-culture (P < 0.05). Moreover, the apoptotic index was significantly higher in control (9.2 ± 1.1) than in non-polarized (4.4 ± 0.8) or polarized cell co-culture (2.4 ± 0.4; P < 0.05).
Table 5.
Cell death in blastocysts drived from vitrified-warmed mouse embryos and developed in sequential media alone or by co-culture with non-polarized or polarized mouse uterine epithelial cell monolayers
| Total blastocyst | % Apoptotic blastocyst | Apoptotic index | |
|---|---|---|---|
| Control | 11 | 11/11 (100)a | 9.2 ± 1.1a |
| Treatment 1 | 17 | 13/17 (76)a | 4.4 ± 0.8b |
| Treatment 2 | 12 | 9/12 (75)a | 2.4 ± 0.4b |
Control: culture in sequential media alone (G-1™ ver3 followed by G-2™ ver3 media); treatment 1: co-culture in sequential media with non-polarized monolayer; treatment 2: co-culture in sequential media with polarized monolayer. Apoptotic index is the number of cells displaying both TUNEL and condensation of the nuclei as a proportion of the total number of cells in a blastocyst. Data are means ± SEM. Values within columns with different superscripts are significantly different (ANOVA, P < 0.05).
Expression of mRNA for selected apoptotic related genes: bax and Bcl-2
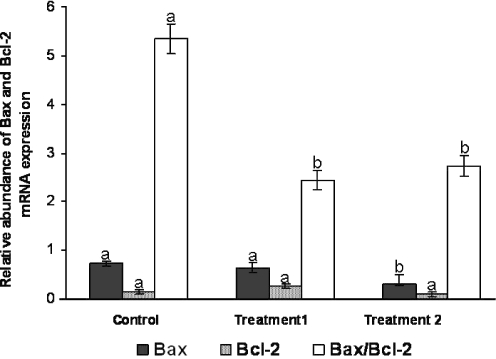
To investigate whether non-polarized or polarized uterine epithelial cells using sequential culture media modulate mRNA expression of apoptotic-related genes in developing in-vitro-derived blastocysts, we subjected the mRNA from the three treatments to semi-quantitative RT-PCR for Bcl-2 and Bax transcripts. Bax was expressed more strongly in the control and non-polarized cell co-culture than in the polarized cell co-culture (P < 0.05). Bcl-2 was significantly similar for all treatments (P < 0.05). In the co-culture treatments the value for the Bax/Bcl-2 mRNA expression was similar, but it was higher in control (P < 0.05; Fig. 2).
Fig. 2.
Relative abundance of Bax and Bcl-2 mRNA expression and Bax/Bcl-2 mRNA expression in blastocysts drived from vitrified-warmed mouse embryos and developed in sequential media alone or by co-culture with non-polarized or polarized mouse uterine epithelial cell monolayers. Control: culture in sequential media alone (G-1TM ver3 followed by G-2TM ver3 media); treatment 1: co-culture in sequential media with non-polarized monolayer; treatment 2: co-culture in sequential media with polarized monolayer. The relative abundance of each mRNA species is the ratio of the intensity of each band to the intensity of the corresponding β-tubulin. Columns within a variate with different letters are significantly different (ANOVA, P < 0.05)
Discussion
The use of cryopreservation provides a unique opportunity to study the early development of the post-thawed embryos in vitro as well as to save viable embryos for later use. A decrease in the cell viability after freezing and thawing has been largely attributed to physical or chemical damages induced during cryopreservation [39]. Various cryopreservation systems have been designed to overcome in vitro embryo losses by using different cryopreservation methods. Among these, vitrification as a simple cryopreservation method of directly submerged in liquid nitrogen after brief exposure to a cryoprotectant agent is substantially less harmful to cells that are conventional cryopreservation methods using traditional slow-freezing [9]. However, the direct and rapid contact with liquid nitrogen, coupled with as small volume of the vitrification solution as is practical, allows higher cooling rates to be achieved and has the positive effect to reduce the concentration of cryoprotectant and the exposure time. Thus, exposure to toxic effects of the cryoprotectant is also decreased [15, 18]. The most commonly employed carrier systems for vitrification including open pulled straw [33], electron microscope copper gride [40] nylon mesh [41] and cryoloop [16, 17], involve storing the embryos in liquid nitrogen, and have a potential risk of infection and movement of pathogens (cross-contamination) [42]. Recently, vitrification protocol based on a closed pulled straw (CPS) has been proven effective for eliminating the possibility of cross-contamination. The tip of a CPS is designed to have a small diameter and the thin wall holds the embryos in a very small volume of vitrification solution allowing very fast cooling and warming rates. CPS had the advantage of achieving a high survival and preserving good patterns of meiotic spindles in vitrified mouse oocytes [32]. Subsequently, this approach was used for embryo vitrification in order to maximize the post-thaw survival in embryos. The developmental capacity of embryos vitrified using CPS was improved compared with those vitrified using conventional and open pulled straws [43].
The present study has investigated the effects of a co-culture system, using polarized or non-polarized uterine epithelial cells together with sequential culture media, on in vitro embryo development in the mouse after vitrification with CPS. In addition, to gain a more complete picture of the consequences modifying in subsequent culture conditions into the functional aspects of blastocyst quality in the mouse, we combined the measurement of embryo developmental competence with such quantitative measures as blastocyst diameter, cell number, apoptotosis and relative mRNA abundance of apoptosis related genes.
From the results of survival analysis, 183 vitrified-warmed two cell mouse embryos were evaluated for their ability to survive the vitrification. The overall survival rate of vitrified-warmed embryos as examined in microscopy and confirmed by PI staining was greeter than 80%. Our results are, in general, in agreement with previous studies which reported 76% post-thaw survival rate on two-cell mouse embryos [9, 43]. Nevertheless, the effect of culture treatments on vitrified-warmed embryos was expressed during in vitro culture.
The results of this study demonstrated that blastulation rate was significantly higher in polarized cell co-culture than the non-polarized cell co-culture and the control. Therefore the polarized cells may have had more effective properties, which assisted the embryo to continue its development. The first studies with human uterine epithelial cells using Matrigel as extracellular matrix and in addition the dual-chambered system showed that the epithelial cells were grown on artificial basement membrane-coated porous filters developed morphological properties characteristics of mouse uterine epithelial cells [44]. The beneficial effects of in vitro cell polarization have also been shown on uterine epithelial cells by analyzing the epithelial cell morphology and the secretion of steroid and possibly additional factors present in the complete fetal bovine serum into the culture medium [30]. However, according to the claims of the putative benefits of the in vitro polarized uterine epithelial cells on uterine stromal cells in a co-culture system [45], it can be postulated that using in vitro-polarized epithelial cells may optimize embryo co-culture.
Our group in a previous study set up a co-culture system in which mouse embryos were cultured on polarized and non-polarized uterine epithelial cells together with sequential culture media. Using the culture protocol described in that study, the polarized cell supported the development of higher proportions of blastocysts and larger proportions of hatched blastocysts from the two-cell stage mouse embryos in comparison to the co-culture with non-polarized cells and culture without cell support [31]. It is demonstrated that autocrine or paracrine growth or survival factors released by the co-culture cells in the surrounding culture medium support embryos in their development [46]. The beneficial effects of cells growing on the ECM-gel-coated filter on embryo development as well as blastocyst hatching is unclear; it may be the result of a change in the type or concentration of embryotrophic factors or the secreted proteolytic enzymes that reportedly plays a role in zona thinning [47]. The polarized cells may have had more effective biophysical and biochemical properties, which assisted the embryo to continue its development.
The present data show that polarized and non-polarized cell co-cultures produced larger blastocysts that had slightly larger and rounder ICM. Richter et al. suggested that in the blastocysts with relatively round ICMs, blastomeres are nearer to each other and display better integrity and are more likely to achieve implantation [35]. This may be true for our embryos in the co-cultures. However, it would be necessary to carry out embryo transfer to recipients to determine whether the size and shape of the embryo affected implantation and the achievement of pregnancy. In addition, we observed that the co-culture increased the number of cells and also reduced the incidence of apoptosis compared to medium alone. The underlying mechanism for the increase in the blastomere count of embryos as well as that for the decrease in apoptosis after co-culture remains unclear. Cell number and apoptosis levels are useful indicators of embryo development and quality. Apoptosis is necessary for normal development [48] but it is more likely to occur during in vitro embryo culture [49], and in intact blastocysts, some cells spontaneously undergo apoptosis during embryonic development [50]. Recently, the number of apoptotic cells in the frozen embryos was shown to increase after thawing compared with that of fresh embryos [11]. Therefore, the damage in the cell organelles and genetic material of embryos resulting from the exposure to cryoprotectants may trigger the apoptotic cascade leading to a decrease in the developmental competence of embryos. Entry to and progression through the apoptotic pathway seem to be controlled by a balanced expression of several conserved genes that have either pro or anti-apoptotic effects. The Bcl-2 gene family is known to include anti-apoptotic and pro-apoptotic subgroups and Bcl-2 gene function to offer protection against apoptosis. In contrast, another group of highly conserved genes are positive regulators of apoptosis; these include Bax proteins in the mouse [51]. In the present study, we found that co-culture decreased Bax gene expression in the blastocysts developing in vitro. This observation may reflect the ability of somatic cells to reduce the apoptotic process in mouse blastocysts. Bcl-2 is a very potent cell-death suppressor in embryos that are destined to develop, and increases in the expression of cell-death-promoting genes would be inhibited by increasing the expression of protective genes (e.g. Bcl-2) in maternal stores [50]. The expression of pro-apoptotic gene Bax was higher in the blastocysts produced in the medium alone than that in those produced in the co-culture systems. This finding is consistent with the notion that mRNA levels for these transcripts are higher in suboptimal embryos.
In conclusion, our study provides the first evidence that co-culture with polarized and non-polarized uterine epithelial cells combined with sequential culture media enhances the in vitro development of the vitrified-warmed mouse embryo. Improving development by co-culture in the present experiment not only resulted in an increase in both blastocyst formation in polarized co-culture and blastocyst cell number as well as a decrease in apoptosis, but also resulted in the decreased expression of pro-apoptotic gene Bax. This co-culture model system may also provide a basis for future research into the improvement of subsequent culture conditions for embryos after cryopreservation. Moreover, it may facilitate investigations to improve the success of embryo cryopreservation programs.
Footnotes
Capsule Polarized uterine epithelial cell co-culture system can improve vitrified-warm mouse embryo development.
References
- 1.Whittingham DG, Leibo SP, Mazur P. Survival of mouse embryos frozen to −196°C and −269°C. Science. 1972;178:411–4. [DOI] [PubMed]
- 2.Balakier H, Cabaca O, Bouman D, Shewchuk AB, Laskin C, Squire JA. Spontaneous blastomere fusion after freezing and thawing of early human embryos leads to polyploidy and chromosomal mosaicism. Hum Reprod. 2000;15:2404–10. [DOI] [PubMed]
- 3.James PS, Wolfe CA, Mackie A, Ladha S, Prentice A, Jones R. Lipid dynamics in the plasma membrane of fresh and cryopreserved human spermatozoa. Hum Reprod. 1999;14:1827–32. [DOI] [PubMed]
- 4.Saunders KM, Parks JE. Effects of cryopreservation procedures on the cytology and fertilization rate of in vitro- matured bovine oocytes. Biol Reprod. 2000;61:178–87. [DOI] [PubMed]
- 5.Stojkovic M, Machado SA, Stojkovic P, Zakhartchenko V, Hutzler P, Goncalves PB, et al. Mitochondrial distribution and adenosine triphosphate content of bovine oocytes before and after in vitro maturation: correlation with morphological criteria and developmental capacity after in vitro fertilization and culture. Biol Reprod. 2000;64:904–9. [DOI] [PubMed]
- 6.Fuko E, Xia L, Downey BR. Ultrastructural changes in bovine oocytes cryopreserved by vitrification. Cryobiology. 1995;32:139–56. [DOI] [PubMed]
- 7.Ahn HJ, Sohn IP, Kwon HC, Jo DH, Park YD, Min CK. Characteristics of the cell membrane fluidity, actin fibers, and mitochondrial dysfunctions of frozen-thawed two-cell mouse embryos. Mol Reprod Dev. 2002;61:466–76. [DOI] [PubMed]
- 8.Chedid S, Van den Abbeel E, Van Steirteghem AC. Effects of cryopreservation on survival and development of interphase- and mitotic-stage 1-cell mouse embryos. Hum Reprod. 1992;7:1451–6. [DOI] [PubMed]
- 9.Uechi H, Tsutsumi O, Morita Y, Takai Y, Taketani Y. Comparison of the effects of controlled-rate cryopreservation and vitrification on 2-cell mouse embryos and their subsequent development. Hum Reprod 1999;14:2827–32. [DOI] [PubMed]
- 10.Betts DH, King WA. Genetic regulation of embryo death and senescence. Theriogenology. 2001;55:171–91. [DOI] [PubMed]
- 11.Marquez-Alvarado YC, Galina CS, Castilla B, Leon H, Moreno-Mendoza N. Evidence of damage in cryopreserved and fresh bovine embryos using the TUNEL technique. Reprod Domest Anim. 2004;39:141–5. [DOI] [PubMed]
- 12.Rajaei F, Karja NW, Agung B, Wongsrikeao P, Taniguchi M, Murakami M, et al. Analysis of DNA fragmentation of porcine embryos exposed to cryoprotectants. Reprod Domest Anim. 2005;40:429–32. [DOI] [PubMed]
- 13.Rall WF, Fahy GM. Ice-free cryopreservation of mouse embryos at −196°C by vitrification. Nature. 1985;313:573–5. [DOI] [PubMed]
- 14.Ali J, Shelton NJ. Design of vitrification solutions for the cryopreservation of embryos. J Reprod Fertil. 1993;99:471–7. [DOI] [PubMed]
- 15.Kassai M. Cryopreservation of mammalian embryos. Mol Biotechnol. 1997;7:173–9. [DOI] [PubMed]
- 16.Lane M, Schoolcraft WB, Gardner DK. Vitrification of mouse and human blastocysts using a novel cryoloop container-less technique. Fertil Steril. 1999;72:1073–8. [DOI] [PubMed]
- 17.Lane M, Bavister BD, Lyons EA, Forest KT. Containerless vitrification of mammalian oocytes and embryos. Nat Biotechnol. 1999;17:1234–6. [DOI] [PubMed]
- 18.Vajta G, Booth PJ, Holm P, Greve T, Callesen H. Successful vitrification of early stage bovine in vitro produced embryos with the Open Pulled Straw (OPS) method. CryoLetters. 1997;18:191–5.
- 19.Liebermann J, Tucker MJ. Effect of carrier system on the yield of human oocytes and embryos as assessed by survival and developmental potential after vitrification. Reproduction. 2002;124:483–9. [DOI] [PubMed]
- 20.Kito S, Noguchi Y, Ohta Y, Ohhata T, Abe M, Shiomi N, et al. Evaluation of developmental competence of vitrified-warmed early cleavage stage embryos and their application for chimeric mouse production. Exp Anim. 2003;52:179–83. [DOI] [PubMed]
- 21.Vajta G, Holm P, Greve T, Callesen H. Overall efficiency of in vitro embryo production and vitrification in cattle. Theroigenology. 1996;45:683–9. [DOI] [PubMed]
- 22.Vajta G, Holm P, Greve T, Callesen H. Factors affecting survival rates of in vitro produced bovine embryos after vitrification and direct in-straw rehydration. Anim Reprod Sci. 1996;45:191–200. [DOI] [PubMed]
- 23.Selman HA, El-Danasouri I. Pregnancies derived from vitrified human zygotes. Fertil Steril. 2002;77:422–3. [DOI] [PubMed]
- 24.Massip A, Mermillod P, Wils C, Dessy F. Effects of dilution procedure and culture conditions after thawing on survival of frozen bovine blastocysts produced in vitro. J Reprod Fertil. 1993;97:65–9. [DOI] [PubMed]
- 25.Kaidi S, Donnay I, Van Langendonckt A, Dessy F, Massip A. Comparison of two co-culture systems to assess the survival of in vitro produced bovine blastocysts after vitrification. Animal Reprod Sci. 1998;52:39–50. [DOI] [PubMed]
- 26.Valojerdi MR, Movahedin M, Hosseini A. Improvement of development of vitrified two-cell mouse embryo by vero cell coculture. J Assist Reprod Genet. 2002;19:31–8. [DOI] [PMC free article] [PubMed]
- 27.Fong CY, Bongso A, Ng SC, Kumar J, Trounson A, Ratnam S. Blastocyst transfer after enzymatic treatment of the zona pellucida: improving in-vitro fertilization and understanding implantation. Hum Reprod. 1998;13:2926–32. [DOI] [PubMed]
- 28.Fong CY, Bongso A. Comparison of human blastulation rates and total cell number in sequential culture media with and without co-culture. Hum Reprod. 1999;14:774–81. [DOI] [PubMed]
- 29.Baghban Eslami Nejad MR, Valojerdi MR, Kazemi Ashtiani S. A comparison of polarized and non-polarized human endometrial monolayer culture systems of murine embryo development. J Exp Clin Assist Reprod. 2005;2:7. [DOI] [PMC free article] [PubMed]
- 30.Bowen JA, Newton GR, Weise DW, Bazer FW, Burghardt RC. Characterization of polarized porcine uterine epithelial model system. Biol Reprod. 1996;55:613–9. [DOI] [PubMed]
- 31.Azadbakht M, Valojerdi MR, Mowla SJ. Development of mouse embryos co-cultured with polarized or non-polarized uterine epithelial cells using sequential culture media. Anim Reprod Sci. 2006;100:141–57. [DOI] [PubMed]
- 32.Chen SU, Lien YR, Cheng YY, Chen HF, Ho HN, Yang YS. Vitrification of mouse oocytes using closed pulled straws (CPS) achieves a high survival and preserves good patterns of meiotic spindles, compared with conventional straws, open pulled straws (OPS) and grids. Hum Reprod. 2001;16:2350–6. [DOI] [PubMed]
- 33.Vajta G, Holm P, Kuwayama M, Booth PJ, Jacobsen H, Greve T, et al. Open pulled straw (OPS) vitrification: A new way to reduce cryoinjuries of bovine oocytes and embryos. Mol Reprod Dev. 1998;51:53–8. [DOI] [PubMed]
- 34.Cossarizza A, Kalashnikova G, Grassilli E, Chiappelli F, Salvioli S, Capri M, et al. Mitochondrial modification during rat thymocyte apoptosis: a study at the single cell level. Exp Cell Res. 1994;214:323–30. [DOI] [PubMed]
- 35.Richter KS, Harris DC, Daneshmand ST, Shapiro BS. Quantitative grading of a human blastocyst: optimal inner cell mass size and shape. Fertil Steril. 2001;76:1157–67. [DOI] [PubMed]
- 36.Thouas GA, Korfiatis NA, French AJ, Jones GM, Trounson AO. Simplified technique for differential staining of inner cell mass and trophectoderm cells of mouse and bovine blastocyst. Reprod Biomed Online. 2001;3:25–9. [DOI] [PubMed]
- 37.Brison DR, Schultz RM. Increased incidence of apoptosis in transforming growth factor alpha-deficient mouse blastocysts. Biol Reprod. 1998;59:136–44. [DOI] [PubMed]
- 38.Gilliland G, Perrin S, Bun HF. PCR protocols: a guide to methods and applications. New York: Academic; 1990.
- 39.Ashwood-Smith MJ, Morris GW, Fowler R, Appleton TC, Ashoren R. Physical factors are involved in the destruction of embryos and oocytes during freezing and thawing procedure. Hum Reprod. 1988;3:795–802. [DOI] [PubMed]
- 40.Park SP, Kim EY, Oh JH, Nam HK, Lee KS, Park SY, et al. Ultra rapid freezing of human multipronuclear zygotes using electron microscope grids. Hum Reprod. 2000;15:1787–90. [DOI] [PubMed]
- 41.Matsumoto H, Jiang JY, Tanaka T, Sasada H, Sato E. Vitrification of large quantities of immature bovine oocytes using nylon mesh. Cryobiology. 2001;42:139–44. [DOI] [PubMed]
- 42.Leture-Konirsch H, Collin G, Sifer C, Devaux A, Kuttenn F, Madelenat P, et al. Safety of cryopreservation straws for human gametes and embryos: a study with human immunodeficiency virus-1 under cryopreservation conditions. Hum Reprod. 2003;18:140–4. [DOI] [PubMed]
- 43.Ramezani M, Valojerdi MR, Parivar K. Effect of three vitrification methods on development of two-cell mouse embryos. Cryoletters. 2005;26:85–92. [PubMed]
- 44.Classen-Linke I, Kusche M, Knauthe R, Beier HM. Establishment of a human endometrial cell culture system and characterization of its polarized hormone responsive epithelial cells. Cell Tissue Res. 1997;287:171–85. [DOI] [PubMed]
- 45.Wegner CC, Carson DD. Mouse uterine stromal cells secrete a 30-kilodalton protein in response to coculture with epithelial cells. Endocrinology. 1992;131:2565–72. [DOI] [PubMed]
- 46.Xu JS, Cheung TM, Chan ST, Ho PC, Yeung WS. Temporal effect of human oviductal cell and its derived embryotrophic factors on mouse embryo development. Biol Reprod. 2001;65:1481–8. [DOI] [PubMed]
- 47.Sawada H, Yamazaki K, Hoshi M. Trypsin-like hatching protease from mouse embryos: evidence for the presence in culture medium and its enzymatic properties. J Exp Zool. 1990;254:83–7. [DOI] [PubMed]
- 48.Wyllie AH. The genetic regulation of apoptosis. Curr Opin Genet Dev. 1995;5:97–104. [DOI] [PubMed]
- 49.Kamjoo M, Brison DR, Kimber SJ. Apoptosis in the preimplantation mouse embryo: effect of strain difference and in vitro culture. Mol Reprod Dev. 2002;61:67–77. [DOI] [PubMed]
- 50.Jurisicova A, Latham KE, Casper RF, Varmuza SL. Expression and regulation of genes associated with cell death during murine preimplantation embryo development. Mol Reprod Dev. 1998;51:243–53. [DOI] [PubMed]
- 51.Exley GE, Tang C, McElhinny AS, Warner CM. Expression of caspase and BCL-2 apoptotic family members in mouse preimplantation embryos. Biol Reprod. 1999;61:231–9. [DOI] [PubMed]