Abstract
Purpose
This study was to investigate the effect of sodium selenite (SS) on in vitro maturation of mouse preantral follicles.
Methods
The isolated preantral follicles were cultured in TCM 199 medium supplemented with different concentrations (0, 5, 10, 15 ng/ml) of SS and 3 mg/ml bovine serum albumin (BSA) or 5% Fetal Bovine Serum (FBS). The ovulation was induced by addition of 1.5 IU/ml human chorionic gonadotropin. The size and development of follicles and oocytes were assessed by calibrated eyepiece.
Results
The survival rates of follicles in FBS supplemented groups containing 5 and 10 ng/ml SS (88.23%, 90.83%) were higher than other groups (P < 0.05 and P < 0.001 respectively). The mean diameter of follicles (199.84 ± 15.58 μm) and the percentage of MII oocyte (33.08%) were higher in FBS supplemented group containing 10 ng/ml SS (P < 0.001).
Conclusion
The sodium selenite and FBS improve the in vitro growth and maturation of mouse preantral follicles.
Keywords: In vitro maturation, Oocyte, Preantral follicles, Sodium selenium, Serum free media
Introduction
Many systems have been developed for in vitro culture and maturation of preantral follicles to studying the oogenesis, folliculogenesis and oocytes–somatic cell interactions and it has shown that the isolated follicles were cultured and matured successfully [1–5].
The supplementations of the media with serum, growth factors, cytokines and hormones have improved the follicular maturation [6–11], e.g., previously Haidari et al. demonstrated that Leukemia inhibitory factor and co-culture system synergize to promote optimal follicular growth in the fresh and vitrified preantral follicles [9]. Negar et al. showed that epidermal growth factors increased the in vitro maturation (IVM) and fertilization of goat cumulus oocyte complexes [11].
During in vitro cell culture, the cells are maintained under higher concentrations of O2 than those that occur in vivo and the free radicals are produced continuously in aerobic cells. The high levels of free radicals cause cells damage and loss of their function [12]. Moreover, in vivo cells are protected by defense systems or antioxidants that remove these agents to prevent oxidative damage [13–17].
During in vitro culture and maturation of follicles, the development of follicles and subsequent embryo may be affected by the free radicals [18–20] and in this regard the investigators have been introducing the addition of appropriate antioxidant such as ascorbic acid and selenium [13–15, 21–24].
Selenium (Se) is an essential trace element that is necessary for the maintenance of various physiological processes [24]. In a cell culture system, selenium in the form of sodium selenite (SS) protects the cells from oxidative damage by reducing the production of free radical and inhibits lipid peroxidation [22–25].
Previous experiments showed that the presence of selenium as insulin-transferrin-selenium (ITS; at 5 nm/ml concentration) supplementation was support follicular growth and maturation of oocyte in vitro [26–28].
In this regard Jeong et al. 2007 evaluated the effects of ITS in defined supplemented IVM media on the developmental competence of porcine oocytes and they showed that the addition of ITS during IVM improved the developmental competence of porcine oocytes [26]. According to our knowledge there is poor attention to usage other dosage of selenium to improvement of follicular development in vitro.
Fetal calf serum (FCS), fetal bovine serum (FBS) and bovine serum albumin (BSA) are the most common protein supplements of IVM media, however the controversially reports were published about their usage in follicular maturation media in different species [28–31].
Wright et al. showed the follicles were cultured for 10 days with human serum albumin and ITS were significantly larger, more developed and showed significantly less atresia than those cultured with serum alone [28]. In contrast Leibfried-Ratlegge et al. reported that addition of FCS to the medium increased the maturation of hamster and cow oocyte than BSA [31].
Thus the purpose of this study was designed to evaluate the effects of different concentrations of sodium selenite on the development of preantral follicles in TCM-199 serum-free media and the other subject of the present study was the comparison between the BSA and FCS as different protein sources on the development of follicles in combination with SS.
Materials and methods
Chemicals
All the reagents were obtained from Sigma-Aldrich (Germany) unless otherwise specified.
Animals and collection of ovarian tissue
12–14 day-old female NMRI mice (n = 15) were cared for and used according to the guide for the care and use of laboratory animals of university and housed under a 12-h light/12-h dark regime at 22–24°C, and 55% humidity.
Mice were killed by cervical dislocation and their ovaries were dissected free of fat and mesentery and immediately transferred to dissection medium, consisting of HEPES-modified Tissue Culture Medium (TCM 199) supplemented with 5% FBS (Gibco, UK), 100 IU/ml penicillin and 100 mg/ml streptomycin.
Experimental design
After mechanical isolation of 120–150 μm preantral follicles from fresh mouse ovaries (see Follicle Isolation and Selection), they were divided into two main experimental groups: BSA and FBS supplemented cultured groups then subdivided into control and different concentrations (5, 10, 15 ng/ml) of sodium selenite treated groups [32].
Preantral follicle isolation
Preantral follicles from ovaries were isolated by mechanical dissection under a stereomicroscope, at ×10 magnification, using 29-gauge needles to ensure that follicular structure remained intact. Isolated follicles were selected according to the following criteria: (1) intact follicle with one or two layers of granulosa cells and some adhering theca cells; (2) visible, round and central oocyte and (3) follicle diameter between 120 and 150 μm. Then the isolated follicles were transferred to fresh culture medium.
In vitro culture of preantral follicles
Preantral follicles were cultured individually in a culture dish (35 mm Petri dishes) contain a 20 μl droplets of culture medium under detoxified mineral oil in a humidified atmosphere of 5% CO2 in air at 37°C for 12 days.
The culture medium consisted of TCM-199 supplemented with 0.33 mM sodium pyruvate, 100 mIU/ml recombinant follicle stimulating hormone (rFSH or Gonal-f; Serono, Switzerland), 1% insulin (Gibco, UK), transferrin (Gibco, UK), 100 μg/ml penicillin and 50 μg/ml streptomycin. In culture media of two main experimental groups different concentrations of sodium selenite (5, 10, 15 ng/ml) with 3 mg/ml BSA or 5% FBS were added. The TCM-199 culture media supplemented with BSA or FBS and without SS considered as control group.
Every 48 h of culturing, 10 μl of culture medium from each drop was replaced by fresh medium. Measurement of follicle and oocyte diameter was assessed using precalibrated ocular micrometer at ×100 magnification on day 2 and 4 of culturing.
The survival rate of the follicles was checked by evaluation of follicle morphology under inverted microscope.
In vitro ovulation induction
At day 12 of culture final oocyte maturation and ovulation was induced by addition of 1.5 IU/ml human chorionic gonadotropin (hCG; Organon) to the media. Released oocytes were scored as germinal vesicle (GV), germinal vesicle breakdown (GVBD) when the GV was absent and as metaphase II (MII) when the first polar body was extruded. The proportion of GV, GVBD and MII was assessed 48 h after hCG addition in all groups of study.
Statistical analysis
The survival, degeneration and developmental rates and the antral formation of follicles were assessed by Tukey, ANOVA and follicular diameter analyzed by Means, ANOVA. P < 0.05 was considered to be statistically significant.
Results
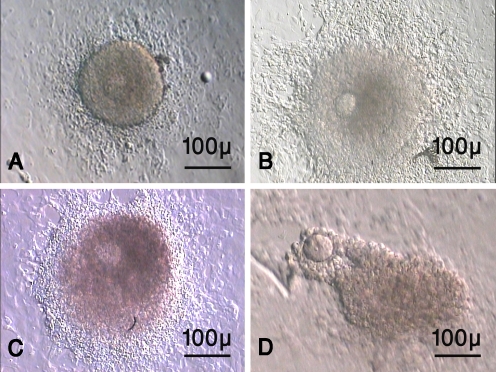
The morphology of the cultured isolated follicles was demonstrated on Fig. 1. The follicles were immobilized by growing and adhesion of granulosa and theca cells (Fig. 1a–b). The outgrowth of granulosa cells was irregular and also they had diffuse appearance. The antral cavities were seen as clear cavities within follicles (Fig. 1c). After hCG supplementation the release of oocyte was took place (Fig. 1d).
Fig. 1.
The morphology of mouse preantral follicles during in vitro maturation. The cultured isolated follicle on day 6 (a), day 8 (b) and day 10 of culturing (c). The oocyte ovulated of cultured preantral follicle 16 h after hCG administration (d). As the figure shows the granulosa and thecal cells outgrowth were prominent (Fig. 1a–b) and the antral cavities are seen as clear cavities (Fig. 1c)
The diameter of cultured isolated preantral follicles
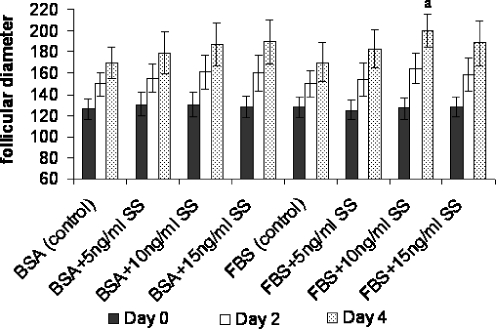
The diameter of preantral follicles in all groups of study was increased during in vitro culture and their data were summarized in Fig. 2. The diameter of cultured follicles in medium supplemented with different concentrations of SS (0, 5, 10, 15 ng/ml) and BSA were 169.33 ± 14.56, 179.18 ± 19.77, 187.07 ± 20.51, 189.22 ± 20.95 micrometer and in FBS supplemented groups were 170.16 ± 17.97, 182.79 ± 18.72, 199.84 ± 15.58, 188.23 ± 21.54 micrometer respectively. As results showed, the mean diameter of follicles which were cultured in FBS supplemented groups containing 10 ng/ml SS were significantly higher than other groups on day 4 of culturing (P < 0.001).
Fig. 2.
The follicular diameter (μm) of cultured preantral follicles in serum-free and serum supplemented media containing different concentrations of sodium selenite (ng/ml). a there was significant difference with other groups (P < 0.001). BSA Bovine serum albumin, FBS fetal bovine serum, SS sodium selenite. The day on the figure showed duration after culturing and the first day of culturing was considered as day 0
The diameter of oocyte
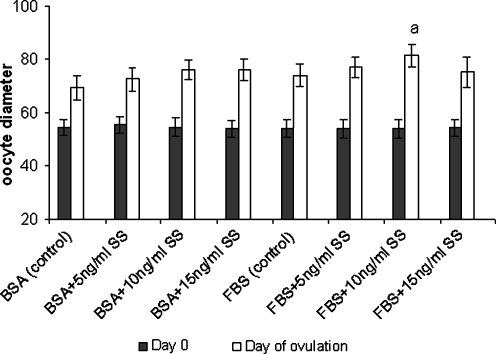
The diameter of oocyte in all groups of study was increased during in vitro culture and their data were summarized in Fig. 3. The diameter of ovulated oocyte derived from follicles that cultured in medium supplemented with different concentrations of SS (0, 5, 10, 15 ng/ml) and BSA were 69.30 ± 4.43, 72.45 ± 4.22, 75.95 ± 3.93, 76.05 ± 4.16 micrometer and in FBS supplemented groups were 74.0 ± 4.11, 77.07 ± 3.87, 81.32 ± 4.30, 76.13 ± 5.72 micrometer respectively. As results showed, the mean diameter of oocytes which were cultured in FBS supplemented groups containing 10 ng/ml SS were significantly higher than other groups (P < 0.01).
Fig. 3.
The oocyte diameter (μm) of cultured preantral follicles in serum-free and serum supplemented media containing different concentrations of sodium selenite (ng/ml). a there was significant difference with other groups (P < 0.01). BSA Bovine serum albumin, FBS fetal bovine serum, SS sodium selenite. Note: The day on the figure showed duration after culturing and the first day of culturing was considered as day 0
The survival rate of cultured follicles
By day 12 of culture, the survival rates of isolated follicles cultured in medium supplemented with different concentrations of SS (0, 5, 10, 15 ng/ml) and BSA were 69.37%, 73.74%, 74.10%, 76.45% and that of follicles cultured in FBS supplemented media were 76.53%, 88.23%, 90.83%, 78.93% respectively (Table 1). There were significant differences between the survival rates of follicles were cultured in FBS supplemented groups containing 5 and 10 ng/ml SS with other groups (P < 0.05 and P < 0.001 respectively).
Table 1.
The development of cultured preantral follicles in serum-free and serum supplemented media containing different concentrations of sodium selenite (ng/ml) after 12 days
| Groups | N. of follicles | Survived follicles N (%) | Degenerated follicles N (%) | Antrum formation N (%) | Developmental stage of oocyte | ||||
|---|---|---|---|---|---|---|---|---|---|
| GV | MI | MП | |||||||
| BSA | FBS | SS | N (%) | N (%) | N (%) | ||||
| + | − | 0 | 52 | 36 (69.37) | 16 (30.63) | 7 (19.38) | 18 (50) | 14 (38.75) | 4 (11.25) |
| + | − | 5 | 53 | 39 (73.45) | 14 (26.55) | 8 (21.88) | 19 (48.75) | 14 (36.24) | 6 (15.19) |
| + | − | 10 | 62 | 46 (74.10) | 16 (25.90) | 11 (23.89) | 21 (46) | 18 (38.83) | 7 (15.17) |
| + | − | 15 | 74 | 56 (76.45) | 18 (23.55) | 12 (20.78) | 23 (40.89) | 25 (44.36) | 8 (14.75) |
| − | + | 0 | 65 | 50 (76.53) | 15 (23.47) | 12 (24.35) | 24 (48.52) | 18 (35.47) | 8 (16.01) |
| − | + | 5 | 60 | 53 (88.23)* | 7 (11.77) | 16 (29.57) | 20 (37.99) | 18 (34.26) | 15 (27.75)* |
| − | + | 10 | 76 | 69 (90.83)** | 7 (9.17) | 21 (30.83) | 22 (31.79) | 24 (35.13) | 23 (33.08)** |
| − | + | 15 | 45 | 36 (78.93) | 9 (21.07) | 9 (27.08) | 16 (45.83) | 14 (37.50) | 6 (16.67) |
There was significant difference with other groups in the same column (*P < 0.05; **P < 0.001)
BSA Bovine serum albumin, FBS fetal bovine serum, SS sodium selenite, GV germinal vesicle, MI metaphase I, metaphase MП
Antrum formation
Antrum formation was observed in follicles from day 8. The rate of antrum formation in follicles cultured in BSA supplemented media containing 0, 5, 10, 15 ng/ml SS were 19.38%, 21.88%, 23.89%, 20.78% and that of follicles cultured in FBS supplemented media were 24.35%, 29.57%, 30.83%, 27.08% respectively. There was no significant difference between groups (Table 1).
Rate of released MII oocyte
The rate of released MII oocyte in follicles were cultured in medium supplemented with different concentrations of SS (0, 5, 10, 15 ng/ml) and BSA media were 11.25%, 15.19%, 15.17% and 14.75% and that of cultured in FBS supplemented media were 16.01%, 27.76%, 33.08% and 16.67% respectively (Table 1). There were significant differences between the developmental rates of 5 and 10 ng/ml SS treated follicles cultured in serum culture system with others in each group (5 ng/ml, P < 0.05 and 10 ng/ml, P < 0.001).
Discussions
Successful oocyte maturation, fertilization and embryonic development depend on the coordination between growth and development of the ovarian follicle. Follicular growth and differentiation is a process characterized by morphological and functional changes that influenced by intra- and extra-ovarian factors [33].
Follicular culture requires strictly defined culture media to sustain its viability and quality. Although several follicular culture media are now available, they do not provide optimal milieu and research focused on follicular culture improvement [6].
Our results showed that during in vitro maturation of isolated follicles, the size and diameter of follicles and oocyte was increased in all groups. However follicles and those oocytes cultured in fetal bovine serum supplemented media containing 10 ng/mL SS were shown larger diameter than other groups. On the other hand, the growth of the oocytes and follicle was coordinated during culturing period. Also the survival rate of isolated follicles cultured in fetal bovine serum supplemented culture media containing 5 and 10 ng/mL SS was higher than other groups.
Our suggestion is that the effects of SS on the growth and survival of follicles and oocytes is dose dependent and it acts as proliferative and antioxidant factor.
In agreement with our results Basini et al. demonstrated SS stimulates the proliferation of bovine granulosa cells from small follicles [34] and Cavilla et al. showed SS promoted human oocyte growth during in vitro maturation [35].
It was shown that SS prevents cell damage by reduce reactive oxygen species and inhibits lipid peroxidation [22, 23].
Saito et al. showed selenium deficiency caused a significant increase in reactive oxygen species and peroxidation inside the cells. It is considered that selenium-deficient conditions cause oxidative DNA damage that eventually leads to cancer formation [23].
Uhm SJ et al. demonstrated that supplementation of porcine embryos culture media with SS at a concentration of 25 ng/ml significantly increases their developmental ability and reduces their apoptosis in vitro [36].
The use of serum as a supplement in the IVM media for follicles seems to have controversial results because the substances of the sera varied from different sources and its component may have beneficial or harmful effects [28–31, 37]. However, the other subject of the present study was the comparison between the BSA and FCS on the development of mouse preantral follicles in the presence of SS. Our results demonstrated that SS at 5 and 10 ng/ml concentrations when combined with FBS in follicular culture media had a beneficial effect on the in vitro maturation of mouse ovarian follicle than BSA. We suggest that FBS may contains hormones, growth factors, vitamins, peptides, proteins, and an antioxidant which effective to improvement of follicular development than defined BSA and this effect seems to be better shown when FBS supplemented with SS.
In agreement with our results Gigli et al. showed different concentrations of FBS alone were much less effective at maintaining follicular health and supporting the initiation and progression of follicular growth than ITS supplemented condition [16]. Also it was shown that additions of selenium in the form of ITS stimulated chorionic gonadotropin secretion in human blastocyst and relieve some of the inhibitory effects of FBS on primary cell culture and improved the developmental competence of porcine oocytes [26, 36].
In contast Wright et al. showed follicles which cultured for 10 days with human serum albumin and ITS were significantly larger, more developed and showed significantly less atresia than those cultured with serum alone [28]. These results suggested that the effect of SS as a supplement in follicular in vitro maturation depends on culture media.
In conclusion, our results demonstrated that SS at concentrations of 10 ng/ ml in FBS supplemented media increases the maturation rate of mouse preantral follicles in vitro and additional analysis needs to understand its mechanism.
Acknowledgements
We thank Mr. Pour Beyranvand and Ms, Ebrahimi for providing excellent technical assistance. This work was supported by a grant from Tarbiat Modares University and Iran National Science Foundation.
Footnotes
Capsule Sodium selenite at concentrations of 10 ng/ ml in FBS supplemented media increases the in vitro maturation of mouse preantral ovarian follicle.
References
- 1.Bishonga C, Takahashi Y, Katagiri S, Nagano M, Ishikawa A. In vitro growth of mouse ovarian preantral follicles and the capacity of their oocytes to develop to blastocyst stage. J Vet Med Sci. 2001;63:619–624. doi:10.1292/jvms.63.619. [DOI] [PubMed]
- 2.Hasegawa A. In vitro growth and maturation of mouse oocyte-granulosa cell complex from cryopreserved ovaries and achievement of pup birth. Reprod Med Biol. 2007;6:77–83. doi:10.1111/j.1447-0578.2007.00169.x. [DOI] [PMC free article] [PubMed]
- 3.Haidari K, Salehnia M, Valoujerdi MR. The effect of leukemia inhibitory factor and co-culture on the in vitro maturation and ultrastructure of vitrified and non-vitrified isolated mouse preantral follicles. Fertil Steril. 2008; In press. [DOI] [PubMed]
- 4.Cortvrindt R, Smitz J, Van Steirteghem AC. In vitro maturation, fertilization and embryo development of immature oocytes from early preantral follicles from prepuberal mice in a simplified culture system. Hum Reprod. 1996;11:2656–66. [DOI] [PubMed]
- 5.Kreeger PK, Fernandes NN, Woodruff TK, Shea LD. Regulation of mouse follicle development by follicle-stimulating hormone in a three-dimensional in vitro culture system is dependent on follicle stage and dose. Biol Reprod. 2005;73:942–50. doi:10.1095/biolreprod.105.042390. [DOI] [PMC free article] [PubMed]
- 6.Demeestere I, Centner J, Gervy C, Englert Y, Delbaere A. Impact of various endocrine and paracrine factors on in vitro culture of preantral follicles in rodents. Reproduction. 2005;130:147–56. doi:10.1530/rep.1.00648. [DOI] [PubMed]
- 7.Adriaens I, Cortvrindt R, Smitz J. Differential FSH exposure in preantral follicle culture has marked effects on folliculogenesis and oocyte developmental competence. Hum Reprod. 2004;19:398–408. doi:10.1093/humrep/deh074. [DOI] [PubMed]
- 8.Wu j, Xu B, Wang W. Effects of luteinizing hormone and follicle stimulating hormone on the developmental competence of porcine preantral follicle oocytes grown in vitro. J Assist Reprod Genet. 2007;24:419–24. doi:10.1007/s10815-007-9154-5. [DOI] [PMC free article] [PubMed]
- 9.Haidari K, Salehnia M, Valoujerdi MR. The effects of different concentration of leukemia inhibitory factor on the development of isolated preantral follicle from fresh and vitrified mouse ovaries. Iran Biomed J. 2006;10:185–90.
- 10.Tareq K, Miah AG, Salma U, Yoshida M, Tsujii H. Effect of amino acids and dipeptides on accumulation of ammonia in the medium during in vitro maturation and fertilization of porcine oocytes. Reprod Med Biol. 2007;6:165–70. doi:10.1111/j.1447-0578.2007.00180.x. [DOI] [PMC free article] [PubMed]
- 11.Nagar D, Purohit GN. Effect of epidermal growth factor on maturation and fertilization in vitro of goat follicular oocytes in a serum free or serum supplemented medium. Veterinarski Arh. 2005;75:459–67.
- 12.Chwa M, Atilano SR, Reddy V, Jordan N, Kim DW, Kenney MC. Increased stress-induced generation of reactive oxygen species and apoptosis in human keratoconus fibroblasts. Invest Ophthalmol Vis Sci. 2006;47:1902–10. doi:10.1167/iovs.05-0828. [DOI] [PubMed]
- 13.Cassano E, Tosto L, Balestrieria M, Zicarelli L, Abrescia P. Antioxidant defense in the follicular fluid of water buffalo. Cell Physiol Biochem. 1999;9:106–16. doi:10.1159/000016307. [DOI] [PubMed]
- 14.Carbone MC, Tatone C, Delle Monache S, Marci R, Caserta D, Colonna R, et al. Antioxidant enzymatic defences in human follicular fluid: characterization and age-dependent changes. Mol Hum Reprod. 2003;9:639–43. doi:10.1093/molehr/gag090. [DOI] [PubMed]
- 15.Oyawoye O, Abdel Gadir A, Garner A, Constantinovici N, Perrett C, Hardiman P. Antioxidants and reactive oxygen species in follicular fluid of women undergoing IVF: relationship to outcome. Hum Reprod. 2003;18:2270–4. doi:10.1093/humrep/deg450. [DOI] [PubMed]
- 16.Gigli I, Byrd DD, Fortune JE. Effects of oxygen tension and supplements to the culture medium on activation and development of bovine follicles in vitro. Theriogenology. 2006;66:344–53. doi:10.1016/j.theriogenology.2005.11.021. [DOI] [PubMed]
- 17.Sugino N. Reactive oxygen species in ovarian physiology. Reprod Med Biol. 2005;4:31–44. doi:10.1111/j.1447-0578.2005.00086.x. [DOI] [PMC free article] [PubMed]
- 18.Tatemoto H, Sakurai N, Muto N. Protection of porcine oocytes against apoptotic cell death caused by oxidative stress during in vitro maturation: role of cumulus cells. Biol Reprod. 2000;63:805–10. doi:10.1095/biolreprod63.3.805. [DOI] [PubMed]
- 19.Tatemoto H, Ootaki K, Shigeta K, Muto N. Enhancement of developmental competence after in vitro fertilization of porcine oocytes by treatment with ascorbic acid 2-O-α-glucoside during in vitro maturation. Biol Reprod. 2001;65:1800–6. doi:10.1095/biolreprod65.6.1800. [DOI] [PubMed]
- 20.Jancar N, Kopitar AN, Ihan A, Klun IV, Bokal EV. Effect of apoptosis and reactive oxygen species production in human granulosa cells on oocyte fertilization and blastocyst development. J Assist Reprod Genet. 2007;24:91–7. doi:10.1007/s10815-006-9103-8. [DOI] [PMC free article] [PubMed]
- 21.Tatemoto H, Okuda T, Sogo N, Muto N. Male pronuclear formation and blastocyst formation are improved by supplementation of ascorbic acid 2-O-α-glucoside during in vitro maturation cultured of denuded porcine oocytes. J Reprod Dev. 2001;47:329–39. doi:10.1262/jrd.47.329. [DOI]
- 22.Ebert R, Ulmer M, Zeck S, Meissner-Weigl J, Schneider D, Stopper H, et al. Selenium supplementation restores the antioxidative capacity and prevents cell damage in bone marrow stromal cells in vitro. Stem Cells. 2006;24:1226–35. doi:10.1634/stemcells.2005-0117. [DOI] [PubMed]
- 23.Saito Y, Yoshida Y, Akazawa T, Takahashi K, Niki E. Cell death caused by selenium deficiency and protective effect of antioxidants. J Biol Chem. 2003;278:39428–34. doi:10.1074/jbc.M305542200. [DOI] [PubMed]
- 24.Zhang J, Robinson D, Salmon P. A novel function for selenium in biological system: selenite as a highly effective iron carrier for Chinese hamster ovary cell growth and monoclonal antibody production. Biotechnol Bioeng. 2006;95:1188–97. doi:10.1002/bit.21081. [DOI] [PubMed]
- 25.Tatemoto H, Muto N, Sunagawa I, Shinjo A, Nakada T. Protection of porcine oocytes against cell damage caused by oxidative stress during in vitro maturation: role of superoxide dismutase activity in porcine follicular fluid. Biol Reprod. 2004;71:1150–7. doi:10.1095/biolreprod.104.029264. [DOI] [PubMed]
- 26.Jeong YW, Hossein MS, Bhandari DP, Kim YW, Kim JH, Park SW, et al. Effects of insulin-transferrin-selenium in defined and porcine follicular fluid supplemented IVM media on porcine IVF and SCNT embryo production. Anim Reprod Sci. 2008;106:13–24. [DOI] [PubMed]
- 27.Raghu HM, Nandi S, Reddy SM. Effect of insulin, transferrin and selenium and epidermal growth factor on development of buffalo oocytes to the blastocyst stage in vitro in serum-free, semidefined media. Vet Rec. 2002;151:260–5. [DOI] [PubMed]
- 28.Wright CS, Wright CS, Hovatta O, Margara R, Trew G, Winston RM, et al. Effects of follicle-stimulating hormone and serum substitution on the in-vitro growth of human ovarian follicles. Hum Reprod. 1999;14:1555–62. doi:10.1093/humrep/14.6.1555. [DOI] [PubMed]
- 29.Marques MG, Nicacio AC, de Oliveira VP, Nascimento AB, Caetano HV, Mendes CM, et al. In vitro maturation of pig oocytes with different media, hormone and meiosis inhibitors. Anim Reprod Sci. 2007;97:375–81. doi:10.1016/j.anireprosci.2006.02.013. [DOI] [PubMed]
- 30.Funahashi H, Day BN. Effects of different serum supplements in maturation medium on meiotic and cytoplasmic maturation of pig oocytes. Theriogenology. 1993;39:965–73. doi:10.1016/0093-691X(93)90433-6. [DOI] [PubMed]
- 31.Leibfried-Rutledge ML, Critser ES, First NL. Effects of fetal calf serum and bovine serum albumin on in vitro maturation and fertilization of bovine and hamster cumulus-oocyte complexes. Biol Reprod. 1986;35:850–85. doi:10.1095/biolreprod35.4.850. [DOI] [PubMed]
- 32.Hill KE, Chittum HS, Lyons PR, Boeglin ME, Burk RF. Effect of selenium on selenoprotein P expression in cultured liver cells. Biochim Biophys Acta. 1996;1313:29–34. doi:10.1016/0167-4889(96)00047-X. [DOI] [PubMed]
- 33.Webb R, Garnsworthy PC, Campbell BK, Hunter MG. Intra-ovarian regulation of follicular development and oocyte competence in farm animals. Theriogenology. 2007;68:S22–9. doi:10.1016/j.theriogenology.2007.04.036. [DOI] [PubMed]
- 34.Basini G, Tamanini C. Selenium stimulates estradiol production in bovine granulosa cells: possible involvement of nitric oxide. Dome Anim Endo. 2000;18:1–17. doi:10.1016/S0739-7240(99)00059-4. [DOI] [PubMed]
- 35.Cavilla JL, Kennedy CR, Byskov AC, Hartshorne GM. Human immature oocytes grow during culture for IVM. Hum Reprod. 2008;23:37–45. doi:10.1093/humrep/dem178. [DOI] [PubMed]
- 36.Uhm SJ, Gupta MK, Yang JH, Lee SH, Lee HT. Selenium improves the developmental ability and reduces the apoptosis in porcine parthenotes. Mol Reprod Dev. 2007;74:1386–94. doi:10.1002/mrd.20701. [DOI] [PubMed]
- 37.Jiude M, Guangming W, Michael FS, Tod CM, Tom CC, Randall SP, et al. Effects of culture medium, serum type and various concentrations of follicle stimulating hormone on porcine preantral follicular development and antrum formation in vitro. Biol Reprod. 2002;67:1197–203. doi:10.1095/biolreprod67.4.1197. [DOI] [PubMed]