Abstract
Purpose
To describe the ultrastructure of spermatozoa from a patient with complete asthenozoospermia that resulted in live births following blastocyst culture.
Materials and methods
Analyses of spermatozoa from a 36 year old patient were performed using light and electron microscopy. The hypo-osmotic swelling test was used to select spermatozoa for intracytoplasmic sperm injection. Embryos were cultured to the blastocyst stage.
Results
100% of the spermatozoa had dynein arm deficiency with secondary defects varying from 3–17%. Six oocytes were injected; five fertilized normally and one was digynic. All five zygotes formed good quality blastocysts. Three blastocysts were cryopreserved and two blastocysts were transferred. Twin females were born at 37 weeks.
Conclusions
The hypo-osmotic swelling test can be used to select viable immotile ejaculated spermatozoa from a patient with dynein arm deficiency and can produce excellent fertilization rates and blastocyst development resulting in live births.
Keywords: Blastocyst culture, Dynein arm deficiency, Hypo-osmotic swelling test, Primarily ciliary dyskinesia, Sperm ultrastructure
Introduction
Primary ciliary dyskinesia (PCD), also known as immotile cilia syndrome (ICS), is identified by immotility of the ciliated cells in the body such as epithelial airway cells and spermatozoa. PCD is heterogeneous and has been shown to be inherited in an autosomal recessive pattern [1]. Individuals presenting with this disease will have a chronic cough and recurrent upper respiratory tract infections which will lead to bronchiectasis. Males will most often present with infertility or sub-fertility.
The severity of symptoms and the age at which the condition is diagnosed is quite variable, even though the symptoms are present from birth [2]. Approximately 50% of patients will fall within a subset of PCD known as Kartagener’s syndrome (KS). In addition to bronchiectasis and chronic sinusitis, these patients will also present with situs inverus; indicating the cilia of the patient was affected during embryonic development [3].
Successful treatment of PCD with the birth of healthy babies following embryo transfer on day three or earlier has been reported using sub-zonal insemination (SUZI) [4–6] and intracytoplasmic sperm injection (ICSI) [7–13]. The hypo-osmotic swelling (HOS) test [14] was used in conjunction with ICSI of immotile spermatozoa with success in several cases as well [15–17].
We believe this is the first report of a viable twin pregnancy for a couple where the husband had PCD and the embryos were grown to the blastocyst stage prior to embryo transfer. Viable ejaculated spermatozoa were selected using the HOS test prior to ICSI. Several spermatozoa were analyzed with transmission electron microscopy (TEM) to confirm the structural flagellar defects responsible for the complete asthenozoospermia.
Materials and methods
Patients
A 36 year old male was referred to our clinic following seven years of infertility. He had 0% motility on several semen evaluations. He also had a history of chronic bronchitis and recurrent pneumonia. He was referred to a pulmonary specialist and showed no signs of situs inversus and was therefore diagnosed with PCD and not KS. His 28 year old wife presented with normal menstrual cycles and a history of pelvic inflammatory disease. A prior hysterosalpingogram from 2005 showed one uterine tube blocked.
Semen samples and electron microscopy studies
Semen samples were obtained by masturbation following 2 to 3 days of abstinence. Following liquefaction, samples were analyzed using the protocol of the World Health Organization Laboratory Manual [18].
Transmission electron microscopy preparation
The washed spermatozoa samples were concentrated to a pellet and fixed in glutaraldehyde in PBS buffer for 30 min. The pellet was washed twice in PBS and secondarily fixed in osmium tetroxide for 1 h. The pellet was rinsed with de-ionized water and melted agar was added to the pellet. The sample was placed in a refrigerator at 5°C, overnight. The next day, the pellet was diced into smaller pieces and transferred to glass vials for gradient series dehydration with ethanol followed by 100% acetone. The samples were embedded in PolyBed 812 resin (Polysciences, Warrington, PA, USA) and the resin was allowed to harden at 60°C for 3 days. The specimen blocks were sectioned using a Leica Ultracut R ultra-microtome (Leica Microsystems Inc, Bannockburn, IL, USA). The cut sections were then placed on small copper grids and stained with uranyl acetate and lead citrate. Images were visualized using the JEOL 200CX transmission electron microscope (Tokyo, Japan).
Ovarian stimulation and ICSI procedures
After an ovarian stimulation with an antagonist protocol using Gonal-F (Serono, Rockland, MD, USA) low dose human chorionic gonadotropin (hCG) (Abraxis Pharmaceutical Products, Schaumburg, IL, USA), and Ganirelix (Organon, Roseland, NJ, USA), 11 oocytes were recovered on day 10 of the cycle following 10,000 IU of hCG (Abraxis Pharmaceutical Products, Schaumburg, IL, USA) given 36 h prior to vaginal oocyte retrieval (VOR). All 11 oocytes had the cumulus removed with 80 IU of hyaluronidase (Sage, Trumbull, CT, USA) 3 h after VOR. Six of the oocytes were at the MII stage and used for insemination with ICSI. The semen was collected by masturbation and was washed twice in 5 mg/mL human serum albumin (HSA) in human tubal fluid (HTF)/4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) (InVitroCare, Frederick, MD, USA). 25 mL of 5 mg/mL HSA in HTF/HEPES was combined with 25 mL of sterile water to make a HOS media in order to identify viable spermatozoa. The washed spermatozoa were placed in the HOS media and they were considered viable if they had coiled flagella. Viable spermatozoa were then aspirated into an injection pipette (Humagen, Charlottesville, VA, USA) and transferred to 10% PVP (Sage, Trumbull, CT, USA). The mid-piece of each spermatozoon was disrupted and re-aspirated for injection. The MII oocytes were injected with the viable spermatozoa 5 h following VOR. The injected oocytes were placed in culture media (Sage, Trumbull, CT, USA) and assessed for fertilization 15 h later.
Results
The spermatozoa concentrations varied in several different analyses from 24 to 40 M/mL with all having complete asthenozoospermia. Viability with eosin-nigrosin staining (Conception Technologies, San Diego, CA, USA) averaged 40%; while Kruger’s strict morphology averaged 4%. One semen sample was sent off-site to be analyzed (Repromedix, Woburn, MA, USA) for the ability of the sperm to undergo decondensation, deoxyribonucleic acid (DNA) synthesis, and recondensation following oocyte penetration [19]. The results were 98.4%, which indicated that most of the patient’s spermatozoa were able to undergo those processes successfully.
Transmission Electron Microscopy
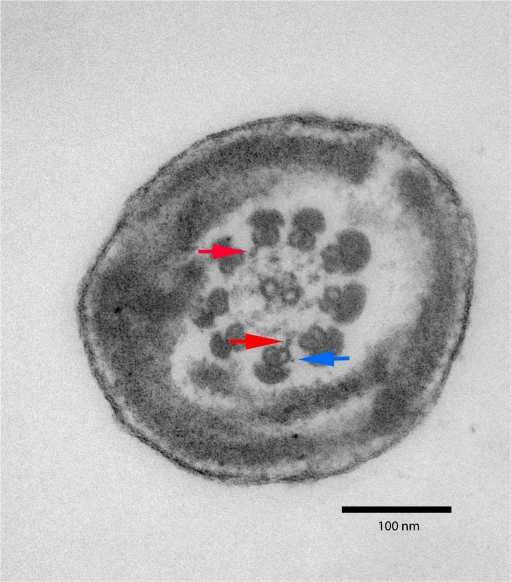
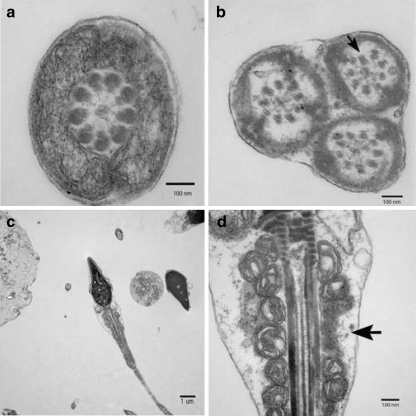
200 individual spermatozoon flagellum and mid-piece sections were examined for ultrastructural anomalies. All spermatozoa flagella sections examined showed total or partial lack of the inner and outer dynein arms (Fig 1). Complete axoneme disruption was also a common defect. Other defects were seen to a lesser extent in conjunction with the absence of the dynein arms (Fig 2). Results are summarized in Table 1.
Fig. 1.
Transmission electron micrograph of a cross-section of a spermatozoon flagellum with almost complete abscence of dynein arms at 200,000× magnification. Note the presence of a truncated outer dynein arm (blue arrow) and inner dynein arms (red arrow)
Fig. 2.
Transmission electron micrograph of various spermatozoa from the patient with PCD. A) A cross section through a mid-piece region of flagellum. B) Three axonemes surrounded by the same outer membrane. Notice the partial axonemal disruption (Arrow). C) Transverse section of a spermatozoon. D) Same spermatozoon flagellum at a higher magnification. Notice the disruption of the mitochondrial sheath (Arrow)
Table 1.
Ultrastructural defects visualized with transmission electron microscopy
| Defect | Percentage of cells with defect |
|---|---|
| Total or partial lack of dynein arms | 100% |
| Total disruption of the axoneme | 17% |
| Missing central pair | 4% |
| Displacement of the fibrous sheath or mitochondrial sheath | 10% |
| Missing radial spokes | 3% |
| Multiple tails | 3% |
Embryo Culture
Five of the six oocytes injected fertilized normally and one was digynic. 24 h post insemination, three ongoing embryos were in syngamy and two were still at the pronuclear stage. On day three at 63 h post insemination, all five were high quality 8-cell embryos with two of the embryos already compacting. All embryos were transferred to extended culture media (Irvine Scientific, Santa Ana, CA, USA) at 67 h post insemination and grown to day six. Two high quality hatching blastocysts (5AB) [20] were transferred to the patient’s uterus at 135 h post insemination. The three remaining embryos were all good quality expanded blastocysts (4BB) and were cryopreserved at 138 h post insemination. A pregnancy test was performed 7 days later and confirmed a positive pregnancy. An early pregnancy scan at 6 1/2 weeks of gestation revealed a viable twin pregnancy with cardiac activity. The healthy twin girls were born weighing 5 lbs 13 oz and 6 lbs 13 oz; following 37 weeks of gestation.
Discussion
This report shows that even in severe cases of asthenozoospermia related to dynein arm deficiency, ICSI [21] can overcome the inability of the spermatozoa to reach the ovum and produce healthy offspring. Additionally, the asthenozoospermia that results from the lack of the dynein arms within the spermatozoon flagellum does not adversely effect the development of the resulting embryo. Examining the ultrastructural defects of the cilia in patients with PCD is valuable from an investigative standpoint and in conjunction with genetic studies may be of great benefit to the PCD patient. The cilia ultrastructure and genetic profile of the PCD patient is not only important in terms of reproduction, but it may also lead to different treatment options for the respiratory issues associated with this disease. The key to successful in vitro fertilization treatment for these patients is selection of viable spermatozoa that can lead to high quality embryo development. We have added to the base of literature showing that the HOS test is a good tool to select viable spermatozoa and we have also shown that embryos from PCD patients can be successfully grown to the blastocyst stage prior to embryo transfer.
The HOS test identifies spermatozoa with intact plasma membranes and does not damage the spermatozoa. It is a simple, reliable, and non-toxic test, which allows for the selection of a viable spermatozoon prior to oocyte injection. Casper et al. showed the HOS test can be used successfully to select viable spermatozoa and increase the fertilization rate nearly 2-fold over random selection alone [22].
Cayan and colleagues described the use of immotile testicular spermatozoa for ICSI on two couples where the males had ICS/KS. In the first case, the HOS test was used to select spermatozoa and the cycle resulted in the birth of a healthy female following a frozen embryo transfer with three embryos. In the second case, 98% of the spermatozoa were missing their inner and outer dynein arms. The HOS test was not used to inject spermatozoa in the second case and no pregnancy was achieved [15]. Okada et al. used immotile spermatozoa that lacked the central microtubules from four males with ICS and polycystic kidney disease. The spermatozoa used for injection were selected using the HOS test and 38.6% of the oocytes fertilized normally and although all the couples had embryos transfers, none resulted in a viable pregnancy. One male who only had the defect in 80% of his sperm underwent a second cycle of ICSI with motile spermatozoa which resulted in a pregnancy [23].
Westlander et al. reported two cases where the HOS test was again used to select viable spermatozoa. In the first cycle of the first case, immotile ejaculated spermatozoa were used and fertilization did not occur. In the second cycle of the first case, testicular spermatozoa was used and resulted with a 75% fertilization rate and a viable pregnancy. In the second case, 50% of the oocytes were injected with immotile testicular spermatozoa resulting in a 56% fertilization rate and 50% of the oocytes were injected with immotile ejaculated spermatozoa resulting in a 44% fertilization rate [16]. In another case where the HOS test was used for selection of spermatozoa from a husband with ICS, two grade A embryos at 7- and 8-cells were transferred on day three and resulted in a pregnancy that miscarried at 21 weeks. The subsequent FET resulted in the transfer of two grade A morulae that resulted in a single intrauterine pregnancy [17].
The HOS test is a more reliable method for selecting viable spermatozoa than random spermatozoa selection. Some studies conducted with immotile spermatozoa from ejaculates using ICSI without the addition of the HOS test have failed to produce a viable pregnancy. [6, 24, 25] Other reports have shown that injection of immotile spermatozoa resulted in complete fertilization failure [26, 27].
Nijs et al. showed immotile ejaculated spermatozoa fertilized fewer oocytes following ICSI when compared to spermatozoa which gained motility following incubation. Additionally, the embryos derived from totally immotile spermatozoa produced lower quality embryos than embryos produced from motile spermatozoa. Ongoing pregnancies were only produced from spermatozoa with delayed motility or immotile spermatozoa retrieved from the testes only. For a single KS patient SUZI produced a healthy pregnancy whereas ICSI did not [6]. Other successful fertilizations from immotile sperm via sub-zonal insemination have been obtained as well [4, 28] without subsequent pregnancies. The Terriou et al. report indicated two sets of triplet pregnancies but these were from patients that had 5% non-progressive motility in the semen samples [28].
In the cases of fertilization failure and cleavage arrest, it is possible the result was due to some inherent anomaly with the spermatozoa which prevented normal development, rather than a technical aspect of ICSI such as touching the spermatozoon flagellum prior to injection [29] or selection of non-viable spermatozoa, although either of the latter instances could be the case. TEM was not done on any samples so the ultrastructure of the spermatozoa is unknown. Our clinic routinely uses the sperm DNA decondensation test [19] to access the fertilization potential of spermatozoa and fertilization in this case was 100% with one abnormal digynic zygote. This is the first report to show complete fertilization from a PCD patient and that high quality embryos from immotile ejaculated spermatozoa can be grown to the blastocyst stage prior to transfer with great success. The sperm DNA decondensation test may be a good predictor of fertilization potential and embryo development. Further experiments with the sperm DNA decondensation test in conjunction with varying ultrastructural defects may help determine which ultrastructural defects ultimately affect embryo development and make viable pregnancies more difficult.
In contrast to the reports that have yielded an unfavorable result, several reports have shown the successful treatment of PCD using ICSI without the HOS test, but fertilization rates varied from 50% to 73.4%. Olmedo and colleagues used ejaculated spermatozoa from a male with a combination of dysplasia of the fibrous sheath and dynein arm deficiency. Three embryos were transferred at the two-cell stage which resulted in the birth of a healthy baby girl [10]. Von Zumbusch et al. treated two couples with KS. Fertilization rate for the couples was 66% and 50% respectively and both couples had embryo transfers on 2 day after VOR resulting in the birth of healthy babies. The authors concluded that “results seem to ethically justify the use of assisted reproductive technology in similar cases [13].” Finally, Barros and colleagues reported the birth of two healthy children following random selection of ejaculated spermatozoa in one of four patients [8].
Several ultrastructural phenotypes have been shown in patients with PCD. The first reported ultrastructural defect for human spermatozoa was a lack of dynein arms along with some irregularities of accessory fibers and fibrous sheath. Biochemical tests, rates of oxygen consumption, and lactic acid production for the immotile spermatozoa were similar to that of motile sperm [30]. The lack or reduction of both inner and outer dynein arms was again seen by other authors [3, 31]. Our patient showed nearly complete lack of dynein arms in all flagella analyzed. Without the dynein arms to attach to the microtubule pairs, the ability of the sperm to move is not possible; which accounts for our patient’s asthenozoospermia.
The other alterations seen in our patient’s TEMs have been reported in other papers. Additional aberrations have included absence of the radial spokes [32], peripheral microtubule defects, dysplasia for the fibrous sheath [12], non-specific axoneme defects [33], complete ciliary aplasia, orientation defects [34], and absence of the central microtubule pair [28, 31, 35]. Wolff et al. also reported a case of immotile cilia syndrome where the axonemes of the spermatozoa tails were complete except they lacked the two central microtubules. However, the patient did not present with any other symptoms of ICS such as recurrent airway infections, bronchiectases or situs inversus [36].
In our patient, these secondary aberrations may be associated with his PCD but probably not responsible for the asthenozoospermia since all the spermatozoa examined lacked dynein arms in addition to the other defects. Isolated ultrastructural defects are rare. Usually there will be a combination of multiple aberrations such as dysplasia of the fibrous sheath, dynein deficiency, and unassembled mitochondria at the mid-piece [37, 38]. In one of the few papers to look at more than just one or a few patients; 247 severely asthenozoospermic patients where assessed for ultrastructural defects. The ultrastructural studies showed two main alterations: 83% had non-specific flagellar anomalies (NSFA), affecting variable numbers of spermatozoa; 17% had dysplasia of the fibrous sheath which affected between 70% and 100% of the spermatozoa in several cases [12]. Yokota et al. showed defects in the dynein arms, central microtubules, and total axoneme defects in one report [39] and still another report showed disorganization of mitochondria in the mid-piece’s capsule and irregular arrangement of the axoneme’s thick fibers in addition to two to four axonemes surrounded by the same cellular structure was also seen by other investigators [40].
Other TEM examinations have shown that defects can vary within the spermatozoa population itself and between spermatozoa and other cilia within the body. A patient with immotile spermatozoa and normally functioning cilia through the rest of his body was reported where the spermatozoa lacked dynein arms but the other cilia had normal ultrastructure [41]. There were two separate reports of patients with repeated respiratory tract infections indicative of PCD, yet both patients had motile spermatozoa [42, 43].
The previously mentioned TEM studies show that PCD is obviously a multifactorial condition and can affect any of the sub-structures of the flagella. It is usually inherited in an autosomal recessive pattern [1]; however, it has also been shown to be inherited in an X-linked or autosomal fashion [44]. PCD occurs somewhere around 1:15,000–30,000 live births. The range varies widely and may be an underestimate because not all cases are diagnosed [45].
PCD is heterogeneous and caused by mutations in several different genes on several different chromosomes [46]. Many genes have been screened for mutations and 2 have been found to affect the ultrastructure of dyneins in cilia in PCD patients. The first gene to be identified was DNAI1 and located on chromosome 9p13–21. It is highly expressed in the testes and trachea and contains 20 exons [47]. The second gene is the DNAH5 gene is located on chromosome 5p15-5p14 [48]. A third gene that has not been proven to affect dyneins but is still a good candidate is DNAH11 on chromosome 7p21 [49].
It is conceivable that variations of the genetic alterations that affect the spermatozoa axonemes may also affect the embryo itself. Centrosomes/centrioles of spermatozoa give rise to the tail axoneme during spermiogenesis [50]. In humans, the spermatozoon deposits one of its centrosomes in the cytoplasm of the oocyte and forms the sperm aster. The aster then provides a focal point for the new microtubule assembly process (MAP) of the embryo. These MAPs are necessary for normal embryonic fertilization and subsequent embryo cleavage [51].
As technology allows us to bypass conditions that would normally prevent conception, it becomes necessary for us to better understand the mechanisms that cause infertility in sub-fertile populations so we do not unintentionally pass along genetic defects to offspring. It is possible that with better understanding of the genetic, molecular, and proteomic aspects of the function of spermatozoa that poor motility may be treated or cured using other methods such as gene therapy; rather than simply bypassing the problems through the use of ICSI. Although both baby girls appear healthy and without signs of respiratory disease, there is a possibility they are more than likely carriers for the disease. The children should be monitored for respiratory problems as they grow and familial genetic profiles may help determine the genes responsible for the father’s PCD.
Acknowledgements
Special thanks to Amanda Rabka for assistance with the semen analyses and the spermatozoa preparation.
Footnotes
Capsule We report the ultrastructure of spermatozoa from a primary ciliary dyskinesia patient with dynein arm deficiency that resulted in a twin birth following blastocyst culture.
References
- 1.Blouin JL, Meeks M, Radhakrishna U, Sainsbury A, Gehring C, Saïl GD, et al. Primary ciliary dyskinesia: a genome-wide linkage analysis reveals extensive locus heterogeneity. Eur J Hum Genet. 2000;8:109–18. doi:10.1038/sj.ejhg.5200429. [DOI] [PubMed]
- 2.Coren ME, Meeks M, Morrison I, Buchdahl RM, Bush A. Primary ciliary dyskinesia: age at diagnosis and symptom history. Acta Paediatr. 2002;91:667–9. doi:10.1080/080352502760069089. [DOI] [PubMed]
- 3.Eliasson R, Mossberg B, Camner P, Afzelius BA. The immotile-cilia syndrome. A congenital ciliary abnormality as an etiologic factor in chronic airway infections and male sterility. N Engl J Med 1977;297:1–6. [DOI] [PubMed]
- 4.Bongso TA, Sathananthan AH, Wong PC, Ratnam SS, Ng SC, Anandakumar C, et al. Human fertilization by micro-injection of immotile spermatozoa. Hum Reprod. 1989;4:175–9. [DOI] [PubMed]
- 5.Wolf JP, Feneux D, Escalier D, Rodrigues D, Frydman R, Jouannet P. Pregnancy after subzonal insemination with spermatozoa lacking dynein arms. J Reprod Fertil. 1993;97:487–92. doi:10.1530/jrf.0.0970487. [DOI] [PubMed]
- 6.Nijs M, Vanderzwalmen P, Vandamme B, Segal-Bertin G, Lejeune B, Segal L, et al. Fertilizing ability of immotile spermatozoa after intracytoplasmic sperm injection. Hum Reprod. 1996;11:2180–5. [DOI] [PubMed]
- 7.Stalf T, Sánchez R, Köhn FM, Schalles U, Kleinstein J, Hinz V, et al. Pregnancy and birth after intracytoplasmic sperm injection with spermatozoa from a patient with tail stump syndrome. Hum Reprod. 1995;10:2112–4. [DOI] [PubMed]
- 8.Barros A, Sousa M, Oliveira C, Silva J, Almeida V, Beires J. Pregnancy and birth after intracytoplasmic sperm injection with totally immotile sperm recovered from the ejaculate. Fertil Steril. 1997;67:1091–4. doi:10.1016/S0015-0282(97)81444-6. [DOI] [PubMed]
- 9.Kahraman S, Işik AZ, Vicdan K, Ozgür S, Ozgun OD. A healthy birth after intracytoplasmic sperm injection by using immotile testicular spermatozoa in a case with totally immotile ejaculated spermatozoa before and after Percoll gradients. Hum Reprod. 1997;12:292–3. doi:10.1093/humrep/12.2.292. [DOI] [PubMed]
- 10.Olmedo SB, Nodar F, Chillik C, Chemes HE. Successful intracytoplasmic sperm injection with spermatozoa from a patient with dysplasia of the fibrous sheath and chronic respiratory disease. Hum Reprod. 1997;12:1497–9. doi:10.1093/humrep/12.7.1497. [DOI] [PubMed]
- 11.Olmedo SB, Rawe VY, Nodar FN, Galaverna GD, Acosta AA, Chemes HE. Pregnancies established through intracytoplasmic sperm injection (ICSI) using spermatozoa with dysplasia of fibrous sheath. Asian J Androl. 2000;2:125–30. [PubMed]
- 12.Chemes HE, Olmedo SB, Carrere C, Oses R, Carizza C, Leisner M, et al. Ultrastructural pathology of the sperm flagellum; association between flagellar pathology and fertility prognosis in severely asthenozoospermic men. Hum Reprod. 1998;13:2521–6. doi:10.1093/humrep/13.9.2521. [DOI] [PubMed]
- 13.Von Zumbusch A, Fiedler K, Mayerhofer A, Jebberger B, Ring J, Vogt HJ. Birth of healthy children after intracytoplasmic sperm injection in two couples with male Kartagener’s syndrome. Fertil Steril. 1998;70:643–6. doi:10.1016/S0015-0282(98)00246-5. [DOI] [PubMed]
- 14.Jeyendran RS, Van der Ven HH, Perez-Pelaez M, Crabo BG, Zaneveld LJ. Development of an assay to assess the functional integrity of the human sperm membrane and its relationship to other semen characteristics. J Reprod Fertil. 1984;70:219–28. doi:10.1530/jrf.0.0700219. [DOI] [PubMed]
- 15.Cayan S, Conaghan J, Schriock ED, Ryan IP, Black LD, Turek JP. Birth after intracytoplasmic sperm injection with use of testicular sperm from men with Kartagener/immotile cilia syndrome. Fertil Steril. 2001;76:612–4. doi:10.1016/S0015-0282(01)01974-4. [DOI] [PubMed]
- 16.Westlander G, Barry M, Petrucco O, Norman R. Different fertilization rates between immotile testicular spermatozoa and immotile ejaculated spermatozoa for ICSI in men with Kartagener’s syndrome: case reports. Hum Reprod. 2003;18:1286–8. doi:10.1093/humrep/deg240. [DOI] [PubMed]
- 17.Peeraer K, Nijs M, Raick D, Ombelet W. Pregnancy after ICSI with ejaculated immotile spermatozoa from a patient with immotile cilia syndrome: a case report and review of the literature. Reprod Biomed Online. 2004;9:659–963. [DOI] [PubMed]
- 18.World Health Organization. WHO laboratory manual for the exxmination of human semen and sperm-cervical mucus interaction. 4th ed. Cambridge: Cambridge University Press; 1999.
- 19.Brown DB, Nagamani M. Use of xenopus laevis frog egg extract in diagnosing human male unexplained infertility. Yale J Biol Med. 1992;65:29–38. [PMC free article] [PubMed]
- 20.Towards reproductive certainty: infertility and genetics beyond. Gardner, DK, Schoolcraft WB, Jansen R and Mortimer D (eds.) Carnforth: Parthenon; 1999 pp 378.
- 21.Palermo G, Joris H, Devroey P, Van Steirteghem AC. Pregnancies after intracytoplasmic injection of single spermatozoon into an oocyte. Lancet. 1992;340:17–8. doi:10.1016/0140-6736(92)92425-F. [DOI] [PubMed]
- 22.Casper RF, Meriano JS, Jarvi KA, Cowan L, Lucato ML. The hypo-osmotic swelling test for selection of viable sperm for intracytoplasmic sperm injection in men with complete asthenozoospermia. Fertil Steril. 1996;65:972–6. [DOI] [PubMed]
- 23.Okada H, Fujioka H, Tatsumi N, Fujisawa M, Gohji K, Arakawa S, et al. Assisted reproduction for infertile patients with 9 + 0 immotile spermatozoa associated with autosomal dominant polycystic kidney disease. Hum Reprod. 1999;14:110–3. doi:10.1093/humrep/14.1.110. [DOI] [PubMed]
- 24.Nagy ZP, Liu J, Joris H, Verheyen G, Tournaye H, Camus M, et al. The result of intracytoplasmic sperm injection is not related to any of the three basic sperm parameters. Hum Reprod. 1995;10:1123–9. [DOI] [PubMed]
- 25.Vandervorst M, Tournaye H, Camus M, Nagy ZP, Van Steirteghem A, Devroey P. Patients with absolutely immotile spermatozoa and intracytoplasmic sperm injection. Hum Reprod. 1997;12:2429–33. doi:10.1093/humrep/12.11.2429. [DOI] [PubMed]
- 26.Abu-Musa A, Hannoun A, Khabbaz A, Devroey P. Failure of fertilization after intracytoplasmic sperm injection in a patient with Kartagener’s syndrome and totally immotile spermatozoa: case report. Hum Reprod. 1999;14:2517–8. doi:10.1093/humrep/14.10.2517. [DOI] [PubMed]
- 27.Liu J, Nagy Z, Joris H, Tournaye H, Smitz J, Camus M, et al. Analysis of 76 total fertilization failure cycles out of 2,732 intracytoplasmic sperm injection cycles. Hum Reprod. 1995;10:2630–6. [PubMed]
- 28.Terriou P, Giorgetti C, Hans E, Spach JL, Salzmann J, Carlon N, et al. Subzonal sperm insemination and total or extreme asthenozoospermia: an effective technique for an uncommon cause of male infertility. Fertil Steril. 1993;60:1057–61. [DOI] [PubMed]
- 29.Vanderzwalmen P, Bertin G, Lejeune B, Nijs M, Vandamme B, Schoysman R. Two essential steps for a successful intracytoplasmic sperm injection: injection of immobilized spermatozoa after rupture of the oolema. Hum Reprod. 1996;11:540–7. [DOI] [PubMed]
- 30.Afzelius BA, Eliasson R, Johnsen O, Lindholmer C. Lack of dynein arms in immotile human spermatozoa. J Cell Biol. 1975;66:225–32. doi:10.1083/jcb.66.2.225. [DOI] [PMC free article] [PubMed]
- 31.Walt H. Sperm flagella and cilia with pathologic motility and ultrastructure. Schweiz Med Wochenschr. 1984;114:1442–50. [PubMed]
- 32.Sturgess JM, Chao J, Wong J, Aspin N, Turner JA. Cilia with defective radial spokes: a cause of human respiratory disease. N Engl J Med. 1979;300:53–6. [DOI] [PubMed]
- 33.Alosilla Fonttis A, Napolitano R, Tomás MA. Successful ICSI in a case of severe asthenozoospermia due to 93% non-specific axonemal alterations and 90% abnormal or absent mitochondrial sheaths. Reprod Biomed Online. 2002;5:70–2. [DOI] [PubMed]
- 34.Mitchell V, Rives N, Albert M, Peers MC, Selva J, Clavier B, et al. Outcome of ICSI with ejaculated spermatozoa in a series of men with distinct ultrastructural flagellar abnormalities. Hum Reprod. 2006;21:2065–74. doi:10.1093/humrep/del130. [DOI] [PubMed]
- 35.Sturgess JM, Chao J, Turner JA. Transposition of ciliary microtubules: another cause of impaired ciliary motility. N Engl J Med. 1980;303:318–22. [DOI] [PubMed]
- 36.Wolff H, Schaller M, Panhans A, Korting HC, Meurer M. The immotile cilia syndrome. A rare form of male infertility. Hautarzt 1994;45:611–4. doi:10.1007/s001050050137. [DOI] [PubMed]
- 37.Chemes HE, Brugo S, Zanchetti F, Carrere C, Lavieri JC. Dysplasia of the fibrous sheath: an ultrastructural defect of human spermatozoa associated with sperm immotility and primary sterility. Fertil Steril. 1987;48:664–9. [DOI] [PubMed]
- 38.Chemes HE, Morero JL, Lavieri JC. Extreme asthenozoospermia and chronic respiratory disease: a new variant of the immotile cilia syndrome. Int J Androl. 1990;13:216–22. doi:10.1111/j.1365-2605.1990.tb00979.x. [DOI] [PubMed]
- 39.Yokota T, Ohno N, Tamura K, Seita M, Toshimori K. Ultrastructure and function of cilia and spermatozoa flagella in patient with kartagener’s syndrome. Intern Med. 1993;32:593. doi:10.2169/internalmedicine.32.593. [DOI] [PubMed]
- 40.Papadimas J, Tarlatzis BC, Bili H, Sotiriadis T, Koliakou K, Bontis J, et al. Therapeutic approach of immotile cilia syndrome by intracytoplasmic sperm injection: a case report. Fertil Steril. 1997;67:562–5. doi:10.1016/S0015-0282(97)80087-8. [DOI] [PubMed]
- 41.Wilton LJ, Teichtahl H, Temple-Smith PD, De Kretser DM. Kartagener’s syndrome with motile cilia and immotile spermatozoa: axonemal ultrastructure and function. Am Rev Respir Dis. 1986;134:1233–6. [DOI] [PubMed]
- 42.Greenstone M, Rutman A, Pavia D, Lawrence D, Cole PJ. Normal axonemal structure and function in Kartagener’s syndrome: an explicable paradox. Thorax. 1985;40:956–7. [DOI] [PMC free article] [PubMed]
- 43.Jonsson MS, McCormick JR, Gillies CG, Gondos B. Kartagener’s syndrome with motile spermatozoa. N Engl J Med. 1982;307:1131–3. [DOI] [PubMed]
- 44.Narayan D, Krishnan SN, Upender M, Ravikumar TS, Mahoney MJ, Dolan TF Jr, et al. Unusual inheritance of primary ciliary dyskinesia (Kartagener’s syndrome). J Med Genet. 1994;31:493–6. [DOI] [PMC free article] [PubMed]
- 45.Bush A, Chodhari R, Collins N, Copeland F, Hall P, Harcourt J, et al. Primary ciliary dyskinesia: current state of the art. Arch Dis Child. 2007;92:1136–40. doi:10.1136/adc.2006.096958. [DOI] [PMC free article] [PubMed]
- 46.Geremek M, Witt M. Primary ciliary dyskinesia: genes, candidate genes and chromosomal regions. J Appl Genet. 2004;45:347–61. [PubMed]
- 47.Pennarun G, Chapelin C, Escudier E, Bridoux AM, Dastot F, Cacheux V, et al. The human dynein intermediate chain 2 gene (DNAI2): cloning, mapping, expression pattern, and evaluation as a candidate for primary ciliary dyskinesia. Hum Genet. 2000;107:642–9. doi:10.1007/s004390000427. [DOI] [PubMed]
- 48.Omran H, Häffner K, Völkel A, Kuehr J, Ketelsen UP, Ross UH, et al. Homozygosity mapping of a gene locus for primary ciliary dyskinesia on chromosome 5p and identification of the heavy dynein chain DNAH5 as a candidate gene. Am J Respir Cell Mol Biol. 2000;23:696–702. [DOI] [PubMed]
- 49.Bartoloni L, Blouin JL, Pan Y, Gehrig C, Maiti AK, Scamuffa N, et al. Mutations in the DNAH11 (axonemal heavy chain dynein type 11) gene cause one form of situs inversus totalis and most likely primary ciliary dyskinesia. Proc Natl Acad Sci USA. 2002;99:10282–6. doi:10.1073/pnas.152337699. [DOI] [PMC free article] [PubMed]
- 50.Sathananthan AH, Kola I, Osborne J, Trounson A, Ng SC, Bongso A, et al. Centrioles in the beginning of human development. Proc Natl Acad Sci USA. 1991;88:4806–10. doi:10.1073/pnas.88.11.4806. [DOI] [PMC free article] [PubMed]
- 51.Nagy ZP. Sperm centriole disfunction and sperm immotility. Mol Cell Endocrinol. 2000;166:59–62. doi:10.1016/S0303-7207(00)00298-7. [DOI] [PubMed]