Abstract
Purpose
To conduct a prospective randomized study in order to investigate the effect of recombinant HCG (rHCG) on oocyte nuclear and cytoplasm maturity compared to urinary HCG (uHCG), for inducing ovulation in women treated with ICSI for male factor infertility.
Materials and Methods
We compared 89 patients randomly assigned to one of the two study groups. Group A consisted of 42 women who received a subcutaneous (s.c.) injection of 250 μg rHCG and group B consisted of 47 patients receiving an intramuscular (i.m.) injection of 10,000 IU uHCG.
Results
Patients treated with rHCG showed a rate of metaphase II oocytes, a number of metaphase II oocytes with mature cytoplasm and a rate of metaphase II oocytes with mature cytoplasm calculated from total MII oocytes statistically higher than in patients treated with uHCG. However this differences were not associated with a significantly better clinical outcome.
Conclusion
Our data show that in women treated with ICSI for male factor infertility, rHCG increases the rate of metaphase II oocytes, the number and the rate of MII oocytes with mature cytoplasm compared to uHCG. A larger study comparing transfer cycles of embryos all derived from oocytes with mature cytoplasm and transfer cycles of embryos all derived from oocytes with immature cytoplasm may be needed to clarify clinical correlations.
Keywords: IVF, Ovulation induction, Recombinant HCG, Urinary HCG, Oocyte maturity
Introduction
The preovulatory surge of luteinizing hormone (LH) provides the physiologic stimulus for final oocyte maturation and induces ovulation, during which the ovarian follicle releases the mature oocyte that is picked up by the infundibular fimbria of the oviduct.
In assisted conception, urinary (u) HCG has been used for several years to mimic the endogenous LH surge as there are considerable structural similarities between HCG and human (h) LH, and hence both hormones stimulate the same receptor [1]. HCG is readily available in the urine of pregnant women, whereas only low concentrations of LH are found in the urine of post-menopausal women. Urinary preparations, however, are associated with a number of disadvantages, including an uncontrolled source, lack of purity and batch-to-batch variation in activity leading to variable clinical results [2]. Recombinant HCG (rHCG) was recently introduced as an alternative to urinary HCG for the final maturation of oocytes in women treated with IVF and ICSI. rHCG is derived from genetically engineered Chinese hamster ovary cells through recombinant DNA technology. This product has a high purity that facilitates characterization and quantitation by phsycochemical means, reducing the need for animal bioassays [3]. However, little information is reported in the literature on the nuclear and cytoplasmic maturation of the oocytes in women receiving recombinant HCG. Both nuclear and cytoplasmic maturation should be completed in a coordinated mode to ensure optimal conditions for subsequent fertilization. Nuclear maturation is usually closely attended by a general maturation of the cytoplasm that is characterized by an increase in number of organelles scattered throughout the ooplasm. The presence of a first polar body indicates that nuclear maturation has been reached (metaphase II (MII) stage). Mature oocyte should have an intact first polar body and homogeneously fine granular and light-colored ooplasm [4]. Disturbances or asynchrony of the processes of nuclear and cytoplasmic maturation may occur and resulted in different morphological abnormalities [5, 6, 7]. Sometimes we may find MII oocytes that have completed their nuclear maturation with extrusion of the first polar body, but they show cytoplasmic anomalies, such as excessive granularity or presence of cytoplasmic inclusions, that may be poor prognostic factors as they may be signs of oocyte cytoplasmic immaturity [5, 6, 8, 9].
In standard IVF it is often difficult to assess the cytoplasmic morphology of the oocytes and the exact stage of maturation as the oocytes are always surrounded by the cumulus or corona cells at the time of collection. As only mature oocytes are suitable for ICSI, the cumulus cells are routinely removed for accurate assessment of the nuclear maturity and to facilitate handling during the microinjection procedure. A more precise assessment of the oocytes stage and the cytoplasmic morphology is therefore possible.
The aim of this work was to conduct a prospective randomized study in order to investigate the effect of rHCG on oocyte nuclear and cytoplasm maturity compared to urinary HCG (uHCG), for inducing ovulation in women treated with ICSI for male factor infertility.
Materials and methods
The study was conducted at the IVF/ICSI program of the GENESIS - Grimaldi Medical Group IVF Center, Rome, Italy, between January and December 2003 on infertile couples due to male factor undergoing ICSI treatment.
The study was reviewed and approved by the institutional review board at the GENESIS - Grimaldi Medical Group IVF Center. All patients undergoing ICSI and participating in the study gave informed consent.
Patients included in the study had regular spontaneous menstrual cycles (26–39 days) and were aged < 42years. All patients had acceptable follicular phase serum concentrations of FSH (≤10IU/L), LH (<10IU/L) and oestradiol (<60pg/ml), body mass index (BMI) ≤ 30 kg/m2, presence of both ovaries and normal uterine cavity, no assisted reproduction treatment attempts for at least two full menstrual cycles. Patients were excluded from the study if they had any clinically significant systemic disease, polycystic ovarian syndrome (PCOS), a previous history of severe ovarian hyperstimulation syndrome (OHSS), abnormal gynaecological bleeding of unknown origin, a previous history of intolerance to any of the agents used in the study. This was a prospective, randomized study.
All women were treated with a long stimulation protocol in which GnRH-analogue (Buserelin subcutaneous, 0.4 mg daily) was given as a pre-treatment and recombinant FSH (rFSH) administration was started when pituitary desensitisation was confirmed by the presence of small antral follicles (diameter 2–6 mm), an endometrial thickness < 5 mm and serum estradiol level lower than 50 pg/ml. From the 7th day of stimulation in both groups, daily monitoring of follicle size by ultrasound was performed, and plasma levels of estradiol were measured. The dose of rFSH was adjusted according to the individual response of each patient. Ovulation was triggered with HCG when plasma oestradiol levels reached between 1,000 and 4,500 pg/ml and at least four follicles > 16 mm diameter were visualised on ultrasonography. Fifty patients were randomly allocated to each of the two study groups, according to a computer-generated number sequence. Group A consisted of women who received a subcutaneous (s.c.) injection of 250 µg rHCG , and group B consisted of patients who received an intramuscular (i.m.) injection of 10,000IU uHCG. Oocyte retrieval was performed under ultrasound guidance by the transvaginal route 34 to 37 h after HCG administration. Either local or general anesthesia was used. The number of oocytes retrieved was recorded. Approximately two to four hours after retrieval, following incubation of < 30s in culture medium containing 80 IU/ml hyaluronidase, oocytes were individually treated by gentle pipetting to remove the cumulus and corona radiata cells. Oocyte maturation stage and morphology were assessed under an inverted microscope at X200 or X400 magnification. Nuclear maturity was assessed by extrusion of the first polar body in the perivitelline space. Maturity of the cytoplasm was defined as clear cytoplasm with uniform structure, homogeneous fine granularity and, absence of cytoplasmic inclusions. Oocytes were classified as follows: (i) metaphase II oocyte (mature oocyte), characterized by the presence of first polar body and an ooplasm of a light color and fine homogeneous granularity (Fig. 1), (ii) metaphase I oocyte (considered nearly mature or intermediate in maturation), characterized by absence of both the germinal vesicle and the first polar body, round and even in form, with a homogeneously granular and light-colored ooplasm in case of late MI oocyte, sometimes with minor central granularity in case of early MI oocytes (Fig. 2), (iii) prophase I oocyte (immature oocyte) characterized by its distinct germinal vesicle and refractile nucleolus, with irregular shape, darkened center and coarse granular ooplasm (Fig. 3). Oocytes were classified according to Veeck [4].
Fig. 1.

Metaphase II oocyte
Fig. 2.
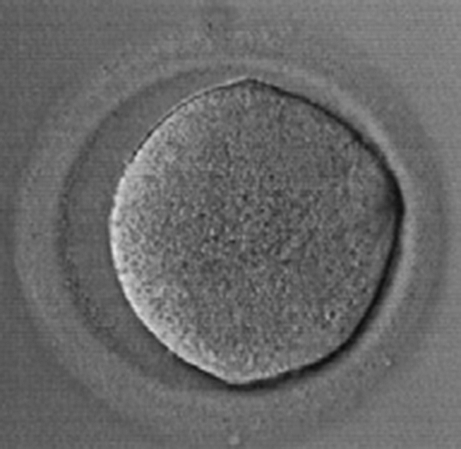
Metaphase I oocyte
Fig. 3.

Prophase I oocyte
The rate of metaphase II oocytes was calculated and the MII oocytes were assessed for cytoplasmic morphology and maturity. MII oocytes with a light color and fine homogeneous granulate ooplasm were considered oocytes with normal morphology and classified as oocytes that have completed their cytoplasmic maturation [6]. MII oocyte with excessive granularity or presence of cytoplasmic inclusions were considered oocytes with citoplasmic anomalies and classified as oocytes that have not completed their cytoplasmic maturation and defined as MII oocytes with immature cytoplasm [6].
Semen samples were prepared with the Swim-up method. Intracytoplasmic sperm injection was performed in all patients according to the published procedures [10, 11]. The holding and injection pipettes were made from glass capillaries with an outside diameter of 1 mm and inside diameter of 0.6 mm. ICSI procedures were carried out on the heated stage (37°C) of an inverted microscope at X400 magnification using the Hoffman Modulation Contrast System. The system included Narishige micromanipulators and a video graphic printer. Immediately before the injection procedure 1µl of the sperm suspension was diluted in a microdroplet containing 4µl of 10% polyvinylpyrrolidone (PVP), placed in the center of a Petri dish while the oocytes were placed in surrounding microdroplets containing 5µl of HEPES(N-2-Hydroxyethylpiperazine-N'-2-Ethanesulfonic Acid)-buffered culture medium covered by oil. A single spermatozoon of apparently normal morphology was then selected and immobilized, compressing the sperm tail between the injection pipette and the bottom of the Petri dish, before the injection into the oocyte cytoplasm [12]. The immobilized spermatozoon, tail first, was then aspirated into the injection pipette and moved from the PVP droplet into one of the peripheral droplets containing an oocyte. The oocyte was rotated to locate the first polar body at the 6 or 12 o’clock position, held by gentle suction on the holding pipette and the equatorial plane located in focus. The spermatozoon was then ejected slowly, close up to the tip and the edge of the pipette, which was then gently pushed deep into cytoplasm at the three o’clock position. To ensure that the spermatozoon was deposited inside the oocyte cytoplasm and not in the perivetlline space, gentle suction was applied from the injection pipette until the oolemma breaks. The cytoplasm was aspirated after rupture. The cytoplasm organelles and the spermatozoon were then ejected back into the cytoplasm slowly with the smallest amount of medium possible. Thereafter the injection pipette was gently withdrawn and the oocyte released from the holding pipette. The injected oocyte was then rinsed in HEPES-free culture medium and culture [13].
Only MII oocytes were microinjected. Both MII oocytes with mature cytoplasm and MII oocytes with immature cytoplasm were injected in the two groups. Only a small number of MII oocytes with coarse morphological anomalies were not considered for microinjection. All ICSI procedures were performed by the same embryologist.
Oocytes with two pronuclei and two polar bodies 18h after ICSI were considered as normally fertilized. Assessment of embryonic development was undertaken after 44h post insemination. The embryos obtained were categorized on day two or three into three categories, depending on their morphological appearance. Grade A had equal and regular blastomeres without the presence of cytoplasm fragments; grade B had unequal blastomeres with or without cytoplasm fragments; and grade C were totally fragmented embryos which were not transferred [14]. Embryos were transferred 48–72h after insemination with an embryo transfer catheter. All transfers were performed by the same physician in order to avoid interoperator variability. All pregnancies were confirmed by a rising titer of serum HCG from 12 days after embryo transfer. The same luteal phase support was used in both groups: 200 mg twice a day of progesterone vaginally from the day after oocyte retrieval. The fertilization rate, pregnancy rate and implantation rate were also calculated. Statistical analysis was done using T test and Chi square as required, considering P < 0.05 as significant.
Results
We included in the study a total of 100 infertile couples undergoing ICSI treatment for male factor who were randomly assigned to the two study groups, 50 to the rHCG group (group A) and 50 to the uHCG group (group B). Eight patients in group A and three patients in group B were protocol violators and were excluded from the study. Thus, there were 89 women in the all-patient population with 42 and 47 patients in the rHCG and uHCG groups respectively. There were no statistically significant differences between the two study groups for any demographic characteristic assessed. The mean (±SD) age of women was 35.5 ± 4.9 years and 36.8 ± 4.4 years in the rHCG group and uHCG group respectively; the body mass index 21.5 ± 5.9 and 23.3 ± 5.9kg/m2 in the rHCG group and uHCG group respectively and median duration of infertility 4.2 ± 1.1 and 4.3 ± 1.3years in the rHCG group and uHCG group respectively. The two treatment groups were comparable for any of the initial screening hormone concentrations without statistically significant differences (mean ± SD, rHCG group versus uHCG group: FSH, 7.5 ± 2.4 versus 6.9 ± 2.5mIU/mL; LH, 5.7 ± 3 versus 5.3 ± 2.8mIU/mL; estradiol 48 ± 20.4 versus 50.6 ± 21.7pg/mL) (Table 1).
Table 1.
Demographic characteristics and hormonal basal parameters of patients
| Parameters | Group A (rHCG) | Group B (uHCG) | P value |
|---|---|---|---|
| (n = 42) | (n = 47) | ||
| Age (yr) | 35.5 ± 4.9 | 36.8 ± 4.4 | 0.17 |
| BMI (kg/m2) | 21.5 ± 5.9 | 23.3 ± 5.9 | 0.08 |
| Median duration of infertility (years) | 4.2 ± 1.1 | 4.3 ± 1.3 | 0.22 |
| Basal FSH (mIU/mL) | 7.5 ± 2.4 | 6.9 ± 2.5 | 0.27 |
| Basal LH (mIU/mL) | 5.7 ± 3 | 5.3 ± 2.8 | 0.48 |
| Basal estradiol (pg/mL) | 48 ± 20.4 | 50.6 ± 21.7 | 0.55 |
Parameters expressed as mean ± SD.
Similarly there were no statistically significant differences between treatment groups in the duration of stimulation ([mean ± SD] 11.1 ± 1.2days in Group A versus 11.3 ± 1.3days in group B), in the total dosages of rFSH used ([mean ± SD] 2721 ± 1048IU in Group A versus 2972 ± 1057IU in group B), in the estradiol dosage on day of HCG administration ([mean ± SD] 1709 ± 848pg/mL in Group A versus 1691 ± 773pg/mL in group B), and in the number of follicles at HCG day ([mean ± SD] 10.2 ± 5.1 in Group A versus 8.8 ± 5.0 in group B)
There were no statistically significant difference also in the number of oocytes retrieved ([mean ± SD] 8.4 ± 5.0 in Group A versus 7.1 ± 5.2 in group B). The mean number of MII oocytes was higher in Group A (7.4 ± 4.6) than in Group B (5.7 ± 3.8) but the difference did not reached statistical significance. The rate of metaphase II oocytes in women treated with rHCG was significantly higher (88.1%) than in patients treated with uHCG (80.8%) (P < 0.01). The mean number of MII oocytes with mature cytoplasm was significantly higher in Group A (7.3 ± 4.2) than in Group B (4.7 ± 3.1) (P < 0.01). The rate of metaphase II oocytes with cytoplasmic maturity calculated from total MII oocytes was significantly higher in women treated with rHCG (89.1%) than in women treated with uHCG (81.5%) (P < 0.01).
Only MII oocytes were microinjected. Both MII oocytes with mature cytoplasm and MII oocytes with immature cytoplasm were injected in the two groups. Only a small number of MII oocytes with coarse morphological anomalies were not considered for microinjection.
There were no statistically significant differences between the two groups in the median number of injected oocytes ([mean ± SD] 7.1 ± 4.3 in Group A versus 5.5 ± 3.5 in group B) fertilization rate (87.2% in Group A versus 86.7% in Group B), top quality embryo (grade A) rate (34% in Group A versus 40.4% in group B), pregnancy rate (50% in group A versus 51% in group B), implantation rate (23% in Group A versus 21.2% in group B), or miscarriage rate (19% in Group A versus 20.8% in Group B) (Table 2).
Table 2.
Data of patients treated with rHCG or uHCG
| Parameters | Group A (rHCG) | Group B (uHCG) | P value |
|---|---|---|---|
| (n = 42) | (n = 47) | ||
| Duration of stimulation (d) | 11.1 ± 1.2 | 11.3 ± 1.3 | 0.56 |
| Total dose of FSH required (IU) | 2721 ± 1048 | 2872 ± 1057 | 0.5 |
| Estradiol on day of HCG administration (pg/mL) | 1709 ± 848 | 1691 ± 773 | 0.92 |
| Follicles day HCG | 10.2 ± 5.1 | 8.8 ± 5 | 0.19 |
| Retrived oocytes | 8.4 ± 5 | 7.1 ± 5.2 | 0.23 |
| Metaphase II oocytes (MII) | 7.4 ± 4.6 | 5.7 ± 3.8 | 0.06 |
| % MII oocytes | 88.1% (312/354) | 81.8% (270/334) | <0.01* |
| MII oocytes with mature cytoplasm | 7.3 ± 4.2 | 4.7 ± 3.1 | <0.01* |
| %MII with mature cytoplasm calculated from total MII oocytes | 89.1% (278/312) | 81.8% (221/270) | <0.01* |
| Fertilization rate | 87.2% | 86.7% | 0.99 |
| Injected oocytes | 7.1 ± 4.3 | 5.5 ± 3.5 | 0.06 |
| % Top quality embryo (grade A) | 34% (65/191) | 40.4% (75/210) | 0.8 |
| Transferred embryos | 2.7 ± 0.9 | 2.9 ± 1.0 | 0.23 |
| B-hCG positive (%) | 50%(21/42) | 51% (24/47) | 0.92 |
| Implantation rate | 23% | 21.2% | 0.81 |
| Abortion rate | 19% | 20.8% | 0.88 |
Parameters expressed as mean ± SD or percentages, as appropriate.
Discussion
Urinary preparations have many disadvantages, including an uncontrolled source, the presence of urinary contaminants and batch-to-batch inconsistency, leading to undesiderable variations in response, not just between patients, but also within the same patient from cycle to cycle and high incidence of local allergic reactions [15, 16, 17]. Recombinant technology allows to remove all urinary contaminants leading to a safe subcutaneous administration of a compound better tolerated, with fewer injection-site reactions than uHCG and also, with less batch-to-batch variation [18].
Previous studies found that rHCG, 250 µg, and uHCG, 5000 or 10000 IU are equally effective in inducing follicular maturation in women undergoing ART [19, 3, 20].
In this study we analyzed the effect of rHCG on oocyte nuclear and cytoplasm maturity compared to urinary HCG (uHCG), for inducing ovulation in women treated with ICSI for male factor infertility. We chose this group of patients because only oocyte denudation from cumulus cells needed to perform intacytoplasmic microinjection allows an adequate study of oocyte maturity.
In our study we note that rHCG treatment resulted in a statistically higher rate of MII oocytes (nuclear maturity) and in a statistically higher number and rate of MII oocytes with complete cytoplasmic maturation, defined as presence of clear cytoplasm with uniform structure and homogeneous granulate ooplasm. This finding is consistent with a previous study that found statistically higher numbers of mature oocytes in patients treated with rHCG compared to those treated with uHCG [21]. Uhler et al [22] in an age-matched retrospective analysis also showed an increase in the number of mature oocytes in patients treated with rHCG compared with patients receiving uHCG, although this data did not reach statistical significance. The observation of lower oocyte maturity in the uHCG group is not definitely understood. Urine derived HCG is known to contain HCG degradation products [23]. This may suggest that HCG degradation products in the urinary preparation have subtle interference with the active HCG molecules and therefore with the HCG-induced cumulus-oocyte maturation. The difference in the biological activity of rHCG in human compared to that of uHCG may be suggested to explain some of our results [24].
Our study showed no significant difference between both preparations in terms of clinical outcome. However, to correctly compare the effect of the different oocytes cytoplasmic maturity induced by rHCG and uHCG in terms of implantation and pregnancy rate, it would be necessary to compare results of transfer cycles in which all the transferred embryos were derived from oocytes with mature cytoplasm, with transfer cycles in which all the transferred embryos were derived from oocytes with immature cytoplasm.
In conclusion our data show that in women treated for ICSI, rHCG increases the rate of metaphase II oocytes, the number and the rate of MII oocytes with mature cytoplasm compared to uHCG. A larger study comparing transfer cycles of embryos all derived from oocytes with mature cytoplasm and transfer cycles of embryos all derived from oocytes with immature cytoplasm may be needed to clarify clinical correlations.
References
- 1.Pierce JG, Parsons TF. Glycoprotein hormones: structure and function. Annu Rev Biochem. 1981;50:465–95. doi:10.1146/annurev.bi.50.070181.002341. [DOI] [PubMed]
- 2.Zegers-Hochschild F, Fernandez E, Mackenna A, Fabres C, Altieri E, Lopez T. The empty follicle syndrome: a pharmaceutical industry syndrome. Hum Reprod. 1995;10:2262–5. [DOI] [PubMed]
- 3.Chang P, Kenley S, Burns T, Denton G, Currie K, DeVane G, et al. Recombinant human chorionic gonadotropin (rhCG) in assisted reproductive technology: results of clinical trial comparing two doses of rhCG (Ovidrel) to urinary hCG (profasi) for induction of final follicular maturation on in vitro fertilization-embryo transfer. Fertil Steril. 2001;76:67–74. doi:10.1016/S0015-0282(01)01851-9. [DOI] [PubMed]
- 4.Veeck LL. Atlas of the human oocyte and early conceptus. Baltimore: Williams and Wilkins; 1986.
- 5.Veeck LL. Oocyte assessment and biological performace. Ann N Y Acad Sci. 1988;541:259–74. doi:10.1111/j.1749-6632.1988.tb22263.x. [DOI] [PubMed]
- 6.Veeck LL. Atlas of the human oocyte and early conceptus vol 2. Baltimore: Williams and Wilkins; 1991.
- 7.Fasolino A, Longo V, Capece G, Trotta R, Franco F, Fasolino MC. La valutazione della maturazione ovocitaria in pazienti sottoposte ad ICSI e trattate con r-FSH. Minerva Ginecol. 2001;53:157–63. [PubMed]
- 8.Bedford JM, Kim HH. Sperm/egg binding patterns and oocyte cytology in retrospettive analysis of fertilization failure in vitro. Hum Reprod. 1993;8:453–63. [DOI] [PubMed]
- 9.Serhal PF, Ranieri DM, Kinis A, Marchant S, Davies M, Khadum IM. Oocyte morphology predicts outcome of intracytoplasmic sperm injection. Hum Reprod. 1997;12:1267–70. doi:10.1093/humrep/12.6.1267. [DOI] [PubMed]
- 10.Palermo G, Joris H, Devroej P, Van Steirtegem AC. Pregnancies after intracytoplasmic injection of a single spermatozoon into an oocyte. Lancet. 1992;340:17–8. doi:10.1016/0140-6736(92)92425-F. [DOI] [PubMed]
- 11.Van Steirteghem AC, Nagy Z, Joris H, Liu J, Staessen C, Smitz J, Wisanto A, Devroey P. High fertilization and implantation rates after intracytoplasmic sperm injection. Hum Reprod. 1993;8:1061–6. [DOI] [PubMed]
- 12.Fishel S, Lisi F, Rinaldi L, Green S, Hunter A, Dowell K, Thornton S. Systematic examination of immobilizing spermatozoa before intracytoplasmic sperm injection in the human. Hum Reprod. 1995;10(3):497–500. [DOI] [PubMed]
- 13.Ubaldi F, Rienzi L. Micromanipulation techniques in human infertilità: PZD, SUZI, ICSI, MESA, PESA, FNA and TESE. In Biotechnology of Human Reproduction. 2003. Parthenon Publisching. 315–336.
- 14.Veeck LL. Fertilization and early embryonic development. Curr Opin Obstet Gynecol. 1992;4(5):702–11. doi:10.1097/00001703-199210000-00010. [PubMed]
- 15.Polson DW, Mason HD, Saldahna MBY, Franks S. Ovulation of a single dominant follicle during treatment with low-dose pulsatile FSH in women with polycystic ovary syndrome. Clin Endocrinol (Oxf). 1987;26:205–12. doi:10.1111/j.1365-2265.1987.tb00778.x. [DOI] [PubMed]
- 16.Albano C, Smitz J, Camus M, et al. Pregnancy and birth in an in vitro fertilization cycle after controlled ovarian stimulation in a woman with a history of allergic reactions to human menopausal gonadotrophin. Hum Reprod. 1996;11:1632–4. [DOI] [PubMed]
- 17.Whitman-Elia GF, Banks K, O’Dea LS. Recombinant follicle stimulating hormone in a patient hypersensitive to urinary-derived gonadotrophins. Gynecol Endocrinol. 1998;12:209–12. doi:10.3109/09513599809015547. [DOI] [PubMed]
- 18.Al-Inany HG, Aboughar M, Mansour R, Proctor M. Recombinant versus urinary human chorionic gonadotropin for ovulation induction in assisted conception. Cochrane Database Syst Rev. 2005;18(2):CD003719. Apr. [DOI] [PubMed]
- 19.Discoll GL, Tyler JP, Hangan JT, Fisher PR, Birdsall MA, Kniight DC. A prospective, randomized, controlled, double-blind, double-dummy comparison of recombinant and urinary HCG for inducing oocyte maturation and follicular luteinization in ovarian stimulation. Hum Reprod. 2000;15:1305–10. doi:10.1093/humrep/15.6.1305. [DOI] [PubMed]
- 20.Ludwig M, Doody KJ, Doody KM. Use of recombinant human chorionic gonadotropin in ovulation induction. Fertil Steril. 2003;79:1051–9. doi:10.1016/S0015-0282(03)00173-0. [DOI] [PubMed]
- 21.European Recombinant Human Chroionic Gonadotrophin Study Group. Induction of final follicular maturation and early luteinization in women undergoing ovulation induction for assisted reproduction treatment-recombinant HCG versus urinary HCG. Hum Reprod. 2000;15:1446–51. doi:10.1093/humrep/15.7.1446. [DOI] [PubMed]
- 22.Uhler ML, Beltsos AN, Grotjan HE, Lederer KJ, Lifchez AS. Age-matched comparison of recombinant and urinary HCG for final follicular maturation. Reprod Biomed Online. 2006;13(3):315–20. [DOI] [PubMed]
- 23.Wehmann RE, Nisula BC. Characterization of a discrete degradation product of the human chorionic gonadotropin β-subunit in humans. J Clin Endocrinol Metab. 1980;51(1):101–5. [DOI] [PubMed]
- 24.Penarrubia J, Balasch J, Fabregues F, et al. Recurrent empty follicle syndrome successfully treated with recombinant human chorionic gonadotrophin. Hum Reprod. 1999;14:1703–6. doi:10.1093/humrep/14.7.1703. [DOI] [PubMed]


