Abstract
Purpose
We focused on the improvements of prenatal diagnosis by the analysis of DNA from maternal plasma, using Huntington disease as a model of disease.
Methods
We studied plasma from a pregnancy at risk of having a fetus affected with Huntington disease by the use of two direct analysis of the mutation and polymorphic STRs.
Results
Direct methods were not informative. Analysis with STRs revealed the presence of the allele that does not co-segregate with the disease, thus the fetus was healthy.
Conclusions
This strategy is very useful to face complex cases when the direct study is not informative not only for Huntington disease but also for many other disorders.
Keywords: Huntington disease, Maternal plasma, Non-invasive, Prenatal diagnosis, STRs
Introduction
The potential use of fetal DNA from maternal plasma for prenatal diagnosis has been evaluated since the last decade. Due to the overriding presence of maternal DNA in plasma, we are only able to detect fetal sequences of paternal origin. For years, the detection of male specific DNA sequences has been mainly used for the assessment of fetal sex, Rh factor [1, 2] and pathological conditions such as preeclampsia [3]. Nowadays, the principal use for prenatal diagnosis is the detection of fetal aneuploidies, most of which are maternally inherited. The second main purpose for prenatal diagnosis is the diagnosis of single gene disorders. Our group has focused on the non-invasive use of fetal DNA in this field [4–6], and some investigators have also considered this idea [7].
The aim of this work is to prove that fetal DNA detection in maternal plasma could be very useful for dominant and paternally inherited disorders. We have been working in this area using QF-PCR [5, 6] in Huntington disease (HD) because we are a centre of reference of this disorder.
The prenatal diagnosis of HD requires a DNA extraction and analysis from chorion villus sample (CVS) between 10–13 weeks of gestation. This technique includes a 0.5–1% risk of abortion. HD is an autosomal dominant neurodegenerative disease with adult onset for which there is no cure. The responsible mutation is an expanded trinucleotide (CAG) in the IT-15 gene (Huntingtin gene) located at 4p16.3. Presymptomatic, predictive, and prenatal testing is possible by direct mutation analysis. Using haplotype analysis, it is also possible to perform exclusion testing so that the chromosome at a 50% risk can be identified as present in the fetus or not.
Unaffected individuals show between 6–35 (CAG) repeats, affected patients show more than 35 repeats. Right next to this region, there is a polymorphic CCG tract with 7–12 repeats in normal population [8].
Direct analysis can be performed by two different primer sets. The primer sets that size the CAG region, including the CCG polymorphism, can lead to some inconsistencies in disease vs. normal size ranges. Furthermore, an associated CAA trinucleotide deletion, which follows the CAG tract described in some patients, can prevent the primer from hybridizing, resulting in a false homozygosity.
In our previous work we successfully performed the diagnosis with fetal DNA from maternal plasma by the use of primers flanking the CAG + CCG repeats region. However, our experience in HD diagnosis showed that direct analysis is not informative when parents share allele size, when we find size range inconsistencies and when the inherited paternal allele is very large (expanded allele). Moreover, the low quantity of fetal DNA makes detection of fetal mutation difficult and increases the chance of false results or none at all.
In this study we have tried to solve a complex case of non-invasive HD prenatal diagnosis where direct method were not useful, using different simple techniques. We used two different primer sets: CAG and CAG + CCG region primers. We used informative STRs closely linked to the HD gene as an indirect analysis using DNA of the parents and affected relatives. In this way, we were able to detect the presence of the healthy paternally inherited allele, resulting in an HD unaffected fetus.
Materials and methods
We studied a woman at 12 weeks of gestation married to a HD patient attending our unit for prenatal diagnosis. We took 10 ml of peripheral blood from the pregnant woman. The sample was collected in EDTA sterile tube, previous to CVS, and under informed consent. We also collected 10 ml of peripheral blood from the husband and affected relatives.
DNA extraction
Plasma was obtained from 0.8 ml of maternal plasma with the QIAamp DNA Blood Mini Kit (QIAGEN; Hilden, Germany) according to the manufacturer’s recommended protocol and eluted in a final volume of 100 μl. We also extracted DNA from whole blood with the Bio Robot EZ1 (QIAGEN; Hilden, Germany).
PCR amplification
Direct analysis PCR were performed in a GeneAmp PCR System 2700 (Applied Biosystems), with a final volume of 50 μl, 20 μl of plasma DNA, along with 1.5 mM of magnesium chloride, 2.5 μl of 10× buffer (Roche, Indianapolis, USA), 200 mM dNTPs (labClinics techlineSM, USA) and 1.5 U of Fast Start taq Polymerase (Roche) per reaction and 10 pmol of each primer:
PCR for the CAG region F: 5′(6-FAM-ATGGCGACCCTGGAAAGCTGATGAA-3′) and R: (5′-CGGCGGTGGCGGCTGTTG-3′) (Applied Biosystems, Foster City, USA). Cycling conditions were 94°C 5 min, 55°C 1 min, 72°C 1 min, then 50 cycles of 94°C 45 s 65°C 40 s and final extension of 72°C 10 min.
Primers for the CAG + CCG region were: F:5′(6-FAM-ATGGCGACCCTGGAAAGCTGATGAA-3′) and R: (5′-GGCGGCTGAAGAAGCTGAGGA-3). Cycling conditions were 94°C 5 min, 55°C 1 min, 72°C 1 min, 50 cycles of 94°C 45 s 58°C 40 s and final extension of 72°C 10 min.
PCRs for STR typing were performed in a volume of 50 μl with 10 pmol of each primer. PCRs were carried out in a GeneAmp PCR System 2700 (Applied Biosystems) for 20 cycles at 94°C for 30 s, 55°C for 30 s and 72°C for 90 s, and 20 cycles at 89°C for 30 s, 65°C for 30 s and 72°C for 90 s, with a final extension time of 30 min at 72°C.
We used STRs located near the IT-15 gene (1 cM from gene), D4S3034, D4S126, D4S127, D4S412. We chose D4S412 as the most informative: D4S412: F: 5′(6-FAM-ACTACCGCCAGGCACT-3′) and R: (5′- CTAAGATATGAAAACCTAAGGGA-3′).
DNA fragments for all reactions were electrophoresed in an ABI Prism 3100 Genetic Analyzer and analyzed with the GeneMapper 3.5 software package (Applied Biosystems, Foster City, USA).
Results
Using primers for the CAG region, results showed two alleles of 18 and 21 repeats in the mother DNA. Analysis of the father showed 21 and 44 repeats. Using maternal plasma we could find one allele of 21 repeats that could have been confusing. We continued with the second pair of primers comprising the CAG + CCG region and we found two alleles of 18 and 21 repeats in the mother. Analysis of father DNA revealed two alleles of 21 and 45 repeats each. Both direct approaches were therefore discarded.
We used these STRs for the indirect analysis: D4S412, D4S3034, D4S126 and D4S127 STRs. To abridge, we show results from D4S412 as the most informative.
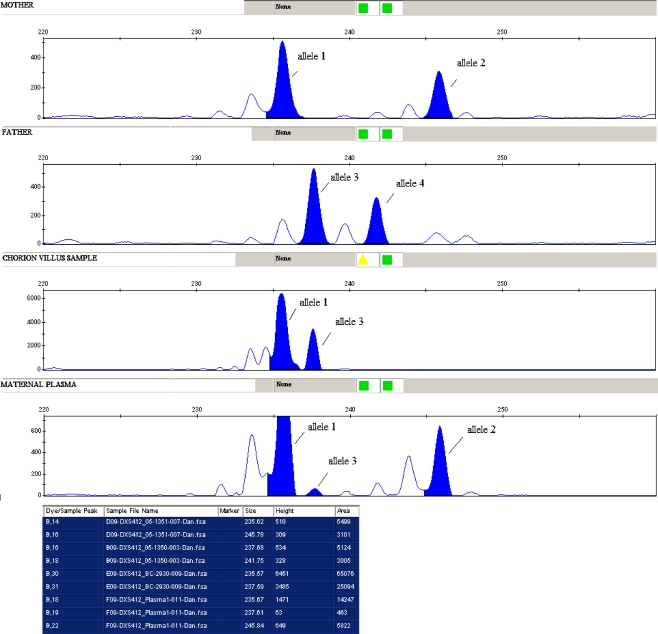
We amplified DNA from whole blood from the mother and father. Results from paternal DNA showed a 238 bp allele and a 242 bp allele. Upon analysis of affected relatives, we found out that a 242 bp allele was the one that co-segregates with the disease.
In maternal whole blood DNA analysis, we found a 236 bp and 246 bp alleles. Using DNA from plasma we found a 236 bp and 246 bp alleles and a small peak of 238 bp that could correspond to the fetal allele inherited from the father (Fig. 1) and did not appear in the analysis of maternal DNA from the whole blood. We concluded that the inherited allele was the 238 bp that does not co-segregate with the disease, thus the fetus was healthy.
Fig. 1.
D4S412 analysis for maternal DNA (allele 1 and 2), paternal DNA (allele 3 and 4), chorion villus sample (allele 1 and 3). In the maternal plasma an allele 1 and 2 is showed and a small peak corresponding to paternal allele 3
These results were later confirmed with the analysis of DNA from CVS.
Discussion
The use of fetal DNA in maternal plasma is leading to the development of many strategies for non invasive prenatal diagnosis (NIPD). The low amount of fetal DNA and the high background of maternal DNA sequences complicate the labor seriously. Our proposal is to try to avoid this drawback using all our resources. Direct method is the safest diagnosis procedure for HD. In this work direct method with maternal plasma was not informative which made the diagnosis difficult. Here, we show results from one STRs, (D4S412 1% risk of recombination) and we emphasize that it is essential to use two or more STRs proximal and distal to the gene to avoid any misdiagnosis due to a recombination event.
Our experience showed that each family case could need a different technical approach. We consider that it is worth using all the available tools in the laboratory to obtain a reliable diagnosis.
We have performed a NIPD using direct and indirect analysis resulting in a reliable NIPD. The highlight of this report is to show the advantage of having different strategies to ensure the diagnosis for any dominant disorder by the use of maternal plasma. This strategy is very useful to confirm direct studies, the technology is low cost and easily available in any genetic laboratory.
A great advantage is the early time of gestation, in which we can perform this analysis. Results can be obtained in 48 h, so that any result can be confirmed in a short time. At this moment fetal DNA can be detected at 7 weeks of gestation by the use of real time PCR in our laboratory [9]. This early time of gestation would allow undergoing chorion villus sampling in case it would be required.
Our group is now performing further HD prenatal studies to evaluate the accuracy of these methods.
Because this is an adult onset disorder, indirect analysis as an exclusion testing remains a valuable tool for couples who do not wish to define their own risk, but nonetheless wish to have children who will not develop HD [10]. The described assay could be a helpful tool in those cases. Jasper et al. [11] have developed a similar protocol for preimplantation diagnosis that provided couples with the opportunity to minimize the likelihood of disease transmission to their children, without the requirement for predictive testing. This strategy could be offered as a confirmatory tool in preimplantation field and as a pre-test to those couples afraid of the abortion risk of invasive prenatal techniques.
Advances and improvements in the sensitivity of fetal DNA detection are increasing the diagnostic applications. Huang et al. [12] described an assay that involves an automated system that yields higher quantities of cell-free DNA of fetal origin that appeared to be of greater purity. Recent studies have also described a detection of fetal single gene point mutations in the FGFR3 gene (achondroplasia) in DNA from maternal plasma. This study uses either the matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS) assay with single allele base extension reaction (SABER) approach or the size fractionation of cf-DNA in maternal plasma [13].
Our assays indicate that fetal DNA in maternal plasma could be a useful source for a safe prenatal diagnosis, applicable in clinical practice for many disorders.
Conclusions
This strategy is very useful to face complex cases using maternal plasma, when the direct study is not informative not only for Huntington disease but also for many others disorders.
Acknowledgements
F.I.S. supported this project (PI040218). We thank Foundation Conchita Rábago for their support to MC González-González and Ana Bustamante Aragonés. F.I.S. and Foundation Conchita Rábago approved and agreed to be named in the acknowledgments of this paper.
Footnotes
Capsule Prenatal diagnosis of single gene disorders such as Huntington disease are possible using maternal blood and different simple laboratory techniques.
References
- 1.Lo YM. Fetal RhD genotyping from maternal plasma. Ann Med. 1999;31(5):308–122. [DOI] [PubMed]
- 2.Johnson KL, Dukes KA, Vidaver J, LeShane ES, Ramirez I, Weber WD, Bischoff FZ, Hahn S, Sharma A, Dang DX. Interlaboratory comparison of fetal male DNA detection from common maternal plasma samples by real-time PCR. Clin Chem. 2004;50(3):516–21. doi:10.1373/clinchem.2003.024380. [DOI] [PubMed]
- 3.Lo YM, Leung TN, Tein MS, Sargent IL, Zhang J, Lau TK, Haines CJ, Redman CW. Quantitative abnormalities of fetal DNA in maternal serum in preeclampsia. Clin Chem. 1999;45(2):184–8. [PubMed]
- 4.Gonzalez-Gonzalez MC, Garcia-Hoyos M, Trujillo MJ, Rodriguez de Alba M, Lorda-Sanchez I, Diaz-Recasens J, Gallardo E, Ayuso C, Ramos C. Prenatal detection of a cystic fibrosis mutation in fetal DNA from maternal plasma. Prenat Diagn. 2002;22:946–8. doi:10.1002/pd.439. [DOI] [PubMed]
- 5.Gonzalez-Gonzalez MC, Trujillo MJ, Rodriguez de Alba M, Garcia-Hoyos M, Lorda-Sanchez I, Diaz-Recasens J, Ayuso C, Ramos C. Huntington disease-unaffected fetus diagnosed from maternal plasma using QF-PCR. Prenat Diagn. 2003;23:232–4. doi:10.1002/pd.570. [DOI] [PubMed]
- 6.Gonzalez-Gonzalez MC, Trujillo MJ, Rodriguez de Alba M, Ramos C. Early Huntington disease prenatal diagnosis by maternal semiquantitative fluorescent-PCR. Neurology. 2003;60:1214–5. [DOI] [PubMed]
- 7.Sekizawa A, Saito H. Prenatal screening of single-gene disorders from maternal blood. Am J Pharmacogenomics. 2001;1(2):111–7. doi:10.2165/00129785-200101020-00004. [DOI] [PubMed]
- 8.Andrew SE, Goldberg YP, Theilmann J, Zeisler J, Hayden MRA. CCG repeat polymorphism adjacent to the CAG repeat in the Huntington disease gene: implications for diagnostic accuracy and predictive testing. Hum Mol Genet. 1994;3(1):65–7. doi:10.1093/hmg/3.1.65. [DOI] [PubMed]
- 9.Bustamante-Aragones A, Rodriguez de Alba M, Gonzalez-Gonzalez C, Trujillo-Tiebas MJ, Diego-Alvarez D, Vallespin E, Plaza J, Ayuso C, Ramos C. Foetal sex determination in maternal blood from the seventh week of gestation and its role in diagnosing haemophilia in the foetuses of female carriers. Haemophilia. 2008;14(3):593–8. doi:10.1111/j.1365-2516.2008.01670.x. [DOI] [PubMed]
- 10.Creighton S, Almqvist EW, MacGregor D, Fernandez B, Hogg H, Beis J, Welch JP, Riddell C, Lokkesmoe R. Predictive, pre-natal and diagnostic genetic testing for Huntington’s disease: the experience in Canada from 1987 to 2000. Clin Genet. 2003;63(6):462–475. assay. Prenat Diagn. 2007;27(1):11–17. doi:10.1002/pd.1608 [DOI] [PubMed]
- 11.Jasper MJ, Hu DG, Liebelt J, Sherrin D, Watson R, Tremellen KP, Hussey ND. Singleton births after routine preimplantation genetic diagnosis using exclusion testing (D4S43 and D4S126) for Huntington’s disease. Fertil Steril. 2006;85(3):597–602. doi:10.1016/j.fertnstert.2005.08.050. [DOI] [PubMed]
- 12.Huang DJ, Zimmermann BG, Holzgreve W, Hahn S. Improvement of methods for the isolation of cell-free fetal DNA from maternal plasma: comparison of a manual and an automated method. Ann N Y Acad Sci U S A. 2006;1075:308–12. doi:10.1196/annals.1368.041. [DOI] [PubMed]
- 13.Li Y, Page-Christiaens GC, Gille JJ, Holzgreve W, Hahn S. Non-invasive prenatal detection of achondroplasia in size-fractionated cell-free DNA by MALDI-TOF MS assay. Prenat Diagn. 2007;27(1):11–7. doi:10.1002/pd.1608. [DOI] [PubMed]