Abstract
Objective
To determine the predictive value of euploid embryos in women with recurrent implantation failure undergoing repeated IVF-ET cycles with PGD (PGD).
Design
Cohort of IVF-PGD cycles in a tertiary care ART facility.
Materials and method(s)
Fifty-five consecutive patients with repeated implantation failure (more than three failed IVF-ET cycles) underwent two or more PGD cycles for aneuploidy testing. Mean maternal age was 37.6 ± 5.3 years. Biopsies were performed on day 3. One blastomere was removed from each pre-embryo, fixed and analyzed by multicolor and multi-probe FISH for chromosomes X and Y, 13, 15, 16, 17, 18, 21, and 22.
Result(s)
Forty-three of 55 patients (78%) undergoing PGD had at least one euploid embryo for transfer. Of these 31 patients (72%) also had at least one euploid embryo available for transfer with the second cycle. Of the 12 (28%) patients with no euploid embryos available for transfer with the second IVF/PGD cycle, five had a third cycle of PGD and two of these had euploid embryos available for transfer. Seventeen of the 31 patients (55%) who had euploid embryos on the second PGD cycle conceived. The ongoing pregnancy and implantation rates in patients with at least one euploid embryo were 40% and 18%, respectively. Twelve of the 55 patients (22%) had no euploid embryos available for transfer on the first PGD cycle, but on the second PGD cycle, six (50%) of these had euploid embryos for transfer. Only two pregnancies were achieved among this group of women, yielding a pregnancy rate of 17%, but both conceptions resulted in miscarriage. Of the six patients with no euploid embryos available after the second PGD cycle, four patients had a third IVF/PGD cycle, but none had euploid embryos available for transfer. Also, among women with euploid embryos available only in either the first or second PGD cycle, but not both, no ongoing pregnancy was achieved. No woman who had a PGD cycle productive of no euploid embryos had an ongoing pregnancy. Significant differences were found in terms of ongoing pregnancy (40%, P < 0.05) and implantation rates (18%, P < 0.05) in women with euploid embryos available for transfer with the first and second IVF/PGD cycles, compared to women with no euploid embryos available for transfer with either the first or second cycle. The positive predictive value of the first euploid cycle predicting a second euploid cycle was 72%, 95% CI 0.66–0.78. The negative predictive value of an aneuploid cycle was 50%, 95% CI 0.27–0.72. The sensitivity and specificity of the first PGD cycle predicting the second was 84%, 95% CI 0.77–0.91 and 33%, 95% CI 0.18–0.48, respectively.
Conclusion(s)
Even with a history of recurrent implantation failure, the availability of euploid embryos, especially on two, consecutive PGD cycles is associated with high ongoing pregnancy and implantation rates. Conversely, the absence of euploid embryos for transfer predicts poor reproductive outcome, even if subsequent cycles do yield euploid embryos.
Keywords: Aneuploidy, Recurrent IVF failure, Multicolor FISH, Preimplantation genetic diagnosis
Introduction
The introduction of preimplantation genetic diagnosis (PGD) in reproductive medicine has enabled chromosomal analysis of embryos prior to embryo transfer. Chromosomal aberrations in embryos contribute not only to spontaneous abortions, but also recurrent failed implantation among women with unexplained infertility, regardless of maternal age [1, 2]. Aneuploidy is the leading cause of spontaneous abortions and mental retardation among women of advancing age [3, 4]. What remains unclear is the recurrence of aneuploidy with repeated attempts and the predictive value of subsequent reproductive outcome.
We studied women with repeated implantation failure who underwent two or more consecutive PGD cycles for aneuploidy testing of embryos prior to embryo transfer after a failed first attempt. The aim of the study is to determine the predictive value of the initial PGD cycle attempt based on the presence or absence of euploid embryos on subsequent cycles with respect to reproductive outcome.
Materials and methods
Data collection and analysis from our IVF database were approved by Institutional review board at Women and Infants Hospital of Rhode Island, Brown University. Data from a cohort of women undergoing IVF/PGD cycles from September of 2001 to December of 2005 were analyzed and fifty-five consecutive patients with unexplained repeated implantation failure (more than three failed IVF-ET cycles) who underwent two or more consecutive IVF/PGD cycles for aneuploidy testing of pre-embryos prior to embryo transfer were identified from the initial IVF/PGD cohort of women. The mean maternal age for the study group was 37.6 ± 5.3 years. The mean number of prior failed IVF cycles for the study group was 4.8 cycles. A workup to rule out possible causes of repeated implantation failure was negative to include uterine imaging and parental karyotypes. Results from the first PGD cycle were used to group women. Women were categorized into two groups, a good prognosis and poor prognosis group after the first IVF/PGD cycle attempt based on the availability of at least one euploid embryo for transfer. Women with at least one euploid embryo for transfer were identified as the good prognosis group and women with all aneuploid embryos were identified as the poor prognosis group. There is no statistical difference in terms of maternal age in both groups.
The ovarian stimulation protocols used consisted of either a standard GnRH antagonist or GnRH agonist long-protocol with gonadotropins as determined by the managing physician [5]. No difference was noted in terms of embryonic aneuploidy between agonist and antagonist cycles. In brief, oocyte retrieval was performed 36 h after the administration of human chorionic gonadotropin [6]. Oocytes were fertilized by either conventional insemination or Intracytoplasmic sperm injection for male factor infertility, prior history of poor fertilization and unexplained infertility.
On day 3, one blastomere was removed from each embryo with four or more cells using acidic Tyrode’s solution and micromanipulation. Blastomeres were fixed with methanol acetic acid solution and analyzed by multicolor and multi-probe fluorescence in situ hybridization (FISH) for chromosomes X and Y, 13, 15, 16, 17, 18, 21, and 22 as described by Munne et al. [7]. Biopsied embryos were cultured in blastocyst medium containing 10% SSS until the PGD results were available. Only embryos reported as normal for the chromosomes analyzed were transferred on day 4 or 5.
In both groups either one or two euploid embryos were transferred based on the availability of euploid embryos post analysis. Supernumerary high-quality euploid embryos were cryopreserved per patient request. Statistical analysis of all data point was performed using chi-square analysis and two-sided Student’s t test where appropriate.
Results
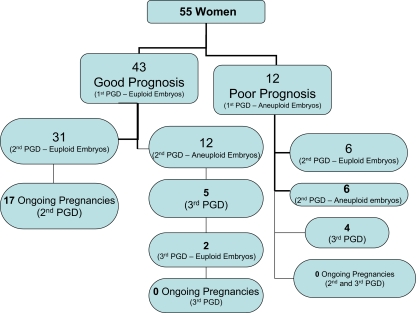
Of the 55 patients included in the study, 43 (78%) had at least one euploid embryo during the first PGD cycle (Fig. 1). Of these, 31 patients (72%) also had at least one euploid embryo available for transfer with the second PGD cycle, while 12 patients (28%) had none. Of the 12 patients with no euploid embryos available for transfer with the second cycle, five had a third cycle of PGD and two of these, had euploid embryos available for transfer. Seventeen of the 31 patients (55%) who had euploid embryos on the second IVF/PGD cycle conceived. The ongoing pregnancy and implantation rates in women with at least one euploid embryo in the first or second cycles were 40% and 18%, respectively. The average number of embryos transferred was 1.8 in women who achieved a pregnancy. No pregnancy was achieved in women who had a third PGD cycle, despite the availability of apparently euploid embryos for transfer (Fig. 1).
Fig. 1.
Summary of PGD Cycle Outcome
Of the 12 patients (22%) with no euploid embryos for transfer on the first PGD cycle, six patients had euploid embryos for transfer in the second PGD cycle. Two pregnancies resulted producing a corresponding pregnancy rate of 17%, but both miscarried. Of the six patients with no euploid embryos available for the second IVF/PGD cycle, four had a third IVF/PGD cycle, but none had euploid embryos available for transfer on PGD cycle 3 (Fig. 1).
No woman who had a PGD cycle productive of no euploid embryos had an ongoing pregnancy. Significant differences were found in terms of ongoing pregnancy (40%, P < 0.05) and implantation rates (18%, P < 0.05) in women with euploid embryos available for transfer with the first and second IVF/PGD cycles, compared to women with no euploid embryos available for transfer with either the first or second cycle. The positive predictive value of the first euploid cycle predicting a second euploid cycle was 72%, 95% CI 0.66–0.78. The negative predictive value of an aneuploid cycle was 50%, 95% CI 0.27–0.72. The sensitivity and specificity of the first PGD cycle predicting the second was 84%, 95% CI 0.77–0.91 and 33%, 95% CI 0.18–0.48, respectively. The negative and positive likelihood ratio was 0.5 and 1.25, respectively.
Discussion
An increased frequency of aneuploid embryos has been observed in a subgroup of women with recurrent implantation failure regardless of their reproductive age undergoing assisted reproductive technologies [1, 2, 4]. The incorporation of preimplantation genetic diagnosis into assisted reproductive technologies has afforded these women the chance to select and transfer only chromosomally normal embryos, as conventional methods to identify the best euploid embryo for transfer by morphological criteria alone are suboptimal [8–14]. Though increasingly being applied clinically the reproducibility of PGD for aneuploidy screening in women with repeated implantation failure remains unclear. Further, controversy still exists in the accuracy of interpreting embryonic aneuploidy from a single day 3 blastomere biopsy.
Our study focused on women with repeated implantation failure who underwent two or more consecutive IVF/PGD cycles for aneuploidy testing of pre-embryos prior to embryo transfer after a failed first attempt. These women could be categorized into two groups based on PGD results, based on the availability of at least one euploid embryo for transfer. Data from our study suggests that chromosomal analysis of pre-embryos is highly predictive of outcome, based both on the presence of euploid embryos available for transfer, and on pregnancy rate on subsequent cycles.
Our data shows that in women with unexplained recurrent implantation failure, two consecutive PGD cycles showing euploid embryo(s) is strongly associated with high ongoing pregnancy and implantation rates, 40% and 18%, respectively. Our data is in keeping with others that report the selection of chromosomally normal embryos in women with implantation failure, favorable IVF outcomes can be achieved with an associated pregnancy rate of 34% and 19.8% implantation rate, comparable to young fertile controls [15]. Conversely, our data shows that patients with no euploid embryos in a PGD cycle are highly unlikely to achieve an ongoing pregnancy in subsequent cycles. Indeed, no patient who had a cycle with only abnormal embryos went on to have an ongoing pregnancy in subsequent cycles. Hence, the presence of euploid embryos in an initial PGD cycle is highly predictive of euploid embryos being available for the second cycle and of favorable reproductive outcome. In contrast, women who have one or more PGD cycles with no euploid embryos predicts poor reproductive outcome, even if they go on to have euploid embryos on a subsequent cycle. This data should help council patients with unexplained recurrent implantation failure who face the difficult decision of whether to pursue treatments which depend on their own eggs or to desist to pursue egg donation or adoption. Acknowledging the background limitations and accuracy of determining embryonic aneuploidy from single day 3 blastomere biopsy. Our recommendation is that regardless of age, patients with unexplained recurrent implantation failure who have no euploid embryos for transfer after consecutive PGD attempts should be counseled that subsequent successful IVF using their own eggs is unlikely to result in a viable pregnancy.
References
- 1.Munne S, Sandalinas M, Magli C, Gianaroli L, Cohen J, Warburton D. Increased rate of aneuploid embryos in young women with previous aneuploid conceptions. Prenat Diagn. 2004;24:638–43. [DOI] [PubMed]
- 2.Baart EB, Martini E, van den Berg I, Macklon NS, Galjaard RJH, Fauser BCJ, Van Opstal D. Preimplantation genetic screening reveals a high incidence of aneuploidy and mosaicism in embryos from young women undergoing IVF. Hum Reprod. 2006;21:223–33. [DOI] [PubMed]
- 3.Hassold T, Hunt P. To err (meiotically) is human: the genesis of human aneuploidy. Nat Rev Genet. 2001;2:280–91. [DOI] [PubMed]
- 4.Munne S, Fischer J, Warner A, Chen S, Zouves C, Cohen J, Referring Centers PGD Group. Preimplantation genetic diagnosis significantly reduces pregnancy loss in infertile couples: a multicenter study. Fertil Steril. 2006;85:326–32. [DOI] [PubMed]
- 5.Voullaire L, Wilton L, McBain J, Callaghan T, Williamson R. Chromosome abnormalities identified by comparative genomic hybridization in embryos from women with repeated implantation failure. Mol Hum Reprod. 2002;8:1035–41. [DOI] [PubMed]
- 6.Sauer MV, Thornton MH, Schoolcraft W, Frishman GN. Comparative efficacy and safety of cetrorelix with or without mid-cycle recombinant LH and leuprolide acetate for inhibition of premature LH surges in assisted reproduction. Reprod Biomed Online. 2004;9:487–93. [DOI] [PubMed]
- 7.Van Landuyt L, De Vos A, Joris H, Verheyen G, Devroey P, Van Steirteghem A. Blastocyst formation in vitro fertilization versus intracytoplasmic sperm injection cycles: influence of the fertilization procedure. Fertil Steril. 2005;83:1397–1403. [DOI] [PubMed]
- 8.Munne S, Magli C, Bahce M, et al. Preimplantation diagnosis of the aneuploidies most commonly found in spontaneous abortions and live births: XY, 13, 14, 15, 16, 18, 21, 22. Prenat Diagn. 1998a;18:1459–66. [DOI] [PubMed]
- 9.Baltaci V. relationship between embryo quality and aneuploidies. Reprod BioMed Online. 2006;12:77–82. [DOI] [PubMed]
- 10.Sandalinas M, Sadowy S, Alikani M, Calderon G, Cohen J, Munne S. Developmental ability of chromosomally abnormal human embryos to develop to the blastocyst stage. Hum Reprod. 2001;16:1954–8. [DOI] [PubMed]
- 11.Magli MC, Jones GM, Gras L, Gianaroli L, Korman I, Trounson AO. Chromosome mosaicism in day 3 aneuploid embryos that develop to morphologically normal blastocysts in vitro. Hum Reprod. 2000;15:1781–6. [DOI] [PubMed]
- 12.Gianaroli L, Magli MC, Ferraretti AP, Fiorentino A, Garrisi J, Munne S. Preimplantation genetic diagnosis increase the implantation rate in human in vitro fertilization by avoiding the transfer of chromosomally abnormal embryos. Fertil Steril. 1997;68:1128–31. [DOI] [PubMed]
- 13.Wilton L. preimplantation genetic diagnosis for aneuploidy screening in early human embryos: a review. Prenat Diagn. 2002;22:512–8. [DOI] [PubMed]
- 14.Staessen C, Platteau P, Van Assche E, Michiels A, Tournaye H, Camus M, Devroey P, Liebaers I, Van Steirteghem A. Comparison of blastocyst transfer with and without preimplantation genetic diagnosis for aneuploidy screening in couples with advanced maternal age: a prospective randomized controlled trial. Hum Reprod. 2004;19:2849–58. [DOI] [PubMed]
- 15.Pehlivan T, Rubio C, Rodrigo L, Romero J, Remohi J, Simon C, Pellicer A. Impact of preimplantation genetic diagnosis on IVF outcome in implantation failure patients. Reprod BioMed Online. 2002;6:232–7. [DOI] [PubMed]