Abstract
Purpose
For this study, the impact of basal FSH levels on ART outcomes was assessed.
Methods
From June 2003 to May 2006, 191 ART cycles were performed in our hospital. All cases were treated with GnRH-a long protocol. The patients were classified according to their basal FSH level as follows: group A: FSH < 10 IU/l, group B: 10 ≦ FSH < 15 IU/l, and group C: 15 IU/l ≦ FSH. ART outcomes were compared among the three groups.
Results
The number of retrieved oocytes in group A was significantly higher than in group B, but fertilized oocytes and the pregnancy rates were comparable. The pregnancy rate in group C was not significantly lower than those found in either group A or B, but the trend was lower.
Conclusion
Oocytes retrieved from the patients who showed basal FSH levels below 15 IU/l were found to possess significant pregnancy potential.
Keywords: ART, Basal FSH, Pregnancy rate
Introduction
In assisted reproductive technology (ART), success depends on obtaining as many high-quality oocytes as possible. In a previous study, we reported that it was possible to evaluate the maturity of the retrieved oocytes and the quality of the embryos by indirect methods: measuring or monitoring intra-ovarian changes and evaluating the incidence of apoptotic granulosa cells [1], measuring the oxidative stress in granulosa cells [2], studying alternations in the cell cycles of granulosa cells [3], or measuring the concentration of hyaluronan in follicular fluids [4].
In a recent study, we reported that the measurement of perifollicular arterial blood flow enabled an evaluation of the quality of the collected oocytes and ovulated oocytes. This was found to be useful in the evaluation of both the quality of the oocytes and in the developmental competence of the corresponding oocytes [5–7].
Testing follicle stimulating hormone (FSH) levels on day 3 of the cycle is a common predictor for success in ART treatment [8], and is routinely used to determine the ovarian reserve in many IVF units prior to starting ART treatment. Many women with elevated basal FSH have been discouraged from undergoing ART treatment because an elevated basal FSH level had indicated a reduced ovarian reserve [9], which lowered their chances for success [10]. Recently, El-Toukhy et al. reported that lower age does not necessarily protect against the adverse effects of reduced ovarian reserve. In that report, it was suggested that an elevated day-3 basal FSH level is associated not only with a low response, but also with oocytes of poor quality, which in turn can lead not only to a reduction in pregnancy rates but also to a rise in miscarriage rates [11]. However, recent reports have suggested that the basal FSH level was useful as an indicator of a quantitative rather than qualitative ovarian response for ovarian hyperstimulation—in other words, basal FSH level did not reflect on the quality of oocytes [10, 12, 13]. Moreover, a multicentre meta-analysis reported that the clinical value of testing for basal FSH was restricted to a small number of patients, and that basal FSH levels should not be used as a routine test for the prediction of IVF outcome [13]. Therefore, evaluation of basal FSH levels prior to the start of ART treatment is a controversial issue. This study examined whether or not basal FSH levels will reflect the ART outcomes.
Materials and methods
Patients and ovarian hyperstimulation
From June 2003 to May 2006, 191 cycles in 191 patients, who underwent ART treatment in the Division of Reproductive Medicine, Department of Perinatal Medicine and Maternal Care, National Center for Child Health and Development, were examined for this study. Informed consent was obtained from all patients and the study was approved by the Institutional Review Board of the National Center for Child Health and Development. The indications for ART treatment included tubal factor infertility (26.7%), unexplained infertility (39.8%), endometriosis (17.8%), and male factor infertility (15.7%). All male factor infertility cases were treated by intracytoplasmic sperm injection (ICSI). All 191 cycles of the 191 patients were hyperstimulated according to our previous report [14]. Briefly, the ovarian hyperstimulation protocol with GnRH-agonist was performed as follows: 600 μg of busereline acetate (Sprecur nasal, Mochida, Tokyo or Buserecure, Fuji Pharma, Tokyo) was administered daily intranasally from the midluteal phase of the pretreatment cycle to the day of human chorionic gonadotropin (hCG) injection. On the third day, of each patient’s menstrual cycle, either 225 international units (IU) of recombinant follicle stimulating hormone (rec-FSH; Foristim ®, Organon, Osaka, Japan), or 300 IU of human menopausal gonadotropin (hMG; Humegon®, Organon, Osaka, Japan), was administered on two consecutive days, and either 150 IU of rec-FSH or 225 IU of hMG was given daily until a dominant follicle reached 16mm in diameter. From September 2005, patients were received rec-FSH. HCG (10,000 IU; Gonatropin®; Mochida, Tokyo, Japan) was administered 35 h before retrieval of oocytes.
IVF/ICSI procedure and ET
The IVF procedure has been previously described [15]. Oocytes were retrieved using transvaginal ultrasound guidance. All follicles with a mean diameter of >15 mm were aspirated separately, using an 18-gauge needle connected to a tube and a 20-ml syringe for suction. The needle was removed after the aspiration of each follicle. The aspiration was interrupted and a new syringe was used if blood appeared in the tube connected to the syringe, thus avoiding any blood contamination. Culture media was not used for washing the follicle. Semen was collected by masturbation. After washing, sperm were allowed to swim upward for 20–30 min. In cases without male factor infertility, insemination was performed by incubating each oocyte with 50–100 thousand motile sperm within 3–4 h after their collection. For male factor infertility patients, ICSI was performed according to the previous report [16]. Oocytes were examined under a dissecting microscope 16–18 h after insemination or ICSI. The presence of two pronuclei with an extrusion of the second polar body was used as evidence of successful fertilization. Embryos that developed more than 8 cells and showed fragmentation of 10% or less 72 h after retrieval of oocytes were defined as morphologically good embryos.
Embryos were then replaced transcervically into the mother’s uterus 72 h after the insemination or ICSI. The number of transferred embryos was limited to two in order to prevent a multiple birth pregnancy. For luteal support, 3,000 IU of hCG was injected on the next day of ET, and on the 4th, 7th, 10th days after ET, and an oral estrogen and progesterone combination drug was administered for 10 days after ET. Hydroxyprogesterone caproate instead of hCG was given every 4 days to patients with higher risks of induced ovarian hyperstimulation syndrome. A pregnancy was defined as such, when the development of the gestational sac was visualized by transvaginal ultrasound imaging on the 21st day after oocyte retrieval.
Hormonal assay and comparison of ART parameters
Basal FSH levels were checked on the 3rd or 4th day of pretreatment cycles using commercially available ELISA kits (IMMULIZE 2000; Diagnostic Products Corporation, LA CA). The patients were divided into three groups according to FSH levels: the patients with FSH levels less than 10 IU/l were classified to group A; patients with more than 10 IU/l, yet less than 15 IU/l, were classified to group B; and, patients with an FSH level of more than 15 IU/l were classified to group C. The numbers of retrieved oocytes, fertilized oocytes, and transferred embryos, the fertilization rate, the embryo transfer rate, the pregnancy rate, and the miscarriage rate were compared among the three groups. Furthermore, in the subgroup—under 40 years of age—the pregnancy rate was also compared. A miscarriage was defined as a pregnancy loss before 22 weeks of gestation, and the fertilization rate was defined as the number of two pronuclear (2PN) embryos divided by the number of retrieved oocytes for each treatment cycle x 100 (%).
Data were analyzed with the Statistics Package for Social Sciences (SPSS, Surrey, UK). Statistical analysis was performed using an unpaired t test and square test and statistical significance was set at p < 0.05.
Results
The background and indication of ART treatment are summarized in Table 1. In a total of 191 cycles, 151 cycles were in group A, and 26 and 14 were in group B and C, respectively. The mean age in groups A, B and C were 36.2 ± 3.5, 36.8 ± 4.4 and 38.1 ± 4.3 (mean ± SD), respectively, and there was no significant difference among the three groups. The duration of infertility, the number of previous ART attempts and the indications of ART treatment are shown in Table 1 with no significant difference among the three groups.
Table 1.
The background and indications of ART treatment among three groups
| Group A | Group B | Group C | |
|---|---|---|---|
| Number of patients | 151 | 26 | 14 |
| Number of stimulated cycles | 151 | 26 | 14 |
| Age (years) | 36.2 ± 3.5 (25–45) | 36.8 ± 4.4 (28–44) | 38.1 ± 4.3 (29–44) |
| Duration of infertility (years) | 3.5 ± 2.8 (0.3–15) | 2.8 ± 2.1 (0–8) | 4.6 ± 4.6 (0.5–16) |
| Number of previous ART attempts (times) | 0.8 ± 1.9 (0–10) | 0.8 ± 1.7 (0–8) | 1.6 ± 2.2 (0–7) |
| Percentage of ICSI cycles (%) | 11.3 | 3.8 | 14.3 |
| Indication (%) | |||
| Tubal factor | 28.5 | 23.1 | 14.3 |
| Male factor | 15.9 | 7.7 | 28.6 |
| Unexplained infertility | 39.7 | 42.3 | 35.7 |
| Endometriosis | 15.9 | 26.9 | 21.4 |
Values are mean ± SD
Parentheses are ranges.
The outcome of ART treatment among the three groups is summarized in Table 2. The basal FSH levels in group A, B and C were 7.0 ± 1.5, 12.0 ± 1.6, and 28.2 ± 18.5 (IU/l; mean ± SD), respectively. The duration of stimulation (days; mean ± SD) and the required gonadotropin doses until oocyte retrieval (ampoules; mean ± SD) were 8.0 ± 2.6 and 24.3 ± 8.0 in group A, 8.2 ± 2.0 and 26.1 ± 6.0 in group B, and 9.6 ± 3.6 and 30.6 ± 10.7 in group C, respectively. The patients in group C, needed a significantly longer stimulation period and a significantly higher number of ampoules of required gonadotropins for oocyte retrieval in comparison to group A (p < 0.05 and p < 0.01, respectively). The number of retrieved oocytes, fertilized oocytes and transferred embryos in group A (mean ± SD) were 8.7 ± 6.1, 4.6 ± 3.9 and 1.8 ± 0.8, respectively, with significantly higher parameters than in group C (2.2 ± 2.0, 1.4 ± 2.0 and 0.9 ± 0.7, respectively, p < 0.001). Those in group B were 5.5 ± 6.4, 4.0 ± 6.2, and 1.6 ± 0.9, respectively, showing a significant difference in comparison with group A in regards to the number of retrieved oocytes and with group C in regards to transferred embryos. Regarding oocyte retrieval rates per cycle, no significant difference was confirmed among the three groups (99.3% in group A, 92.3% in group B and 100% in group C).
Table 2.
The outcomes of ART treatment among three groups
| Group A | Group B | Group C | |
|---|---|---|---|
| Basal FSH level (IU/l) | 7.0 ± 1.5 (1.65–9.96) | 12.0 ± 1.6 (10–14.7) | 28.2 ± 18.5 (15–85) |
| Duration of stimulation (days) | 8.0 ± 2.6 (4–25) | 8.2 ± 2.0 (6–14) | 9.6 ± 3.6 (5–19)a |
| The required gonadotropin doses until oocyte retrieval (ampoules) | 24.3 ± 8.0 (8–76) | 26.1 ± 6.0 (18–41) | 30.6 ± 10.7 (17–59)b |
| Number of retrieved oocytes | 8.7 ± 6.1 (0–33) | 5.5 ± 6.4 (0–32)c | 2.2 ± 2.0 (1–8)d |
| Oocyte retrieval rate (%) | 99.3 | 92.3 | 100 |
| Number of fertilized oocytes | 4.6 ± 3.9 (0–20) | 4.0 ± 6.2 (0–32) | 1.4 ± 2.0 (0–8)d |
| Fertilization rate (%) | 90.1 | 91.7 | 85.7 |
| Number of transferred embryos | 1.8 ± 0.8 (0–3) | 1.6 ± 0.9 (0–3) | 0.9 ± 0.7 (0–2)d,e |
| Embryo transfer rate (%) | 86.8 | 80.8 | 71.4 |
| Pregnancy rate (%; per ET) | 31.3 | 38.1 | 10 |
| Miscarriage rate (%) | 26.8 | 50 | 0 |
Values are mean ± SD
Parentheses are ranges
aA vs C; p < 0.05
bA vs C, p < 0.01
cA vs B, p < 0.05
dA vs C, p < 0.001
eB vs C, p < 0.05
All treatment cycles commenced with ovarian hyperstimulation using GnRH-a long protocol during the study period, and 162 cycles (84.8%) received embryo transfer. The embryo transfer rates in group A, B and C were 86.8, 80.8 and 71.4%, respectively, and there was no significant difference among the three groups. The group with the higher FSH level tended to have a lower embryo transfer rate (Table 2). Pregnancy rates per embryo transfer were 31.3% in group A, 38.1% in group B and 10.0% in group C, with no significant difference among the three groups. Miscarriage rates were 26.8% in group A, and 50.0% in group B, and there were no miscarriages in group C due to the low number of pregnancy cases (Table 2).
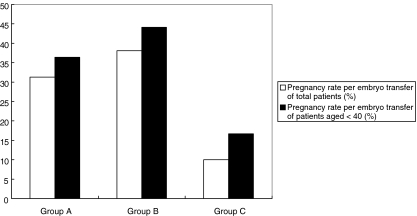
Furthermore, for those patients less than 40 years of age, there were 127 cycles in group A and 19 cycles in group B. Pregnancy rates per embryo transfer were 36.4% in group A, and 44.1% in group B, with no significant difference between the two groups (Fig. 1).
Fig. 1.
This figure indicates pregnancy rate per embryo transfer in each group. The open square indicates the pregnancy rate of all patients regardless of maternal age. Those in group A, B and C are 27.2, 27.2 and 11.1%, respectively. The pregnancy rate in group C was lower than that in group A and B (p < 0.05). The black bar the pregnancy rate for those less than 40 years of age. Pregnancy rates per embryo transfer were 28.7%, in group A and 38.7% in group B, with no significant difference between the groups. There was no pregnancy recorded in group C
Discussion
Basal FSH in the early follicular phase, as reported previously, is a useful indicator of ovarian reserve [8, 9, 17]. In many ART treatment facilities in our country, basal FSH level is often used as a predictor of ovarian function. In the cases of elevated basal FSH levels, a poor outcome for ART treatment is predicted because patients with high basal FSH levels are generally thought to be poor responders to ovarian induction [17]. Indeed, Muasher et al. reported that the results of ART could be predicted from FSH levels in an early follicular phase [8]. Furthermore, El-Toukhy et al. mentioned that baseline levels of FSH reflected quality of eggs as well as ovarian reserve [9]. In recent years, more studies have shown that even with a high basal FSH, younger patients can achieve good results with ART [10, 18], and these studies also suggest that a reduction in pregnancy rate is due to reduced ovarian reserve rather than poor oocyte quality [12].
In this study, for the group with basal FSH levels between 10 IU/l and 15 IU/l (group B), the amount of gonadotropin required, and the stimulation period needed until oocyte retrieval, were comparable to that of the group with FSH < 10 IU/l (group A), but there was an increasing trend. Furthermore, the number of retrieved oocytes in group B, significantly decreased in comparison with group A. The number fertilized oocytes and transferred embryos in group B were comparable to that of group A, but there was a decreasing trend. The pregnancy rate per embryo transfer was 31.3% in group A and 38.1% in group B. From the results of this study, the basal FSH level reflects the ovarian response to the gonadotropin stimulation, rather than pregnancy potential for each oocyte. Furthermore, even infertility patients with basal FSH levels under 15 IU/l can expect to have a good ART outcome, especially if their maternal age is under 40. These results are in agreement with previous reports [10, 12, 18, 19].
In conclusion, the results of this study suggest that, even with FSH levels between 10 and 15 IU/l, a woman of less than 40 years of age can achieve pregnancy. Also, basal FSH levels do not reflect the pregnancy potential of the embryo in cases of infertility for a woman less than 40 years of age whose basal FSH level is less than 15 IU/l—if their embryo is collectable.
References
- 1.Nakahara K, Saito H, Saito T, Ito M, Ohta N, Sakai N, Tezuka N, Hiroi M, Watanabe H. Incidence of apoptotic bodies in membrana granulosa of the patients participating in an in vitro fertilization program. Fertil Steril. 1997;67:302–8. [DOI] [PubMed]
- 2.Seino T, Saito H, Kaneko T, Takahashi T, Kawachiya S, Kurachi H. Eight-hydroxy-2′-deoxyguanosine in granulosa cells is correlated with the quality of oocytes and embryos in an in vitro fertilization-embryo transfer program. Fertil Steril. 2002;77:1184–90. [DOI] [PubMed]
- 3.Toya M, Saito H, Ohta N, Saito T, Kaneko T, Hiroi M. Moderate and severe endometriosis is associated with alterations in the cell cycle of granulosa cells in patients undergoing in vitro fertilization and embryo transfer. Fertil Steril. 2000;73:344–50. [DOI] [PubMed]
- 4.Saito H, Kaneko T, Takahashi T, Kawachiya S, Saito T, Hiroi M. Hyaluronan in follicular fluids and fertilization of oocytes. Fertil Steril. 2000;74:1148–52. [DOI] [PubMed]
- 5.Nakagawa K, Ozawa N, Takamatsu K, Takahashi Y, Irahara M, Yoshimura Y, Saito H. A reduction in intraovarian arterial blood flow resistance after ovulation is necessary to achieve pregnancy in natural cycle. J Assist Reprod Genet. 2005;22:9–14. [DOI] [PMC free article] [PubMed]
- 6.Nakagawa K, Takahashi Y, Ito M, Horikawa T, Ohgi S, Irahara M, Saito H. Intraovarian arterial blood flow resistance in oligomenorrheal infertile women. J Assist Reprod Genet. 2006;23:105–10. [DOI] [PMC free article] [PubMed]
- 7.Nakagawa K, Ohgi S, Kojima R, Itoh M, Horikawa T, Irahara M, Saito H. Reduction of perifollicular arterial blood flow resistance after hCG administration is a good indicator of the recovery of mature oocytes in ART treatment. J Assist Reprod Genet 2006;23:433–8. [DOI] [PMC free article] [PubMed]
- 8.Muasher SJ, Oehninger S, Simonetti S, Matta J, Ellis LM, Liu HC, Jones GS, Rosenwaks Z. The value of basal and/or stimulated serum gonadotropin levels in prediction of stimulation response and in vitro fertilization outcome. Fertil Steril. 1988;50:298–307. [DOI] [PubMed]
- 9.Lenton EA, Sexton L, Lee S, Cooke ID. Progressive changes in LH and FSH and LH: FSH ratio in women throughout reproductive life. Maturitas. 1988;10:35–43. [DOI] [PubMed]
- 10.van Rooij IA, Bancsi LF, Broekmans FJ, Looman CW, Habbema JD, te Velde ER. Women older than 40 years of age and those with elevated follicle-stimulating hormone levels differ in poor response rate and embryo quality in in vitro fertilization. Fertil Steril. 2003;79:482–8. [DOI] [PubMed]
- 11.El-Toukhy T, Khalaf Y, Hart R, Taylor A, Braude P. Young age does not protect against the adverse effects of reduced ovarian reserve—an eight year study. Hum Reprod. 2002;17:1519–24. [DOI] [PubMed]
- 12.Abdalla H, Thum MY. An elevated basal FSH reflects a quantitative rather than qualitative decline of the ovarian reserve. Hum Reprod. 2004;19:893–8. [DOI] [PubMed]
- 13.Bancsi LF, Broekmans FJ, Mol BW, Habbema JD, te Velde ER. Performance of basal follicle-stimulating hormone in the prediction of poor ovarian response and failure to become pregnant after in vitro fertilization: a meta-analysis. Fertil Steril. 2003;79:1091–100. [DOI] [PubMed]
- 14.Nakagawa K, Ohgi S, Kojima R, Sugawara K, Itoh M, Horikawa T, Irahara M, Saito H. Recombinant-FSH has more effective potent than urinary human menopausal gonadotropin in ovarian hyperestimulation for ART treatment. Reprod Med Biol. 2007;6:27–32. [DOI] [PMC free article] [PubMed]
- 15.Nakagawa K, Ohgi S, Kojima R, Itoh M, Horikawa T, Irahara M, Saito H. Reduction of perifollicular arterial blood flow resistance after hCG administration is a good indicator of the recovery of mature oocytes in ART treatment. J Assist Reprod Genet. 2006;23:433–8. [DOI] [PMC free article] [PubMed]
- 16.Nakagawa K, Yamano S, Moride N, Yamashita M, Yoshizawa M, Aono T. Effect of activation with Ca ionophore A23187 and puromycin on the development of human oocytes that failed to fertilize after intracytoplasmic sperm injection. Fertil Steril. 2001;76:148–52. [DOI] [PubMed]
- 17.Surrey ES, Schoolcraft WB. Evaluating strategies for improving ovarian response of the poor responder undergoing assisted reproductive techniques. Fertil Steril. 2000;73:667–76. [DOI] [PubMed]
- 18.Esposito MA, Coutifaris C, Barnhart KT. A moderately elevated day 3 FSH concentration has limited predictive value, especially in younger women. Hum Reprod. 2002;17:118–23. [DOI] [PubMed]
- 19.van Rooij IA, de Jong E, Broekmans FJ, Looman CW, Habbema JD, te Velde ER. High follicle-stimulating hormone levels should not necessarily lead to the exclusion of subfertile patients from treatment. Fertil Steril. 2004;81:1478–85. [DOI] [PubMed]