Abstract
Purpose
To assess the relationship between low-grade inflammation, measured as basal high sensitivity (hs)-CRP, and IVF outcome.
Methods
We recruited a total of 220 women undergoing infertility work up prior to IVF. Patients were selected for a BMI < 30 kg/m2 with an upper age limit of 40 years. Serum hs-CRP levels were measured on day 3 of a spontaneous menstrual cycle preceding ovarian stimulation. A sensitive two-site ELISA was used for analysis. Dose of gonadotrophins required, follicles days 8 and 10, number of oocytes collected, number of oocytes fertilised and pregnancy outcome were recorded.
Results
Median hs-CRP was 1.08 mg/L (0.43–3.00 mg/L). The hs-CRP was significantly related to BMI (r = 0.386, P < .001) but not to age and smoking habit. There were no significant relationships between basal hs-CRP and any of the measured IVF outcomes.
Conclusions
These findings demonstrate that serum hs-CRP concentration is not a predictive marker of cycle or pregnancy outcome in women undergoing IVF treatment.
Keywords: Controlled ovarian hyperstimulation, hs-CRP, Inflammation, IVF, Pregnancy rate
Introduction
A variety of factors including chronological age, body mass index (BMI) and smoking, have a detrimental effect on reproductive performance. Increase in maternal age is known to have a negative impact on fertility secondary to reduced ovarian reserve [1, 2]. Numerous studies concluded that obesity, as defined by a BMI > 25 kg/m2 negatively correlates with fertility potential, affecting ovulation, pregnancy rates and outcomes in natural and treated cycles [3, 4]. There is also evidence that smoking negatively impacts on all areas of fertility from follicle development to fertilization and embryo cleavage. When in vitro fertilization (IVF) is used, fewer follicles are stimulated, fewer oocytes are retrieved, implantation rates are lower, more cycles are cancelled and live birth rates are significantly lower in smokers when compared to non-smokers [5–8]. Interestingly, passive smoking is found to be just as detrimental to fertility as active smoking, halving the pregnancy rate [9].
C-reactive protein (CRP) is an acute phase reactant that is important in the non-specific host defence against inflammation and is synthesised in the liver. In periods of infection and trauma, CRP levels can be raised several fold, but small increases above the baseline have been found to be predictive of low-grade inflammation. A more sensitive CRP assay allows determination of lower levels of CRP. This is known as high-sensitivity CRP (hs-CRP) and has been established as an inflammatory marker in a wide range of medical conditions. Also, increased levels of hs-CRP have been significantly correlated with advancing age [10], BMI [11–13] and smoking [14–16], all of which may be associated with impaired fertility.
Chronic low-grade inflammation may be a common mechanism whereby these predisposing factors cause subfertility. As yet, a handful of studies have investigated CRP levels during assisted conception, making it difficult to know the expected normal levels in different subgroups of patients [17–20]. Levin and colleagues [17] reported that higher CRP levels during IVF stimulation were associated with failure of conception. Conversely, other authors [19, 20] found that CRP measurements were not a predictive marker of IVF success.
The aim of this prospective exploratory study was to assess the relationship between basal inflammation, as evidenced by high level serum CRP, and IVF cycle outcome in an unselected population of women undergoing controlled ovarian stimulation.
Materials and methods
Between September 2005 and April 2006, 220 consecutive women undergoing infertility work-up prior to controlled ovarian hyperstimulation (COH) for IVF/ICSI were recruited at the Regional IVF Unit, St. Mary’s Hospital, Manchester, UK. All the subjects had to fulfil the following inclusion criteria: (1) regular menstrual cycles (28–35 days), (2) first ovarian stimulation treatment, (3) both ovaries visualised on transvaginal ultrasound, (4) no use of hormone therapy in the 6 months before entering the study, (5) no history of premature ovarian failure and/or autoimmune diseases, (6) no previous ovarian surgery, (7) no exposure to cytotoxic drugs or pelvic radiation therapy, and (8) BMI > 19 < 30 kg/m2. Written informed consent was obtained. The study had approval from the Bolton Local Research Ethics Committee (UK-NHS 05/Q1409/50).
Data regarding self reported smoking, chronological age and BMI were recorded on all subjects using a study-specific case report form. Patients and partners were classed as smokers or non-smokers irrespective of the number of cigarettes smoked per day.
All patients underwent COH using a standard long step-down protocol with GnRH analogue and human Menopausal Gonadotrophin (hMG), as previously published [21]. Convention for IVF with or without ICSI was assessed according to the sperm parameters on the day of oocyte retrieval. Follicle tracking was performed by an independent assessor on day 8 and on day 10 of stimulation treatment. Adverse effects to gonadotrophin administration were recorded, as appropriate. Biochemical pregnancy was defined as a positive urine pregnancy test 17 days post embryo transfer, while clinical pregnancy was defined as the presence of fetal heart beat on ultrasound scan performed at >6 weeks of gestation.
On day 3 of a spontaneous menstrual cycle before commencing ovarian stimulation, blood samples for the measurement of serum hs-CRP levels were obtained by venepuncture at approximately 0800 hours. The serum was frozen at −70°C until thawed and assayed in batches. Serum high sensitivity CRP was determined in duplicate by a solid-phase ELISA assay [22]. The sensitivity was 0.1 mg/l, the within-batch coefficient of variation was 5.9% and the between-batch coefficient of variation was 6.1%.
Statistical analysis
Results are expressed either as means ± standard deviation (SD) with range, or as medians with interquartile range (IQR). Correlations between parameters were performed using the Spearman rank test. Difference between groups was analysed by the Mann–Whitney U test. Statistical analysis was performed using StatsDirect Version 2.5.8 (Stats Direct Ltd., UK) from Microsoft Excel® 2003. A 5% significance level was used (P < .05).
Results
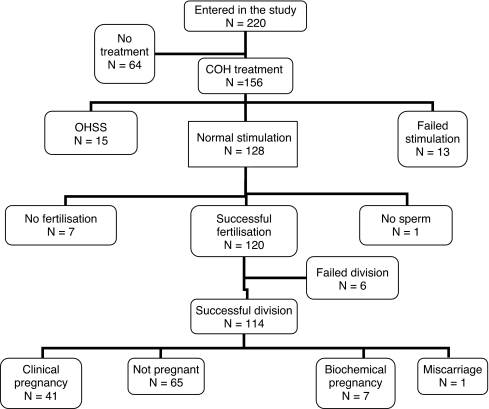
Chronological age and BMI of the 220 recruited patients were 32.6 ± 3.9 years (range 22.9–41.5 years) and 24.4 ± 3.68 kg/m2 (range 16–38 kg/m2), respectively. A total of 156 out of the 220 subjects commenced ovarian stimulation treatment before the end of the study period. Of these, 128 patients had successful stimulation, 15 were cancelled due to the increased risk of ovarian hyperstimulation syndrome (oestradiol levels ≥ 21,000 pmol/L) and 13 had their cycles cancelled because of failed stimulation (oestradiol levels < 1,000 pmol/L on day 6 of stimulation and/or less than four developing follicles seen on ultrasound on day 8 of stimulation). One hundred and twenty-seven patients had IVF with or without ICSI for fertilization, and one had to be cancelled as there was no sperm available on the day. Successful fertilization occurred in 120 patients and embryo cleavage in 114 of them. Forty-one patients achieved a clinical pregnancy, seven had only a biochemical pregnancy and one had a first trimester miscarriage, whilst the remaining 65 patients failed to conceive. The quorum in Fig. 1 shows the number of subjects recruited and those entering each stage of IVF treatment.
Fig. 1.
Quorum on study population entering different stages of IVF treatment and respective outcomes
With regard to pregnancy outcomes, 26.3% of patients starting COH treatment and 36% having embryo transfer achieved a clinical pregnancy (Table 1). These subjects had a higher median hs-CRP and mean BMI than those who failed to conceive, but the differences were not statistically significant.
Table 1.
Pregnancy outcomes and hs-CRP levels
| Variable | No. patients | Outcome/embryo transfer | Outcome/COH (%) | hs-CRP | |
|---|---|---|---|---|---|
| (%) | Median | IQR | |||
| Clinical pregnancy | 41 | 36 | 26.3 | 1.35 | 0.48–3.12 |
| Biochemical pregnancy | 7 | 6.1 | 4.5 | 0.53 | 0.47–1.07 |
| Miscarriage | 1 | 0.9 | 0.6 | 0.23 | – |
| Not pregnant | 65 | 57 | 41.7 | 1.17 | 0.44–2.73 |
hs-CRP activities are expressed as milligram per liter
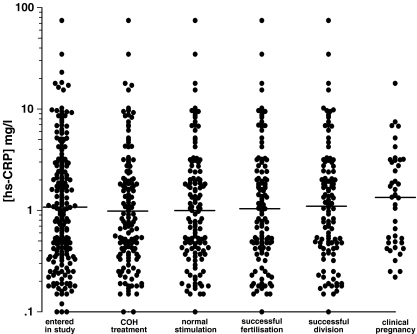
As shown in Table 2, only the BMI showed a significant correlation with hs-CRP levels (r = 0.386, P < .001), while none of the measured IVF cycle outcomes correlated with this inflammation marker. Furthermore, basal hs-CRP levels did not appear to predict successful stages of IVF treatment (Fig. 2). Median hs-CRP levels were 1.08 mg/l (IQR 0.43–3.00) on entry (n = 220), 0.99 mg/l (IQR 0.41–2.59) in the COH treatment group (n = 156), 1.00 mg/l (IQR 0.42–2.43) in the normal stimulation group (n = 128), 1.06 mg/l (IQR 0.44–2.59) in the successful fertilization group (n = 120), 1.12 mg/l (IQR 0.47–2.76) in the successful division group (n = 114) and 1.35 mg/l (IQR 0.48–3.13) in the clinical pregnancy group (n = 41). There were no significant differences when comparing hs-CRP levels between the entry patients group and any of the following groups - COH treatment (P = .513), normal stimulation (P = .526), successful fertilization (P = .718), successful division (P = .983) and clinical pregnancy (P = .595).
Table 2.
Correlation of hs-CRP levels with patient characteristics and IVF cycle outcomes
| Variable | No. patients | r value | P value |
|---|---|---|---|
| Age | 220 | −0.011 | .869 |
| BMI | 220 | 0.386 | <.001 |
| Ampoules | 156 | −0.028 | .727 |
| Follicles day 8 | 137 | −0.029 | .736 |
| Follicles day 10 | 130 | −0.113 | .200 |
| Oocytes collected | 128 | −0.063 | .466 |
| Oocytes fertilized | 120 | −0.009 | .914 |
Correlations were performed using the Spearman rank test
Fig. 2.
Scattergram of hs-CRP values in patients progressing to each stage of treatment. Medians are indicated
In the whole study population, median hs-CRP was 1.09 mg/l (IQR 0.42–2.98) in cases when neither partner smoked, 1.70 mg/l (IQR 0.57–4.06) when both partners smoked, 0.92 mg/l (IQR 0.49–2.86 when the partner only smoked and 0.48 mg/l (IQR 0.24–3.10) when the patient only smoked. Although the median CRP was higher in cases when both partners smoked compared to the non-smoking group, this increase did not reach statistical significance (P = .453).
Discussion
The role of C-reactive protein as putative marker of success in IVF treatment has not been fully investigated. The available studies present considerable differences in design, subjects and methods, reaching contradictory results [17–20].
Gonadotrophin hyperstimulation, oocyte retrieval and blastocyst-endometrium interaction (i.e., early stages of implantation) are thought to induce a temporary inflammatory state as evidenced by a rise in CRP levels and inflammatory cytokines. Levin and colleagues [17] were amongst the first to report significantly higher hs-CRP levels in women admitted to the hospital with a diagnosis of ovarian hyperstimulation syndrome. These and other authors [19] speculated that the inflammatory process results from stimulation treatment and may be responsible for the molecular mechanisms leading to the various degrees of the syndrome. Almagor and colleagues [18] took serial measurements of CRP during the IVF cycle and found that the levels increased 1 week after oocyte retrieval. Pregnant women after IVF treatment were shown to have higher CRP levels than non-pregnant women at 4 weeks of gestation, suggesting that the maternal inflammatory response starts from the early stages of embryo implantation [23].
Some might argue that measurements of CRP levels in the context of ongoing inflammatory states are not a direct reflection of the baseline systemic environment. Therefore, CRP should be measured in neutral condition. In the present study, we have failed to observe any statistically significant correlation between day 3 hs-CRP and IVF cycle outcomes or clinical pregnancy. Our findings concur with other studies [19, 20] which found no correlation between circulating levels of hs-CRP prior to ovarian stimulation with gonadotrophins and IVF outcome. In contrast, Levin and colleagues [17], who measured hs-CRP at different time points, noted that hs-CRP concentrations on day 3 correlated with IVF treatment failure. Noteworthily, the authors included only a very small number of subjects (n = 28) in their analysis, comparing eight women who conceived to 20 women who failed to conceive.
In subfertile women undergoing IVF, the lack of correlation between hs-CRP with chronological age and smoking is not surprising as they are often healthy and relatively young subjects. In the study showing a correlation between CRP levels and IVF outcome, the patients with unsuccessful treatment had higher CRP levels and were older compared to those who achieved a pregnancy [17].
In the last decade, the relationship between body weight, subfertility and inflammatory markers like hs-CRP has been explored in some studies [3, 4, 24]. White adipose tissue plays a central role in the low grade inflammatory state that is characteristic of obesity [25, 26]. In fact, adipose tissue is a potent source of inflammatory interleukins and other circulating cytokines, the secretion of which increase with adiposity [25–27]. In agreement with previous studies [20, 28], we have found a significant correlation between basal hs-CRP and BMI. Furthermore, although the mean BMI of women who conceived was higher than those who had a unsuccessful cycle (26.3 ± 3.15 and 25.0 ± 2.68 kg/m2), this failed to reach statistical significance. Nevertheless, the subtle difference in BMI may perhaps explain the higher levels of hs-CRP in the group of patients who had a clinical pregnancy.
Whether the correlations would have been any different if we had included subjects with BMI ≥ 30 kg/m2 cannot be proven. Certainly, obesity plays a negative role on conception and pregnancy. Weight loss, which is associated with decreased macrophage infiltration, improves the inflammatory profile [29] and enhances metabolic and reproductive potential [30]. It is recommended that women have a normal BMI before commencing any form of fertility treatment in order to maximise their chances to successful treatment outcome and reduce potential fetal-maternal complications of pregnancy.
With regard to chronological age, although some studies have suggested a correlation with circulating CRP [11, 31], we and others [19] have failed to demonstrate such a relationship. A plausible explanation for this lies with the age range of women undergoing fertility treatment and recruited in the present study, as opposite to that of a general female population. Concomitant age-linked medical conditions (e.g., arthritis and other rheumatologic conditions), which are very seldom in healthy young women seeking fertility, are also responsible for chronic low grade inflammation.
Conclusions
To the best of our knowledge this is the largest study to investigate the role of hs-CRP in women of reproductive age undergoing IVF treatment. As no correlation could be demonstrated, we may conclude that baseline hs-CRP is of no use as predictive marker of IVF outcome in routine clinical practice. Larger, properly powered studies are warranted to confirm these preliminary findings. Whether the measurement of CRP levels during ovarian stimulation may identify subgroups of patients who might benefit from prophylactic short-term anti-inflammatory drugs to reduce the adverse effects of IVF treatment and improve pregnancy rates remain to be established. Until then, clinicians should not recommend IVF patients to take unproven and unnecessary pharmacological compounds that may have no beneficial effect on success rate.
Acknowledgments
Supported by a grant from the CMMC Trust Research Scheme (grant number 9671, 2004/05), UK.
Footnotes
Capsule
Basal low-grade inflammation does not predict IVF success as shown by the lack of correlation between day 3 hs-CRP and IVF outcome measures.
References
- 1.Navot D, Bergh PA, Williams MA, Garrisi GJ, Guzman I, Sandler B, et al. Poor oocyte quality rather than implantation failure as a cause of age-related decline in female fertility. Lancet 1991;337:1375–7. doi:10.1016/0140-6736(91)93060-M. [DOI] [PubMed]
- 2.Lim AS, Tsakok MF. Age-related decline in fertility: a link to degenerative oocytes? Fertil Steril. 1997;68:265–71. doi:10.1016/S0015-0282(97)81513-0. [DOI] [PubMed]
- 3.Wittemer C, Ohl J, Bailly M, Bettahar-Lebugle K, Nisand I. Does body mass index of infertile women have an impact on IVF procedure and outcome? J Assist Reprod Genet. 2000;17:547–52. doi:10.1023/A:1026477628723. [DOI] [PMC free article] [PubMed]
- 4.Krizanovska K, Ulcova-Gallova Z, Bouse V, Rokyta Z. Obesity and reproductive disorders. Sb Lek. 2002;103:517–26. [PubMed]
- 5.Nizard J. What are the epidemiological data on maternal and paternal smoking? J Gynecol Obstet Biol Reprod (Paris). 2005;34:347–52. [PubMed]
- 6.Klonoff-Cohen H. Female and male lifestyle habits and IVF: what is known and unknown. Hum Reprod Update. 2005;11:179–203. doi:10.1093/humupd/dmi016. [DOI] [PubMed]
- 7.Lintsen AM, Pasker-de Jong PC, de Boer EJ, Burger CW, Jansen CA, Braat DD, et al. Effects of subfertility cause, smoking and body weight on the success rate of IVF. Hum Reprod. 2005;20:1867–75. doi:10.1093/humrep/deh898. [DOI] [PubMed]
- 8.Augood C, Duckitt K, Templeton AA. Smoking and female infertility: a systematic review and meta-analysis. Hum Reprod. 1998;13:1532–9. doi:10.1093/humrep/13.6.1532. [DOI] [PubMed]
- 9.Neal MS, Hughes EG, Holloway AC, Foster WG. Sidestream smoking is equally as damaging as mainstream smoking on IVF outcomes. Hum Reprod. 2005;20:2531–5. doi:10.1093/humrep/dei080. [DOI] [PubMed]
- 10.Pradhan A, Manson JE, Rifai N, Buring JE, Ridker PM. C-Reactive Protein, interleukin 6, and risk of developing type 2 Diabetes Mellitus. JAMA 2001;286:327–34. doi:10.1001/jama.286.3.327. [DOI] [PubMed]
- 11.Laing I, Yates AP, Mather E, Pickersgill M. Serum C-Reactive Protein (CRP) is related to age and body mass index (BMI) in patients with polycystic ovary syndrome (PCOS) but not to insulin sensitivity. Endocr Abstr. 2003;6:OC12.
- 12.Schindler TH, Cardenas J, Prior JO, Facta AD, Kreissl MC, Zhang XL, et al. Relationship between increasing body weight, insulin resistance, inflammation, adipocytokine leptin, and coronary circulatory function. J Am Coll Cardiol. 2006;47:1188–95. doi:10.1016/j.jacc.2005.10.062. [DOI] [PubMed]
- 13.Wu DM, Chu NF, Shen MH, Wang SC. Obesity, plasma high sensitivity c-reactive protein levels and insulin resistance status among school children in Taiwan. Clin Biochem. 2006;39:810–5. doi:10.1016/j.clinbiochem.2006.05.007. [DOI] [PubMed]
- 14.Hoekstra T, Geleijnse JM, Schouten EG, Kluft C. Smoking and CRP: results of the Arnhem Elderly Study. 2nd Hot Topic Workshop on CRP, Leiden, The Netherlands. CRP 2001;1:018.
- 15.Yasue H, Hirai N, Mizuno Y, Harada E, Itoh T, Yoshimura M, et al. Low-grade inflammation, thrombogenicity, and atherogenic lipid profile in cigarette smokers. Circ J. 2006;70:8–13. doi:10.1253/circj.70.8. [DOI] [PubMed]
- 16.Yanbaeva DG, Dentener MA, Creutzberg EC, Wesseling G, Wouters EF. Systemic effects of smoking. Chest 2007;131:1557–66. doi:10.1378/chest.06-2179. [DOI] [PubMed]
- 17.Levin I, Gamzu R, Mashiach R, Lessing JB, Amit A, Almog B. Higher C-Reactive protein levels during IVF stimulation are associated with ART failure. J Reprod Immunol. 2007;75:141–4. doi:10.1016/j.jri.2007.03.004. [DOI] [PubMed]
- 18.Almagor M, Hazav A, Yaffe H. The levels of C-reactive protein in women treated by IVF. Hum Reprod. 2004;19:104–6. doi:10.1093/humrep/deh036. [DOI] [PubMed]
- 19.Orvieto R, Chen R, Ashkenazi J, Ben-Haroush A, Bar J, Fisch B. C-Reactive protein levels in patients undergoing controlled ovarian hyperstimulation for IVF cycle. Hum Reprod. 2004;19:357–9. doi:10.1093/humrep/deh089. [DOI] [PubMed]
- 20.Wunder DM, Kretschmer R, Bersinger NA. Concentrations of leptin and C-reactive protein in serum and follicular fluid during assisted reproductive cycles. Hum Reprod. 2005;20:1266–71. doi:10.1093/humrep/deh767. [DOI] [PubMed]
- 21.Nardo LG, Cheema P, Gelbaya T, Horne G, Fitzgerald CT, Pease EH, et al. The optimal length of ‘coasting protocol’ in women at risk of ovarian hyperstimulation syndrome undergoing in vitro fertilization. Hum Fertil. 2006;9:175–80. doi:10.1080/14647270600787575. [DOI] [PubMed]
- 22.Highton J, Hessian P. A solid-phase enzyme immunoassay for C-reactive protein: clinical value and the effect of rheumatoid factor. J Immunol Methods. 1984;68:185–92. doi:10.1016/0022-1759(84)90149-2. [DOI] [PubMed]
- 23.Sacks GP, Seyani L, Lavery S, Trew G. Maternal C-reactive protein levels are raised at 4 weeks gestation. Hum Reprod. 2004;19:1025–30. doi:10.1093/humrep/deh179. [DOI] [PubMed]
- 24.Anty R, Bekri S, Luciani N, Saint-Paul MC, Dahman M, Iannelli A, et al. The inflammatory C-Reactive Protein is increased in both liver and adipose tissue in severely obese patients independently from the metabolic syndrome, Type 2 diabetes, and NASH. Am J Gastroenterol. 2006;101:1824–33. doi:10.1111/j.1572-0241.2006.00724.x. [DOI] [PubMed]
- 25.Fain JN. Release of interleukins and other inflammatory cytokines by human adipose tissue is enhanced in obesity and primarily due to the nonfat cells. Vitam Horm. 2006;74:443–77. doi:10.1016/S0083-6729(06)74018-3. [DOI] [PubMed]
- 26.Yudkin JS, Stehouwer CD, Emeis JJ, Coppack SW. C-reactive protein in healthy subjects: associations with obesity, insulin resistance, and endothelial dysfunction: a potential role for cytokines originating from adipose tissue. Arterioscler Thromb Vasc Biol. 1999;19:972–8. [DOI] [PubMed]
- 27.Coppack SW. Pro-inflammatory cytokines and adipose tissue. Proc Nutr Soc. 2001;60:349–56. [DOI] [PubMed]
- 28.Visser M, Bouter LM, McQuillan GM, Wener MH, Harris TB. Elevated C-reactive protein levels in overweight and obese adults. JAMA 1999;282:2131–5. doi:10.1001/jama.282.22.2131. [DOI] [PubMed]
- 29.Bastard JP, Maachi M, Lagathu C, Kim MJ, Caron M, Vidal H, et al. Recent advances in the relationship between obesity, inflammation, and insulin resistance. Eur Cytokine Netw. 2006;17:4–12. [PubMed]
- 30.Balen AH, Anderson RA. Impact of obesity on female reproductive health: British Fertility Society, Policy and Practice Guidelines. Hum Fertil. 2007;10:195–206. doi:10.1080/14647270701731290. [DOI] [PubMed]
- 31.Kwok S, Selby PL, McElduff P, Laing I, Mackness B, Mackness MI, et al. Progestogens of varying androgenicity and cardiovascular risk factors in postmenopausal women receiving oestrogen replacement therapy. Clin Endocrinol (Oxf). 2004;61:760–7. doi:10.1111/j.1365-2265.2004.02166.x. [DOI] [PubMed]