Abstract
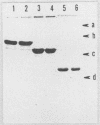
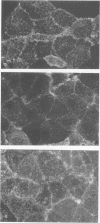
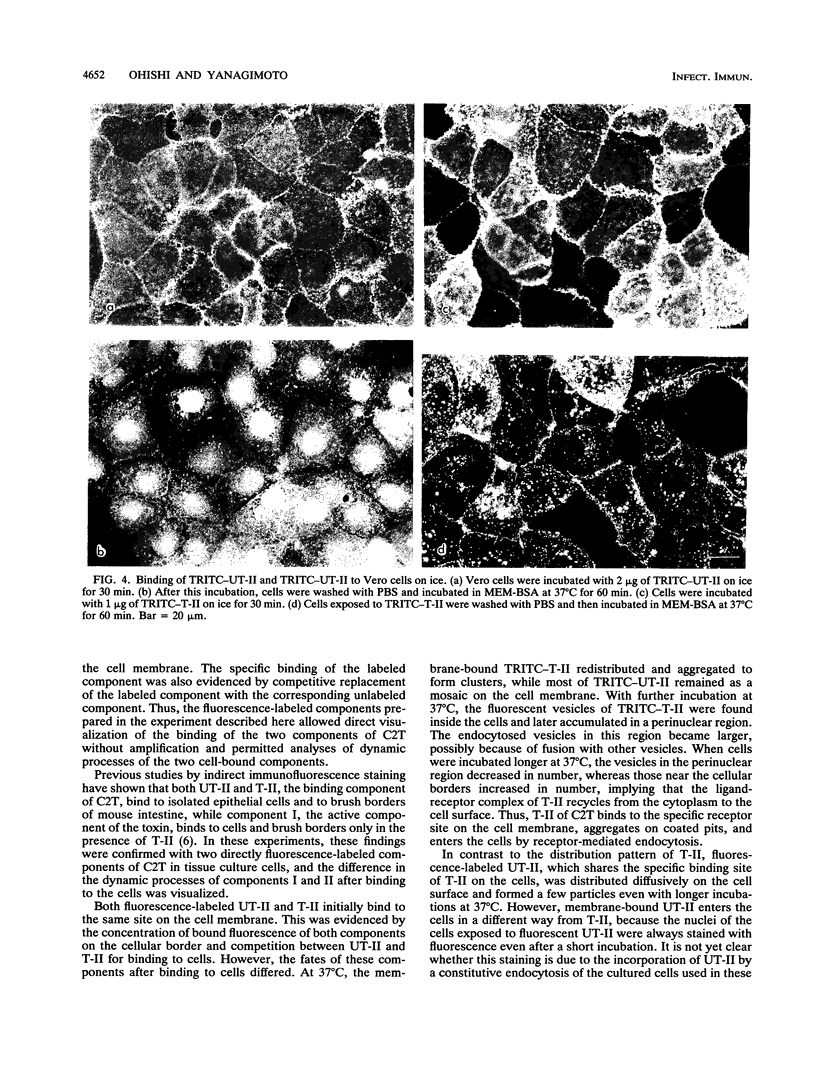
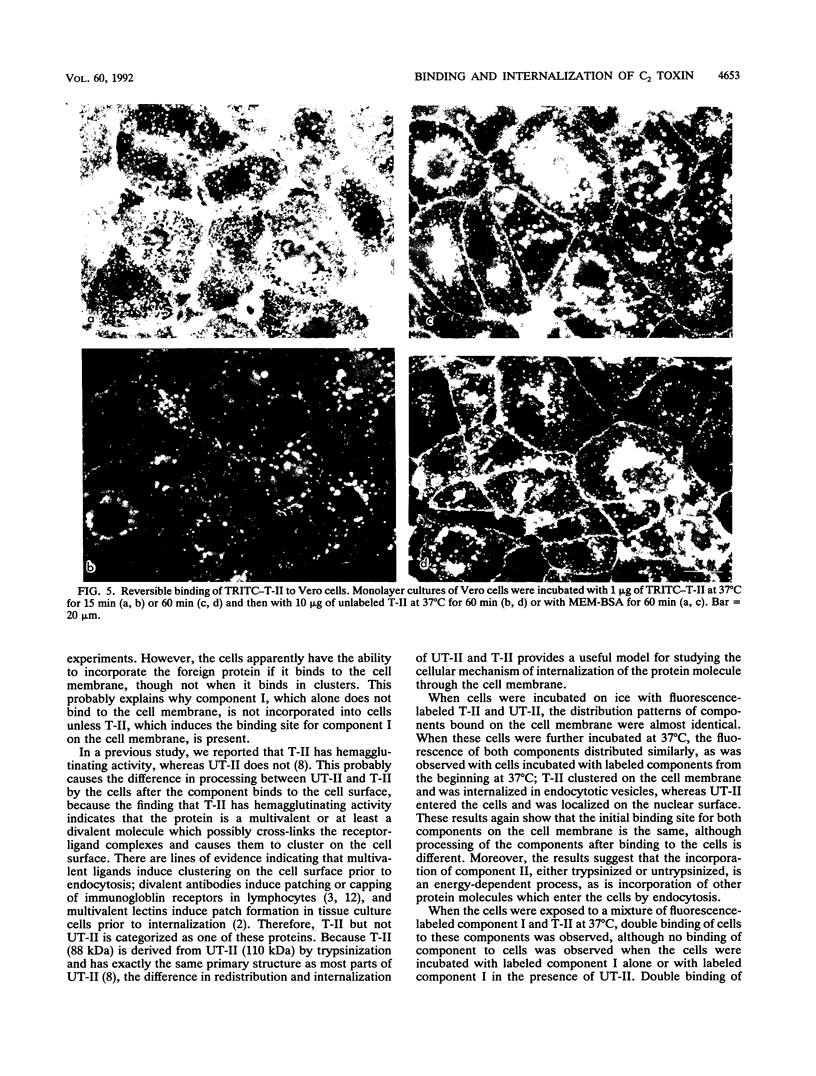
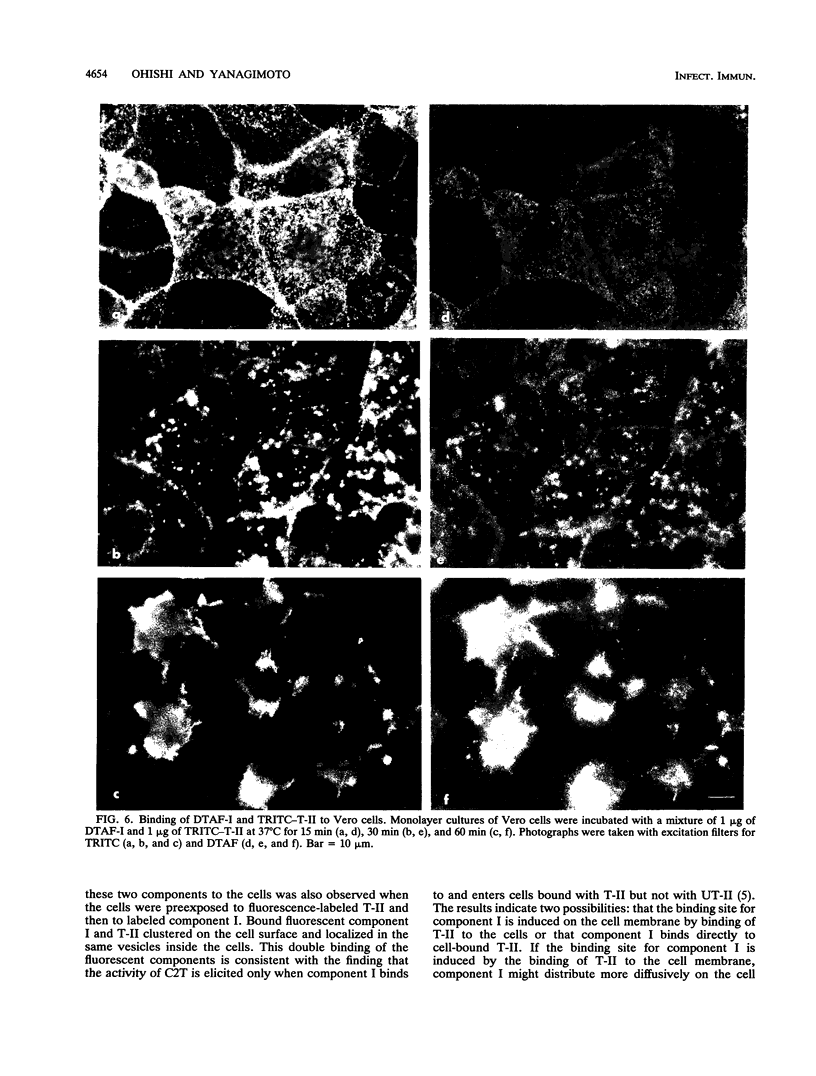
Binding and internalization of two nonlinked components of botulinum C2 toxin were visualized in tissue culture cells with components directly labeled with fluorescence. The binding of both untrypsinized and trypsinized component II (UT-II and T-II, respectively) to common specific sites on the cell membrane was evidenced by competitive binding between fluorescence-labeled and unlabeled components. The distribution patterns of fluorescence-labeled T-II and UT-II after binding to cells at 37 degrees C were different; T-II clustered on the cell membrane and entered the cells in endosomes, whereas UT-II entered the cells inefficiently and not in vesicles and was distributed on the nuclear surface. The difference may be due to the multivalent property of T-II, which is not shared with UT-II. Fluorescence-labeled component I, which binds only to cells bound with T-II, entered cells by the same route as T-II did; both colocated on the same clusters on the cell membrane and also in the same vesicles in the cytoplasm. The present results suggest that component I of C2 toxin, which ADP-ribosylates cytoplasmic actin, directly binds to T-II but not to UT-II on the cell membrane and is internalized into cells together with T-II in the same endosomes.
Full text
PDF

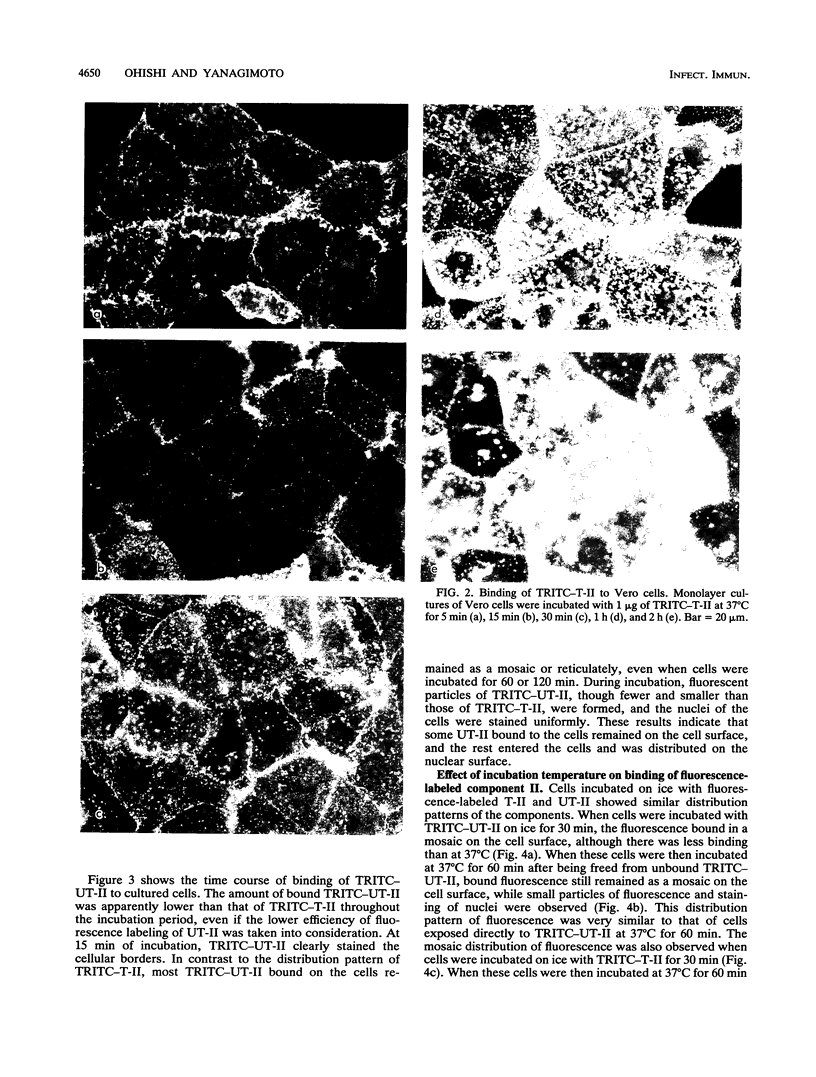
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- De Petris S., Raff M. C., Mallucci L. Ligand-induced redistribution of concanavalin A receptors on normal, trypsinized and transformed fibroblasts. Nat New Biol. 1973 Aug 29;244(139):275–278. doi: 10.1038/newbio244275a0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Miyake M., Ohishi I. Response of tissue-cultured cynomolgus monkey kidney cells to botulinum C2 toxin. Microb Pathog. 1987 Oct;3(4):279–286. doi: 10.1016/0882-4010(87)90061-1. [DOI] [PubMed] [Google Scholar]
- Ohishi I. Activation of botulinum C2 toxin by trypsin. Infect Immun. 1987 Jun;55(6):1461–1465. doi: 10.1128/iai.55.6.1461-1465.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohishi I., Iwasaki M., Sakaguchi G. Purification and characterization of two components of botulinum C2 toxin. Infect Immun. 1980 Dec;30(3):668–673. doi: 10.1128/iai.30.3.668-673.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohishi I., Miyake M. Binding of the two components of C2 toxin to epithelial cells and brush borders of mouse intestine. Infect Immun. 1985 Jun;48(3):769–775. doi: 10.1128/iai.48.3.769-775.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohishi I. NAD-glycohydrolase activity of botulinum C2 toxin: a possible role of component I in the mode of action of the toxin. J Biochem. 1986 Aug;100(2):407–413. doi: 10.1093/oxfordjournals.jbchem.a121728. [DOI] [PubMed] [Google Scholar]
- Ohishi I. Response of mouse intestinal loop to botulinum C2 toxin: enterotoxic activity induced by cooperation of nonlinked protein components. Infect Immun. 1983 May;40(2):691–695. doi: 10.1128/iai.40.2.691-695.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohishi I., Tsuyama S. ADP-ribosylation of nonmuscle actin with component I of C2 toxin. Biochem Biophys Res Commun. 1986 Apr 29;136(2):802–806. doi: 10.1016/0006-291x(86)90511-5. [DOI] [PubMed] [Google Scholar]
- de Petris S., Raff M. C. Normal distribution, patching and capping of lymphocyte surface immunoglobulin studied by electron microscopy. Nat New Biol. 1973 Feb 28;241(113):257–259. doi: 10.1038/newbio241257a0. [DOI] [PubMed] [Google Scholar]