Abstract
Background:
Several epidemiologic studies have suggested periodontitis as a risk factor for coronary heart disease (CHD), but results have been inconsistent.
Methods and Results:
We evaluated the association between clinical and radiographic measures of periodontitis, edentulism and incident CHD (angina, myocardial infarction or fatal CHD) among 1,203 men in the VA Normative Aging and Dental Longitudinal Studies who were followed with triennial comprehensive medical and dental exams up to 35 yrs (median: 24 yrs). Cox proportional hazards models with time-varying effects of exposure and potential confounders were fit. We found a significant, dose-dependent association between periodontitis and CHD incidence among men younger than 60 yrs of age (HR 2.12, 95% CI 1.26, 3.60 comparing highest vs. lowest category of radiographic bone loss, p for trend = 0.02), independent of age, BMI, smoking, alcohol intake, diabetes, fasting glucose, total cholesterol, HDL cholesterol, triglycerides, hypertension, systolic and diastolic blood pressure, education, marital status, income and occupation. No association was found among men older than 60 yrs. Similar results were found using the sum of probing pocket depths as a measure of periodontitis. Among men 60+ yrs, edentulous men tended to have higher risk of CHD compared to dentate men in the lowest bone loss (HR 1.61, 95% CI 0.95, 2.73) and lowest pocket depth categories (HR 1.72, 95% CI 1.03, 2.85), independent of confounders.
Conclusions:
Chronic periodontitis is associated with incidence of CHD among younger men, independent of established cardiovascular risk factors.
Keywords: coronary disease, epidemiology, infection, inflammation, risk factors
Introduction
Several cohort studies have found an association between chronic periodontitis and the risk of coronary heart disease (CHD), independent of a variety of potential confounders 1, 2. However, other studies did not find significant associations after adjustments for important confounding factors 3, 4. These inconsistencies have led to concerns and uncertainties regarding the validity of the periodontitis – CHD association and its strength. Such concerns include residual confounding by smoking 5 and potential misclassification of periodontitis in studies not employing periodontal probing for assessment of periodontitis 1, 3, 4, 6, 7. Indeed, the attenuation of relative risk estimates due to such misclassification can be quite dramatic 8, and this may at least partially explain some of the null results found in several large cohorts.
A different but related issue pertains to the operationalization and definition of chronic periodontitis as the exposure of interest. Periodontitis is clinically assessed by measuring periodontal probing depth and attachment level at various sites of the dentition or by measuring alveolar bone loss from radiographs. Definitions of periodontitis in clinical research are based on either measure or on combinations thereof and vary widely 9. The appropriate choice of clinical exposure measure for studies into the association between periodontitis and CHD is controversial 10. In addition, some authors have argued that the serological response to periodontal pathogens may be a better exposure measure, as it also captures the host inflammatory response 11. Both causal and non-causal pathways have been proposed that could explain an association between periodontitis and CHD 2, 12. Although emerging evidence from intervention studies suggest that successful treatment of periodontitis may have beneficial effects on surrogate cardiovascular endpoints such as inflammatory serum markers 13 and endothelial function 14, the relevance of these findings on a population level remains uncertain given the inconsistent epidemiologic evidence for a periodontitis – CHD association.
The purpose of the present study was to evaluate chronic periodontitis and edentulism (complete absence of natural teeth) as risk factors for incident coronary heart disease among men using data from the VA Normative Aging and Dental Longitudinal Studies.
Materials and Methods
Study population
Study subjects were participants in the U.S. Department of Veteran Affairs (VA) Normative Aging Study (NAS) who were also enrolled in the Dental Longitudinal Study (DLS). The NAS is an ongoing closed panel longitudinal study that initially enrolled 2,280 healthy male volunteers from the greater Boston area beginning in 1961. Men were not VA patients and have continued to receive their medical and dental care in the private sector. Subjects were examined approximately every 3 years by trained VA staff physicians, including a medical history, a physical examination, and a variety of biochemical laboratory tests. Diseases and conditions were entered into the database according to the 8th Revision of the International Classification of Diseases (ICD-8).
Beginning in 1966, a subset of 1,231 volunteers aged 21 to 84 years was enrolled in the DLS 15. The first DLS examination was used as the baseline for the present analyses. We excluded 12 men who had developed CHD prior to their first DLS examination.
The protocol was approved by the Department of Veterans Affairs Committee on Human Studies, and procedures followed were in accordance with institutional guidelines. All subjects conferred their informed consent prior to their entry into the study.
Outcome assessment
Myocardial infarction, angina pectoris, and fatal CHD were considered CHD events and were ascertained in the NAS using the same criteria employed in the Framingham Heart Study 16. Myocardial infarction (MI) was diagnosed based on ECG findings, elevation of serum enzymes and chest discomfort consistent with MI, or autopsy. Angina pectoris was defined as recurrent chest discomfort related to exertion or excitement lasting up to 15 minutes that was responsive to rest or nitroglycerin. Fatal CHD was defined as a primary cause of death attributed to CHD based on ICD-8 codes (410-414). Person-time accrued until 2004 was included in these analyses.
Exposure assessment
A trained and calibrated periodontist conducted comprehensive oral examinations triennially, including full-mouth radiographs and periodontal probing at each tooth. At each examination, periodontitis was assessed both radiographically and clinically. Firstly, alveolar bone loss was assessed on each tooth on all interproximal surfaces. A bone loss score was assigned to each mesial and distal tooth site in 20% increments (score 0: no bone loss, score 1: bone loss ≤ 20%, score 2: bone loss >20% and ≤40%, score 3: bone loss >40% and ≤60%, score 4: bone loss >60% and ≤80%, score 5: bone loss >80%). Secondly, the maximum probing pocket depth was recorded for each tooth by calibrated examiners and recorded as a score (0-3 mm, >3-5 mm, >5 mm). More detailed descriptions of the periodontal measures utilized in the DLS and their reproducibility have been previously published 17, 18. Briefly, weighted kappas for interexaminer reproducibility of probing depth and bone loss scores indicated good reproducibility (k > 0.4)19 based on repeat assessments on 24 and 25 subjects, respectively.
Other variables
Blood pressure was measured in each arm on seated subjects using standard mercury sphygmomanometry. Mean readings from both arms were used for systolic and diastolic blood pressure. Body mass index was calculated from measured weight and height.
Laboratory parameters determined from fasting serum samples included concentrations of total cholesterol, HDL-cholesterol (beginning in 1981), triglycerides and glucose. In addition a 2-h oral glucose tolerance test was performed. Men were classified as diabetic if they had a physician diagnosis of diabetes, or if they had a fasting glucose >= 126 mg/dL, or if their 2h glucose tolerance test was >= 200 mg/dL.
Information on history of cigarette smoking was obtained by interview. Information on smoking intensity, duration and time since cessation was used to calculate a comprehensive smoking index as previously described 20.
Daily alcohol consumption was derived from replies to the Cornell Medical Index Health Questionnaire, where subjects responded to whether they usually drank two or more alcoholic drinks per day (yes/no). Maximum level of education completed was categorized into less than high school education, completed high school or beyond high school education. Occupation was recorded in 9 categories and refers to former occupation for those men who were retired. Income was assessed at the DLS baseline examination only. Marital status was categorized as a binary variable, married or remarried versus divorced, widowed, single or separated. With the exception of income, all dental and non-dental variables were updated at each triennial exam.
Data analysis
Summary statistics of baseline characteristics were calculated for the entire cohort and separately for men who had incident CHD or fatal CHD. Person-time for each participant was calculated from their first DLS visit to first CHD event, death, or last NAS visit, whichever occurred first. Two separate outcome definitions were used, total CHD (non-fatal or fatal) and fatal (CHD). Each participant contributed only one endpoint for each analysis. Therefore, for the total CHD analyses, once a participant was diagnosed with CHD they were excluded from analyses. For the fatal CHD analyses, only fatal CHD events were considered cases, therefore non-fatal cases continued to contribute person-time until death or censored.
Cox proportional hazards models were used to calculate hazard ratios and 95% confidence intervals for the association between periodontitis or edentulism and incidence of CHD. Two separate exposure definitions of periodontitis were used. Firstly, mean whole mouth radiographic bone loss score was calculated as a cumulative measure of periodontitis history. Secondly, ‘cumulative probing depth’ was calculated as the whole mouth sum of pathologically increased probing depth (> 3 mm) using the midpoints of each recorded category (4mm, 6mm). Tests of linear trend were performed for each model by entering mean bone loss score or cumulative pocket depths as continuous variables. In addition, the exposure measures were categorized. The reference categories were pre-specified and included men with no or minimal bone loss (mean bone loss score of 0.5 or less) and no pathologic pocketing (no periodontal pockets > 3 mm, i.e., cumulative probing depth < 4 mm), respectively. Data on periodontitis and edentulism were updated at each DLS examination and modeled as time-varying effects, i.e., subjects who became edentulous during follow-up contributed person-time to both exposure categories (periodontitis and edentulism). Multivariate models adjusted for age, education, income and occupation at baseline and time-varying effects of smoking, body mass index, high density lipoprotein cholesterol, total cholesterol, triglycerides, hypertension, mean systolic blood pressure, mean diastolic blood pressure, diagnosis of diabetes, fasting glucose level, 2 hour glucose level, alcohol consumption and marital status.
Because of prior evidence for effect modification by age 1, 21-23, all models included interaction terms with age. First, models were stratified by means of a dichotomous cut-off (<60, 60+ years). For these models, men could change categories during the study, i.e., they contributed person-time to both age strata if they became older than the cut-off age during the study. In addition, we used quadratic spline regression 24 to evaluate the effect of time-varying age on the periodontitis / CHD association on a continuous scale, defining knots at the age tertile cut-offs of 57 and 67 years. Likelihood ratio tests were used for testing for interactions with age using interaction terms.
All models were evaluated to determine departure from the proportional hazards assumptions using scaled Schoenfeld residual plots for the final multivariate models. We further conducted a sensitivity analysis restricted to never smokers. We also evaluated the potential for survivorship bias as all men had to be systemically healthy at baseline to be enrolled in the study. For that purpose, we conducted a sensitivity analysis comparing results for men 60+ years between those men that were 60+ years at baseline vs. men younger than 60 years at baseline. All analyses were performed using STATA 9.0 (Stata Corp., College St., TX). The authors had full access to and take full responsibility for the integrity of the data. All authors have read and agree to the manuscript as written.
Results
A total of 1,203 men free of CHD enrolled in the DLS. Over a follow-up time of up to 35 years (median: 24 years), a total of 364 men were diagnosed with CHD (either fatal or non-fatal), and 109 men died from CHD. Only 6 men were lost to follow-up, i.e. no information on either non-fatal CHD incidence or CHD mortality could be collected, and these men contributed no person-time. However, we compared baseline characteristics between 383 men who did not attend a NAS/DLS exam for at least 10 years prior to death or prior to the most recent exam cycle and 820 men who did. Men who did not attend a NAS/DLS exam for at least 10 years were significantly older and more likely to be smokers. However, independent of age, there were no significant differences with respect to baseline periodontitis and prevalence of edentulism.
Baseline characteristics for the entire cohort and for men who later developed CHD and fatal CHD are shown in Table 1. At baseline, men who were later diagnosed with CHD and in particular those who died from CHD, tended to be older, have higher serum concentrations of total cholesterol and triglycerides, lower serum concentrations of HDL cholesterol (measured at examination cycle 5), have higher systolic blood pressure, higher prevalence of hypertension and diabetes mellitus and fewer remaining teeth. There was a modest correlation between mean bone loss score and cumulative pocket depth (Spearman ρ = 0.27).
Table 1.
Baseline characteristic of men with or without incident total or fatal coronary heart disease during follow-up
| No incident CHD | Total CHD | Fatal CHD | |
|---|---|---|---|
| N | 839 | 364 | 109 |
| Age | 48 ± 9 | 50 ± 9 | 55 ± 10 |
| BMI | 26.0 ± 3.0 | 26.5 ± 3.2 | 26.4 ± 3.4 |
| Total Cholesterol | 221 ± 44 | 233 ± 52 | 235 ± 49 |
| HDL* | 49 ± 14 | 46 ± 14 | 42 ± 12 |
| Triglycerides | 148 ± 66 | 169 ± 114 | 178 ± 130 |
| Hypertension | 10% | 14% | 17% |
| Systolic Blood Pressure | 123 ± 15 | 127 ± 16 | 131 ± 19 |
| Diastolic Blood Pressure | 76 ± 9 | 78 ± 9 | 78 ± 10 |
| Diabetes † | 6% | 7% | 10% |
| Current Smokers | 25% | 22% | 24% |
| Alcohol Use (2+ drinks/day) | 23% | 20% | 19% |
| Education (less than high school) | 11% | 12% | 25% |
| Occupation (professional) Income ‡ |
17% | 19% | 15% |
| ≤ $14,999 | 30% | 32% | 31% |
| $15,000 – $24,999 | 51% | 49% | 51% |
| ≥ $25,000 | 19% | 19% | 18% |
| Number of teeth | |||
| >20 | 78% | 74% | 63% |
| 15-20 | 9% | 7% | 5% |
| 10-15 | 3% | 5% | 6% |
| <10 | 5% | 6% | 10% |
| Edentulous | 5% | 8% | 17% |
Figures are percentage or mean± s.d.
HDL-cholesterol available beginning in 1981
Diabetes based on diagnosis by physician or elevated fasting or 2h glucose test
Income at DLS baseline
The association between chronic periodontitis and total CHD was modified by age (p=0.006 and p=0.003 for interaction between age and mean bone loss score and cumulative probing depth, respectively). Among men younger than 60 years, there was a positive association between chronic periodontitis and CHD incidence (Table 2). Men with a mean bone loss score > 1.5 (i.e., approximate average bone loss > 20%) were 112% (95%: 26%, 260%) more likely to develop CHD compared to men with a mean bone loss score ≤ 0.5 (i.e., average bone loss ≤ 5%), independent of other CHD risk factors. For each 20% increase in mean bone loss, the rate of CHD increased by 39% (95% CI: 5%, 83%, p = 0.02). Similarly, there was a statistically significant linear trend for increased rates of CHD with increasing cumulative pocket depth (HR: 1.10, 95% CI: 1.05%, 1.17% per 10 mm increase in cumulative pocket depth, p<0.001). Men with a cumulative pocket depth > 40 mm had 94% (95% CI: 23%, 205%) higher rates of CHD compared to younger men with no pathological pockets. Spline regression evaluating effect-modification by age on a continuous scale suggested that the association between periodontitis and CHD was strongest among the youngest men and decreased fairly linearly with age, with no association present among men older than approximately 60 to 65 years of age (Fig. 1). This is consistent with the results from the stratified analyses, where there was no association among older men (Table 2).
Table 2.
Association between periodontitis and total incident coronary heart disease by age (<60, 60+ years)
| Age < 60 years | Age 60+ years | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Mean Bone Loss Score (MBLS) |
# teeth remaining |
Coronary Heart Disease |
MBLS |
# teeth remainin |
Coronary Heart Disease |
|||||||
| Category | Median | median | # events | Person-yrs | HR (95% CI) * | HR (95% CI) † | median | median | # events | Person-yrs | HR (95% CI) * | HR (95% CI) † |
| ≤ 0.5 | 0.24 | 27 | 47 | 7,745 | 1.00 (Reference) | 1.00 (Reference) | 0.31 | 26 | 42 | 2,338 | 1.00 (Reference) | 1.00 (Reference) |
| 0.5 - ≤ 1 | 0.71 | 25 | 51 | 4,127 | 1.86 (1.25, 2.77) | 1.68 (1.13, 2.52) | 0.76 | 24 | 56 | 3,282 | 0.90 (0.60, 1.35) | 0.84 (0.56, 1.26) |
| 1 - ≤ 1.5 | 1.21 | 22 | 25 | 2,099 | 1.77 (1.09, 2.89) | 1.55 (0.94, 2.56) | 1.22 | 21 | 53 | 2,426 | 1.13 (0.75, 1.69) | 0.98 (0.65, 1.48) |
| > 1.5 | 1.81 | 19 | 22 | 1329 | 2.48 (1.49, 4.12) | 2.12 (1.26, 3.60) | 1.88 | 15 | 34 | 1,491 | 1.13 (0.71, 1.78) | 0.98 (0.61, 1.56) |
| Trend | p=0.001 | p=0.02 | p=0.56 | p=0.99 | ||||||||
| Edentulous | 0 | 9 | 683 | 2.06 (1.01, 4.20) | 1.90 (0.92, 3.93) | 0 | 25 | 793 | 1.95 (1.18, 3.22) | 1.61 (0.95 2.73) | ||
|
Cumulative Probing Depth (CPD) |
# teeth remaining |
Coronary Heart Disease |
CPD |
# teeth remaining |
Coronary Heart Disease |
|||||||
| Category | median | median | # events | Person-yrs | HR (95% CI) * | HR (95% CI) † | median | (median) | # events | Person-yrs | HR (95% CI) * | HR (95% CI) † |
| 0 - <4 mm | 0 | 24 | 41 | 5,752 | 1.00 (Reference) | 1.00 (Reference) | 0 | 23 | 48 | 2,823 | 1.00 (Reference) | 1.00 (Reference) |
| 4 - ≤20 mm | 12 | 25 | 36 | 3,952 | 1.32 (0.84, 2.06) | 1.26 (0.80, 1.98) | 12 | 22 | 63 | 3,009 | 1.17 (0.80, 1.71) | 1.11 (0.77, 1.62) |
| 20 - ≤40 mm | 30 | 26 | 29 | 2,797 | 1.44 (0.89, 2.32) | 1.42 (0.88, 2.29) | 30 | 23 | 47 | 1,956 | 1.30 (0.87, 1.95) | 1.23 (0.82, 1.85) |
| > 40 mm | 60 | 26 | 37 | 2,372 | 2.09 (1.34, 3.26) | 1.94 (1.23, 3.05) | 60 | 24 | 26 | 1,680 | 0.83 (0.51, 1.34) | 0.73 (0.45, 1.19) |
| Test of trend | p<0.001 | p<0.001 | p=0.57 | p=0.33 | ||||||||
| Edentulous | 0 | 9 | 683 | 1.83 (0.89, 3.77) | 1.71 (0.82, 3.55) | 0 | 25 | 793 | 2.04 (1.26, 3.33) | 1.72 (1.03, 2.85) | ||
adjusted for age
adjusted for age, BMI, HDL-cholesterol, total cholesterol, triglycerides, hypertension, mean systolic and diastolic blood pressure, diabetes, fasting glucose, smoking, alcohol intake, occupation & education, income and marital status
Fig. 1.
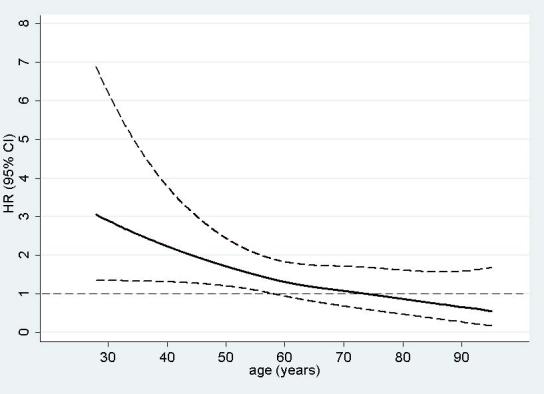
Hazard Ratio (HR) and 95% confidence interval (CI) per one unit increase in mean bone loss score (20% bone loss) as a function of age
There was a non-significant association between edentulism and CHD incidence among younger men, although only 9 edentulous men experienced CHD events in this age group. Edentulous men 60+ years old had 61% (95% CI: −5%, 173%) and 72% (3%, 185%) higher rates of CHD than men with a mean bone loss score ≤0.5 and men with no pathological pockets, respectively (Table 2).
Because of the small number of fatal CHD events in men younger than 60 years, estimates were very imprecise and are not reported here. Among older men, no significant association was found between either measure of periodontitis and fatal CHD (Table 3). However, edentulous men had significantly increased risk of fatal CHD compared to men with a mean bone loss score ≤0.5 (HR: 4.21, 95% CI 1.57, 11.3) and men without pathologically increased pocket depth (HR: 2.97, 95% CI 1.41, 6.25).
Table 3.
Association between periodontitis and fatal incident coronary heart disease for men 60+ years
| Mean Bone Loss Score | # teeth remaining |
Fatal Coronary Heart Disease |
||||
|---|---|---|---|---|---|---|
| Category | median | Median | # events | Person-yrs | HR (95% CI) * | HR (95% CI) † |
| ≤ 0.5 | 0.31 | 26 | 6 | 2,633 | 1 | 1 |
| 0.5 - ≤ 1 | 0.77 | 24 | 17 | 3,937 | 1.63 (0.64, 4.15) | 1.48 (0.57, 2.82) |
| 1 - ≤ 1.5 | 1.22 | 21 | 17 | 3,004 | 1.95 (0.76, 4.98) | 1.59 (0.61, 4.15) |
| > 1.5 | 1.88 | 15 | 14 | 1,877 | 2.32 (0.88, 6.15) | 2.08 (0.77, 5.62) |
| Trend | p=0.05 | p=0. 19 | ||||
| Edentulous | - | 0 | 17 | 989 | 6.45 (2.50, 16.6) | 4.21 (1.57, 11.3) |
| Cumulative Probing Depth | # teeth remaining |
Fatal Coronary Heart Disease |
||||
| Category | median | median | # events | Person-yrs | HR (95% CI) * | HR (95% CI) † |
| 0 - < 4 mm | 0 | 22 | 15 | 3,257 | 1 | 1 |
| 4 - ≤ 20 mm | 12 | 22 | 20 | 3,675 | 1.10 (0.56, 2.15) | 1.28 (0.63, 2.62) |
| > 20 mm | 38 | 24 | 19 | 4,442 | 0.84 (0.42, 1.66) | 1.09 (0.53, 2.24) |
| Trend | p=0.20 | p=0.43 | ||||
| Edentulous | - | 0 | 17 | 989 | 3.58 (1.77, 7.21) | 2.97 (1.41, 6.25) |
adjusted for age
adjusted for age, BMI, HDL-cholesterol, total cholesterol, triglycerides, hypertension, mean systolic and diastolic blood pressure, diabetes, fasting glucose, smoking, alcohol intake, occupation & education, income and marital status
Finally, results were consistent among never smokers as well as between men 60 years and older who were younger or older than 60 years when recruited into the study.
Discussion
In this long-term longitudinal cohort study, we found a positive, dose-dependent association between chronic periodontitis and incidence of coronary heart disease among men younger than 60 years, independent of established cardiovascular risk factors. Among older men, no association between periodontitis and incidence of CHD was found.
Several causal and non-causal pathways have been postulated to explain the observed association between periodontitis (or other chronic infections) and CHD 2, 12. Causal pathways may involve direct and indirect effects of the periodontal infection 12, while genetic and other host factors that increase the susceptibility to both atherosclerosis/thrombosis and chronic periodontitis would be an alternate, non-causal pathway (Fig. 2).
Fig. 2. Possible pathways explaining the periodontitis/CHD association (modified after Beck et al. 2 and Danesh et al. 12).

The dotted arrows describe the causal association of interest, as chronic periodontitis may be a cause of cardiovascular disease through direct (bacteremia) and indirect (systemic inflammation) effects 12. Oral bacteria are considered a necessary cause of chronic periodontitis (pathway 1) 25. Smoking is a strong environmental risk factor for chronic periodontitis (2) 26 and is also an important risk factor for cardiovascular disease (4), which may in part be mediated through its effect on systemic inflammation (3), e.g. elevated CRP concentrations 27. Common susceptibility to the inflammatory diseases, including periodontitis and cardiovascular disease (7,8,9), is determined by genetic (5) and environmental factors, some of which may also be established cardiovascular risk factors (6). There is compelling evidence for a strong genetic base for both periodontitis 28 as well as cardiovascular disease 29, some of which is likely mediated through inflammatory mechanisms (5). On the other hand, diabetes is an established cardiovascular risk factor that may increase the susceptibility to both periodontitis and cardiovascular disease through the increased formation of advanced glycolysation end products, which are pro-inflammatory (6) 30.
Note that even perfect adjustment for established cardiovascular risk factors such as smoking and diabetes, a pro-inflammatory phenotype (at least as far as determined by genetic factors) that predisposes to both periodontitis and CHD may confound the periodontitis/CHD association (non-causal pathway).
It is important to note that the epidemiologic studies available today are not able to differentiate between these causal and non-causal pathways, even if they perfectly control for all established cardiovascular risk factors. Thus, both causal and non-causal pathways may have a role in the observed association (Fig. 2).
Several cross-sectional studies suggest that periodontitis is associated with systemic markers of inflammation, including serum CRP 31, 32 and plasma fibrinogen 32, 33. In addition, several uncontrolled and controlled intervention studies suggest that periodontal treatment may reduce inflammatory biomarkers such as CRP 13, although results are equivocal 34. In addition, a recent randomized controlled trial suggested that successful periodontal therapy may improve endothelial function 14. Furthermore, bacterial DNA and viable periodontal pathogens have been isolated from human atheromas 35. These results provide indirect evidence for a causal role of periodontitis in the pathogenesis of CHD via direct and indirect pathways (Fig. 2). However, the relative importance of such causal mechanisms as compared to confounding by common pro-inflammatory susceptibility factors is uncertain.
Tooth loss, and in particular complete tooth loss (edentulism), should reduce the increased risk for CHD associated with the causal effects of periodontitis, by reducing or eliminating the exposure to periodontal inflammation. Indeed, a small uncontrolled study of 67 patients with advanced periodontitis suggested that serum concentrations of inflammatory biomarkers may decrease following full-mouth tooth extractions 36. However, a large representative cross-sectional study of the US population found similar serum CRP-concentrations in edentulous subjects as in subjects with chronic periodontitis 31. Complete tooth loss may be a marker of previous periodontitis. The authors therefore suggested that the increased CRP concentrations among the edentulous may be a marker of a common underlying pro-inflammatory trait that may predispose to both CHD and periodontitis/tooth loss (Fig. 2)31. Our finding of a higher CHD incidence among older edentulous men compared to older dentate men who are periodontally healthy is also consistent with this hypothesis. It should be noted that edentulism may contribute to CHD risk through alternative pathways such as nutrition; however, dietary changes associated with tooth loss appear to be small and their relevance for CHD risk is questionable 37. Alternatively, edentulism may be a surrogate for socio-economic status and residual confounding may contribute to the observed association. For example, edentulism may partially reflect chronically worse access to care that may have resulted in less effective treatment of cardiovascular risk factors, including hypertension, diabetes and hyperlipidemia.
The finding that the association between periodontitis and CHD incidence is limited to younger men is consistent with previous studies 1, 21, and this modification of the effect of periodontitis by age is also a consistent finding in studies on ischemic stroke 22, 23. Our results suggest that the association decreases continuously with increasing age, and approaches the null at about 60 to 65 years of age. Men with a higher susceptibility to periodontitis will exhibit a given degree of periodontal destruction at an earlier age than men with lower susceptibility to inflammatory periodontitis. In other words, periodontitis at a younger age is a marker of higher disease susceptibility. Therefore, the finding of a significant association between periodontitis and CHD incidence among young men, its continuous decrease with increasing age and no association among older men in the present study is also consistent with the hypothesis of common pro-inflammatory susceptibility factors explaining a large part of the observed association.
Treatment of periodontitis typically results in a reduction or elimination of periodontal pockets but does not typically result in a regeneration of lost bone. Hence, alveolar bone loss is a better measure of periodontitis history, while the cumulative pocket depth measure utilized in this study is a better measure of current exposure to periodontal inflammation. Furthermore, the latter measure adequately accounts for the reduction in inflammatory exposure due to tooth loss 33. This is also illustrated by the decrease in the number of remaining teeth across categories of mean bone loss score compared to the slight increase in the number of remaining teeth with increasing cumulative pocket depth (Table 2). Therefore, one would expect cumulative pocket depth to be a better clinical measure of periodontitis if the periodontal inflammation itself was a causal risk factor for CHD. However, in the present study, bone loss and pocket depth measures yielded similar results.
The ultimate question whether periodontal treatment can reduce the risk of coronary heart disease can only be answered in a randomized controlled clinical trial. Recently, Tonetti et al. reported results from a randomized clinical trial indicating that periodontal treatment significantly improved endothelial function and other surrogate markers of CHD risk 14. However, the feasibility of a trial on true CHD endpoints may be questioned as both severe periodontitis and incident coronary heart disease are relatively uncommon among persons younger than 50, while this and other epidemiologic studies suggest that there is no association among older persons in which both conditions are more common.
The availability of repeated clinical and radiographic measures of periodontitis over a long follow-up period is an important strength of the present study when compared to other available cohort studies. Furthermore, we were able to control for several important CHD risk factors and confounders using time-varying covariates. Some of the available cohort studies have been criticized for lack of adequate control for smoking 5. We used a novel comprehensive smoking index that simultaneously accounts for intensity, duration and time since cessation of smoking to minimize residual confounding 20. In addition, sensitivity analyses restricting to never smokers yielded consistent estimates (data not shown). However, residual confounding - in particular by dimensions of socio-economic status not fully captured by the measures available in the present study 38 or due to changes in income not captured by our time-invariant measure – may still be a concern. This is particularly relevant for estimates of the effects of edentulism as fully adjusted estimated were markedly attenuated compared to age-adjusted estimates.
Further limitations of this study include its moderate sample size which limited the precision of estimates. Hence, considerable uncertainty remains as to the strength of the association between periodontitis and CHD. Furthermore, this cohort consisted almost exclusively of white men and generalizability of these findings to other populations is uncertain.
In conclusion, the results of the present study suggest that chronic periodontitis is associated with incidence of CHD among younger men, independent of established cardiovascular risk factors. However, after complete tooth loss, risk for total and fatal CHD is elevated compared to periodontally healthy dentate older men. Although periodontitis may be a causal risk factor for CHD, the present results may suggest that an increased pro-inflammatory susceptibility common to both periodontitis and CHD may be important on a population level.
Acknowledgements
none
Funding Sources
The VA Normative Aging Study and VA Dental Longitudinal Study are components of the Massachusetts Veterans Epidemiology Research and Information Center, supported by the VA Cooperative Studies Program. This work was supported by NIH grant R03 DE-016357 from the National Institute of Dental and Craniofacial Research awarded to Dr. Dietrich. Dr. Garcia is a recipient of a VA Career Development Award in Health Services Research from the VA HSR&D Service, a VA Merit Review Award and NIH grant K24 DE-00419 from the National Institute of Dental and Craniofacial Research.
Footnotes
Publisher's Disclaimer: “This is an un-copyedited author manuscript that was accepted for publication in Circulation, copyright The American Heart Association. This may not be duplicated or reproduced, other than for personal use or within the  Fair Use of Copyrighted Materials
Fair Use of Copyrighted Materials (section 107, title 17, U.S. Code) without prior permission of the copyright owner, The American Heart Association. The final copyedited article, which is the version of record, can be found at http://circ.ahajournals.org. The American Heart Association disclaims any responsibility or liability for errors or omissions in this version of the manuscript or in any version derived from it by the National Institutes of Health or other parties.”
(section 107, title 17, U.S. Code) without prior permission of the copyright owner, The American Heart Association. The final copyedited article, which is the version of record, can be found at http://circ.ahajournals.org. The American Heart Association disclaims any responsibility or liability for errors or omissions in this version of the manuscript or in any version derived from it by the National Institutes of Health or other parties.”
Disclosures
none
Contributor Information
Thomas Dietrich, Dept. of Health Policy and Health Services Research, Dept. of Periodontology and Oral Biology, Boston University Goldman School of Dental Medicine, Boston, USA and Dept. of Oral Surgery, The School of Dentistry, University of Birmingham, Birmingham, UK.
Monik Jimenez, Dept. of Health Policy and Health Services Research, Boston University Goldman School of Dental Medicine, Dept. of Oral Health Policy and Epidemiology, Harvard School of Public Health, Boston, USA.
Elizabeth A. Krall Kaye, Dept. of Health Policy and Health Services Research, Boston University Goldman School of Dental Medicine, Boston, USA.
Pantel S. Vokonas, VA Normative Aging Study, VA Boston Healthcare System, and Dept. of Medicine, Boston University School of Medicine, Boston, USA.
Raul I. Garcia, VA Dental Longitudinal Study, VA Boston Healthcare System, and Dept. of Health Policy and Health Services Research, Boston University Goldman School of Dental Medicine, Boston, USA.
References
- 1.DeStefano F, Anda RF, Kahn HS, Williamson DF, Russell CM. Dental disease and risk of coronary heart disease and mortality. Bmj. 1993;306:688–691. doi: 10.1136/bmj.306.6879.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beck J, Garcia R, Heiss G, Vokonas PS, Offenbacher S. Periodontal disease and cardiovascular disease. J Periodontol. 1996;67:1123–1137. doi: 10.1902/jop.1996.67.10s.1123. [DOI] [PubMed] [Google Scholar]
- 3.Hujoel PP, Drangsholt M, Spiekerman C, DeRouen TA. Periodontal disease and coronary heart disease risk. JAMA. 2000;284:1406–1410. doi: 10.1001/jama.284.11.1406. [DOI] [PubMed] [Google Scholar]
- 4.Howell TH, Ridker PM, Ajani UA, Hennekens CH, Christen WG. Periodontal disease and risk of subsequent cardiovascular disease in U.S. male physicians. J Am Coll Cardiol. 2001;37:445–450. doi: 10.1016/s0735-1097(00)01130-x. [DOI] [PubMed] [Google Scholar]
- 5.Hujoel PP, Drangsholt M, Spiekerman C, DeRouen TA. Periodontitis-systemic disease associations in the presence of smoking--causal or coincidental? Periodontol 2000. 2002;30:51–60. doi: 10.1034/j.1600-0757.2002.03005.x. [DOI] [PubMed] [Google Scholar]
- 6.Joshipura KJ, Rimm EB, Douglass CW, Trichopoulos D, Ascherio A, Willett WC. Poor oral health and coronary heart disease. J Dent Res. 1996;75:1631–1636. doi: 10.1177/00220345960750090301. [DOI] [PubMed] [Google Scholar]
- 7.Morrison HI, Ellison LF, Taylor GW. Periodontal disease and risk of fatal coronary heart and cerebrovascular diseases. J Cardiovasc Risk. 1999;6:7–11. doi: 10.1177/204748739900600102. [DOI] [PubMed] [Google Scholar]
- 8.Dietrich T, Garcia RI. Associations between periodontal disease and systemic disease: evaluating the strength of the evidence. J Periodontol. 2005;76:2175–2184. doi: 10.1902/jop.2005.76.11-S.2175. [DOI] [PubMed] [Google Scholar]
- 9.Tonetti MS, Claffey N. Advances in the progression of periodontitis and proposal of definitions of a periodontitis case and disease progression for use in risk factor research. J Clin Periodontol. 2005;32:210–213. doi: 10.1111/j.1600-051X.2005.00822.x. [DOI] [PubMed] [Google Scholar]
- 10.Andriankaja OM, Genco RJ, Dorn J, Dmochowski J, Hovey K, Falkner KL, Scannapieco F, Trevisan M. The use of different measurements and definitions of periodontal disease in the study of the association between periodontal disease and risk of myocardial infarction. J Periodontol. 2006;77:1067–1073. doi: 10.1902/jop.2006.050276. [DOI] [PubMed] [Google Scholar]
- 11.Beck JD, Eke P, Heiss G, Madianos P, Couper D, Lin D, Moss K, Elter J, Offenbacher S. Periodontal Disease and Coronary Heart Disease: A Reappraisal of the Exposure. Circulation. 2005;112:19–24. doi: 10.1161/CIRCULATIONAHA.104.511998. [DOI] [PubMed] [Google Scholar]
- 12.Danesh J, Collins R, Peto R. Chronic infections and coronary heart disease: is there a link? The Lancet. 1997;350:430. doi: 10.1016/S0140-6736(97)03079-1. [DOI] [PubMed] [Google Scholar]
- 13.D'Aiuto F, Parkar M, Andreou G, Suvan J, Brett PM, Ready D, Tonetti MS. Periodontitis and systemic inflammation: control of the local infection is associated with a reduction in serum inflammatory markers. J Dent Res. 2004;83:156–160. doi: 10.1177/154405910408300214. [DOI] [PubMed] [Google Scholar]
- 14.Tonetti MS, D'Aiuto F, Nibali L, Donald A, Storry C, Parkar M, Suvan J, Hingorani AD, Vallance P, Deanfield J. Treatment of Periodontitis and Endothelial Function. N Engl J Med. 2007;356:911–920. doi: 10.1056/NEJMoa063186. [DOI] [PubMed] [Google Scholar]
- 15.Kapur KK, Glass RL, Loftus ER, Alman JE, Feller RP. The Veterans Administration longitudinal study of oral health. Aging Hum Devel. 1972;3:125–137. [Google Scholar]
- 16.Shurtleff D. Some characteristics related to the incidence of cardivascular disease and death: Framingham Study, 18 year follow-up. Vol. 74. US Dept. of Health, Education and Welfare; Bethesda: 1974. [Google Scholar]
- 17.Glass RL, Loftus ER, Kapur KK, Alman JE. Analyses of components of periodontal disease. J Dent Res. 1973;52:1238–1244. doi: 10.1177/00220345730520061301. [DOI] [PubMed] [Google Scholar]
- 18.Feldman RS, Douglass CW, Loftus ER, Kapur KK, Chauncey HH. Interexaminer agreement in the measurement of periodontal disease. J Periodontal Res. 1982;17:80–89. doi: 10.1111/j.1600-0765.1982.tb01133.x. [DOI] [PubMed] [Google Scholar]
- 19.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159–174. [PubMed] [Google Scholar]
- 20.Dietrich T, Hoffmann K. A comprehensive index for the modeling of smoking history in periodontal research. J Dent Res. 2004;83:859–863. doi: 10.1177/154405910408301107. [DOI] [PubMed] [Google Scholar]
- 21.Geismar K, Stoltze K, Sigurd B, Gyntelberg F, Holmstrup P. Periodontal disease and coronary heart disease. J Periodontol. 2006;77:1547–1554. doi: 10.1902/jop.2006.050405. [DOI] [PubMed] [Google Scholar]
- 22.Joshipura KJ, Hung HC, Rimm EB, Willett WC, Ascherio A. Periodontal disease, tooth loss, and incidence of ischemic stroke. Stroke. 2003;34:47–52. doi: 10.1161/01.str.0000052974.79428.0c. [DOI] [PubMed] [Google Scholar]
- 23.Grau AJ, Becher H, Ziegler CM, Lichy C, Buggle F, Kaiser C, Lutz R, Bultmann S, Preusch M, Dorfer CE. Periodontal disease as a risk factor for ischemic stroke. Stroke. 2004;35:496–501. doi: 10.1161/01.STR.0000110789.20526.9D. [DOI] [PubMed] [Google Scholar]
- 24.Witte JS, Greenland S. A nested approach to evaluating dose-response and trend. Annals of Epidemiology. 1997;7:188. doi: 10.1016/s1047-2797(96)00159-7. [DOI] [PubMed] [Google Scholar]
- 25.Darveau RP, Tanner A, Page RC. The microbial challenge in periodontitis. Periodontology 2000. 1997;14:12–32. doi: 10.1111/j.1600-0757.1997.tb00190.x. [DOI] [PubMed] [Google Scholar]
- 26.Tomar SL, Asma S. Smoking-attributable periodontitis in the United States: findings from NHANES III. National Health and Nutrition Examination Survey. J Periodontol. 2000;71:743–751. doi: 10.1902/jop.2000.71.5.743. [DOI] [PubMed] [Google Scholar]
- 27.Dietrich T, Garcia RI, de Pablo P, Schulze PC, Hoffmann K. The effects of cigarette smoking on C-reactive protein concentrations in men and women and its modification by exogenous oral hormones in women. Eur J Cardiovasc Prev Rehabil. 2007;14:694–700. doi: 10.1097/HJR.0b013e328270b913. [DOI] [PubMed] [Google Scholar]
- 28.Kinane DF, Shiba H, Hart TC. The genetic basis of periodontitis. Periodontol 2000. 2005;39:91–117. doi: 10.1111/j.1600-0757.2005.00118.x. [DOI] [PubMed] [Google Scholar]
- 29.Arnett DK, Baird AE, Barkley RA, Basson CT, Boerwinkle E, Ganesh SK, Herrington DM, Hong Y, Jaquish C, McDermott DA, O'Donnell CJ. Relevance of genetics and genomics for prevention and treatment of cardiovascular disease: a scientific statement from the American Heart Association Council on Epidemiology and Prevention, the Stroke Council, and the Functional Genomics and Translational Biology Interdisciplinary Working Group. Circulation. 2007;115:2878–2901. doi: 10.1161/CIRCULATIONAHA.107.183679. [DOI] [PubMed] [Google Scholar]
- 30.Goldin A, Beckman JA, Schmidt AM, Creager MA. Advanced Glycation End Products: Sparking the Development of Diabetic Vascular Injury. Circulation. 2006;114:597–605. doi: 10.1161/CIRCULATIONAHA.106.621854. [DOI] [PubMed] [Google Scholar]
- 31.Slade GD, Offenbacher S, Beck JD, Heiss G, Pankow JS. Acute-phase inflammatory response to periodontal disease in the US population. J Dent Res. 2000;79:49–57. doi: 10.1177/00220345000790010701. [DOI] [PubMed] [Google Scholar]
- 32.Wu T, Trevisan M, Genco RJ, Falkner KL, Dorn JP, Sempos CT. Examination of the Relation between Periodontal Health Status and Cardiovascular Risk Factors: Serum Total and High Density Lipoprotein Cholesterol, C-reactive Protein, and Plasma Fibrinogen. Am. J. Epidemiol. 2000;151:273–282. doi: 10.1093/oxfordjournals.aje.a010203. [DOI] [PubMed] [Google Scholar]
- 33.Schwahn C, Volzke H, Robinson DM, Luedemann J, Bernhardt O, Gesch D, John U, Kocher T. Periodontal disease, but not edentulism, is independently associated with increased plasma fibrinogen levels. Results from a population-based study. Thromb Haemost. 2004;92:244–252. doi: 10.1160/TH04-02-0092. [DOI] [PubMed] [Google Scholar]
- 34.Ioannidou E, Malekzadeh T, Dongari-Bagtzoglou A. Effect of periodontal treatment on serum C-reactive protein levels: a systematic review and meta-analysis. J Periodontol. 2006;77:1635–1642. doi: 10.1902/jop.2006.050443. [DOI] [PubMed] [Google Scholar]
- 35.Kozarov EV, Dorn BR, Shelburne CE, Dunn WA, Jr., Progulske-Fox A. Human Atherosclerotic Plaque Contains Viable Invasive Actinobacillus actinomycetemcomitans and Porphyromonas gingivalis. Arterioscler Thromb Vasc Biol. 2005;25:e17–18. doi: 10.1161/01.ATV.0000155018.67835.1a. [DOI] [PubMed] [Google Scholar]
- 36.Taylor BA, Tofler GH, Carey HM, Morel-Kopp MC, Philcox S, Carter TR, Elliott MJ, Kull AD, Ward C, Schenck K. Full-mouth tooth extraction lowers systemic inflammatory and thrombotic markers of cardiovascular risk. J Dent Res. 2006;85:74–78. doi: 10.1177/154405910608500113. [DOI] [PubMed] [Google Scholar]
- 37.Hung HC, Colditz G, Joshipura KJ. The association between tooth loss and the self-reported intake of selected CVD-related nutrients and foods among US women. Community Dent Oral Epidemiol. 2005;33:167–173. doi: 10.1111/j.1600-0528.2005.00200.x. [DOI] [PubMed] [Google Scholar]
- 38.Braveman PA, Cubbin C, Egerter S, Chideya S, Marchi KS, Metzler M, Posner S. Socioeconomic Status in Health Research: One Size Does Not Fit All. JAMA. 2005;294:2879–2888. doi: 10.1001/jama.294.22.2879. [DOI] [PubMed] [Google Scholar]


