Abstract
The macroevolutionary events leading to neural innovations for social communication, such as vocalization, are essentially unexplored. Many fish vocalize during female courtship and territorial defense, as do amphibians, birds, and mammals. Here, we map the neural circuitry for vocalization in larval fish and show that the vocal network develops in a segment-like region across the most caudal hindbrain and rostral spinal cord. Taxonomic analysis demonstrates a highly conserved pattern between fish and all major lineages of vocal tetrapods. We propose that the vocal basis for acoustic communication among vertebrates evolved from an ancestrally shared developmental compartment already present in the early fishes.
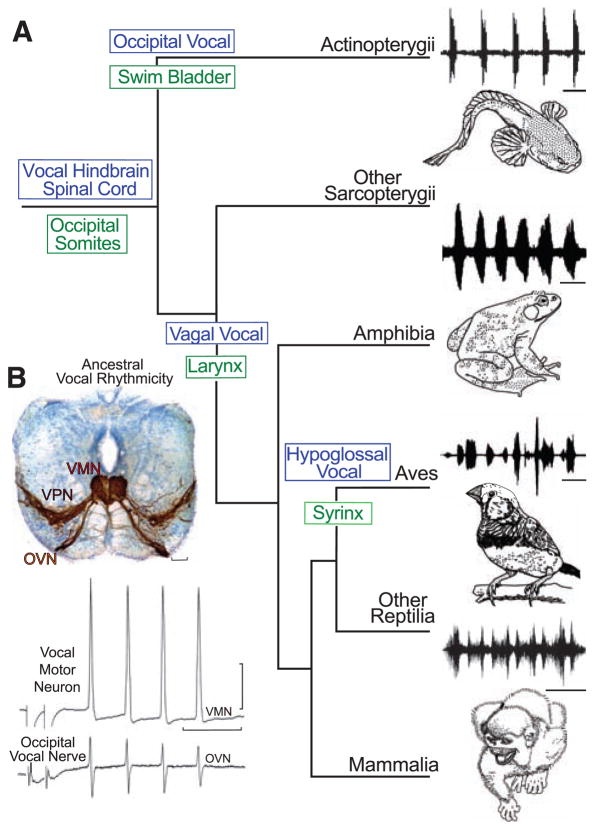
Although the genetic basis for human speech receives much attention [e.g., (1, 2)], the fundamental issue of the ancestral origins of neural networks for vocal signaling is essentially unexplored. Social, context-dependent acoustic communication occurs in most of the major vertebrate lineages, including fishes (Fig. 1A). Teleost fish, the most species-rich of all vertebrate groups (3), have a simple repertoire of vocalizations complemented by vocal and auditory pathways that are organized similarly to those of amphibians, reptiles, birds, and mammals (4). Batrachoidid fish (midshipman and toadfish), in particular, have an expansive vocal-acoustic network, including a rhythmically firing, pacemaker–motor neuron circuit that directly determines the contraction rate of vocal muscles attached to the swim bladder and, in turn, the temporal properties of calls (5, 6) (Fig. 1B and fig. S1; movies S1 to S3). Because batrachoidids also have readily studied larval stages (7), they were chosen to investigate the hypothesis that fish and terrestrial vertebrates share an ancestral origin of their vocal motor networks. Here, we show that the vocal systems of fishes and tetrapods develop very similarly in a segment-like region that forms a transitional compartment between the caudal hindbrain and rostral spinal cord.
Fig. 1.
Evolution of vocal behaviors. (A) Cladogram of living bony vertebrates (3), with oscillogram of a vocalization from a representative species, shows nodal (ancestral) states for vocal characters (10). Vocalizations (top to bottom): midshipman fish agonistic “grunts,” bullfrog advertisement call, estrildid finch song, and squirrel monkey cackle. Scale bars (top to bottom) are 500 ms, 1.0 s, 250 ms, and 200 ms. Vocal mechanisms are unknown for lobe-finned fish (other Sarcopterygii), although well-known for nonavian (other) Reptilia (30). (B) Vocal pacemaker circuit. Among batrachoidid fish (midshipman and toadfish), there is a direct translation between the temporal properties of the vocal circuitry and natural calls (4, 5). (Top) Transverse section at the caudal hindbrain-spinal cord transition of a toadfish shows transneuronal, neurobiotin-labeling of midline vocal motor neurons (VMNs), adjacent pacemaker neurons (VPN), and motor axons exiting via nerve root that gives rise to the occipital vocal nerve (OVN); vocal neurons have extensive lateral processes (5, 6). Scale bar is 100 μm. (Bottom) The rhythmic, oscillatory-like activity of a vocal motor neuron [(top trace) average of four DC-coupled intracellular records] is aligned with occipital nerve activity [(bottom trace) average of four intracranial records] to indicate relative timing [see (5) for same temporal pattern of pacemaker neurons]. Response was evoked by midbrain electrical microstimulation in midshipman fish [paired stimulus artifact far left; modified from (5) with permission]. Horizontal scale bar is 20 ms; vertical scale bar is 20 mv and 1 mv, respectively, for intracellular and nerve records.
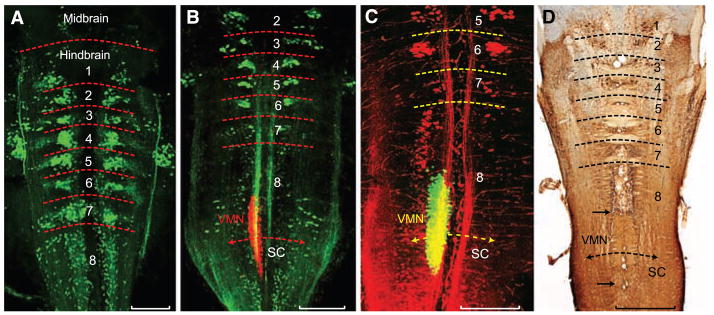
Comparative evidence shows that the developing hindbrain of vertebrates has eight segments or rhombomeres (8). A segmental organization has been shown, in part, by identifying hindbrain reticular neurons that project to the spinal cord (9). We identified a similar pattern of reticulo-spinal pathways in larval batrachoidid fish, labeling the rostral spinal cord with either fluorescent dextran-amines or Alexa biocytin and then visualizing retrogradely filled hindbrain neurons with laser-scanning confocal microscopy (10). Larvae had eight rhombomeres (rh1 to rh8), with rh1 to rh7 distinguished by clusters, and rh8 by an elongated column, of reticulospinal neurons (Fig. 2A). Rhombomere 8 is of special interest for two reasons. First, it is proposed to give rise to pattern-generating circuitry in all vocal vertebrates (11). Second, rh8 forms a transitional zone with the spinal cord that is typically two to three times the size of each rhombomere in segments 2 to 6 (8, 12), and rh8 is subdivided in teleosts (9) and birds (13) into multiple, segment-like regions. A segmental pattern for rh8 was revealed here in larval batrachoidids by using fluorescent tracers with nonoverlapping wavelengths to simultaneously map the reticulospinal scaffold and vocal motor neurons [labeled via occipital nerves innervating developing vocal muscle, see (7)]. A dense midline column of vocal motor neurons (Fig. 2, B and C) was revealed, lying immediately caudal to a rostral subdivision of rh8’s reticulospinal column. The easily delimited vocal motor nucleus (VMN) prefigures its expansive size in postlarval stages (Fig. 2D, also see Fig. 1B). The early larval VMN was more than twice as long as individual rhombomeres within rh2 to rh6, comparable to the two-rhombomere (bimeric) extent of some cranial motor nuclei, namely the trigeminal and abducens nuclei, which span, respectively, rh2 to rh3 and rh5 to rh6 in teleost fish and birds (8, 12, 13).
Fig. 2.
Segmental organization of hindbrain reticulospinal and vocal neurons. (A to C) Confocal projections in horizontal plane of fluorescently labeled neurons in midshipman larvae [n = 21; 8 to 14 days post fertilization (dpf), 7- to 10-mm standard length]. Horizontal hatching approximates midbrain, hindbrain rhombomere (1 to 8), and spinal cord (SC) boundaries. Scale bars are 0.2 mm. (A) Clusters of reticulospinal neurons labeled with Alexa biocytin 488 (green) at a stage before vocal muscle reaches swim bladder (~8 dpf). (B) Simultaneous visualization of reticulospinal neurons and vocal motor neurons (VMNs) labeled with, respectively, Alexa 488 dextran-amine (green) and Alexa 546 dextran-amine (red) at a stage when vocal muscles attach to the swim bladder (~14 dpf). (C) Same stage as (B) highlights rostral subdivision of rh8 reticulospinal column (Alexa 546 dextran-amine, red) and VMN (Alexa 488 dextran-amine, green). Yellow in (B) and (C) is composite overlap (red and green), but no double label. (D) Horizontal section showing biocytin-filled, reticulospinal clusters in postlarval midshipman (80 mm, ~200 dpf). Paired black arrows denote VMN’s rostral-caudal extent. Scale bar is 0.5 mm.
To further test the possibility that the VMN in larval fish develops within rh8, its position relative to other neuronal groups was examined. The labeling of vocal and nonvocal muscles with different detxranamines showed that the caudal half of the VMN was aligned with the rostral portion of a motor column, extending into rostral spinal segments, that innervates pectoral girdle and trunk muscles (PEC and EP, respectively) (Fig. 3, A and B); direct labeling of the occipital nerve roots that innervate these muscles (7, 14) confirmed this pattern (Fig. 3C). We then compared the locations of vocal neurons with those of vagal motor neurons, the caudal extent of which provide a landmark for the hindbrain-spinal transition (13). Composite labeling of the vagal-innervated gastrointestinal wall (fig. S1B) and occipital-innervated pectoral girdle muscle, along with vocal neurons, showed two cell groups (Fig. 3, D to F). The gastrointestinal neurons (XMNs) corresponded to a caudal subgroup of vagal motor neurons (15) that extended along the rostral half of the VMN, whereas the pectoral neurons (LPs) represented the far rostral pole of the occipital-spinal column (Fig. 3C). An antibody to choline acetyltransferase demarcated the entire vagal motor column in a pattern consistent with the dextranamine labels (fig. S2), thus reinforcing its identification. The simultaneous mapping of nonvocal and vocal motor neurons led to the hypothesis that the VMN in fish develops in a distinct compartment formed by hindbrain rh8 and rostral spinal cord.
Fig. 3.
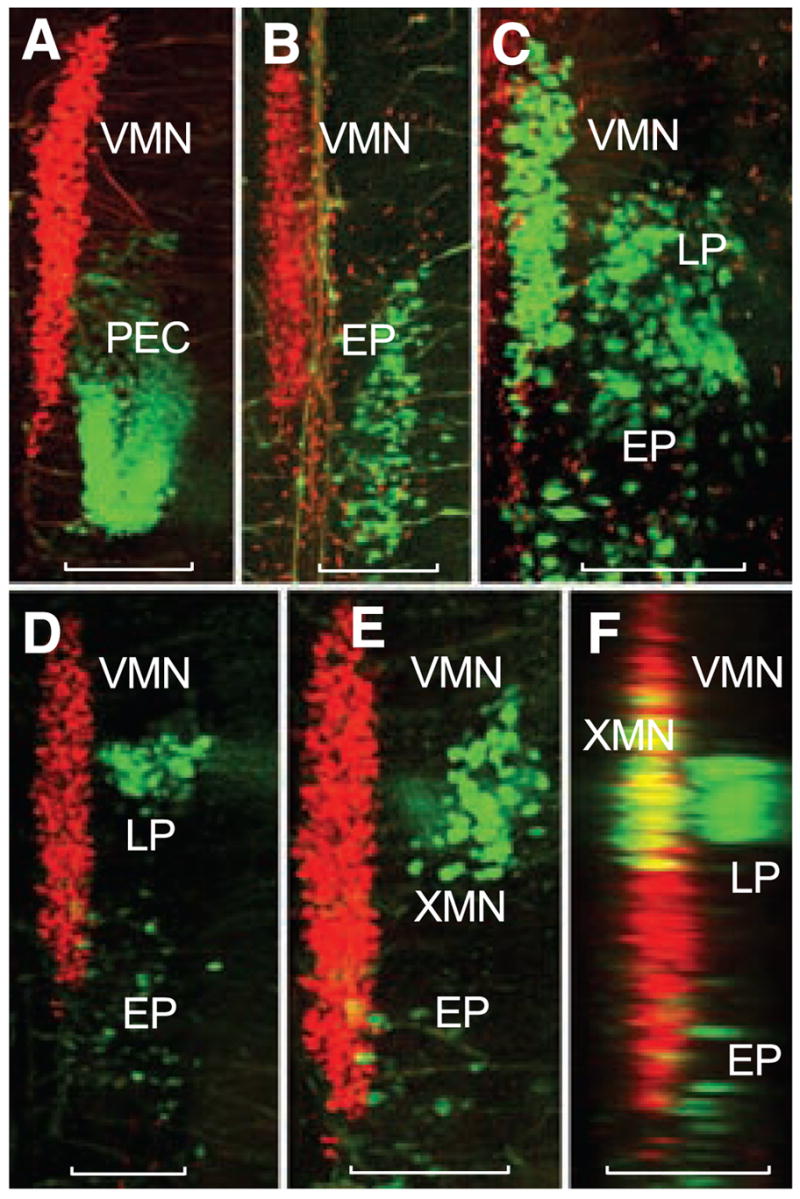
Segmental organization of vocal and non-vocal motor neurons. Simultaneous labeling of vocal and either nonvocal muscles or nerve roots with different wavelength fluorescent dextran-amines provides visualization of vocal motor neurons (VMNs) and nonvocal motor neurons in midshipman larvae (~14 dpf, n = 28). Confocal projections in horizontal (A to E) and sagittal (F) planes. Nonvocal motor neurons are designated by target muscle: EP, epaxialis; LP, levator pectoralis; PEC, pectoral fin. XMN indicates vagal motor neurons innervating wall of gastrointestinal tract. Neurons appearing red and green were labeled with, respectively, fluorescent dextran-amines 546 and 488. Scale bars are 0.1 mm. (A and B) PEC and EP neurons lateral to caudal vocal motor neurons. (C) LP and EP lateral to caudal vocal motor neurons after intracranial label of ipsilateral occipital roots; partial view of contralateral vocal motor neurons filled (far left) by labeling contralateral occipital root. (D) LP neurons lateral to vocal motor neurons with sparsely distributed EP neurons labeled during the exposure. (E) Higher magnification view just dorsal to (D) showing XMN lateral, rather than directly dorsal, to vocal motor neurons because of tissue flat mounting (see fig. S1). (F) Sagittal reconstruction of (D) and (E) showing XMN and LP relative to more medial vocal motor neurons. Yellow is composite overlap (red and green), but no double label.
The fully differentiated vocal circuit includes both pacemaker and motor neurons that are readily labeled via transneuronal transport of neurobiotin applied to the vocal branch of the occipital nerve (6). Only vocal motor neurons are labeled at the earliest larval stages, followed by pacemaker and prepacemaker neurons at successively later stages (16). The developing vocal circuit of larval fish was mapped here relative to the segmental organization of the hindbrain at the earliest stage that includes prepacemaker and pacemaker neurons (10). Alignment of a horizontal series of neurobiotin-filled vocal (Fig. 4, A and B) and reticulospinal (Fig. 4, C and D) neurons in larvae at the same stage showed vocal prepacemaker neurons and the rostral pacemaker–motor neuron circuit in caudal rh8. The caudal pacemaker–motor circuit extended into the rostral spinal cord. The dorsoventral position of the vocal pacemaker circuit was corroborated in transverse series (Fig. 4E); other experiments verified its location relative to nonvocal motor neurons. Because biotin dextranamine is not transported transneuronally (6, 16), both VMN and a contralateral, nonvocal subgroup could be labeled in the same larva. As observed at early stages (Fig. 3), the caudal part of the VMN was coextensive with occipital-spinal neurons innervating nonvocal muscles [EP in (Fig. 4F)]. In sum, the topography of vocal and nonvocal neurons in larvae with a pacemaker circuit showed that both vocal pacemaker and motor neurons develop in the same rh8-spinal compartment.
Fig. 4.
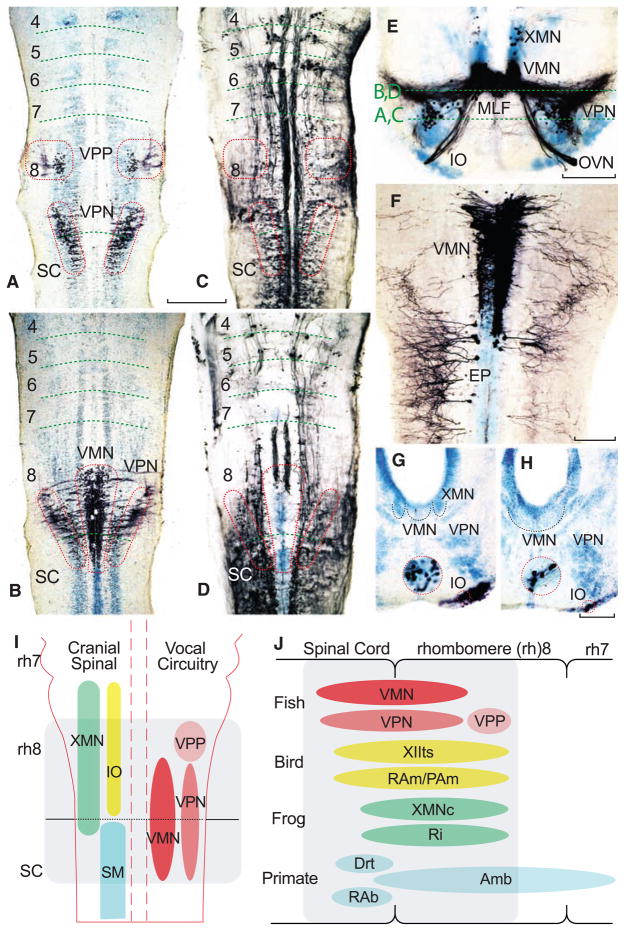
Segmental organization of vocal pacemaker–motor neuron network along with summary diagrams of larval fish and vocal vertebrates. (A to H) Alignment of either neurobiotin- or dextran-biotin–filled (which appear black) vocal and nonvocal neurons in toadfish (A to F) and midshipman (G and H) larvae (n = 25; 1.8 to 2.0 cm, ~30 to 40 dpf). Cresyl violet counterstain illustrates other neuronal groups. Paired horizontal sections at two dorsal-ventral levels, indicated in (E), compare neurobiotin-filled vocal prepacemaker (VPP), pacemaker (VPN), and motor (VMN) neurons (A and B) relative to neurobiotion-filled reticulospinal neurons (C and D). Red hatching in (C) and (D) approximates location of the VPP, VPN, and VMN in (A) and (B). Horizontal hatching in (A to D) estimates rhombomere (4 to 8) and spinal cord (SC) boundaries. Scale bar is 0.2 mm for (A to D). (E) Transverse section through rostral, neurobiotin-filled vocal pacemaker–motor neurons and exiting occipital vocal tract and nerve root (OVN), including a few labeled vagal motor neurons (XMN) that innervate swim bladder smooth muscle (15). (IO, inferior olive; MLF, medial longitudinal fasciculus.) Scale bar is 0.1 mm. (F) Horizontal section of VMN (right side) and trunk (epaxialis, EP; left side) motor neurons labeled with 10-kD biotin-dextran-amine. VMN exhibits dendritic spread ipsilaterally and into contralateral VMN. Scale bar is 0.1 mm. (G and H) Transverse sections showing neurobiotin-labeled inferior olive (IO) coextensive with rostral (G) and middle (H) VMN levels (cresyl violet–stained VMN, outlined with black dotted line, is adjacent to XMN, also outlined, and/or VPN). Red-ringed inserts are higher magnification views of IO. Scale bars are 0.1 and 0.03 mm. (I) Schematic in horizontal plane showing relative positions of vocal and nonvocal neurons comprising a rhombomere (rh) 8 to spinal (SC) compartment. (J) Comparative summary in sagittal plane showing relative positions of vocal neurons in the rh8–spinal compartment. The developmental map for premotor-motor circuitry in fish (this report), as well as for motor neurons in birds (13) and frogs (17), is based on representatives of similar age. Positioning of premotor neurons that participate in vocal patterning in birds (20, 21) and frogs (22), and motor and premotor neurons in mammals (19, 23, 24), including primates (25), is based on adult phenotypes [but see (8) for developmental studies of caudal hindbrain in mammals]. Most laryngeal motor neurons that shape the temporal envelope of mammalian calls originate from caudal nucleus ambiguus (Amb) (23). Abbreviations: Drt, dorsal reticular nucleus; PAm, nucleus parambigualis; Ri, inferior reticular formation; RAb, nucleus retroambiguus; RAm, nucleus retroambigualis; VPP-VPN-VMN, vocal prepacemaker–pacemaker–motor neurons; XMNc, caudal XMN; XIIts, tracheosyringeal division of hypoglossal motor nucleus.
We also identified the inferior olive, a precerebellar nucleus, because it is derived from rh8 in tetrapods (13) and, thus, provides a critical, non–motor neuron landmark for establishing the position of the developing vocal circuit within the caudal hindbrain. Neurobiotin labeling of the larval cerebellum showed the inferior olive’s extent coincident with the vocal prepacemaker and rostral pacemaker–motor circuit (Fig. 4, G and H). Together, the combinatorial mapping of vocal neurons with inferior olive, reticulospinal, and nonvocal motor neurons firmly established the location of the entire vocal pacemaker–motor circuit of larval fish within a compartment that spans hindbrain rh8 and the rostral spinal cord (Fig. 4I).
A taxonomic survey revealed a close alignment of the larval fish pattern with the vocal circuitry of other groups of vocal vertebrates (Fig. 4J). The position of the VMN compares well with that of developing vocal motor neurons that innervate the syrinx in birds (XIIts) (13) and larynx in frogs (XMNc) (17). Although comparable developmental studies have not been done for reptiles and mammals, their adult phenotypes indicate a similar pattern for laryngeal motor neurons (Amb) (18, 19). Premotor neurons that pattern vocal-respiratory mechanisms in birds (RAm/PAm) (20, 21), amphibians (Ri) (22), and mammals (23, 24), including nonhuman primates and humans (Drt/RAb) (25), are topographically comparable to the developing prepacemaker-pacemaker nuclei (VPP-VPN) of fish and, like the VPP-VPN (4), are targets of midbrain vocal neurons (24).
The most parsimonious interpretation of a cladistic analysis (10) is that a vocal hindbrain-spinal compartment originated in a common ancestor of the two major groups of living fishes, the Actinopterygii, which includes the teleosts studied here, and the Sarcopterygii, which includes lobe-finned fishes (lungfish and coelacanth) and tetrapods (Fig. 1A). The vocal muscles of fish and tetrapods also share origins from occipital somites (Fig. 1A) (7, 26, 27), although the innervation of the vocal muscle associated with the sound-generating organ evolved at least three times (Fig. 1A): occipital nerve and swim bladder of fish (Actinopterygii) (4) (fig. S1); vagal nerve and larynx of nonavian tetrapods (17–19, 22–25); and hypoglossal nerve and syrinx of birds (20).
We conclude that our results reveal the ancestral origins of neural pattern generators for vocal-acoustic behaviors that mediate social signaling among all the major vertebrate lineages (Fig. 1A). The phylogenetically robust presence of a vocal compartment that shares an organization comparable to cranial motor nuclei predicts its developmental specification by ancestrally conserved, genetic regulatory pathways including a combination of 5′ Hox gene expression in both mesoderm (in this case, occipital somites) and the region spanning the developing hind-brain and spinal cord (28). Avian studies emphasize similarities with mammals in forebrain audio-vocal pathways (1). We propose that a more ancient neuroectodermal compartment, originating before the radiation of fishes that includes tetrapods, gave rise to the premotor-motor circuitry providing timing signals for a wide range of vertebrate acoustic behaviors (Fig. 1). As Darwin [(29) pp. 23, 331] commented regarding the “musical” sounds of male fish during the breeding season: “when the primeval members of this class [Vertebrata] were strongly excited and their muscles violently contracted, purpose-less sounds would almost certainly have been produced; and these, if they proved in any way serviceable, might readily have been modified or intensified by the preservation of properly adapted variations.”
Supplementary Material
Footnotes
www.sciencemag.org/cgi/content/full/321/5887/417/DC1
Materials
Materials and Methods
Figs. S1 and S2
References
Movies S1 to S3
References and Notes
- 1.White SA, Fisher SE, Geschwind DH, Scharff C, Holy TE. J Neurosci. 2006;26:10376. doi: 10.1523/JNEUROSCI.3379-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vargha-Khadem F, Gadian DG, Copp A, Mishkin M. Nat Rev Neurosci. 2005;6:131. doi: 10.1038/nrn1605. [DOI] [PubMed] [Google Scholar]
- 3.Nelson JS. Fishes of the World. 4. Wiley; Hoboken, NJ: 2006. [Google Scholar]
- 4.Bass AH, McKibben JR. Prog Neurobiol. 2003;69:1. doi: 10.1016/s0301-0082(03)00004-2. [DOI] [PubMed] [Google Scholar]
- 5.Bass AH, Baker R. J Neurobiol. 1990;21:1155. doi: 10.1002/neu.480210802. [DOI] [PubMed] [Google Scholar]
- 6.Bass AH, Marchaterre MA, Baker R. J Neurosci. 1994;14:4025. doi: 10.1523/JNEUROSCI.14-07-04025.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tracy HC. J Comp Neurol. 1959;111:27. [Google Scholar]
- 8.Gilland E, Baker R. Brain Behav Evol. 2005;66:234. doi: 10.1159/000088128. [DOI] [PubMed] [Google Scholar]
- 9.Hanneman E, Trevarrow B, Metkalfe WK, Kimmel CB, Westerfield M. Development. 1988;103:49. doi: 10.1242/dev.103.1.49. [DOI] [PubMed] [Google Scholar]
- 10.Materials and methods are available as supplemental material on Science Online.
- 11.Bass AH, Baker R. Brain Behav Evol. 1997;50(suppl 1):3. doi: 10.1159/000113351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guthrie S. Nat Rev Neurosci. 2007;8:859. doi: 10.1038/nrn2254. [DOI] [PubMed] [Google Scholar]
- 13.Cambronero F, Puelles L. J Comp Neurol. 2000;427:522. doi: 10.1002/1096-9861(20001127)427:4<522::aid-cne3>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 14.Winterbottom RA. Proc Acad Nat Sci Philadelphia. 1974;125:225. [Google Scholar]
- 15.Morita LE, Finger TE. J Comp Neurol. 1987;264:231. doi: 10.1002/cne.902640208. [DOI] [PubMed] [Google Scholar]
- 16.Knapp R, Marchaterre MA, Bass AH. J Neurobiol. 1999;38:475. doi: 10.1002/(sici)1097-4695(199903)38:4<475::aid-neu4>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 17.Straka H, Baker R, Gilland E. J Comp Neurol. 2006;494:228. doi: 10.1002/cne.20801. [DOI] [PubMed] [Google Scholar]
- 18.Kennedy MC. Brain Res. 1981;218:337. doi: 10.1016/0006-8993(81)91311-1. [DOI] [PubMed] [Google Scholar]
- 19.Kitamura S, Okubo J, Ogata K, Sakai A. Exp Neurol. 1987;97:592. doi: 10.1016/0014-4886(87)90116-6. [DOI] [PubMed] [Google Scholar]
- 20.Wild JM. Ann N Y Acad Sci. 2004;1016:438. doi: 10.1196/annals.1298.016. [DOI] [PubMed] [Google Scholar]
- 21.Kubke MF, Yazaki-Sugiyama Y, Mooney R, Wild JM. J Neurophysiol. 2005;94:2379. doi: 10.1152/jn.00257.2005. [DOI] [PubMed] [Google Scholar]
- 22.Zornik E, Kelley DB. J Comp Neurol. 2007;501:303. doi: 10.1002/cne.21145. [DOI] [PubMed] [Google Scholar]
- 23.Rubsamen R, Schweizer H. J Comp Physiol [A] 1986;159:689. doi: 10.1007/BF00612041. [DOI] [PubMed] [Google Scholar]
- 24.Jürgens U. Neurosci Biobehav Rev. 2002;26:235. doi: 10.1016/s0149-7634(01)00068-9. [DOI] [PubMed] [Google Scholar]
- 25.Jürgens U, Ehrenreich L. Brain Res. 2007;1148:90. doi: 10.1016/j.brainres.2007.02.020. [DOI] [PubMed] [Google Scholar]
- 26.Noden DM, Francis-West P. Dev Dyn. 2006;235:1194. doi: 10.1002/dvdy.20697. [DOI] [PubMed] [Google Scholar]
- 27.Huang R, Zhi Q, Izpisua-Belmonte JC, Christ B, Patel K. Anat Embryol (Berlin) 1999;200:137. doi: 10.1007/s004290050268. [DOI] [PubMed] [Google Scholar]
- 28.Deschamps J, van Nes J. Development. 2005;132:2931. doi: 10.1242/dev.01897. [DOI] [PubMed] [Google Scholar]
- 29.Darwin C. In: The Descent of Man, and Selection in Relation to Sex. 1871. John Murray., editor. London, Princeton Univ. Press; Princeton, NJ: 1981. [Google Scholar]
- 30.Moore BA, Russell AP, Bauer AM. J Morphol. 1991;210:227. doi: 10.1002/jmor.1052100303. [DOI] [PubMed] [Google Scholar]
- 31.Thanks to D. Chiu, D. Brown, I. Fein, A. Mensinger, L. Remage-Healey for logistical and technical support; K. Zamudio for help with the cladogram; M. Nelson for drawings; M. Marchaterre for movies; A. Rice for movie editing; E. Adkins-Regan, G. Budney, U. Jürgens, M. Marchaterre for vocal recordings; J. Fetcho, H. Greene, L. Z. Holland, C. Watson, and especially H. Baker, R. Hoy, and J. M Wild for comments on the manuscript. Research support from the NSF and NIH.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.