Abstract
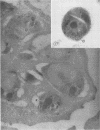
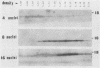
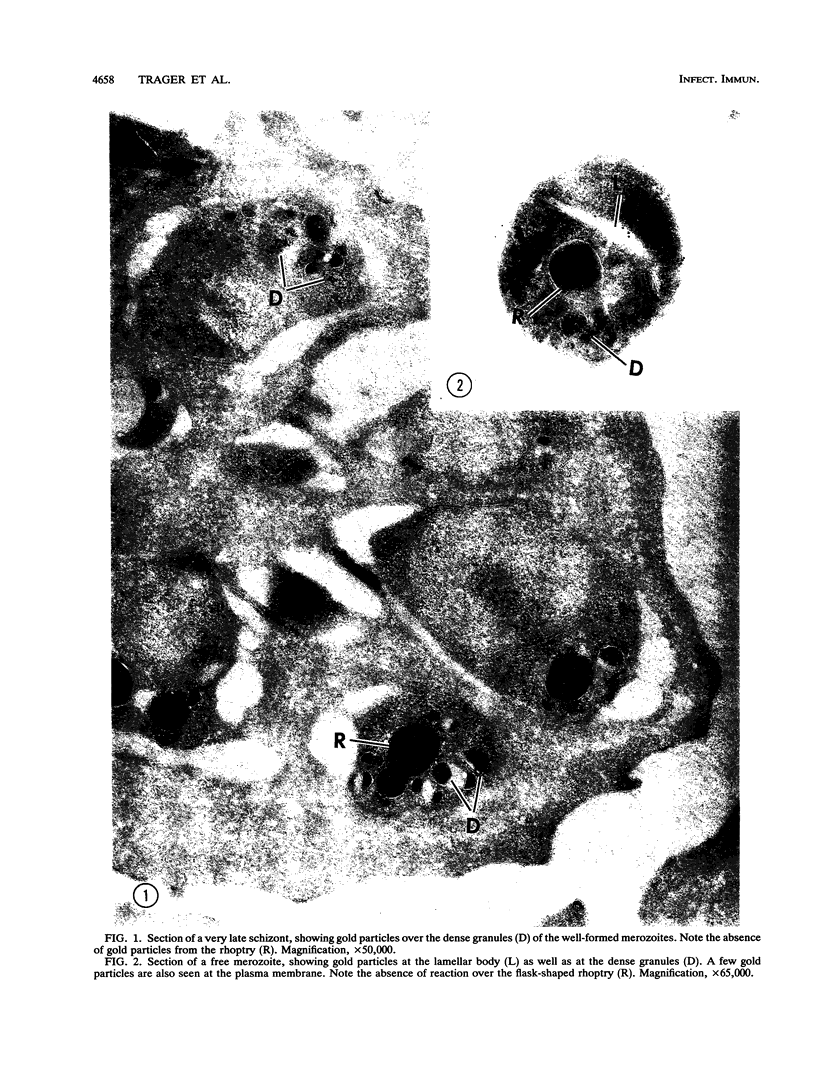
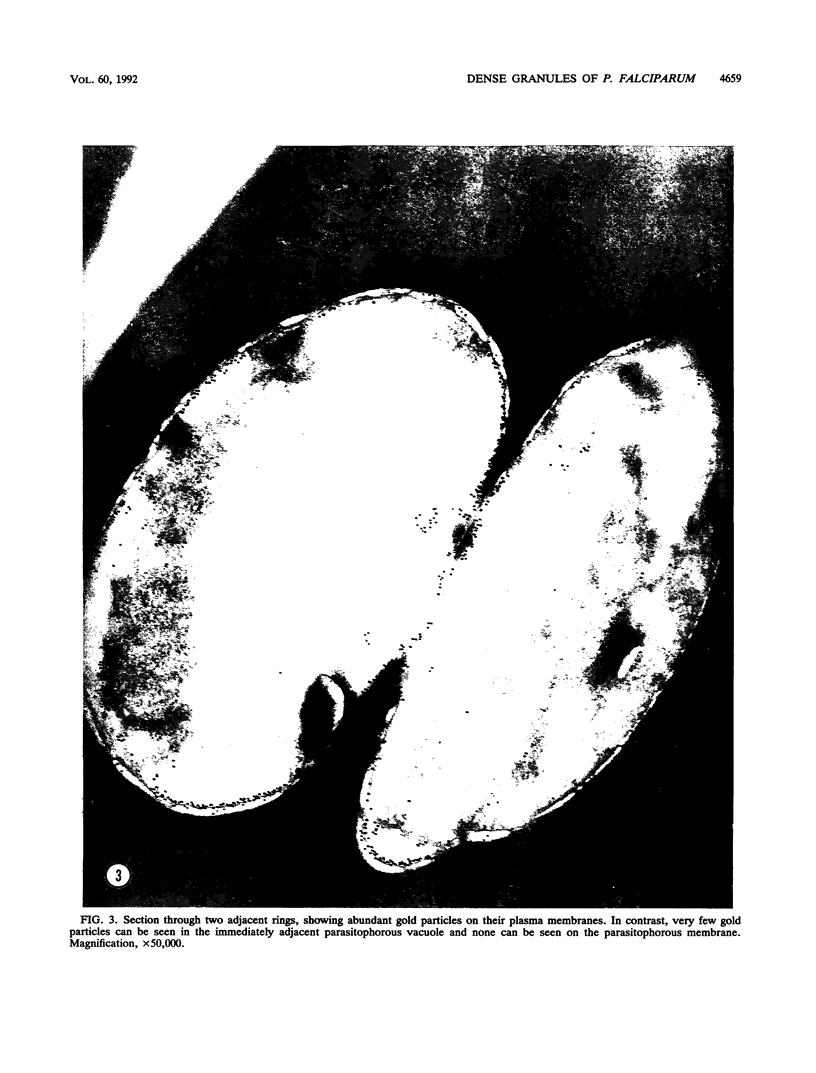
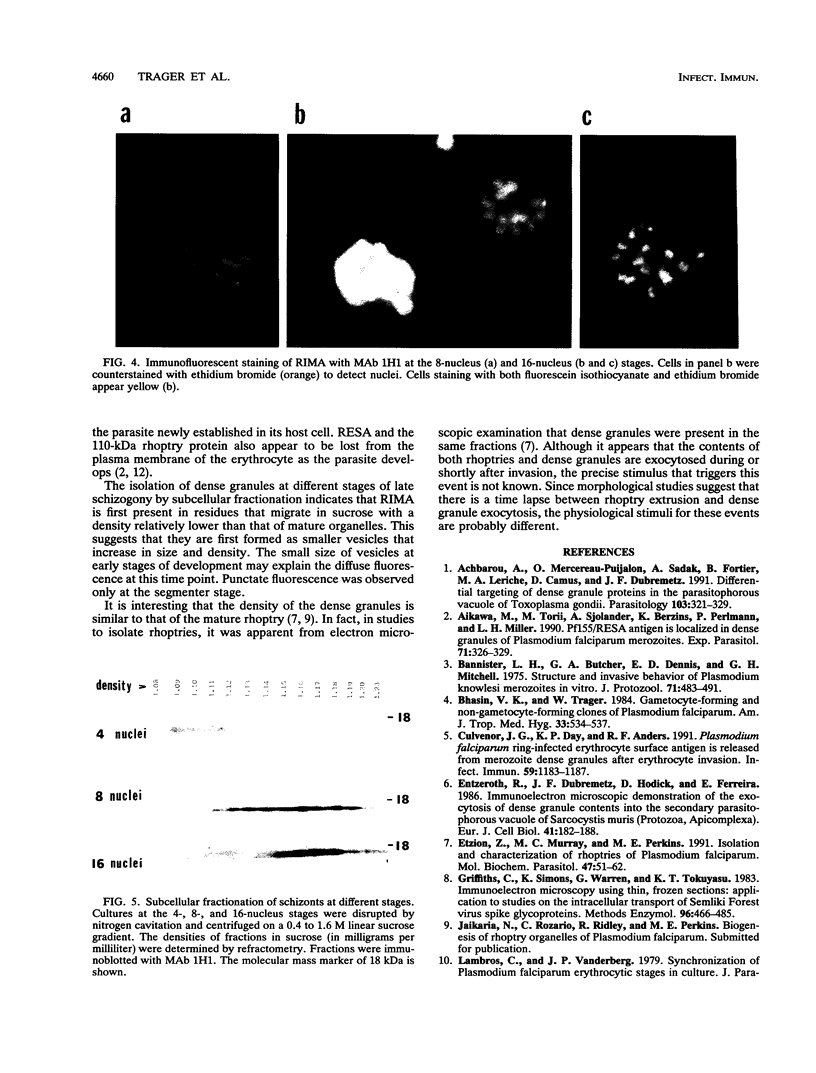
A 14-kDa protein was localized to the dense granules of Plasmodium falciparum by immunoelectron microscopy with monoclonal antibody 1H1. The protein was present in dense granules in late-stage schizonts and free merozoites. After invasion, the protein was localized exclusively on the membrane of the newly invaded ring. The protein is referred to as RIMA, for ring membrane antigen. The 14-kDa protein was synthesized late in schizogony as determined by immunofluorescence microscopy and immunoblotting. At the late schizont stage it was distributed diffusely throughout the intracellular schizont. Only at the segmenter stage was the protein localized in defined spots that correspond to dense granules. Dense granules were isolated from schizont-infected erythrocytes by subcellular fractionation on a sucrose gradient. Fractions containing the 14-kDa protein were detected by immunoblotting with monoclonal antibody 1H1. The 14-kDa protein was first detected in vesicles at the late (8-nucleus) schizont stage. Mature dense granules sedimented with a peak density of 1.17 g/ml, which is similar to the density of rhoptries isolated by the same procedure.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Achbarou A., Mercereau-Puijalon O., Sadak A., Fortier B., Leriche M. A., Camus D., Dubremetz J. F. Differential targeting of dense granule proteins in the parasitophorous vacuole of Toxoplasma gondii. Parasitology. 1991 Dec;103(Pt 3):321–329. doi: 10.1017/s0031182000059837. [DOI] [PubMed] [Google Scholar]
- Aikawa M., Torii M., Sjölander A., Berzins K., Perlmann P., Miller L. H. Pf155/RESA antigen is localized in dense granules of Plasmodium falciparum merozoites. Exp Parasitol. 1990 Oct;71(3):326–329. doi: 10.1016/0014-4894(90)90037-d. [DOI] [PubMed] [Google Scholar]
- Bannister L. H., Butcher G. A., Dennis E. D., Mitchell G. H. Structure and invasive behaviour of Plasmodium knowlesi merozoites in vitro. Parasitology. 1975 Dec;71(3):483–491. doi: 10.1017/s0031182000047247. [DOI] [PubMed] [Google Scholar]
- Bhasin V. K., Trager W. Gametocyte-forming and non-gametocyte-forming clones of Plasmodium falciparum. Am J Trop Med Hyg. 1984 Jul;33(4):534–537. doi: 10.4269/ajtmh.1984.33.534. [DOI] [PubMed] [Google Scholar]
- Culvenor J. G., Day K. P., Anders R. F. Plasmodium falciparum ring-infected erythrocyte surface antigen is released from merozoite dense granules after erythrocyte invasion. Infect Immun. 1991 Mar;59(3):1183–1187. doi: 10.1128/iai.59.3.1183-1187.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Entzeroth R., Dubremetz J. F., Hodick D., Ferreira E. Immunoelectron microscopic demonstration of the exocytosis of dense granule contents into the secondary parasitophorous vacuole of Sarcocystis muris (Protozoa, Apicomplexa). Eur J Cell Biol. 1986 Aug;41(2):182–188. [PubMed] [Google Scholar]
- Etzion Z., Murray M. C., Perkins M. E. Isolation and characterization of rhoptries of Plasmodium falciparum. Mol Biochem Parasitol. 1991 Jul;47(1):51–61. doi: 10.1016/0166-6851(91)90147-x. [DOI] [PubMed] [Google Scholar]
- Griffiths G., Simons K., Warren G., Tokuyasu K. T. Immunoelectron microscopy using thin, frozen sections: application to studies of the intracellular transport of Semliki Forest virus spike glycoproteins. Methods Enzymol. 1983;96:466–485. doi: 10.1016/s0076-6879(83)96041-x. [DOI] [PubMed] [Google Scholar]
- Pasvol G., Wilson R. J., Smalley M. E., Brown J. Separation of viable schizont-infected red cells of Plasmodium falciparum from human blood. Ann Trop Med Parasitol. 1978 Feb;72(1):87–88. doi: 10.1080/00034983.1978.11719283. [DOI] [PubMed] [Google Scholar]
- Sam-Yellowe T. Y., Shio H., Perkins M. E. Secretion of Plasmodium falciparum rhoptry protein into the plasma membrane of host erythrocytes. J Cell Biol. 1988 May;106(5):1507–1513. doi: 10.1083/jcb.106.5.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokuyasu K. T. A technique for ultracryotomy of cell suspensions and tissues. J Cell Biol. 1973 May;57(2):551–565. doi: 10.1083/jcb.57.2.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torii M., Adams J. H., Miller L. H., Aikawa M. Release of merozoite dense granules during erythrocyte invasion by Plasmodium knowlesi. Infect Immun. 1989 Oct;57(10):3230–3233. doi: 10.1128/iai.57.10.3230-3233.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trager W. Plasmodium falciparum in culture: improved continuous flow method. J Protozool. 1979 Feb;26(1):125–129. doi: 10.1111/j.1550-7408.1979.tb02747.x. [DOI] [PubMed] [Google Scholar]
- Zung J. M., Trager W., Gubert E. Initial extracellular forms of Plasmodium falciparum: their ultrastructure and their definition with monoclonal antibodies. Proc Natl Acad Sci U S A. 1991 Jan 1;88(1):89–92. doi: 10.1073/pnas.88.1.89. [DOI] [PMC free article] [PubMed] [Google Scholar]