Abstract
The presence of cervical lymph node metastases in head and neck squamous cell carcinoma (HNSCC) is the strongest determinant of patient prognosis. Owing to the impact of nodal metastases on patient survival, a system for sensitive and accurate detection is required. Clinical staging of lymph nodes is far less accurate than pathological staging. Pathological staging also suffers limitations because it fails to detect micrometastasis in a subset of nodal specimens. To improve the sensitivity of existing means of diagnosing metastatic disease, many advocate the use of molecular markers specific for HNSCC cells. MicroRNA (miRNA) are short noncoding segments of RNA that posttranscriptionally regulate gene expression. Approximately one third of all miRNA will exhibit substantial tissue specificity. Using a quantitative reverse transcription-polymerase chain reaction-based assay, we examined the expression of microRNA-205 (mir-205) across tissues and demonstrated that its expression is highly specific for squamous epithelium. We applied this assay to tissue samples, and we could detect metastatic HNSCC in each positive lymph node specimen, whereas benign specimens did not express this marker. When compared to metastases from other primary tumors, HNSCC-positive lymph nodes were distinguishable by the high expression of this marker. Using an in vitro lymphoid tissue model, we were able to detect as little as one squamous cell in a background of 1 million lymphocytes. By combining the sensitivity of quantitative reverse transcription-polymerase chain reaction with the specificity of mir-205 for squamous epithelium, we demonstrate a novel molecular marker for the detection of metastatic HNSCC.
Introduction
For squamous cell carcinoma of the head and neck, metastasis to regional lymph nodes is the strongest predictor of disease outcome and prognosis [1,2]. Accurate staging of regional lymph node metastases is necessary to improve both locoregional control and patient outcomes. Unfortunately, current routine clinical and pathological methods of detecting lymph node metastasis are suboptimal for identifying the presence of micrometastases and may lead to the understaging of many patients with head and neck squamous cell carcinoma (HNSCC) [3,4]. The poor sensitivity of current clinical and pathological methods for detecting occult micrometastases has led to the clinical strategy of elective neck dissection (END) for patients with a high likelihood of harboring subclinical nodal disease. Yet END is not without morbidity and, in many cases, will constitute overtreatment because only 50% of these patients will be found to harbor metastatic nodal disease [5]. Even for patients whose primary tumor size or site warrants END, routine pathological analysis of dissected nodal specimens will fail to detect microscopic nodal metastases in 8% to 20% of patients [6,7]. Routine pathologic analysis with hematoxylin-eosin (H&E) staining is also prone to sampling errors that can make the detection of micrometastasis difficult [8]. The limitations of routine pathology for detecting micrometastatic disease have made it necessary to explore molecular means of diagnosis that can detect disease through whole or partial node sampling.
Molecular detection of HNSCC cells in a background of lymph node tissue demands a highly specific and sensitive biomarker. Ideally, this biomarker would be abundantly yet exclusively expressed in squamous epithelium, whereas having negligible expression in lymphocytes and lymphatic or vascular stroma. One method for the molecular detection of these biomarkers that has shown promise in recent studies is quantitative reverse transcription-polymerase chain reaction (qRT-PCR) [9–12]. Quantitative reverse transcription-polymerase chain reaction provides the ability to perform rapid quantitative analysis for biomarkers with great sensitivity and from minute amounts of starting material. Because this technology is both rapid and sensitive, it offers the potential to improve clinical decision making, which is often delayed by routine histological means of diagnosis.
Recent studies have focused on the use of qRT-PCR to screen lymph node specimens for gene (mRNA) markers that can distinguish benign lymph nodes from those that harbor metastatic disease [13–15]. One set of mRNA biomarkers that is being used to detect metastatic HNSCC is the cytokeratin proteins [10,16]. These molecules, which are typically expressed in pairs, are specific for cells of epithelial origin and are thought to be conserved during neoplastic cell transformation [17]. Although the detection of cytokeratins and other mRNA markers in metastatic HNSCC nodal samples has proven feasible by both immunohistochemistry and qRT-PCR, there is little data to suggest that mRNA biomarkers retain a high diagnostic accuracy when applied to poorly differentiated tissue samples. Selection of an appropriate gene marker has been hampered by the fact that gene expression in tumors can vary depending on their degree of differentiation, and it often takes multiple gene markers to obtain high diagnostic accuracy [18–22]. Debate currently exists about which gene markers (either alone or in combination) will convey this level of accuracy.
As upstream regulators of mRNA expression, microRNA (miRNA) possess many characteristics that make them appealing diagnostic biomarkers. These small (18–22nt) molecules belong to a class of noncoding, regulatory RNA that modulate the expression of their of gene targets. Each miRNA is estimated to control the expression of hundreds of mRNA species [23]. These molecules also display extraordinary tissue-specificity, and their tissue-specific nature has already been exploited for the purpose of diagnosing and classifying primary cancers and their metastases [24–28]. Evidence suggests that miRNA biomarkes may perform more robustly than mRNA as tumors de-differentiate. In a recent head-to-head comparison, mRNA- and miRNA-based tissue classifiers were compared for their ability to establish the correct diagnosis for poorly differentiated tumors of uncertain origin. In their study, Lu et al. [26] discovered that the miRNA-based classifier established a correct diagnosis with far greater accuracy than the mRNA-based classifier, which proved highly inaccurate for this purpose. In addition to the greater accuracy of miRNA for poorly differentiated tissue, miRNA also maintain their expression profile in fresh-frozen and formalin-fixed, paraffinembedded (FFPE) samples, permitting study of their expression in archived pathologic samples [29,30]. This is in distinction to mRNA, which have limited use for gene expression analysis in archived FFPE tissues owing to mRNA degradation and modification during fixation and processing [31]. One further benefit is that miRNA remain largely intact and are less likely than mRNA to degrade in routinely processed tissue specimens [32]. Given these important advantages, many are now looking to explore the potential of miRNA as tissuespecific biomarkers of cancer and other disease states.
In the current study, we focus on the tissue-specific expression of mir-205, which demonstrates high endogenous expression in squamous epithelium. This microRNA has been reported in previous studies to have variable expression in many human carcinoma cells and, specifically, to be highly overexpressed in HNSCC cell lines [33–37]. In this study, we will examine its range of expression across many human tissues and compare its relative expression in normal and cancerous mucosal tissues of the head and neck. We will also explore the potential use of mir-205 as a biomarker for HNSCC and determine its capacity to aid in the detection of overt and occult metastatic nodal disease.
Materials and Methods
Mouse Tissue
Six-month-old male mice (C57BL/6 strain) were killed by CO2 asphyxiation. The organs were dissected, and 25 mg of each tissue was placed immediately in Qiazol reagent (Qiagen, Valencia, CA). Tissue was lysed with a motorized rotor-stator homogenizer in Qiazol followed by RNA isolation using the miRNEASY minikit (Qiagen). Mouse tissue experiments and expression analyses for each organ were carried out in triplicate. RNA was quantified by spectrophotometry using a Nanodrop 1000 spectrophotometer (NanoDrop Technologies, Wilmington, DE). Each RNA prep was characterized for purity by an OD ratio of 260:280.
Cell Lines
The cell lines used in these experiments were derived from various anatomic sites in patients with primary squamous cell carcinoma. The UM-SCC-1, UM-SCC-6, UM-SCC-87, TU167, UT-SCC-50, UM-SCC-15, and UM-SCC-5 lines were derived from primary squamous carcinoma of the oral cavity, oropharynx, floor-of-mouth, hypopharynx, and larynx. The Jurkat human T-cell leukemia line was obtained from the American Type Culture Corporation (Manassas, VA) and propagated according to established protocol. The primary normal human oral keratinocyte (NHOK) cell line was prepared from keratinized oral epithelial tissues according to methods described elsewhere [38].
Patient Tissue Samples
Snap-frozen tissue from 12 surgically removed, pathologically confirmed, primary HNSCC samples from various subsites in the head and neck and 7 benign mucosal tissue samples derived from the oral cavity or oropharynx were collected from the University of Iowa Department of Pathology after approval by the institutional review board. Patient demographics and tumor characteristics for each primary sample are listed in Table W1. A total of eight histologically determined HNSCC-positive lymph nodes were included in this study. The patient demographics and primary tumor characteristics for each HNSCC-positive specimen are listed in Table W2. For negative controls, lymph nodes were obtained from cancer-free patients who had undergone lymph node removal for the diagnosis or treatment of benign disorders, nd whose specimen was determined by the pathologist to be either “benign” or “benign reactive.” In total, benign nodal samples were obtained from five separate patients. For comparison, we included three lymph node samples each containing metastatic adenocarcinoma of the lung and breast as well as metastatic melanoma. All tumors and metastatic lymph node specimens were classified according to the widely accepted diagnostic histological criteria.
RNA Isolation and cDNA Synthesis
RNA was isolated using the miRNeasy minikit (Qiagen) according to the protocol described by the manufacturer. cDNA was generated from the total RNA sample by reverse transcription using the TaqMan Mir-205 assay kit (Applied Biosystems, Foster City, CA). This kit uses gene-specific stem-loop reverse transcription primers and TaqMan probes to detect mature miRNA transcripts in a two-step qRT-PCR assay. Briefly, each RT reaction contained 10 ng of total RNA, 50 nM stem-looped RT primer, 1x RT buffer, 0.25 mM each of dNTPs, 3.33 U/µl Multiscribe reverse transcriptase, and 0.25 U/µl RNase inhibitor. The 20-µl reactions were incubated in a Thermocycler in a 96-well plate for 30 minutes at 16°C, 30 minutes at 42°C, 5 minutes at 85°C, and then held at 4°C.
Real-time qRT-PCR
Real-time PCR was performed using a standard TaqMan PCR kit protocol on an Applied Biosystems 7500 fast Sequence Detection System. The 20-µl PCR included 1.33 µl of RT product, 10 µl of 1x TaqMan Universal PCR Master Mix (P/N: 4324018; Applied Biosystems), 1.0 µl of the 20x TaqMan MicroRNA assay (contains primer and probe), and 7.67 µl of nuclease-free water. Each 20-µl volume was run in triplicate. The reactions were incubated in a 96-well plate at 95°C for 10 minutes, followed by 40 cycles of 95°C for 15 seconds, and 60°C for 1 minute.
Expression Analysis
Quantitation of mature miRNA expression levels in cell and tissue samples was performed on the ABI 7500 Real-Time PCR System. Each sample was run in triplicate. Endogenous controls were used for the normalization of RNA input: small nucleolar RNA RNU48 and snoRNA202 for human and mouse tissues, respectively. To evaluate the appropriateness of these endogenous controls for use in these tissues, their expression levels were determined in multiple tissue samples. All samples demonstrated low variability in their expression, thus validating their use as normalization controls. MiRNA expression levels were calculated by relative quantitation using the ABI 7500 Real-Time PCR SDS software version 1.4 (Applied Biosystems), and the fold expression changes were determined by 2-ΔΔCT method [39]. The data are presented as the fold-expression change of mir-205 expression in tumors and metastatic lesions relative to their corresponding normal tissues after normalization to the endogenous control.
Results
Tissue-Specific Expression of mir-205
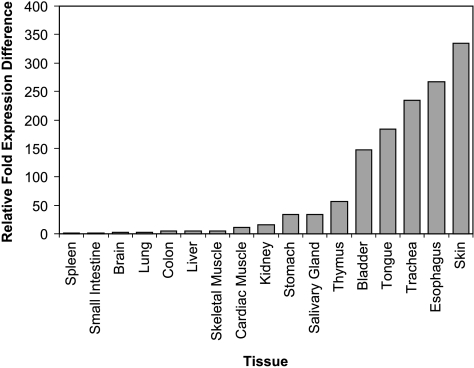
To characterize the range of expression of mir-205 across mammalian tissues, we extracted total RNA from various mouse tissues and performed qRT-PCR analysis for mir-205. Ten nanograms of total RNA from each sample was subjected to qRT-PCR, and the expression of mir-205 was determined after normalization to the endogenous control (snoRNA202). A calibrator sample was selected from among the tissues whose expression of mir-205 was determined through repeat experiments to be negligible. On the basis of the low endogenous expression of mir-205, spleen was selected as the 1x sample for the purpose of calibration, and the expression of mir-205 in the other tissues was measured relative to it. MiRNA expression levels were calculated by relative quantitation using the 2-ΔΔCT method. The data are presented as the n-fold expression difference of each tissue relative to the calibrator sample. The expression of mir-205 is highest in tissues composed of or lined with squamous epithelium and is lower in parenchymal organs such as kidney, liver, and brain (Figure 1). Our experimental findings are in keeping with previously published studies, which suggest that mir-205 exhibits high endogenous expression in a broad range of tissues containing squamous epithelia [36]. Whereas the function of mir-205 in these tissues remains to be elucidated, its predictable pattern of expression validates its potential use as a tissue-specific diagnostic marker.
Figure 1.
Tissue-specific expression of mir-205. The relative fold expression difference of mir-205 in each sample relative to the calibrator sample (spleen).
Expression of mir-205 in HNSCC Cell Lines and Tissue Samples
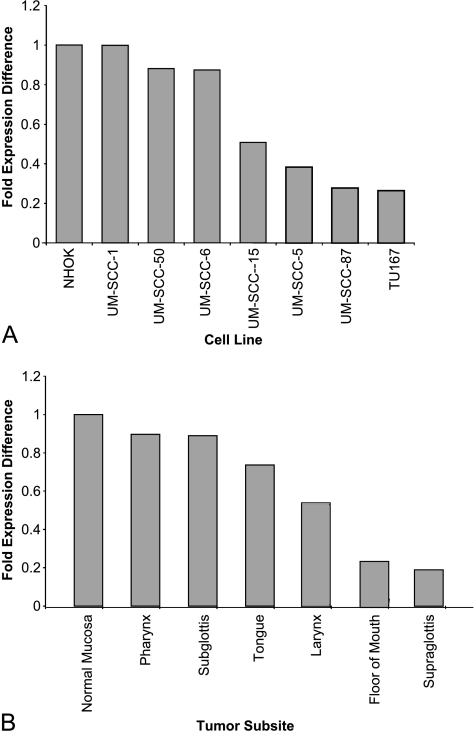
Real-time expression analysis of mir-205 in HNSCC cell lines showed that mir-205 is abundantly expressed, with no significant difference in expression when normalized to the NHOK cell line. Our relative expression analysis as determined by the 2-ΔΔCT method demonstrates a less than twofold variance in the expression of mir-205 in all HNSCC when compared to NHOKs (Figure 2A). Using a threefold expression difference as the minimum cutoff, there is no significant change in the expression of mir-205 as squamous epithelial cells transition from benign to neoplastic. These results are in contradistinction to the assertion by Tran et al. [33] that mir-205 was “exclusively overexpressed” in HNSCC. These results also underscore the importance of proper tissue normalization for relative expression studies.
Figure 2.
Expression of mir-205 in cell lines and tissue samples. (A) The expression of mir-205 in the HNSCC cell lines after normalization to the NHOK cell line. (B) The expression of mir-205 in primary tissue from various anatomical subsites after normalization to normal mucosal tissue.
Results of real-time qRT-PCR amplification of mir-205 in HNSCC tissue samples were concordant with that obtained from the HNSCC cell lines. Total RNA from each tumor specimen was subjected to qRT-PCR for mir-205, and the mir-205 expressions in tumors from the same anatomic subsite were averaged together during the relative expression analysis. Expression of mir-205 showed minimal variance among tumors from different head and neck subsites. There was also little variance among biological replicates. The reduction in relative expression of mir-205 was less than twofold for each tumor when compared to the average expression of the benign mucosal samples (Figure 2B). These findings are consistent with previously published data suggesting that there is a slight reduction in the expression of tissue-specific miRNA between cell lines and their corresponding primary tissue [37]. Because the expression of mir-205 does not change significantly during the neoplastic transformation of squamous cells, its expression will not be useful for the purpose of distinguishing HNSCC cells from normal squamous epithelium. However, its predictable pattern of expression offers unique diagnostic capability insofar as it is not expressed appreciably in lymph node tissue and can thus prove useful for the detection of lymph node metastases.
Detection of Metastatic HNSCC in Lymph Node Tissue Samples
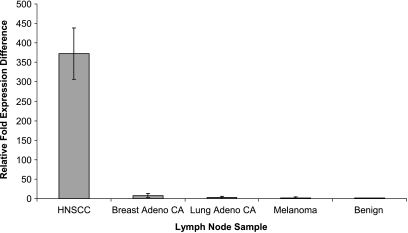
To demonstrate the potential of mir-205 to detect metastatic HNSCC in lymph node tissue samples by qRT-PCR, we analyzed a set of five benign and eight histologically positive nodes for expression of mir-205. Each lymph node designated as “benign” had undergone routine pathological analysis with H&E staining and was determined to have no gross or microscopic evidence of tumor. Total RNA was extracted from each lymph node sample, and the relative expression of mir-205 was measured by qRT-PCR. Using this assay, we were able to distinguish positive from benign nodes on the basis of mir-205 expression. The aggregate mir-205 expression in the eight histologically positive nodes was calibrated against the aggregate expression in the benign nodal samples and then normalized to the endogenous control (RNU48). In each of the eight positive nodes, we were able to detect a significant increase in the relative expression of mir-205 compared to benign samples that had a negligible expression of mir-205 (Figure 3). The data are presented as the average relative fold expression change in the aggregate of each sample type (benign and positive).
Figure 3.
Expression of mir-205 in metastatic lymph node samples. The fold expression difference in mir-205 for each specimen relative to the benign node samples (error bars indicate SD for each sample).
For comparison, we included three samples each of lymph node tissue containing pathologically confirmed adenocarcinoma of the lung and breast and three samples containing metastatic melanoma. Each lymph node sample was processed as described above, and the expression of mir-205 was measured by qRT-PCR. The aggregate mir-205 expression in each sample type was calibrated against the aggregate expression in the benign nodal samples and normalized to the endogenous control. As seen in Figure 3, the expression of mir-205 in the HNSCC-positive lymph node samples was several hundred-fold higher than the other metastatic lesions when normalized to the benign lymph node samples. Of significance, the relative expression difference of mir-205 in the metastatic breast adenocarcinoma sample was above the threefold cutoff value of significance, as this sample averaged an eightfold higher expression of mir-205. On the basis of previously published results, breast adenocarcinoma is expected to express a relatively low level of mir-205 [25,40,41]. Although this relative expression difference was greater than the minimum level of significance, its expression was still much lower than the expression demonstrated by the HNSCC-positive samples. This allows for distinction between the two sample types based on mir-205 expression, as the relative expression in an HNSCC-positive lymph node should be several hundred-fold higher than a breast adenocarcinoma-positive lymph node. The difference in the relative expression of mir-205 between benign and metastatic lymph node samples demonstrates the potential of this marker to detect metastatic squamous cell carcinoma in lymph node specimens as well its ability of to distinguish among metastases from different primary tumor types.
Sensitivity Analysis of mir-205 to Detect HNSCC Cells in a Lymphoid Background
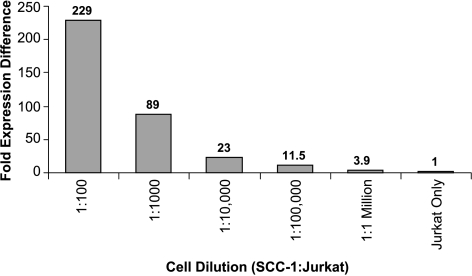
To demonstrate the sensitivity of qRT-PCR for detecting metastatic and micrometastatic HNSCC within lymph nodes, the squamous cell cancer cell line UM-SCC1 was diluted with Jurkat cells, an immortalized, human T-cell leukemia line at ratios ranging from 1:100 to 1:1 million (SCC-1 to Jurkat) cells. This was done to determine the ability of this molecular technique to detect isolated HNSCC metastases at concentrations less than detectable by standard histological techniques. Total RNA was then isolated from each cellular mixture, and 10 ng of total RNA from each mixture was subjected to qRT-PCR for mir-205. No mir-205 was detected by qRT-PCR in the Jurkat cell line when examined alone. MicroRNA-205 could be detected in each cellular mixture, and the fold expression difference for each mixture relative to the Jurkat-only cell normalization standard was more than the minimum threefold cutoff (Figure 4). As little as one SCC-1 cell could be detected from a background of 1 x 106 mononuclear cells by means of its mir-205 expression. These experiments demonstrate the sensitivity of qRT-PCR for detecting this marker from only a few metastatic cells, highlighting the use of this technique for whole or partial node sampling in the detection of micrometastatic disease.
Figure 4.
Sensitivity of qRT-PCR to detect mir-205. The relative expression of each cell dilution when compared to the Jurkat-only sample.
Discussion
Reported here are the results of the first detailed study to examine the use of a highly tissue-specific miRNA to detect metastatic tumor from lymph node samples. Expression profiling of multiple organs and tissues demonstrated that mir-205 is abundantly expressed in squamous epithelial cells and that this expression remains relatively constant as these tissues transition from normal to neoplastic. Significantly, mir-205 is not expressed in normal lymph node tissue, allowing for the use of whole-lymph node processing and quantitative analysis by PCR to detect this biomarker. The sensitivity of pathological analysis by H&E staining for the detection of small tumor deposits in lymph nodes has been improved by the addition of immunohistochemical staining, which has been demonstrated to up-stage many patients who were classified as having no clinically measurable metastatic disease [17]. However, this technique suffers from the limitations of cost and time consumption that make it unsuitable for rapid diagnostic and clinical decision making.
These drawbacks directed the search for a more sensitive means of discovering metastasis with molecular markers that are detectable by more rapid techniques such as qRT-PCR. Early studies focused on the detection of clonal genetic changes that were specific for HNSCC tumor cells, such as mutations in p53 [42]. In recent years, researchers have shifted focus from tumor-specific markers toward tissuespecific markers, as they seek to take advantage of the differential gene expression of HNSCC cells and other tissues such as lymph nodes or serum [11,12,43].
Because microRNA play a crucial role in the development, differentiation, and functioning of organs and because they display remarkable tissue-specificity [44–46], these small molecules are ideal for use as tissue classifiers. We have demonstrated the high endogenous expression of mir-205 in normal and cancerous squamous epithelial cells and its low expression in lymph node tissue. These tissue-specific differences in mir-205 expression make it a worthy biomarker for detecting small numbers of cells (which cannot be detected by traditional means) through PCR amplification. Although our findings suggest that mir-205 is not useful as a marker of malignant transformation of squamous epithelial cells, its consistent expression makes it a reliable surrogate marker for the presence of squamous epithelium. As we have demonstrated, an additional benefit of this squamous epithelial-specific marker is its capacity to distinguish among metastatic lesions from different tumor types. Although further study is needed, which includes more tumor types, our findings hold potential for assisting in the determination of primary tumor site for metastases of unknown origin.
To date, few studies have sought to use the tissue-specific nature of microRNA for the purpose of molecular diagnostics. This is primarily because our knowledge of tissue-specific microRNA is still emerging. Despite this, the demand for sensitive yet specific biomarkers that can be selectively amplified by PCR will likely make microRNA the next step in the evolution of molecular diagnostics. MiRNA biomarkers convey specific advantages over their mRNA counterparts. MiRNA have a more robust profiling performance than mRNA in de-differentiated tumor samples [23,26,27] and because they show improved stability in routinely processed clinical samples, they may be more suitable for study in some tissue samples. Owing to their superior stability, miRNA are also extracted more easily from archived FFPE samples and do not undergo as much degradation during fixation and processing [29,30]. This allows for miRNA profiling in some tissue samples that are unfit for mRNA expression analysis.
The use of miRNA as biomarkers for the diagnosis of HNSCC is showing early promise. In a recent study, Wong et al. [47] found that elevated plasma levels of mir-184 were associated with the presence of primary squamous cell carcinoma of the tongue. Along with the screening potential demonstrated by miRNA, they have also proven useful for the prediction of patient prognosis in some solid tumors [48,49]. Indeed, as we begin to understand more about the expression of miRNA in benign and cancerous mucosal tissues (as well as their associated functions), we will add another potent diagnostic tool to our armamentarium. It remains to be determined whether the addition of other tissue-specific miRNA markers will improve the sensitivity of this technique for identifying occult HNSCC metastases. We believe, however, that our study will give credence to the use of mir-205 as a squamous epithelial-specific marker capable of detecting occult metastatic tumor deposits. These data lay a foundation for additional studies that examine the use of microRNA to help solve the existing diagnostic and staging dilemmas that exist in the treatment of HNSCC.
A comparison study is needed to determine the efficacy of mir-205 relative to existing mRNA markers for the detection of HNSCC metastases. In addition, future study should focus on determining a minimum threshold of detection for this marker, as this would help reduce the risk of false-positive results. Our demonstration of this highly discriminatory assay for the detection of small tumor deposits will hopefully supply the pilot data needed to incorporate this technique into a clinically relevant application that will improve our ability to properly stage patients with metastatic HNSCC.
Supplementary Material
Footnotes
This article refers to supplementary materials, which are designated by Tables W1 and W2 and are available online at www.neoplasia.com.
References
- 1.Takes RP. Staging of the neck in patients with head and neck squamous cell cancer: imaging techniques and biomarkers. Oral Oncol. 2004;40:656–667. doi: 10.1016/j.oraloncology.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 2.Shah JP. Patterns of cervical lymph node metastasis from squamous carcinomas of the upper aerodigestive tract. Am J Surg. 1990;160:405–409. doi: 10.1016/s0002-9610(05)80554-9. [DOI] [PubMed] [Google Scholar]
- 3.Rhee D. The significance of immunohistochemically demonstrated nodal micrometastases in patients with squamous cell carcinoma of the head and neck. Laryngoscope. 2002;112:1970–1974. doi: 10.1097/00005537-200211000-00011. [DOI] [PubMed] [Google Scholar]
- 4.Thomsen JB. Sentinel lymph nodes in cancer of the oral cavity: is central step-sectioning enough? J Oral Pathol Med. 2007;36:425–429. doi: 10.1111/j.1600-0714.2007.00538.x. [DOI] [PubMed] [Google Scholar]
- 5.Greenberg JS. Disparity in pathologic and clinical lymph node staging in oral tongue carcinoma. Implication for therapeutic decision making. Cancer. 2003;98:508–515. doi: 10.1002/cncr.11526. [DOI] [PubMed] [Google Scholar]
- 6.Ferlito A. Lymph node micrometastases in head and neck cancer: a review. Acta Otolaryngol. 2001;121:660–665. doi: 10.1080/00016480152583584. [DOI] [PubMed] [Google Scholar]
- 7.Hamakawa H. Histological study on pN upgrading of oral cancer. Virchows Arch. 2000;437:116–121. doi: 10.1007/s004280000199. [DOI] [PubMed] [Google Scholar]
- 8.Ku NN. Pathologic examination of sentinel lymph nodes in breast cancer. Surg Oncol Clin N Am. 1999;8:469–479. [PubMed] [Google Scholar]
- 9.Barrera JE. Detection of occult cervical micrometastases in patients with head and neck squamous cell cancer. Laryngoscope. 2003;113:892–896. doi: 10.1097/00005537-200305000-00022. [DOI] [PubMed] [Google Scholar]
- 10.Becker MT. Molecular assay to detect metastatic head and neck squamous cell carcinoma. Arch Otolaryngol Head Neck Surg. 2004;130:21–27. doi: 10.1001/archotol.130.1.21. [DOI] [PubMed] [Google Scholar]
- 11.Ferris RL. Molecular staging of cervical lymph nodes in squamous cell carcinoma of the head and neck. Cancer Res. 2005;65:2147–2156. doi: 10.1158/0008-5472.CAN-04-3717. [DOI] [PubMed] [Google Scholar]
- 12.Mitas M. Real-time reverse transcription-PCR detects KS1/4 mRNA in mediastinal lymph nodes from patients with non-small cell lung cancer. Clin Chem. 2003;49:312–315. doi: 10.1373/49.2.312. [DOI] [PubMed] [Google Scholar]
- 13.Nieuwenhuis EJ. Quantitative molecular detection of minimal residual head and neck cancer in lymph node aspirates. Clin Cancer Res. 2003;9:755–761. [PubMed] [Google Scholar]
- 14.Mitas M, et al. Quantitative real-time RT-PCR detection of breast cancer micrometastasis using a multigene marker panel. Int J Cancer. 2001;93:162–171. doi: 10.1002/ijc.1312. [DOI] [PubMed] [Google Scholar]
- 15.Xi L, et al. Molecular staging of lymph nodes from patients with esophageal adenocarcinoma. Clin Cancer Res. 2005;11:1099–1109. [PubMed] [Google Scholar]
- 16.Kwon SY. The usefulness of cytokeratin immunohistochemistry in detection of lymph node micrometastasis in neck dissection specimens. Otolaryngol Head Neck Surg. 2004;131:300–306. doi: 10.1016/j.otohns.2004.02.020. [DOI] [PubMed] [Google Scholar]
- 17.Marley JJ. Expression of human cytokeratin 14 in normal, premalignant and malignant oral tissue following isolation by plaque differential hybridisation. Eur J Cancer. 1994;30B:305–311. doi: 10.1016/0964-1955(94)90030-2. [DOI] [PubMed] [Google Scholar]
- 18.Yamamichi N, et al. Frequent loss of Brm expression in gastric cancer correlates with histologic features and differentiation state. Cancer Res. 2007;67:10727–10735. doi: 10.1158/0008-5472.CAN-07-2601. [DOI] [PubMed] [Google Scholar]
- 19.Xi L. A combination of molecular markers accurately detects lymph node metastasis in non-small cell lung cancer patients. Clin Cancer Res. 2006;12:2484–2491. doi: 10.1158/1078-0432.CCR-05-2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen G. Detection of occult metastasis in lymph nodes from colorectal cancer patients: a multiple-marker reverse transcriptase-polymerase chain reaction study. Dis Colon Rectum. 2004;47:679–686. doi: 10.1007/s10350-003-0118-2. [DOI] [PubMed] [Google Scholar]
- 21.Saglam O. Molecular differentiation of early and late stage laryngeal squamous cell carcinoma: an exploratory analysis. Diagn Mol Pathol. 2007;16:218–221. doi: 10.1097/PDM.0b013e3180d0aab5. [DOI] [PubMed] [Google Scholar]
- 22.Ramaswamy S, et al. Multiclass cancer diagnosis using tumor gene expression signatures. Proc Natl Acad Sci USA. 2001;98:15149–15154. doi: 10.1073/pnas.211566398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lim LP. Microarray analysis shows that some microRNAs downregulate large numbers of target mRNAs. Nature. 2005;433:769–773. doi: 10.1038/nature03315. [DOI] [PubMed] [Google Scholar]
- 24.Sood P. Cell-type-specific signatures of microRNAs on target mRNA expression. Proc Natl Acad Sci USA. 2006;103:2746–2751. doi: 10.1073/pnas.0511045103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liang Y. Characterization of microRNA expression profiles in normal human tissues. BMC Genomics. 2007;8:166. doi: 10.1186/1471-2164-8-166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lu J, et al. MicroRNA expression profiles classify human cancers. Nature. 2005;435:834–838. doi: 10.1038/nature03702. [DOI] [PubMed] [Google Scholar]
- 27.Rosenfeld N, et al. MicroRNAs accurately identify cancer tissue origin. Nat Biotechnol. 2008;26:462–469. doi: 10.1038/nbt1392. [DOI] [PubMed] [Google Scholar]
- 28.Nikiforova MN. MicroRNA expression profiling of thyroid tumors: biological significance and diagnostic utility. J Clin Endocrinol Metab. 2008;93:1600–1608. doi: 10.1210/jc.2007-2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xi Y. Systematic analysis of microRNA expression of RNA extracted from fresh frozen and formalin-fixed paraffin-embedded samples. RNA. 2007;13:1668–1674. doi: 10.1261/rna.642907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Doleshal M. Evaluation and validation of total RNA extraction methods for micro-RNA expression analyses in formalin-fixed, paraffin-embedded tissues. JMol Diagn. 2008;10:203–211. doi: 10.2353/jmoldx.2008.070153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li J. Comparison of miRNA expression patterns using total RNA extracted from matched samples of formalin-fixed paraffin-embedded (FFPE) cells and snap frozen cells. BMC Biotechnol. 2007;7:36. doi: 10.1186/1472-6750-7-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nelson PT. Microarray-based, high-throughput gene expression profiling of micro-RNAs. Nat Methods. 2004;1:155–161. doi: 10.1038/nmeth717. [DOI] [PubMed] [Google Scholar]
- 33.Tran N. MicroRNA expression profiles in head and neck cancer cell lines. Biochem Biophys Res Commun. 2007;358:12–17. doi: 10.1016/j.bbrc.2007.03.201. [DOI] [PubMed] [Google Scholar]
- 34.Feber A. MicroRNA expression profiles of esophageal cancer. J Thorac Cardiovasc Surg. 2008;135:255–260. doi: 10.1016/j.jtcvs.2007.08.055. discussion 260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gottardo F, et al. Micro-RNA profiling in kidney and bladder cancers. Urol Oncol. 2007;25:387–392. doi: 10.1016/j.urolonc.2007.01.019. [DOI] [PubMed] [Google Scholar]
- 36.Ryan DG. MicroRNAs of the mammalian eye display distinct and overlapping tissue specificity. Mol Vis. 2006;12:1175–1184. [PubMed] [Google Scholar]
- 37.Jiang J. Real-time expression profiling of microRNA precursors in human cancer cell lines. Nucleic Acids Res. 2005;33:5394–5403. doi: 10.1093/nar/gki863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kang MK. Replicative senescence of normal human oral keratinocytes is associated with the loss of telomerase activity without shortening of telomeres. Cell Growth Differ. 1998;9:85–95. [PubMed] [Google Scholar]
- 39.Livak KJ. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 40.Blenkiron C, et al. MicroRNA expression profiling of human breast cancer identifies new markers of tumor subtype. Genome Biol. 2007;8:R214. doi: 10.1186/gb-2007-8-10-r214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sempere LF. Altered microRNA expression confined to specific epithelial cell subpopulations in breast cancer. Cancer Res. 2007;67:11612–11620. doi: 10.1158/0008-5472.CAN-07-5019. [DOI] [PubMed] [Google Scholar]
- 42.Brennan JA. Molecular assessment of histopathological staging in squamous-cell carcinoma of the head and neck. N Engl J Med. 1995;332:429–435. doi: 10.1056/NEJM199502163320704. [DOI] [PubMed] [Google Scholar]
- 43.Wadsworth JT. Identification of patients with head and neck cancer using serum protein profiles. Arch Otolaryngol Head Neck Surg. 2004;130:98–104. doi: 10.1001/archotol.130.1.98. [DOI] [PubMed] [Google Scholar]
- 44.Hornstein E. The microRNA miR-196 acts upstream of Hoxb8 and Shh in limb development. Nature. 2005;438:671–674. doi: 10.1038/nature04138. [DOI] [PubMed] [Google Scholar]
- 45.Sempere LF. Expression profiling of mammalian microRNAs uncovers a subset of brain-expressed microRNAs with possible roles in murine and human neuronal differentiation. Genome Biol. 2004;5:R13. doi: 10.1186/gb-2004-5-3-r13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lee YS. Depletion of human micro-RNA miR-125b reveals that it is critical for the proliferation of differentiated cells but not for the down-regulation of putative targets during differentiation. J Biol Chem. 2005;280:16635–16641. doi: 10.1074/jbc.M412247200. [DOI] [PubMed] [Google Scholar]
- 47.Wong TS. Mature miR-184 as potential oncogenic microRNA of squamous cell carcinoma of tongue. Clin Cancer Res. 2008;14:2588–2592. doi: 10.1158/1078-0432.CCR-07-0666. [DOI] [PubMed] [Google Scholar]
- 48.Schetter AJ, et al. MicroRNA expression profiles associated with prognosis and therapeutic outcome in colon adenocarcinoma. JAMA. 2008;299:425–436. doi: 10.1001/jama.299.4.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yanaihara N, et al. Unique microRNA molecular profiles in lung cancer diagnosis and prognosis. Cancer Cell. 2006;9:189–198. doi: 10.1016/j.ccr.2006.01.025. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.