Abstract
Objectives. Poor sleep is associated with chronic widespread pain (CWP). Conversely, good-quality sleep may play a role in the resolution of pain symptoms. Sleep is a multidimensional construct, comprising a number of diverse components. The aims of the current study were to examine the hypotheses that: (i) good sleep quality would predict the resolution of CWP, (ii) restorative sleep would predict the resolution of CWP and (iii) that these relationships would be independent of confounding psychological factors.
Methods. Subjects in a population-based prospective study completed a pain questionnaire at baseline from which subjects with CWP were identified. Baseline sleep was measured using the Estimation of Sleep Problems Scale which measures sleep onset, maintenance, early wakening and restorative sleep. The questionnaire also contained scales examining psychosocial status. Subjects were followed up 15 months later and pain status was assessed.
Results. A total of 1061 subjects reported CWP at baseline of whom 679 (75% of eligible subjects) responded at follow-up. Of those, a total of 300 (44%) no longer satisfied criteria for CWP. Univariate analysis revealed that three of the four sleep components were associated with the resolution of CWP: rapid sleep onset, odds ratio (OR) = 1.7, 95% CI 1.2, 2.5; absence of early wakening, OR = 1.6, 95% CI 1.1, 2.4; and restorative sleep, OR = 2.7, 95% CI 1.5, 4.8. After adjusting for the effect of psychosocial factors, which may have confounded the relationship, only restorative sleep (OR = 2.0, 95% CI 1.02, 3.8) was associated.
Conclusions. Self-reported restorative sleep was independently associated with the resolution of CWP and return to musculoskeletal health.
Keywords: Sleep quality, Chronic widespread pain resolution, Restorative sleep
Introduction
Chronic widespread pain (CWP) is the cardinal feature of fibromyalgia. CWP impacts upon physical and psychological well-being and has important long-term consequences including an increased risk of incident cancer [1] and early mortality [2]. There is limited research examining the association between CWP and sleep quality. However, a robust evidence base has demonstrated an association between poor sleep quality and fibromyalgia. In a clinical population, 73% of fibromyalgia patients reported some sleep disturbance [3]. Population-based studies provide further support for the high prevalence of sleep problems amongst individuals with fibromyalgia, with up to 99% of fibromyalgia subjects reporting some form of sleep difficulty [4]. However, these studies have been cross-sectional and were unable to determine whether the observed sleep disturbance preceded or was a consequence of having chronic pain.
Data gathered using sleep diaries (which recorded the timing and quality of sleep) indicated that, amongst women with fibromyalgia, a night of poorer sleep was followed by a significantly more painful day, and a more painful day was followed by a night of poorer sleep [5]. We have previously reported, in a population-based prospective study of subjects free of CWP, that self-reported poor sleep increased the risk of new symptom onset 15 months later [6]. Experimental research is also supportive of a sequential link between sleep and pain. Induced periods of night-time mini-arousal have been shown to induce symptoms similar to those observed in subjects with CWP [7], while, intriguingly, on removal of these arousal periods, the induced pain symptoms resolved. Furthermore, sleep deprivation studies have shown that in healthy volunteers poor sleep lowers pressure pain threshold, whereas recovery sleep after sleep deprivation increases pain thresholds [8]. Research supports a reciprocal relationship between CWP and sleep quality, where pain results in poor sleep which, in turn, increases pain and results in the persistence of the disease [8]. Conversely, good-quality sleep may play a role in the resolution of pain symptoms and the return to musculoskeletal health.
Sleep is a multidimensional construct, comprising a number of diverse components. Sleep problems usually include one or more of the following complaints: delay of sleep onset, difficulty staying asleep, waking too early and non-restorative sleep (waking feeling unrefreshed). Non-restorative sleep is a prominent feature of the sleep problems reported by patients with fibromyalgia [3, 9]. However, the relationship between non-restorative sleep and the resolution of CWP is not known.
A number of psychological factors that are associated both with sleep quality and pain may confound the relationship. For example, depression and anxiety are both associated with poor sleep quality and CWP [10–12]. As such, we will examine the influence of potential confounders on the association between sleep quality and the resolution of CWP.
The aims of the current study are to examine the hypotheses that: (i) good sleep quality would predict the resolution of CWP; (ii) restorative sleep would predict the resolution of CWP; and (iii) these relationships would be independent of confounding psychological factors.
Methods
Design
Subjects were those who participated in a population-based prospective study of pain. The sampling frame was all individuals aged between 25 and 65 yrs who were registered at one of three general practices in the north-west of England. Subjects completed a questionnaire at baseline that assessed pain status from which those with CWP (see subsequently) were identified. Sleep quality was also assessed at baseline. A number of psychosocial factors are associated with CWP and also with sleep, and may have confounded any relationship observed. To assess and allow us to control for these potential confounders, the questionnaire contained scales examining levels of psychosocial distress, somatic symptom reporting and medical help-seeking behaviour. Fifteen months after the baseline survey, subjects were sent a follow-up questionnaire that assessed pain status. The study was approved by the local NHS Research Ethics Committee.
Baseline questionnaire
Sleep quality
The four-item Estimation of Sleep Problems Scale [13] was used to examine sleep quality. The scale asks about recent problems with sleep and contains items on the most commonly occurring symptoms of poor sleep quality: sleep onset (‘During the past month did you have trouble falling asleep?’); sleep maintenance (‘During the past month did you wake up several times per night?’); early wakening (‘During the past month did you have trouble staying asleep, including waking up far too early?’); and non-restorative sleep (‘During the past month did you wake up after your normal amount of sleep feeling tired and worn out?’). Subjects indicate the number of days in the past month that they have experienced difficulties in each of the four sleep components on a 5-point scale ranging from 0 to 5 (0 = 0 days; 1 = 1–3 days; 2 = 4–7 days; 3 = 8–14 days; 4 = 15–21 days; 5 = 22–31 days). The scale has been used in previous studies of CWP and fibromyalgia [14, 15]. The internal consistency co-efficient for the scale was 0.79 [13].
Pain assessment
The questionnaire asked subjects about the occurrence and chronicity of pain. Four-line drawings (blank body manikins: front, back and sides) accompanied the pain questions and subjects were asked to indicate site(s) of pain they had experienced for 1 day or longer in the past month. These methods have been used previously to determine the location and duration of pain [16, 17]. Using the information on pain status, CWP was defined based on the ACR definition in their criteria for fibromyalgia [18]. These require that pain must have been present for at least 3 months and be present in two contralateral areas of the body, above and below the waist and in the axial skeleton.
Questionnaire: psychosocial assessment
A number of factors associated with CWP may also be risk factors for poor sleep and may therefore potentially confound any relationship observed. Therefore, the baseline questionnaire also contained scales assessing: psychological distress (General Health Questionnaire), somatization (Somatic Symptom Checklist), anxiety and depression (Hospital Anxiety and Depression Scale) and health anxiety and illness behaviour (Illness Attitude Scales).
Hospital Anxiety and Depression Scale
The Hospital Anxiety and Depression (HAD) scale [19] examines levels of anxiety and depression in the last week. The HAD contains a seven-item depression subscale and a seven-item anxiety subscale. Subjects rate each item on a four-point scale scored 0–3 with higher scores associated with a higher probability of having a depressive or anxiety disorder.
General Health Questionnaire
The 12-item version of the General Health Questionnaire (GHQ) [20] was used to examine levels of psychological distress. Each item has four possible responses, for scoring purposes; these are dichotomized (0 or 1) and the 12 scores added together to give a total GHQ score between 0 and 12. Higher scores indicate higher levels of psychological distress.
Somatic Symptoms Checklist
The Somatic Symptoms Checklist (SSC) [21] was originally designed and validated as a screening test for somatization disorder. The scale contains six items (and an additional item for females regarding menstrual cramps). Subjects are required to answer yes or no to questions regarding the occurrence of symptoms (e.g. ‘Have you ever had trouble breathing?’). Scores are summed to provide a total number of somatic symptoms reported. One item, ‘have you ever had difficulties swallowing or had an uncomfortable lump in your throat that stayed with you for at least an hour?’, was excluded from the analysis due to a high proportion of missing answers. To avoid spurious association with CWP the item examining pain in fingers and toes was also excluded.
Illness attitudes scales
The Illness Attitudes Scales (IAS) [22] consist of two subscales: health anxiety and illness behaviour. The health anxiety subscale contains 11 items (e.g. ‘are you worried that you may get a serious illness in the future?’) which are scored on a five-point scale (0–4) with total scores ranging from 0 to 44. The illness behaviour scale contains six items (e.g. ‘how often do you see a doctor?’) also scored on a five-point scale from 0 (‘No’) to 4 (‘Most of the time’), with total scores ranging from 0 to 24.
Follow-up
All subjects who reported CWP at baseline, who agreed to further contact from the study team and provided contact details, were eligible for follow-up. Subjects were sent a questionnaire 15 months after they completed the baseline questionnaire. Pain status was assessed using the same methodology as in the baseline survey described above. Trained observers, who were blind to all baseline data, determined pain status at follow-up and subjects were classified into those with and without CWP.
Statistical analysis
The distribution of sleep scores was not Gaussian. Therefore, for analysis the total sleep score was categorized into thirds based upon the distribution of subjects' scores, resulting in three equal-sized groups for analysis. Similarly, the individual sleep components (onset, maintenance, early wakening and non-restorative) were categorized into thirds: ‘no sleep problems’ (reporting 0 days); ‘moderate level of sleep problems’ (1–7 days); and ‘high level of sleep problems’ (8–31 days). Subjects classified as ‘high level of sleep problems’ were classed as the referent group. We used logistic regression to examine the association between ‘moderate sleep problems’ and ‘no sleep problems’ at baseline and the resolution of CWP at follow-up, adjusting for the effects of age and gender. We then examined the association between each of the psychosocial variables and CWP resolution. For this analysis, each of the psychosocial scales were categorized into thirds based upon the distribution of subjects' scores to form three equal-sized groups with the highest third classed as the referent group. Those psychosocial factors that were independently associated with CWP resolution were included in a final multivariate logistic regression model that examined the association between sleep quality components and CWP resolution.
The majority of the subjects whose CWP had resolved at follow-up still reported some regional pain. To examine the influence of this on the findings, subjects were stratified by pain status at follow-up (‘no pain’ and ‘some pain’) and the multivariate model was re-run to determine whether the relationship observed in the multivariate model was apparent for both groups. Number of pain sites at baseline was added to the final model to determine whether the observed relationship was confounded by pain severity at baseline. The results are presented as odds ratios (ORs) with 95% CI. All analyses were conducted using the STATA statistical software (Texas, USA) [23].
Results
Response rates
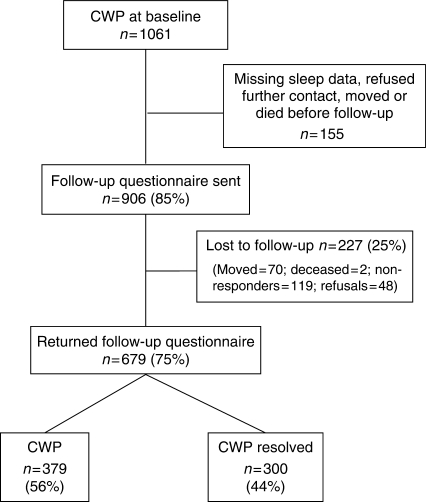
A total of 1061 subjects reported CWP at baseline and formed the cohort for this study. Of those, 906 (85%) provided complete sleep data, consent and contact details for follow-up. A total of 679 subjects returned the follow-up questionnaire and, after adjusting for those who had moved or died and therefore could not receive a questionnaire (n = 72), the response rate was 92% (Fig. 1).
Fig. 1.
Flow chart showing participation of subjects at follow-up.
Sleep quality and the resolution of CWP
A total of 300 (44%) subjects no longer satisfied criteria for CWP at follow-up (Table 1). Subjects who reported symptom resolution were younger than those whose CWP persisted. The groups did not differ on gender.
Table 1.
Prevalence of symptom resolution by age and gender
| Male |
Female |
|||||
|---|---|---|---|---|---|---|
| Age (yrs) | n | CWP resolved (n) | % | n | CWP resolved (n) | % |
| 25–45 | 73 | 32 | 44 | 154 | 93 | 60 |
| 46–55 | 79 | 34 | 43 | 147 | 58 | 39 |
| 56–65 | 73 | 32 | 44 | 153 | 51 | 33 |
| Total | 225 | 98 | – | 454 | 202 | – |
Univariate analysis of total sleep scores revealed that compared to those in the referent group (poor-quality sleep) subjects in the lowest third, i.e. those reporting the best quality sleep, were 60% more likely to have symptoms that resolved (OR = 1.6, 95% CI 1.1, 2.4).
Specific sleep components and resolution of CWP
Examining the independent sleep components revealed that three of the four were significantly associated with the resolution of CWP: rapid sleep onset (OR = 1.7, 95% CI 1.2, 2.5); absence of early wakening (OR = 1.6, 95% CI 1.1, 2.4); and restorative sleep (OR = 2.7, 95% CI 1.5, 4.8) (all analyses adjusted for age and gender).
The role of confounding factors
In comparison with the CWP subjects whose pain persisted, subjects whose CWP resolved had significantly lower baseline levels of anxiety (HAD; P = 0.014), depression (HAD; P = 0.000), psychological distress (GHQ; P = 0.033), somatic symptoms (SSC; P = 0.000), illness behaviour (P = 0.000) and health anxiety (IAS; P = 0.048) (all P-values Mann–Whitney rank sum).
Univariate logistic regression showed that scoring in the middle or lowest third of each of the psychosocial factors, except health anxiety, was associated with CWP resolution. In multivariate analysis, illness behaviour independently predicted symptom resolution (Table 2).
Table 2.
The association between psychological factors and CWP resolution, adjusted for age and gender
| Psychological | CWP persisted | CWP resolved | Univariate model | Multivariate model | |
|---|---|---|---|---|---|
| factor | Categories | (n) | (n) | OR (95% CI) | OR (95% CI) |
| HAD—depression | 8–21 | 132 | 61 | Referent | Referent |
| 4–7 | 127 | 113 | 2.0 (1.3, 3.0) | 1.4 (0.8, 2.3) | |
| 0–3 | 117 | 120 | 2.2 (1.5, 3.4) | 1.2 (0.7, 2.2) | |
| HAD—anxiety | 11–21 | 113 | 72 | Referent | Referent |
| 7–10 | 123 | 96 | 1.3 (0.8, 1.9) | 0.9 (0.5, 1.4) | |
| 0–6 | 138 | 128 | 1.5 (1.0, 2.3) | 0.9 (0.6, 1.6) | |
| GHQ | 4–12 | 135 | 84 | Referent | Referent |
| 1–3 | 79 | 60 | 1.3 (0.8, 1.9) | 0.9 (0.5, 1.6) | |
| 0 | 156 | 151 | 1.6 (1.1, 2.3) | 1.0 (0.6, 1.6) | |
| Somatic symptoms | 2–3 | 67 | 34 | Referent | Referent |
| 1 | 159 | 104 | 1.4 (0.8, 2.2) | 1.0 (0.6, 1.6) | |
| 0 | 142 | 156 | 2.4 (1.5, 3.9) | 1.3 (0.8, 2.2) | |
| Illness behaviour | 12–24 | 153 | 57 | Referent | Referent |
| 7–11 | 113 | 97 | 2.3 (1.5, 3.4) | 2.0 (1.2, 3.1) | |
| 0–6 | 100 | 138 | 3.7 (2.5, 5.5) | 3.2 (2.0, 5.1) | |
| Health anxiety | 15–44 | 118 | 73 | Referent | Referent |
| 9–14 | 112 | 101 | 1.4 (0.9, 2.1) | – | |
| 0–8 | 132 | 114 | 1.4 (0.95, 2.1) | – |
A final multivariate model examined the association between the four sleep components and symptom resolution after adjusting for psychosocial factors, age and gender (Table 3). Only restorative sleep (OR = 2.0, 95% CI 1.02, 3.8) remained independently associated with the resolution of CWP. Including the number of pain sites at baseline did not influence the final model; restorative sleep remained the only factor that was independently associated with symptom resolution (OR = 2.0, 95% CI 1.00, 3.8).
Table 3.
The association between sleep and CWP resolution for both total sleep score and the individual components of sleep, adjusted for age, gender and psychosocial scores
| CWP persisted | CWP resolved | Univariate model (crude) | Univariate model adjusting for age and gender | Multivariate model adjusting for age, gender and psychosocial factors | ||
|---|---|---|---|---|---|---|
| Sleep factor | Sleep score | (n) | (n) | OR (95% CI) | OR (95%CI) | OR (95% CI) |
| Overall | 15–20 | 125 | 76 | Referent | Referent | – |
| 8–14 | 128 | 97 | 1.2 (0.8, 1.8) | 1.2 (0.8, 1.8) | – | |
| 0–7 | 126 | 127 | 1.7 (1.1, 2.4) | 1.6 (1.1, 2.3) | – | |
| No. of days of sleep problems | ||||||
| Onset | 8–31 | 159 | 90 | Referent | Referent | Referent |
| 1–7 | 113 | 111 | 1.7 (1.2, 2.5) | 1.7 (1.2, 2.5) | 1.4 (0.9, 2.2) | |
| 0 | 107 | 99 | 1.6 (1.1, 2.4) | 1.7 (1.2, 2.5) | 0.99 (0.6, 1.6) | |
| Maintenance | 8–31 | 224 | 158 | Referent | Referent | Referent |
| 1–7 | 117 | 103 | 1.2 (0.9, 1.7) | 1.2 (0.9, 1.7) | 1.1 (0.7, 1.9) | |
| 0 | 38 | 39 | 1.5 (0.9, 2.4) | 1.4 (0.8, 2.3) | 0.8 (0.4, 1.7) | |
| Early wakening | 8–31 | 192 | 134 | Referent | Referent | Referent |
| 1–7 | 118 | 85 | 1.0 (0.7, 1.5) | 0.98 (0.7, 1.4) | 0.7 (0.4, 1.1) | |
| 0 | 69 | 81 | 1.7 (1.1, 2.5) | 1.6 (1.1, 2.4) | 1.1 (0.6, 2.0) | |
| Restorative | 8–31 | 241 | 170 | Referent | Referent | Referent |
| 1–7 | 113 | 91 | 1.1 (0.8, 1.6) | 1.2 (0.8, 1.6) | 0.9 (0.6, 1.4) | |
| 0 | 25 | 39 | 2.2 (1.3, 3.8) | 2.7 (1.5, 4.8) | 2.0 (1.0, 3.8) | |
The majority of subjects (n = 232, 77.7%) whose CWP had resolved at follow-up still reported some regional pain and only 68 (23%) subjects were completely pain free. When stratified by having ‘some pain’ or ‘no pain’ at follow-up, we found that the association between restorative sleep and pain resolution was similar between these two groups: pain free at follow-up, OR = 1.9, 95% CI 0.7, 5.3; regional pain at follow-up, OR = 1.8, 95% CI 0.9, 3.7.
Discussion
We have shown that, in this population of CWP subjects, self-reported good-quality sleep is associated with the resolution of symptoms and the return to musculoskeletal health. This study examined the role of four constructs of good-quality sleep: sleep onset; sleep maintenance; early wakening; and restorative sleep. In univariate analyses, three of the four sleep components were associated with the resolution of CWP: rapid sleep onset, absence of early wakening and restorative sleep. However, after controlling for psychosocial factors only restorative sleep was associated.
There are a number of limitations to the current study. First, 78% of those subjects who were classified as having CWP that had resolved at follow-up reported some regional pain. We were concerned that perhaps we were predicting, not the resolution of pain symptoms, but moving out of criteria for CWP. However, the relationships between restorative sleep and having some pain or being completely pain free at follow-up were identical. Reporting some pain at follow-up did not alter the association between CWP resolution and sleep quality. Although these associations are strong, the CIs span unity, which is likely to be a result of the smaller sample size in this stratified analysis [n = 432 (CWP persist = 366, CWP resolve = 66) and n = 592 (CWP persist = 366, CWP resolve = 226), respectively]. Second, pain severity at baseline is an obvious potential confounder of the relationship. We did not have a measure of pain severity at baseline. However, we examined the extent of the pain by controlling for the number of pain sites at baseline. The relationship between pain resolution and restorative sleep persisted after controlling for number of pain sites. Third, this study identified a group of subjects who had CWP at baseline and followed them up 15 months later. We may have missed subjects whose pain had resolved and who then went on to develop new pain before we contacted them at the follow-up. However, given that the definition of CWP requires having pain for more than 3 months it seems probable that only a small proportion of subjects could have experienced a resolution of their CWP and then development of new CWP in that time frame. There were a number of non-responders at baseline and follow-up. For non-response to influence the associations, we have reported you would have to hypothesize that factors associated with responding to a postal survey would confound the association between sleep and pain resolution. This seems unlikely. Finally, this study used a self-report measure which may not accurately reflect actual sleep quality. For example, chronic musculoskeletal pain patients have been shown to under-estimate total sleep time and over-estimate sleep onset latency [24]. Further research using both objective and subjective measures of sleep quality should address this issue because understanding the nature of sleep disturbance in these individuals has implications for the development of effective treatment interventions.
The results of our study are consistent with randomized controlled trials of sodium oxybate, a drug used to prevent the onset of cataplexy and daytime fatigue, and pregabaline, a drug used to treat pain and anxiety, in fibromyalgia. Both drugs produce refreshing sleep and reduced pain in fibromyalgia, and increase slow wave sleep on polysomnography [25, 26, 27]. Thus experimental studies support the link between restorative sleep and symptomatic improvement in fibromyalgia and CWP, but these studies also indicate that the restoration of refreshing sleep may be achieved through different pharmacological and pathophysiological mechanisms. It is not clear whether refreshing sleep is a mechanism for recovery or a marker of recovery but either way restoration of refreshing sleep may be a clinically useful sign of impending improvement in fibromyalgia or CWP.
As the weight of evidence does suggest that sleep is associated with the onset and resolution of CWP, it is interesting and potentially important to speculate on the possible mechanisms. Good sleep has been shown to buffer the relationship between pain and negative affect [28]. Subjects with fibromyalgia or RA recorded sleep quality, daily stressful events, pain and negative and positive affect (mood) at 2½-h intervals during the day. In subjects who reported poor sleep there was a strong association between momentary increases in pain and increases in negative affect. Whereas, there was no association between pain and negative affect in subjects who reported good-quality sleep. Hamilton et al. [28] suggested that good-quality or restorative sleep acts as a biobehavioural resource that minimizes allostatic load. The majority of the studies examining the association between sleep disruption and pain have been descriptive and it is unclear what biological mechanisms underpin the association [29]. In a recent review, a number of potential biological mechanisms were identified [29]. It was suggested that rapid eye movement (REM) sleep deprivation may render the serotonin system functionally unable to support pain inhibition produced by opioidergic activation. Furthermore, sleep is an important component of homeostasis and sleep deprivation affects a number of circadian rhythms, including the hypothalamic pituitary adrenal (HPA) axis [30], alterations that are associated with CWP [31].
To conclude, the present population-based prospective study has demonstrated that sleep is associated with the resolution of CWP. Restorative sleep was independently associated with the resolution of CWP and return to musculoskeletal health. If there is a bi-directional relationship between sleep and the experience of pain symptoms, this would support the development of interventions focusing on improving sleep in CWP sufferers. Although we cannot infer a causal relationship based on these data, the role of sleep quality in the resolution of symptoms warrants further investigation since interventions targeted at sleep problems could form one component of management.

Acknowledgements
We would like to thank the staff and patients at the general practices involved in the study. Thanks also to Stewart Taylor, Karen Schafheutle and Richard Jones for survey administration.
Funding: This study was funded by the Arthritis Research Campaign, Grant number: 17552.
Disclosure statement: The authors have declared no conflicts of interest.
References
- 1.McBeth J, Silman AJ, Macfarlane GJ. Association of widespread body pain with an increased risk of cancer and reduced cancer survival: a prospective, population-based study. Arthritis Rheum. 2003;48:1686–92. doi: 10.1002/art.10973. [DOI] [PubMed] [Google Scholar]
- 2.Macfarlane GJ, McBeth J, Silman AJ. Widespread body pain and mortality: prospective population based study. Br Med J. 2001;323:662–5. doi: 10.1136/bmj.323.7314.662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wolfe F, Pincus T. Standard self-report questionnaires in routine clinical and research practice - an opportunity for patients and rheumatologists. J Rheumatol. 1991;18:643–6. [PubMed] [Google Scholar]
- 4.Theadom A, Cropley M, Humphrey KL. Exploring the role of sleep and coping in quality of life in fibromyalgia. J Psychosom Res. 2007;62:145–51. doi: 10.1016/j.jpsychores.2006.09.013. [DOI] [PubMed] [Google Scholar]
- 5.Affleck G, Urrows S, Tennen H, Higgins P, Abeles M. Sequential daily relations of sleep, pain intensity, and attention to pain among women with fibromyalgia. Pain. 1996;68:363–8. doi: 10.1016/s0304-3959(96)03226-5. [DOI] [PubMed] [Google Scholar]
- 6.Gupta A, Silman AJ, Ray D, et al. The role of psychosocial factors in predicting the onset of chronic widespread pain: results from a prospective population-based study. Rheumatology. 2007;46:666–71. doi: 10.1093/rheumatology/kel363. [DOI] [PubMed] [Google Scholar]
- 7.Moldofsky H, Scarisbrick P. Induction of neurasthenic musculoskeletal pain syndrome by selective sleep stage deprivation. Psychosom Med. 1976;38:35–44. doi: 10.1097/00006842-197601000-00006. [DOI] [PubMed] [Google Scholar]
- 8.Onen SH, Alloui A, Gross A, Eschallier A, Dubray C. The effects of total sleep deprivation, selective sleep interruption and sleep recovery on pain tolerance thresholds in healthy subjects. J Sleep Res. 2001;10:35–42. doi: 10.1046/j.1365-2869.2001.00240.x. [DOI] [PubMed] [Google Scholar]
- 9.Yunus MB, Masi AT, Calabro JJ, Miller KA, Feigenbaum SL. Primary fibromyalgia (fibrositis): clinical study of 50 patients with matched normal controls. Semin Arthritis Rheum. 1981;11:151–71. doi: 10.1016/0049-0172(81)90096-2. [DOI] [PubMed] [Google Scholar]
- 10.Anch AM, Lue FA, MacLean AW, et al. Sleep physiology and psychlogical aspects of fibrositis (fibromyalgia) syndrome. Can J Psychol. 1991;179:184. doi: 10.1037/h0084280. [DOI] [PubMed] [Google Scholar]
- 11.Bigatti SM, Hernandez AM, Cronan TA, Rand KL. Sleep disturbances in fibromyalgia syndrome: relationship to pain and depression. Arthritis Rheum. 2008;59:961–7. doi: 10.1002/art.23828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Palermo TM, Toliver-Sokol M, Fonareva I, Koh JL. Objective and subjective assessment of sleep in adolescents with chronic pain compared to healthy adolescents. Clin J Pain. 2007;23:812–20. doi: 10.1097/AJP.0b013e318156ca63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jenkins CD, Stanton BA, Niemcryk SJ, Rose RM. A scale for the estimation of sleep problems in clinical research. J Clin Epidemiol. 1988;41:313–21. doi: 10.1016/0895-4356(88)90138-2. [DOI] [PubMed] [Google Scholar]
- 14.Croft P, Schollum J, Silman A. Population study of tender point counts and pain as evidence of fibromyalgia. Br Med J. 1994;309:696–9. doi: 10.1136/bmj.309.6956.696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yunus MB, Arslan S, Aldag JC. Relationship between body mass index and fibromyalgia features. Scand J Rheumatol. 2002;31:27–31. doi: 10.1080/030097402317255336. [DOI] [PubMed] [Google Scholar]
- 16.Croft PR, Rigby AS, Boswell R, Schollum J, Silman AJ. The prevalence of chronic widespread pain in the general population. J Rheumatol. 1993;20:710–3. [PubMed] [Google Scholar]
- 17.Hunt IM, Silman AJ, Benjamin S, McBeth J, Macfarlane GJ. The prevalence and associated features of chronic widespread pain in the community using the ‘Manchester’ definition of chronic widespread pain. Rheumatology. 1999;38:275–9. doi: 10.1093/rheumatology/38.3.275. [DOI] [PubMed] [Google Scholar]
- 18.Wolfe F, Smythe HA, Yunus MB, Bennett R, Bombardier C. The American College of Rheumatology 1990 criteria for the classification of fibromyalgia. Arthritis Rheum. 1990;33:160–72. doi: 10.1002/art.1780330203. [DOI] [PubMed] [Google Scholar]
- 19.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67:361–70. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 20.Goldberg DP, Williams P. A user's guide to the general health questionnaire. Windsor: NFER-Nelson; 1988. [Google Scholar]
- 21.Othmer E, DeSouza C. A screening test for somatization disorder (hysteria) Am J Psychiatry. 1985;142:1146–9. doi: 10.1176/ajp.142.10.1146. [DOI] [PubMed] [Google Scholar]
- 22.Kellner R. Abridged manual of the illness attitude scale. New Mexico: Department of Psychiatry, School of Medicine, University of New Mexico; 1987. [Google Scholar]
- 23.STATA. Stata reference manual 3.1. College Station, TX: STATA Corporation; 1993. [Google Scholar]
- 24.Wilson KG, Watson ST, Currie SR. Daily diary and ambulatory activity monitoring of sleep in patients with insomnia associated with chronic musculoskeletal pain. Pain. 1998;75:75–84. doi: 10.1016/S0304-3959(97)00207-8. [DOI] [PubMed] [Google Scholar]
- 25.Scharf MB, Baumann M, Berkowitz DV. The effects of sodium oxybate on clinical symptoms and sleep patterns in patients with fibromyalgia. J Rheumatol. 2003;30:1070–4. [PubMed] [Google Scholar]
- 26.Crofford LJ, Rowbotham MC, Mease PJ, et al. Pregabalin for the treatment of fibromyalgia syndrome: results of a randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2005;52:1264–73. doi: 10.1002/art.20983. [DOI] [PubMed] [Google Scholar]
- 27.Hindmarch I, Dawson J, Stanley N. A double-blind study in healthy volunteers to assess the effects on sleep of pregabalin compared with alprazolam and placebo. Sleep. 2005;28:187–93. doi: 10.1093/sleep/28.2.187. [DOI] [PubMed] [Google Scholar]
- 28.Hamilton NA, Catley D, Karlson C. Sleep and the affective response to stress and pain. Health Psychol. 2007;26:288–95. doi: 10.1037/0278-6133.26.3.288. [DOI] [PubMed] [Google Scholar]
- 29.Lautenbacher S, Kundermann B, Krieg JC. Sleep deprivation and pain perception. Sleep Med Rev. 2006;10:357–69. doi: 10.1016/j.smrv.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 30.Vgontzas AN, Chrousos GP. Sleep, the hypothalamic-pituitary-adrenal axis, and cytokines: multiple interactions and disturbances in sleep disorders. Endocrinol Metab Clin North Am. 2002;31:15–36. doi: 10.1016/s0889-8529(01)00005-6. [DOI] [PubMed] [Google Scholar]
- 31.McBeth J, Silman AJ, Gupta A, et al. Moderation of psychosocial risk factors through dysfunction of the hypothalamic-pituitary-adrenal stress axis in the onset of chronic widespread musculoskeletal pain: findings of a population-based prospective cohort study. Arthritis Rheum. 2007;56:360–71. doi: 10.1002/art.22336. [DOI] [PubMed] [Google Scholar]