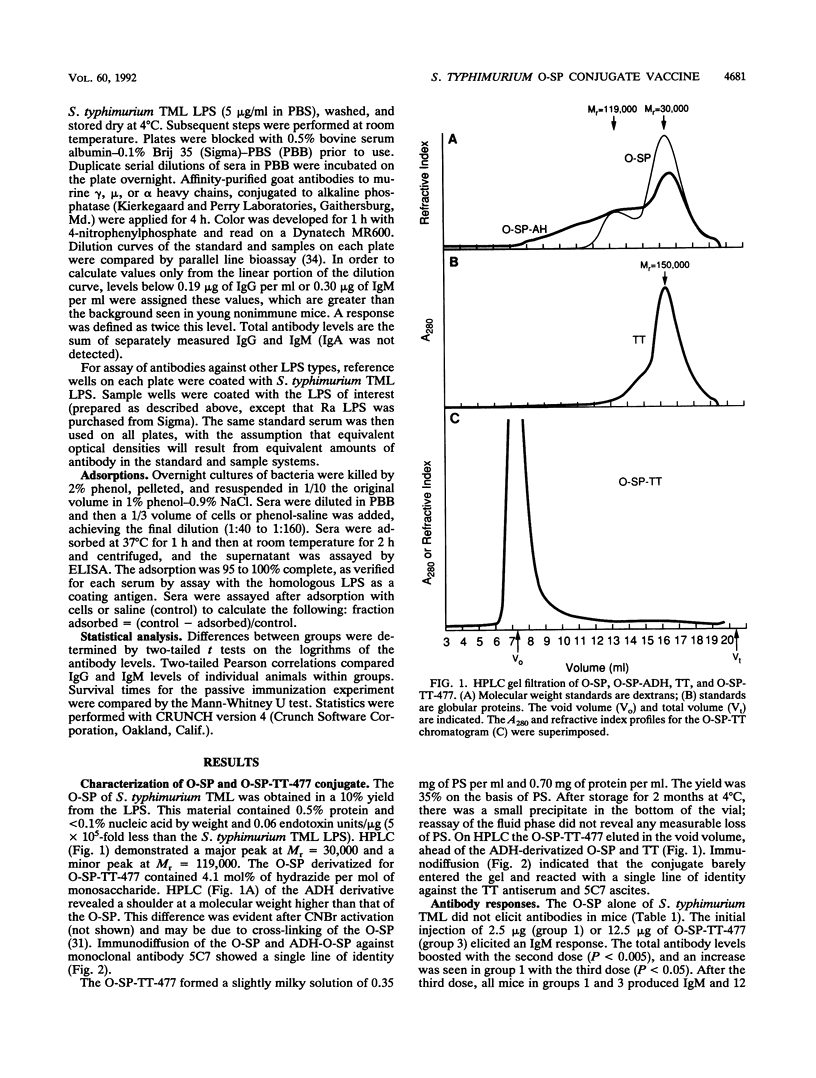
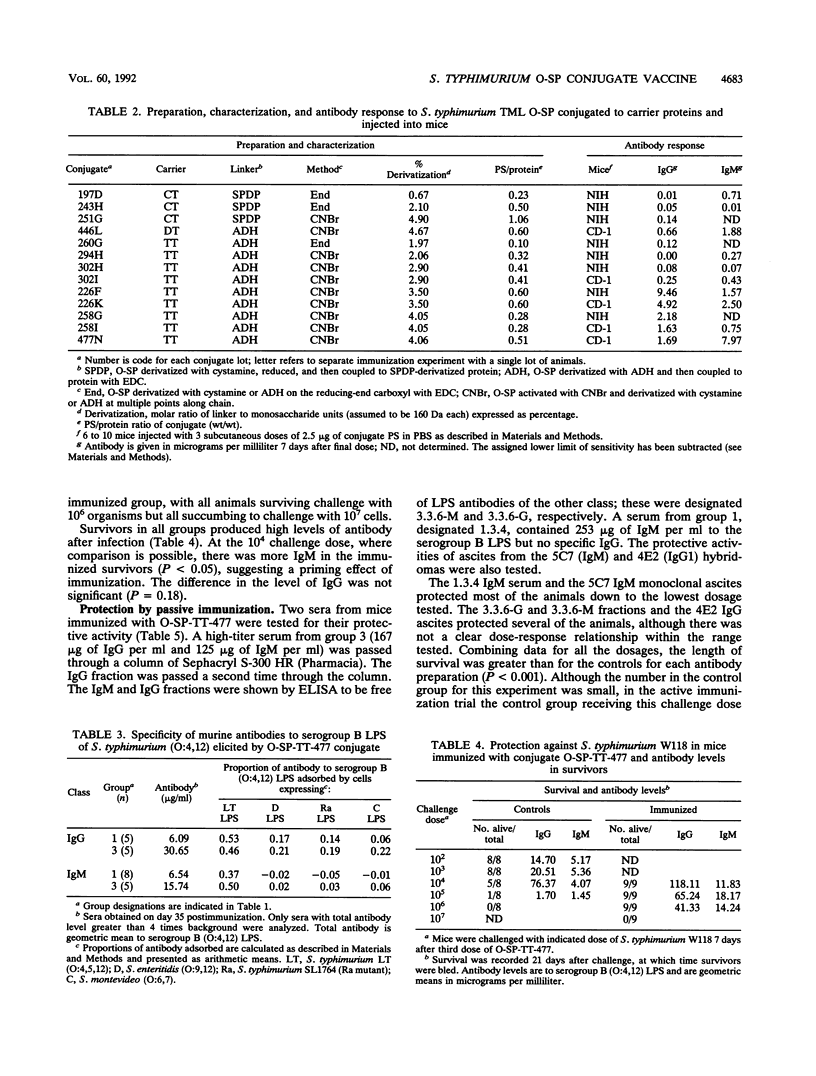
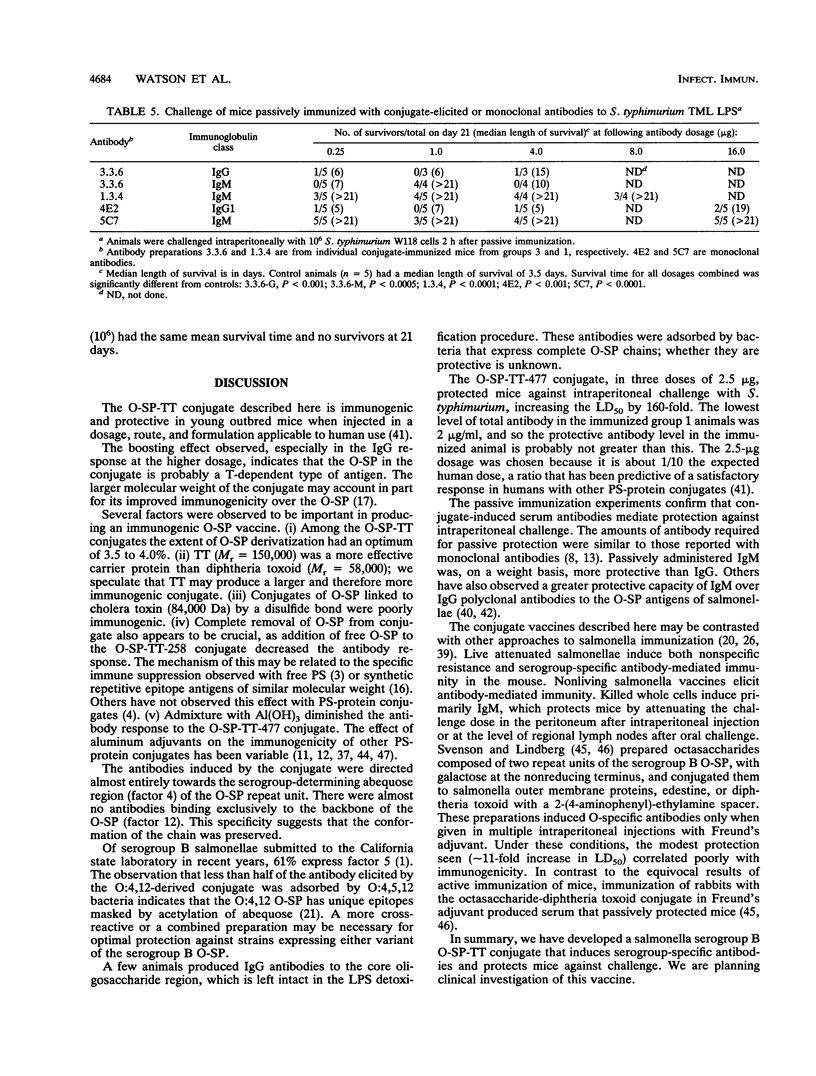
Abstract
Serious infections with salmonellae remain a threat in many human populations. Despite extensive study of salmonella infections in animals and clinical experience with killed cellular vaccines, there are no vaccines against serotypes other than Salmonella typhi licensed for human use. Serum antibodies to the O-specific polysaccharide (O-SP) of salmonellae protect mice against invasive infection. In order to render it immunogenic, we have conjugated the O-SP of Salmonella typhimurium to carrier proteins by various schemes. O-SP conjugated to tetanus toxoid (O-SP-TT) elicited antibodies in outbred mice after three subcutaneous injections without adjuvant. The O-SP alone elicited no detectable antibody. The antibody response to O-SP-TT was boosted by successive doses and consisted of immunoglobulin G (IgG) and IgM. Most mice only produced antibodies specific for the abequose (O:4 factor) region of the O-SP. Occasional animals also produced antibodies to the core oligosaccharide. Immunized mice were protected against intraperitoneal challenge with S. typhimurium, demonstrating a 160-fold increase in the 50% lethal dose. Passive immunization with conjugate-induced IgM or IgG also protected against challenge. These results indicate that an O-SP-TT conjugate, when given by a route and formulation acceptable for human use, protects mice against challenge with S. typhimurium.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adeyokunnu A. A., Hendrickse R. G. Salmonella osteomyelitis in childhood. A report of 63 cases seen in Nigerian children of whom 57 had sickle cell anaemia. Arch Dis Child. 1980 Mar;55(3):175–184. doi: 10.1136/adc.55.3.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker P. J. Regulation of magnitude of antibody response to bacterial polysaccharide antigens by thymus-derived lymphocytes. Infect Immun. 1990 Nov;58(11):3465–3468. doi: 10.1128/iai.58.11.3465-3468.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beuvery E. C., van Delft R. W., Miedema F., Kanhai V., Nagel J. Immunological evaluation of meningococcal group C polysaccharide-tetanus toxoid conjugate in mice. Infect Immun. 1983 Aug;41(2):609–617. doi: 10.1128/iai.41.2.609-617.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaser M. J., Feldman R. A. From the centers for disease control. Salmonella bacteremia: reports to the Centers for Disease Control, 1968-1979. J Infect Dis. 1981 May;143(5):743–746. doi: 10.1093/infdis/143.5.743. [DOI] [PubMed] [Google Scholar]
- Bock K., Meldal M., Bundle D. R., Iversen T., Garegg P. J., Norberg T., Lindberg A. A., Svenson S. B. The conformation of Salmonella O-antigenic polysaccharide chains of serogroups A, B, and D1 predicted by semi-empirical, Hard-Sphere (HSEA) calculations. Carbohydr Res. 1984 Jul 15;130:23–34. doi: 10.1016/0008-6215(84)85267-2. [DOI] [PubMed] [Google Scholar]
- Bock K., Meldal M., Bundle D. R., Iversen T., Pinto B. M., Garegg P. J., Kvanström I., Norberg T., Lindberg A. A., Svenson S. B. The conformation of Salmonella O-antigenic oligosaccharides of serogroups A, B, and D1 inferred from 1H- and 13C-nuclear magnetic resonance spectroscopy. Carbohydr Res. 1984 Jul 15;130:35–53. doi: 10.1016/0008-6215(84)85268-4. [DOI] [PubMed] [Google Scholar]
- CVJETANOVIC B., UEMURA K. THE PRESENT STATUS OF FIELD AND LABORATORY STUDIES OF TYPHOID AND PARATYPHOID VACCINES WITH SPECIAL REFERENCE TO STUDIES SPONSORED BY WORLD HEALTH ORGANIZATION. Bull World Health Organ. 1965;32:29–36. [PMC free article] [PubMed] [Google Scholar]
- Carlin N. I., Svenson S. B., Lindberg A. A. Role of monoclonal O-antigen antibody epitope specificity and isotype in protection against experimental mouse typhoid. Microb Pathog. 1987 Mar;2(3):171–183. doi: 10.1016/0882-4010(87)90019-2. [DOI] [PubMed] [Google Scholar]
- Celum C. L., Chaisson R. E., Rutherford G. W., Barnhart J. L., Echenberg D. F. Incidence of salmonellosis in patients with AIDS. J Infect Dis. 1987 Dec;156(6):998–1002. doi: 10.1093/infdis/156.6.998. [DOI] [PubMed] [Google Scholar]
- Chalker R. B., Blaser M. J. A review of human salmonellosis: III. Magnitude of Salmonella infection in the United States. Rev Infect Dis. 1988 Jan-Feb;10(1):111–124. doi: 10.1093/clinids/10.1.111. [DOI] [PubMed] [Google Scholar]
- Chu C. Y., Liu B. K., Watson D., Szu S. S., Bryla D., Shiloach J., Schneerson R., Robbins J. B. Preparation, characterization, and immunogenicity of conjugates composed of the O-specific polysaccharide of Shigella dysenteriae type 1 (Shiga's bacillus) bound to tetanus toxoid. Infect Immun. 1991 Dec;59(12):4450–4458. doi: 10.1128/iai.59.12.4450-4458.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu C., Schneerson R., Robbins J. B., Rastogi S. C. Further studies on the immunogenicity of Haemophilus influenzae type b and pneumococcal type 6A polysaccharide-protein conjugates. Infect Immun. 1983 Apr;40(1):245–256. doi: 10.1128/iai.40.1.245-256.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colwell D. E., Michalek S. M., Briles D. E., Jirillo E., McGhee J. R. Monoclonal antibodies to Salmonella lipopolysaccharide: anti-O-polysaccharide antibodies protect C3H mice against challenge with virulent Salmonella typhimurium. J Immunol. 1984 Aug;133(2):950–957. [PubMed] [Google Scholar]
- Cygler M., Rose D. R., Bundle D. R. Recognition of a cell-surface oligosaccharide of pathogenic Salmonella by an antibody Fab fragment. Science. 1991 Jul 26;253(5018):442–445. doi: 10.1126/science.1713710. [DOI] [PubMed] [Google Scholar]
- Dintzis H. M., Dintzis R. Z. Profound specific suppression by antigen of persistent IgM, IgG, and IgE antibody production. Proc Natl Acad Sci U S A. 1992 Feb 1;89(3):1113–1117. doi: 10.1073/pnas.89.3.1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dintzis R. Z., Okajima M., Middleton M. H., Greene G., Dintzis H. M. The immunogenicity of soluble haptenated polymers is determined by molecular mass and hapten valence. J Immunol. 1989 Aug 15;143(4):1239–1244. [PubMed] [Google Scholar]
- Ebong W. W. Acute osteomyelitis in Nigerians with sickle cell disease. Ann Rheum Dis. 1986 Nov;45(11):911–915. doi: 10.1136/ard.45.11.911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards E. A., Johnson D. P., Pierce W. E., Peckinpaugh R. O. Reactions and serologic responses to monovalent acetone-inactivated typhoid vaccine and heat-killed TAB when given by jet injection. Bull World Health Organ. 1974;51(5):501–505. [PMC free article] [PubMed] [Google Scholar]
- Eisenstein T. K., Sultzer B. M. Immunity to Salmonella infection. Adv Exp Med Biol. 1983;162:261–296. doi: 10.1007/978-1-4684-4481-0_26. [DOI] [PubMed] [Google Scholar]
- Elkins K., Metcalf E. S. Monoclonal antibodies demonstrate multiple epitopes on the O antigens of Salmonella typhimurium LPS. J Immunol. 1984 Oct;133(4):2255–2260. [PubMed] [Google Scholar]
- Hargrett-Bean N. T., Pavia A. T., Tauxe R. V. Salmonella isolates from humans in the United States, 1984-1986. MMWR CDC Surveill Summ. 1988 Jun;37(2):25–31. [PubMed] [Google Scholar]
- Hormaeche C. E., Joysey H. S., Desilva L., Izhar M., Stocker B. A. Immunity conferred by Aro- Salmonella live vaccines. Microb Pathog. 1991 Feb;10(2):149–158. doi: 10.1016/0882-4010(91)90075-l. [DOI] [PubMed] [Google Scholar]
- Jann B., Reske K., Jann K. Heterogeneity of lipopolysaccharides. Analysis of polysaccharide chain lengths by sodium dodecylsulfate-polyacrylamide gel electrophoresis. Eur J Biochem. 1975 Dec 1;60(1):239–246. doi: 10.1111/j.1432-1033.1975.tb20996.x. [DOI] [PubMed] [Google Scholar]
- Johnson K. G., Perry M. B. Improved techniques for the preparation of bacterial lipopolysaccharides. Can J Microbiol. 1976 Jan;22(1):29–34. doi: 10.1139/m76-004. [DOI] [PubMed] [Google Scholar]
- Kohn J., Wilchek M. Procedures for the analysis of cyanogen bromide-activated Sepharose or Sephadex by quantitative determination of cyanate esters and imidocarbonates. Anal Biochem. 1981 Aug;115(2):375–382. doi: 10.1016/0003-2697(81)90020-8. [DOI] [PubMed] [Google Scholar]
- Lepage P., Bogaerts J., Van Goethem C., Ntahorutaba M., Nsengumuremyi F., Hitimana D. G., Vandepitte J., Butzler J. P., Levy J. Community-acquired bacteraemia in African children. Lancet. 1987 Jun 27;1(8548):1458–1461. doi: 10.1016/s0140-6736(87)92207-0. [DOI] [PubMed] [Google Scholar]
- Levine W. C., Buehler J. W., Bean N. H., Tauxe R. V. Epidemiology of nontyphoidal Salmonella bacteremia during the human immunodeficiency virus epidemic. J Infect Dis. 1991 Jul;164(1):81–87. doi: 10.1093/infdis/164.1.81. [DOI] [PubMed] [Google Scholar]
- Nesbitt A., Mirza N. B. Salmonella septicaemias in Kenyan children. J Trop Pediatr. 1989 Feb;35(1):35–39. doi: 10.1093/tropej/35.1.35. [DOI] [PubMed] [Google Scholar]
- Phillips I., Wharton B. Acute bacterial infection in kwashiorkor and marasmus. Br Med J. 1968 Feb 17;1(5589):407–409. doi: 10.1136/bmj.1.5589.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porro M., Costantino P., Viti S., Vannozzi F., Naggi A., Torri G. Specific antibodies to diphtheria toxin and type 6A pneumococcal capsular polysaccharide induced by a model of semi-synthetic glycoconjugate antigen. Mol Immunol. 1985 Aug;22(8):907–919. doi: 10.1016/0161-5890(85)90077-x. [DOI] [PubMed] [Google Scholar]
- Roantree R. J. Salmonella O antigens and virulence. Annu Rev Microbiol. 1967;21:443–466. doi: 10.1146/annurev.mi.21.100167.002303. [DOI] [PubMed] [Google Scholar]
- Robbins J. B., Schneerson R. Polysaccharide-protein conjugates: a new generation of vaccines. J Infect Dis. 1990 May;161(5):821–832. doi: 10.1093/infdis/161.5.821. [DOI] [PubMed] [Google Scholar]
- Saxén H., Mäkelä O., Svenson S. B. Isotype of protective anti-Salmonella antibodies in experimental mouse salmonellosis. Infect Immun. 1984 Jun;44(3):633–636. doi: 10.1128/iai.44.3.633-636.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxén H., Reima I., Mäkelä P. H. Alternative complement pathway activation by Salmonella O polysaccharide as a virulence determinant in the mouse. Microb Pathog. 1987 Jan;2(1):15–28. doi: 10.1016/0882-4010(87)90111-2. [DOI] [PubMed] [Google Scholar]
- Schneerson R., Robbins J. B., Parke J. C., Jr, Bell C., Schlesselman J. J., Sutton A., Wang Z., Schiffman G., Karpas A., Shiloach J. Quantitative and qualitative analyses of serum antibodies elicited in adults by Haemophilus influenzae type b and pneumococcus type 6A capsular polysaccharide-tetanus toxoid conjugates. Infect Immun. 1986 May;52(2):519–528. doi: 10.1128/iai.52.2.519-528.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svenson S. B., Lindberg A. A. Artificial Salmonella vaccines: Salmonella typhimurium O-antigen-specific oligosaccharide-protein conjugates elicit protective antibodies in rabbits and mice. Infect Immun. 1981 May;32(2):490–496. doi: 10.1128/iai.32.2.490-496.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svenson S. B., Nurminen M., Lindberg A. A. Artificial Salmonella vaccines: O-antigenic oligosaccharide-protein conjugates induce protection against infection with Salmonella typhimurium. Infect Immun. 1979 Sep;25(3):863–872. doi: 10.1128/iai.25.3.863-872.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szu S. C., Stone A. L., Robbins J. D., Schneerson R., Robbins J. B. Vi capsular polysaccharide-protein conjugates for prevention of typhoid fever. Preparation, characterization, and immunogenicity in laboratory animals. J Exp Med. 1987 Nov 1;166(5):1510–1524. doi: 10.1084/jem.166.5.1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb D. K., Serjeant G. R. Systemic Salmonella infections in sickle cell anaemia. Ann Trop Paediatr. 1989 Sep;9(3):169–172. doi: 10.1080/02724936.1989.11748623. [DOI] [PubMed] [Google Scholar]
- Zarkowsky H. S., Gallagher D., Gill F. M., Wang W. C., Falletta J. M., Lande W. M., Levy P. S., Verter J. I., Wethers D. Bacteremia in sickle hemoglobinopathies. J Pediatr. 1986 Oct;109(4):579–585. doi: 10.1016/s0022-3476(86)80216-5. [DOI] [PubMed] [Google Scholar]