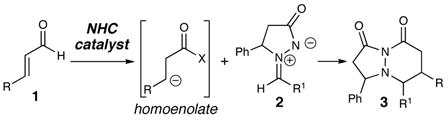
Intermolecular cycloadditions are powerful methods for the convergent synthesis of cyclic compounds from simple precursors.1 While major advances have been made in the area of metal-catalyzed cycloadditions over the past decade,2 there is great potential for these reactions using organic molecules as catalysts.3 In 1968, Dorn and coworkers demonstrated that 3-oxo-pyrazolidin-1-ium-2-ides such as 2 are stable and easily handled compounds.4,5 Fu, Hayashi and Suga have separately shown that these compounds are efficient substrates in metal-catalyzed cycloadditions to furnish five and six-membered heterocycles.6 We have been interested in developing new reactions catalyzed by N-heterocyclic carbenes (NHCs) derived from azolium salts.7 Our recent studies, along with those of Glorius, Bode, and Nair, have shown that the combination of NHCs and α,β-unsaturated aldehydes generate unique homoenolate species.8,9 The use of an organic molecule to catalyze a formal [3 + 3] cycloaddition of azomethine imines has yet to be realized.10 Herein, we report the direct synthesis of pyridazinones (3) by the NHC-catalyzed reaction of aldehyde (1) and azomethine imines (2, eq 1).
(1).
Our proposed pathway for this formal [3 + 3] cycloaddition involves the addition of an NHC to an α,β-unsaturated aldehyde (1) to afford the extended Breslow intermediate (I) after addition and rearrangement (Scheme 1). The homoenolate intermediate undergoes addition to the azomethine imine (2) and subsequently generates enol II. After tautomerization of II, the resulting activated heteroazolium species III releases the NHC catalyst and affords the pyridazinone (3) by an intramolecular acylation.
Scheme 1.
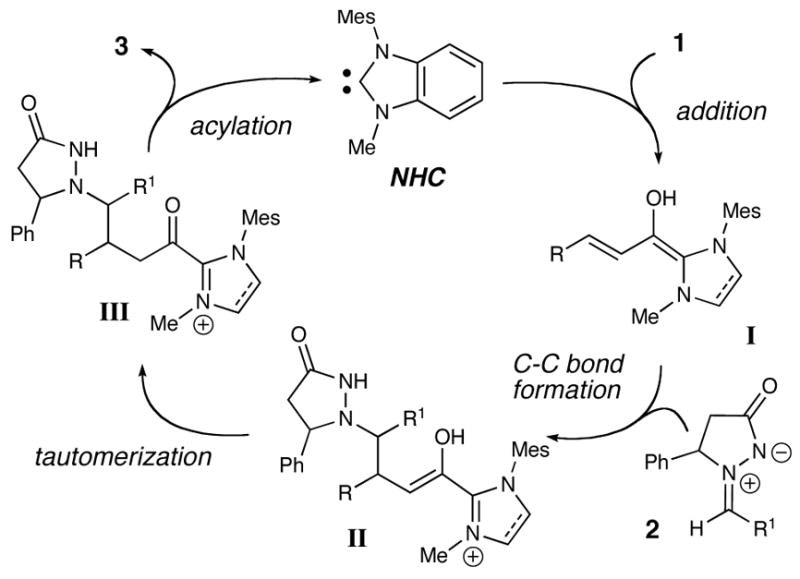
Proposed Catalytic Pathway
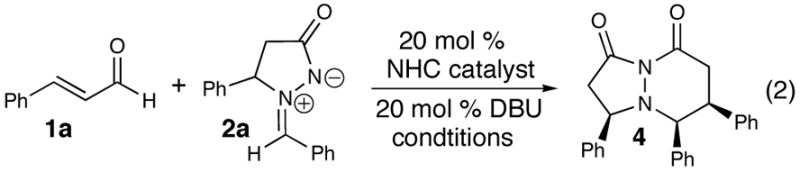
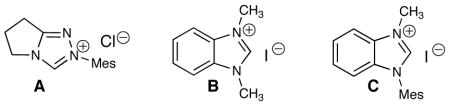
A challenge with NHC catalysis is the presence of two separate electrophiles during a reaction. A successful process must allow for selective interaction between the carbene and the α,β-unsaturated aldehyde. Irreversible addition of the carbene to the secondary electrophile (e.g. azomethine imine) would result in no reaction. Our studies began with cinnamaldehyde (1a), azomethine imine 2a, and heteroazolium salts A–C (Table 1, entries 1–3). To our gratification, all three catalysts derived from heteroazolium salts A–C produced the desired product 4 as a single diastereomer.11 While benzimidazolium salt C afforded the highest yield (39%, entry 3), the process clearly required improvement. Reactions in THF required heating to induce homogeneity (entries 4–5), but did not improve yields compared to CH2Cl2. Carefully monitoring the reaction revealed that shorter times (3 h vs. 24 h) significantly improves the yield of 4 (entries 6–7). Additionally, increasing the temperature of the reaction to 40 °C favors the [3 + 3] cycloaddition manifold with a further increase in yield (79%, entry 8). With these temperature parameters identified, an extensive re-examination of different azolium salts confirmed that the placement of a single N-mesityl substituent on the benzimidazole core is necessary for good yields. Decreasing the catalyst loading of C decreases the yield of 4 to 46% (entry 9).
Table 1.
Optimization of Conditionsa
 | ||||
|---|---|---|---|---|
| entry | catalyst | conditions | yield (%)b | d.r.c |
| 1 | A | CH2CI2, 23 °C, 24 h | 35 | >20:1 |
| 2 | B | CH2CI2, 23 °C, 24 h | 29 | >20:1 |
| 3 | C | CH2CI2, 23 °C, 24 h | 39 | >20:1 |
| 4 | C | THF, 50 °C, 24 h | 15 | >20:1 |
| 5 | C | 10:1 THF/tBuOH, 50 °C, 24h | 23 | >20:1 |
| 6 | C | CH2CI2, 23 °C, 3 h | 45 | >20:1 |
| 7 | C | 10:1 THF/tBuOH, 50 °C, 3h | 59 | >20:1 |
| 8 | C | CH2CI2, 40 °C, 2 h | 79 | >20:1 |
| 9d | C | CH2CI2, 40 °C, 2 h | 46 | >20:1 |
2 equiv. 1a and 1 equiv. 2a.
Isolated yield after purification.
As determined by 1H NMR spectroscopy.
10 mol % C, 10 mol % DBU.
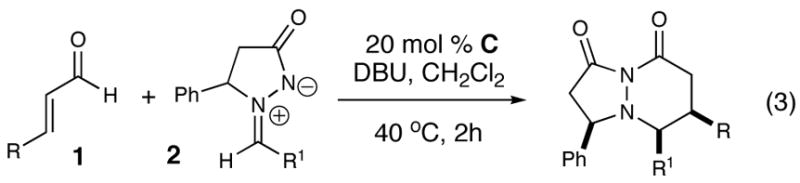
With the optimal parameters established for this formal [3 + 3] cycloaddition process, we turned out attention to investigate the scope of this reaction (Table 2, eq 3). A survey of the α,β-unsaturated aldehyde reveals the process accommodates electron donating groups on the aryl ring (entries 1–5), but electron withdrawing groups (entry 8) do not yield products. The reaction also tolerates β-alkyl substituents and extended dienylic systems to afford 9 and 10 in moderate yields (entries 6–7). An examination of the azomethine imine component indicates that variously substituted aryl groups are competent substrates. Electron-withdrawing groups on the aryl ring of the imine afford good to excellent yields of the pyridazinones (entries 9–12). Placing an electron-donating group on the aryl ring is also a suitable partner (entry 14), albeit in reduced yield (67%). While enolizable or 2-substituted aryl substituents at R1 of 2 do not afford any pyridazinones, all productive reactions are highly diastereoselective (>20:1 dr) favoring the all syn stereoisomers.
Table 2.
Substrate Scopea
 | |||||
|---|---|---|---|---|---|
| entry | R | R1 | product | yield (%)b | d.r.c |
| 1 | Ph (1a) | Ph (2a) | 4 | 79 | >20:1 |
| 2 | 4-OMePh(1b) | Ph (2a) | 5 | 76 | >20:1 |
| 3 | 3-OMePh(1c) | Ph (2a) | 6 | 79 | >20:1 |
| 4 | 2-OMePh (1d) | Ph (2a) | 7 | 94 | >20:1 |
| 5 | 2-naphthyl (1e) | Ph (2a) | 8 | 77 | >20:1 |
| 6 | CH2CH2CH3(1f) | Ph (2a) | 9 | 67 | >20:1 |
| 7 | HC=CHCH3(1g) | Ph (2a) | 10 | 51 | >20:1 |
| 8 | 4-CIPh(1h) | Ph (2a) | 11 | 0 | - |
| 9 | Ph (1a) | 4-BrPh (2b) | 12 | 87 | >20:1 |
| 10 | Ph (1a) | 4-FPh (2c) | 13 | 82 | >20:1 |
| 11 | Ph (1a) | 3-CF3Ph (2d) | 14 | 93 | >20:1 |
| 12 | Ph (1a) | 3-BrPh (2e) | 15 | 78 | >20:1 |
| 13 | Ph (1a) | 3-CH3Ph (2f) | 16 | 76 | >20:1 |
| 14 | Ph (1a) | 3-OMePh (2g) | 17 | 67 | >20:1 |
| 15 | Ph (1a) | cyclohexyl (2h) | 18 | 0 | - |
2 equiv. 1 and 1 equiv. 2,0.25 M.
Isolated yield after purification.
Determined by 500 MHz
1H NMR spectroscopy. Relative stereochemistry of 16 determined by X-ray crystallography and further assigned by analogy. See Supporting Information for details.
The current model for the high levels of syn diastereoselectivity for the products invokes a hydrogen bonding assembly (IV) between the imine and the carbene-aldehyde adduct. The catalyst structure enforces an extended geometry of the carbene-aldehyde adduct (I, as the Z(O) enol) and this nucleophilic intermediate approaches away from the phenyl substituent on the azomethine ring.12 Our initial investigations of the reactivity of the pyridazinone compounds have determined that substituted esters and amides (e.g. 19 and 20) can be accessed in excellent yields by a highly selective ring opening upon addition of methanol or benzyl amine to a solution of the pyridazinone (4).13
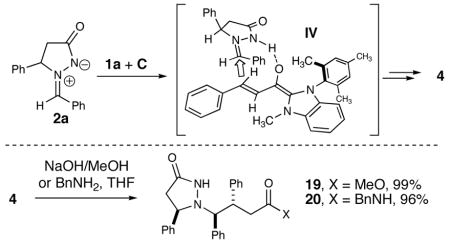
In summary, we have developed the first formal [3 + 3] cycloaddition reaction catalyzed by N-heterocyclic carbenes. The addition of an N-mesityl benzimidazolyl carbene to an α,β-unsaturated aldehyde generates a homoenolate intermediate that undergoes an addition/acylation sequence with an azomethine imine to afford new bicyclic heterocycles with excellent diastereoselectivity. The pyridazinone products can be manipulated to provide esters or amides in excellent yields upon addition of alcohols or amines. Further studies generating nucleophiles with unique properties using N-heterocyclic carbene catalysis are ongoing.
Supplementary Material
Experimental procedures and spectral data for all new compounds (PDF). This material is available free of charge via the Internet at http://pubs.acs.org.
Acknowledgments
Research support was generously provided by NIGMS (RO1 GM73072), Abbott Laboratories, Amgen, 3M, and Boerhinger-Ingelheim. A. C. is the recipient of a Dow Chemical Company Fellowship. We thank FMCLithium, Sigma-Aldrich, and BASF for providing reagents used in this research, Troy Reynolds (NU) for assistance with X-ray crystallography, and Prof. Regan Thomson (NU) for helpful discussions. Funding for the NU Analytical Services Laboratory has been furnished in part by the NSF (CHE-9871268).
References
- 1.For reviews of intermolecular cycloadditions, see: Gothelf KV, Jorgensen KA. Chem Rev. 1998;98:863–909. doi: 10.1021/cr970324e.Pearson WH, Stoy P. Synlett. 2003:903–921.
- 2.For recent examples, see: Gothelf AS, Gothelf KV, Hazell RG, Jorgensen KA. Angew Chem, Int Ed Engl. 2002;41:4236–4238. doi: 10.1002/1521-3773(20021115)41:22<4236::AID-ANIE4236>3.0.CO;2-W.Kano T, Hashimoto T, Maraoka K. J Am Chem Soc. 2005;127:11926–11927. doi: 10.1021/ja0523284.Shirakawa S, Lombardi PJ, Leighton JL. J Am Chem Soc. 2005;127:9974–9975. doi: 10.1021/ja052307+.Cabrera S, Arrayas RG, Carretero JC. J Am Chem Soc. 2005;127:16394–16395. doi: 10.1021/ja0552186.Sibi MP, Stanley LM, Jasperse CP. J Am Chem Soc. 2005;127:8276–8277. doi: 10.1021/ja051650b.Kano T, Hashimoto T, Maraoka K. J Am Chem Soc. 2006;128:2174–2175. doi: 10.1021/ja056851u.
- 3.Northrup AB, MacMillan DWC. J Am Chem Soc. 2002;124:2458–2460. doi: 10.1021/ja017641u.Jen WS, Wiener JJM, MacMillan DWC. J Am Chem Soc. 2000;122:9874–9875.Karlsson S, Hogberg HE. Tetrahedron: Asymmetry. 2002;13:923–926.Chen W, Yuan X-H, Li R, Du W, Wu Y, Ding L-S, Chen Y-C. Adv Synth Catal. 2006;348:1818–1822.For a review, see: Dalko PI, Moisan L. Angew Chem, Int Ed. 2004;43:5138–5175. doi: 10.1002/anie.200400650.
- 4.(a) Dorn H, Otto A. Chem Ber. 1968;101:3287–3301. doi: 10.1002/cber.19681010934. [DOI] [PubMed] [Google Scholar]; (b) Dorn H, Otto A. Angew Chem, Int Ed Engl. 1968;7:214–215. [Google Scholar]
- 5.For a review, see: Schantl JG. Sci Synth. 2004;27:731–824.
- 6.(a) Shintani R, Fu GC. J Am Chem Soc. 2003;125:10778–10779. doi: 10.1021/ja036922u. [DOI] [PubMed] [Google Scholar]; (b) Suarez A, Downey CW, Fu GC. J Am Chem Soc. 2005;127:11244–11245. doi: 10.1021/ja052876h. [DOI] [PubMed] [Google Scholar]; (c) Shintani R, Hayashi T. J Am Chem Soc. 2006;128:6330–6331. doi: 10.1021/ja061662c. [DOI] [PubMed] [Google Scholar]; (d) Suga H, Funyu A, Kakehi A. Org Lett. 2007;9:97–100. doi: 10.1021/ol062675y. [DOI] [PubMed] [Google Scholar]
- 7.(a) Mattson AE, Bharadwaj AR, Scheidt KA. J Am Chem Soc. 2004;126:2314–2315. doi: 10.1021/ja0318380. [DOI] [PubMed] [Google Scholar]; (b) Mattson AE, Scheidt KA. Org Lett. 2004;6:4363–4366. doi: 10.1021/ol0481129. [DOI] [PubMed] [Google Scholar]; (c) Bharadwaj AR, Scheidt KA. Org Lett. 2004;6:2465–2468. doi: 10.1021/ol049044t. [DOI] [PubMed] [Google Scholar]; (d) Myers MC, Bharadwaj AR, Milgram BC, Scheidt KA. J Am Chem Soc. 2005;127:14675–14680. doi: 10.1021/ja0520161. [DOI] [PubMed] [Google Scholar]; (e) Chan A, Scheidt KA. J Am Chem Soc. 2006;128:4558–4559. doi: 10.1021/ja060833a. [DOI] [PubMed] [Google Scholar]; (f) Mattson AE, Bharadwaj AR, Zuhl AM, Scheidt KA. J Org Chem. 2006;71:5715–5724. doi: 10.1021/jo060699c. [DOI] [PubMed] [Google Scholar]; (g) Maki BE, Chan A, Phillips EM, Scheidt KA. Org Lett. 2007;9:371–374. doi: 10.1021/ol062940f. [DOI] [PubMed] [Google Scholar]
- 8.For early homoenolate studies, see: Nickhorn A, Lambert JL. J Am Chem Soc. 1964;84:4604–4605.Freeman JP, Plonka JH. J Am Chem Soc. 1966;88:3662–3663.
- 9.(a) Burstein C, Glorius F. Angew Chem, Int Ed. 2004;43:6205–6208. doi: 10.1002/anie.200461572. [DOI] [PubMed] [Google Scholar]; (b) Sohn SS, Rosen EL, Bode JW. J Am Chem Soc. 2004;126:14370–14371. doi: 10.1021/ja044714b. [DOI] [PubMed] [Google Scholar]; (c) Chan A, Scheidt KA. Org Lett. 2005;7:905–908. doi: 10.1021/ol050100f. [DOI] [PubMed] [Google Scholar]; (d) Sohn SS, Bode JW. Org Lett. 2005;7:3873–3876. doi: 10.1021/ol051269w. [DOI] [PubMed] [Google Scholar]; (e) Nair V, Vellalath S, Poonoth M, Mohan R, Suresh E. Org Lett. 2006;8:507–509. doi: 10.1021/ol052926n. [DOI] [PubMed] [Google Scholar]; (f) Nair V, Vellalath S, Poonoth M, Suresh E. J Am Chem Soc. 2006;128:8736–8737. doi: 10.1021/ja0625677. [DOI] [PubMed] [Google Scholar]; (g) Burstein C, Tschan S, Xie XL, Glorius F. Synthesis. 2006:2418–2439. [Google Scholar]
- 10.For recent formal [3 + 3] cycloadditions, see: Hedley SJ, Moran WJ, Price DA, Harrity JPA. J Org Chem. 2003;68:4286–4292. doi: 10.1021/jo030002c.Ganton MD, Kerr MA. J Org Chem. 2004;69:8554–8557. doi: 10.1021/jo048768f.Hsung RP, Kurdyumov AV, Sydorenko N. Eur J Org Chem. 2004:23–44.Provoost OY, Hedley SJ, Hazelwood AJ, Harrity JPA. Tetrahedron Lett. 2006;47:331–333.Kurdyumov AV, Lin N, Hsung RP, Gullickson GC, Cole KP, Sydorenko N, Swidorski JJ. Org Lett. 2006;8:191–193. doi: 10.1021/ol0523042.Pattenden LC, Wybrow RAJ, Smith SA, Harrity JPA. Org Lett. 2006;8:3089–3091. doi: 10.1021/ol0610789. and references cited therein.
- 11.Azomethine imines lacking the phenyl substituent on the ring afford products, but in reduced yields.
- 12.The Z(O) enol isomer of I in IV minimizes interactions between the N-mesityl group and the imine phenyl ring.
- 13.Laurent E, Lee SK, Pellissier N. J Chem Res Miniprint. 1978:5201–5218. Additional studies on the synthetic utility of these unusual heterocycles are ongoing. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Experimental procedures and spectral data for all new compounds (PDF). This material is available free of charge via the Internet at http://pubs.acs.org.


