Abstract
Immunofluorescence was used to study immunoreactivity (IR) for corticotropin releasing factor (CRF) in the guinea-pig enteric nervous system. CRF-IR was expressed in both the myenteric and submucosal plexuses of all regions of the large and small intestine and the myenteric plexus of the stomach. CRF-IR-nerve fibers were present in the myenteric and submucosal plexuses, in the circular muscle coat and surrounding submucosal arterioles. Most of the CRF-IR fibers persisted in the myenteric and submucosal plexuses after 7 days in organotypic culture. CRF-IR was not co-expressed with tyrosine hydroxylase-IR or calcitonin gene-related peptide-IR fibers. The proportions of CRF-IR cell bodies in the myenteric plexus increased progressively from the stomach (0.6%) to the distal colon (2.8%). Most of the CRF-IR myenteric neurons (95%) had uniaxonal morphology; the remainder had Dogiel type II multipolar morphology. CRF-IR cell bodies in the myenteric plexus of the ileum expressed IR for choline acetyltransferase (56.9%), substance P (55.0%), and nitric oxide synthase (37.9%). CRF-IR never co-localized with IR for calbindin, calretinin, neuropeptide Y, serotonin or somatostatin in the myenteric plexus. CRF-IR cell bodies were more abundant in the submucosal plexus (29.9-38.0%) than in the myenteric plexus. All CRF-IR neurons in submucosal ganglia expressed vasoactive intestinal peptide-IR and were likely to be secretomotor/vasodilator neurons. CRF-IR neurons did not express IR for the CRF1 receptor. CRF1-IR was expressed in neuronal neighbors of those with CRF-IR. Collective evidence suggests that VIPergic secretomotor neurons might provide synaptic input to neighboring cholinergic neurons.
Keywords: gastrointestinal tract, myenteric plexus, submucosal plexus, stress
Clinical and experimental evidence suggests that stress is associated with the onset, symptom exacerbation and reactivation of gastrointestinal disorders, which include the irritable bowel syndrome, ulcerative colitis, Crohn’s disease, gastroesophageal reflux disease and gastric ulcer (Duffy et al., 1991; Greene et al., 1994; Levenstein et al., 1994; Mayer, 2000; Peck and Wood, 2000; Wood et al., 2000; Wood, 2002; Drossman, 2004). Exposure of animals to stress causes erosions and ulceration in the gastric mucosa and inhibits gastric emptying coincident with stimulation of colonic motility, colonic transit, fecal pellet expulsion, mucosal electrolyte secretion and opening of the mucosal barrier to antigenic molecules (Fone et al., 1990; Taché, 1999; 2001; Maillot et al., 2000; Martinez et al., 20002; Million et al., 2002; 2003; Saunders et al., 2002a,b). Moreover, environmental stress factors initiate ulcerative colitis-like disease that is associated with colon cancer in primates (Peck and Wood, 2000; Wood et al., 2000). The mechanistic details of these stress-induced gastrointestinal functional abnormalities are poorly understood. Nevertheless, the available evidence points to corticotropin releasing factor (CRF) signaling pathways in the brain and the gut as significant factors in the stress-evoked gastrointestinal disorders, including changes in gastrointestinal motor and epithelial functions (Taché et al., 2004; Taché and Perdue, 2004).
Results obtained following injection of CRF, CRF-related ligands or CRF receptor antagonists strongly implicate CRF signaling in the brain as a significant factor in stress-related alterations in gastric and colonic physiology. Intracerebroventricular (ICV) injection of CRF inhibits gastric motility and emptying of solid or liquid meals in rats, mice and dogs (Martinez et al., 1997; 2002; 2004; Tache et al., 2001; 2004). The non-selective CRF receptor antagonists α-helical CRF (9-41), astressin or the selective CRF2 receptor antagonist astressin2-B, blocks CRF and stress induced inhibition of gastric transit when injected ICV. On the other hand, ICV injection of the CRF1 receptor antagonist NBI-35965 does not suppress stress-induced changes in gastric emptying (Martinez et al., 1997; 2002; 2004; Chen et al., 2002). This implicates involvement of the CRF2 subtype as the receptor in the brain involved in CRF-evoked inhibition of gastric motility.
Effects of stress on colonic motor function differ significantly from the effects on gastric motility. Water avoidance or cold-restraint stress stimulates colonic motility, which is reflected as accelerated transit and defecation in animal models (Tache et al., 2001). Administration of CRF or urocortin by the ICV route mimics the effects of stress on the colon (William et al., 1987; Lenz et al., 1988; Monnikes et al., 1992; Martinez et al., 1997; 2002; 2004). Central administration of the non-selective CRF receptor antagonist α-helical CRF (9-41), astressin or the selective CRF1 receptor antagonist NBI-35965 suppresses the effects of both stress and central administration of CRF or urocortin on colonic motility. The CRF2 receptor antagonist astress2-B does not attenuate stress responses or centrally mediated actions of CRF or urocortin (Martinez et al., 1997; 2002; 2004; Million et al., 2003). Most of the pharmacological evidence suggests that both the effects of stress and ICV administration of CRF on colonic motility involve stimulation of the CRF1 receptor subtype in the involved pathways in the brain.
Pharmacological evidence suggests that expression of CRF and its receptors in the enteric nervous system (ENS) might have importance for stress related alterations of gastrointestinal physiology equivalent to that of CRF in the brain. Intraperitoneal or intravenous administration of CRF or urocortin inhibits gastric emptying and stimulates colonic motility and transit in the rat and mimics the changes induced by stress or central injection of CRF (Pappas et al., 1985; Nozu et al., 1999; Maillot et al., 2000; Wang et al., 2001; Martinez et al., 2002; Miampamba et al., 2002; Million et al., 2002; Tache and Perdue, 2004). These effects of peripheral administration likely reflect direct actions within the gastrointestinal tract because peripherally administered CRF does not enter the brain (Martins et al., 1996). Furthermore, the effects of peripheral administration of CRF are blocked by CRF receptor antagonists that do not readily cross the blood-brain barrier (Martinez et al., 1997; 2002; Maillot et al., 2000; Wang et al., 2001). Gastric responses to peripherally administered CRF or urocortin are blocked by intravenous injection of the CRF2 receptor antagonist astressin2-B or antisuvagine-30, but not by the CRF1 receptor antagonist CP-154,526 (Wang et al., 2001; Million et al., 2002; Martinez et al., 2002). Colonic motor responses to peripherally administered CRF were blocked by the CRF1 receptor antagonist CP-154,526 and NBI 35965, but not by any of the CRF2 antagonists (Million et al., 2002; 2003; Martinez et al., 2002). Peripheral administrations of urocortin 2 or urocortin 3, both of which are selective agonists for the CRF2 receptor, inhibit gastric emptying without alteration of colonic transit (Million et al., 2002; Martinez et al., 2002). The evidence suggests that delayed gastric emptying evoked by peripherally administered CRF is mediated by activation of the CRF2 receptors and that stimulation of colonic transit is mediated by the activation of the CRF1 receptor.
Apart from its effects on gastrointestinal motility, stress also alters intestinal epithelial functions. Cold restraint, water-avoidance or immobilization stress stimulates intestinal mucosal secretion of water, electrolytes and mucus (Saunders et al., 1994; 1997; 2002a,b; Castagliuolo et al., 1996; Santos et al., 1999). Fluxes of proinflammatory bacterial peptides and large molecules (e.g., mannitol, horseradish peroxidase or EDTA) from lumen to lamina propria are significantly enhanced in mucosal preparations from stressed animals and this suggests breakdown in intestinal barrier function (Saunders et al., 1994; 1997; 2002b; Kiliaan et al., 1998; Santos et al., 1999). These kinds of mucosal changes are reproduced by intraperitoneal injections of CRF (Saunders et al., 1994; 1997; 2002b; Castagliuolo et al., 1996; Kiliaan et al., 1998; Santos et al., 1999). Both stress and CRF-evoked changes in epithelial physiology are suppressed by the CRF receptor antagonist, α-helical CRF (9-41), when it is injected intraperitoneally. Intravenous injection of CRF evokes watery diarrhea with a rapid onset and in a dose-related manner in conscious rats (Saunders et al., 2002a). This diarrheogenic action of CRF, which is likely to be mediated by excitation of secretomotor neurons in the submucosal plexus, is mediated by the CRF1 receptor subtype (Saunders et al., 2002a).
Most of the evidence suggests that the ENS is the site where the peripheral actions of CRF mediate stress associated changes in gastrointestinal motility and mucosal functions. The following is a summary of the evidence: 1) Application of CRF to segments of guinea-pig small intestine in vitro evokes muscle contractions that are blocked by tetrodotoxin, indicating that the CRF-evoked contractions are mediated by stimulation of excitatory musculomotor neurons (Lazer et al., 2003). 2) Exposure of rat isolated colonic segments to CRF enhances neurally-mediated peristaltic activity and this action is prevented by the CRF-receptor antagonist α-helical-CRF (9-41) (Mancinelli et al., 1998). 3) Application of CRF to the myenteric plexus of guinea-pig small intestine evokes excitatory responses in single neurons recorded with microelectrodes (Hanani and Wood, 1992). 4) Intraperitoneal injection of CRF induces elevated expression of c-fos in colonic myenteric neurons, which is blocked by peripheral application of astressin or selective CRF1 receptor antagonists (Miampamba et al., 2002). 5) RT-PCR detects CRF1 m-RNA receptor transcripts in the myenteric plexus and immunohistochemical analysis reveals the expression of the CRF1 receptor subtype by neurons in both myenteric and submucosal plexuses (Chatzaki et al., 2004; Liu et al., 2005). This in vitro evidence, together with the evidence from whole animal studies, suggests that CRF signaling occurs in the enteric neural networks that organize intestinal motility and secretion.
The neuronal cell types that release CRF in the integrative microcircuits of the ENS are inadequately identified. In the human colon, CRF mRNA can be detected near the bases of the crypts of Lieberkühn, where it might be expressed by enterochromaffin cells (Kawahito et al., 1994). CRF-like immunoreactivity has been described in the stomach, duodenum, liver and pancreas of several animal species (Petrusz et al., 1984; Suda et al., 1984; Kawai et al., 1985). A brief report also described the presence of CRF-IR in the myenteric and submucosal plexuses of rat duodenum (Wolter, 1984). Nevertheless, detailed evaluation of the distribution of CRF-IR nerve fibers and the identities of the types of neurons that express CRF in the ENS is unavailable. Extensive information on the properties of guinea-pig small intestinal myenteric and submucosal neurons facilitates research to identify relations between CRF expression and the neurophysiological functions of specific subpopulations of enteric neurons. In the present study, we used double-label immunohistochemistry to evaluate the chemical coding patterns of the neurons that express CRF-IR in the guinea-pig small intestine and to evaluate the distribution of CRF-IR neurons and nerve fibers along the gastrointestinal tract.
MATERIALS AND METHODS
Tissue Preparation
Adult male guinea-pigs (300 - 400 g) of the albino Hartley strain were euthanized by stunning and exsanguination from the cervical blood vessels, as approved by the Ohio State University Laboratory Animal Care and Use Committee and United State Department of Agriculture inspectors. Segments of the duodenum, jejunum, ileum, colon and stomach were immediately removed and placed in chilled Krebs solution containing (in mM): NaCl, 120.9; KCl, 5.9; MgCl2, 1.2; NaH2PO4, 1.2; NaHCO3, 14.4; CaCl2, 2.5; and glucose, 11.5. The solution also contained 1-μM scopolamine and nifedipine to minimize muscle contraction and permit maximal stretching of the preparations. Stomachs were opened along the lesser and greater curvature. Preparations of equal size were removed along the greater curvature of the corpus and antrum. Intestinal specimens were opened along the mesenteric border. The preparations were stretched tautly and pinned-out flat with mucosa side up to Sylgard® at the bottom of the dish. Preparations, which were not to be treated with colchicine, were immediately fixed in Zamboni’s fixative (4% formaldehyde plus 0.2% picric acid in 0.1 M sodium phosphate buffer, pH 7.0) for 3 hours at room temperature.
Colchicine Treatment
To enhance somal immunostaining for CRF and other neuropeptides, some whole-mount preparations were maintained in a culture medium containing colchicine prior to fixation. For these studies, all utensils and solutions were sterilized either in 75% ethanol or autoclaved. The gut segments were cut along their mesenteric borders and pinned flat with mucosal side up in sterile dishes containing ice-cold sterile Krebs solution. The mucosal layer was carefully peeled away with microdissection forceps. The preparations were incubated in tissue culture medium consisting of Dulbecco’s modified Eagle’s medium (DMEM) to which fetal bovine serum (10%), antibiotic antimycotic solution (1 ml/100ml), glucose (12 mM), and colchicine (40 μM) were added. The preparations were placed in a humidified incubator, which was kept at 37°C and aerated with 95% O2/5% CO2, for 24 hours. The tissue was subsequently rinsed thoroughly with Krebs solution and fixed in Zamboni’s fixative for 3 hours at room temperature.
Organotypic Culture
Myenteric and submucosal plexus preparations were maintained in organotypic culture for 7 days to allow time for the nerve fibers of extrinsic origin to degenerate. Segments of intestine were removed from the animals and opened along the mesenteric border as described above. After repeated washes with sterile Krebs solution, the flat preparations were pinned mucosal side up to Sylgard®-lined Petri dishes. The mucosal layer was carefully peeled away with microdissection forceps. The preparations were incubated with sterile culture medium DMEM supplemented with fetal bovine serum (10%), antibiotic antimycotic solution (1 ml/100ml) and glucose (12 mM). The preparations were placed in a humidified incubator, which was kept at 37°C and aerated with 95% O2/5% CO2, for 7 days with daily change of culture medium. The tissues were rinsed thoroughly with Krebs solution and fixed as described above.
Immunohistochemistry
Following fixation, the preparations were washed three times for 10 min each in dimethylsulphoxide followed by three 10-minute washes in phosphate-buffered saline (PBS; 0.9% NaCl in 0.1 M sodium phosphate buffer, pH 7.0). Whole-mounts of the myenteric and submucosal plexuses were dissected from these segments. To permeabilize the tissue, and to minimize nonspecific binding, the preparations were placed in PBS containing 0.3% Triton X-100, 10% normal donkey serum and 0.1% sodium azide for 30 min. The preparations were then placed in a rabbit polyclonal antibody raised against CRF, diluted in PBS containing 10% normal donkey serum, 0.3% Triton X-100 and 0.1% sodium azide, for 24 hours at room temperature. After removal from the primary antibody, the tissues were rinsed 3 × 10 minutes with PBS and incubated in donkey anti-rabbit IgG conjugated to fluorescein isothiocyanate (FITC) for 1 hour at room temperature. Following a final set of rinses, the preparations were mounted on a micro slide and cover-slipped with VECTASHIELD fluorescence mounting medium, which inhibits photobleaching (Vector Laboratories INC., Burlingame, CA).
The proportions of CRF-IR neurons relative to the total neuronal population in the various gastrointestinal regions were evaluated by double labeling with a mouse monoclonal antibody for human neuronal protein HuC/HuD (anti-Hu) and CRF. Anti-Hu antibody binds specifically to antigens that are present exclusively in neurons and is a useful tool for marking all neurons in the ENS (Lin et al., 2002). The preparations were incubated in a mixture of primary antibodies for both CRF and anti-Hu for 24 hours. After incubation with the primary antibodies, the tissues were washed (3 × 10 minutes) in PBS and incubated at room temperature for 1 hour with a mixture of appropriate secondary antibodies, donkey anti-rabbit IgG conjugated with FITC and donkey anti-mouse IgG conjugated with indocarbocyanin (Cy3). The tissues were then rinsed in PBS and cover-slipped with fluorescence mounting medium.
Double labeling of CRF with other specific neurochemical markers was used to identify the cell types that express CRF IR. The primary antisera for the established neurochemical markers in the ENS used in the current study include: calbindin, calretinin, choline acetyltransferase (ChAT), nitric oxide synthase (NOS), neuropeptide Y (NPY), serotonin (5-HT); somatostatin, substance P and vasoactive intestinal peptide (VIP). Sources and dilutions for the primary antibodies are listed in Table 1. Combination of primary antibodies for CRF and one of the other neurochemical markers was done at the same time to achieve double immunolabeling. Following incubation in the primary antibodies, the preparations received 3 × 10 minutes washes in PBS and were then incubated for 1 hour in the mixture of appropriate secondary antibodies conjugated to the contrasting fluorophores FITC or Cy3. The tissues then received 3 × 10 minutes washes in PBS prior to placement in fluorescence mounting medium.
TABLE 1.
Primary Antibodies Used in the Study
| Antigen | Host | Code | Dilution | Source | Demo of Specificity |
|---|---|---|---|---|---|
| Anti-Hu | Mouse | A21271 | 150 | Mol. Probes | Lin et al., 2002 |
| Calbindin | Mouse | C9848 | 1:3,000 | Sigma | Chard et al., 1995 |
| Calbindin | Mouse | 300 | 1;3,000 | Swant | Reiche et al., 1999 |
| Calbindin | Rabbit | CB38 | 1:3,000 | Swant | Reiche et al., 1999 |
| Calretinin | Goat | AB1550 | 1:2,500 | Chemicon | Liu et al., 1997 |
| CGRP | Mouse | T-1604 | 1:200 | Bachem | Szabat et al., 1994 |
| ChAT | Goat | AB144P | 1:50 | Chemicon | Mann et al., 1999 |
| CRF | Rabbit | PBLrC70 | 1:3,000 | Gift, W.Vale | Vale et al., 1983 |
| CRF | Rabbit | C5348 | 1:100 | Sigma | Staining eliminated by pre- treating antibody with CRF |
| CRFl | Goat | SC-13811 | 1:100 | Santa Cruz | King et al., 1997 |
| NeuN | Mouse | MAB377 | 1:500 | Chemicon | Mullen et al., 1992 |
| NOS | Sheep | AB1529 | 1:500 | Chemicon | Hu et al., 2002 |
| NPY | Sheep | AB1583 | 1:5,000 | Chemicon | Hu et al., 2002 |
| Serotonin | Rat | MAB352 | 1:200 | Chemicon | Hu et al., 2002 |
| Somatostatin | Rat | MAB354 | 1:200 | Chemicon | Kawaguchi & Shindou, 1998 |
| Substance P | Rat | MAB356 | 1:200 | Chemicon | Hu et al., 2002 |
| TH | Sheep | AB 1542 | 1:1,000 | Chemicon | Li et al., 2004 |
| VIP | Sheep | AB1581 | 1:100 | Chemicon | Staining eliminated by pre- treating antibody with synthetic peptide VIP(1-28) |
Anti-Hu, anti-human neuronal protein HuC/HuD; CGRP, calcitonin gene-related peptide; ChAT, choline acetyltransferase; CRF, corticotropin releasing factor; CRF1, corticotropin releasing factor type 1 receptor; NeuN, neuronal nuclear protein; NOS, nitric oxide synthase; NPY, neuropeptide Y; TH, tyrosine hydroxylase; VIP, vasoactive intestinal peptide
Control experiments involved exposure to the secondary antibody without prior exposure to the primary antibody for CRF. In additional control experiments, aliquots of the CRF antibody were pre-absorbed in the presence of 1-μM CRF peptide against which the antibody was raised, or of urocortin peptide, and applied to the myenteric and submucosal preparations. In these preparations, no immunoreactivity was observed with antiserum that had been pre-absorbed with CRF, while immunoreactivity persisted in preparations pre-absorbed with urocortin.
Analysis of Immunohistochemically Labeled Preparations
For quantitative analysis, 30 ganglia from 5 to 8 preparations of a given region, taken from different guinea-pigs, were analyzed. All preparations were examined under a Nikon Eclipse E-600 fluorescence microscope (Nikon Inc., Melville, NY) and analyzed with filter combinations that enabled separate visualization of multiple fluorophores. Digital images were obtained with a SPOT RT cooled CCD digital camera (Diagnostic Instruments, Sterling, Heights, MI), stored on disk, and analyzed with SPOT II software. Contrast in the digital images was sometimes enhanced before either converting to JPEG file interchange format (*.jpg) for electronic transfer or printing as photomicrographs with ink jet printers.
RESULTS
Distribution of CRF-IR Neurons in the Stomach and Intestine
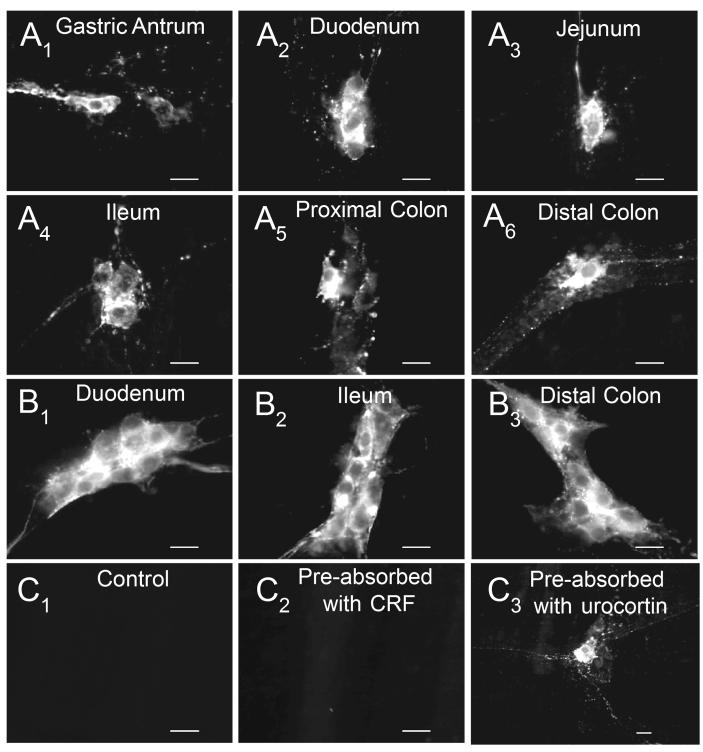
Two different CRF antibodies revealed a similar distribution of CRF-IR in whole-mount preparations from the guinea-pig stomach, duodenum, jejunum and ileum and proximal and distal colon. CRF-IR nerve cell bodies were present in the myenteric and submucosal plexuses of the intestine and the myenteric plexus of the stomach (Fig. 1A,B). Immunoreactive nerve fibers occurred in the myenteric ganglia (Fig. 2A), along interganglionic connectives (Fig. 2A), in the tertiary plexus (Fig. 2B), in the circular muscle layer (Fig. 2C), in the submucosal plexus and around submucosal arteroles (Fig. 2D). Exposure of preparations to secondary antiserum without prior exposure to primary antisera for CRF resulted in no immunostaining (Fig. 1C1). Immunoreactivity for CRF was lost if the antisera was pre-absorbed with CRF (Fig. 1C2), and immunoreactivity persisted with antisera pre-absorbed with urocortin (Fig. 1C3).
Fig. 1.
Distribution of CRF-IR cell bodies in the guinea-pig gastrointestinal tract. (A1-6) Myenteric plexus of the gastric antrum, duodenum, jejunum, ileum, proximal and distal colon. (B1-3) Submucosal plexus of the duodenum, ileum, and distal colon. (C1-3) Controls. (C1) Omission of the primary antibody for CRF. (C2) CRF-IR, antiserum pre-absorbed with CRF. (C3) CRF-IR, antiserum pre-absorbed with urocortin. Scale bars = 20 μm.
Fig. 2.
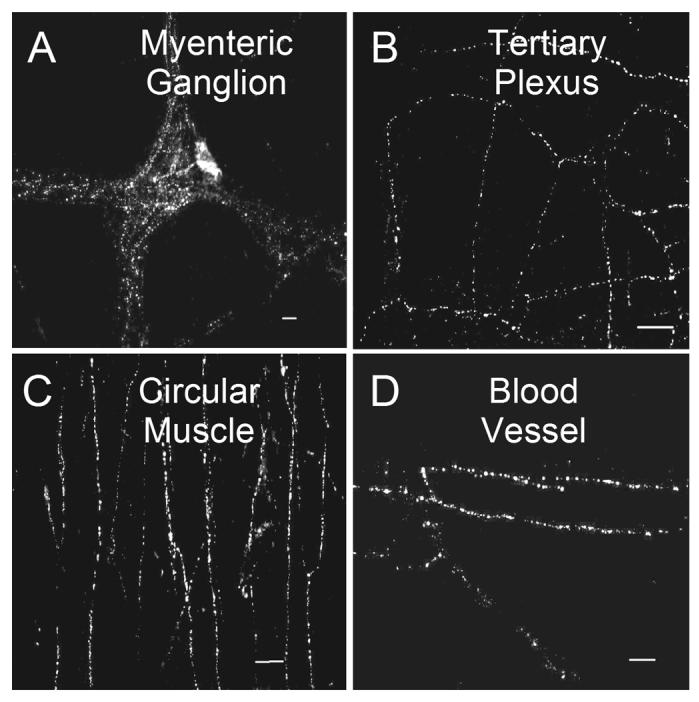
Distribution of CRF-IR nerve fibers in whole-mount myenteric and submucosal plexus preparations. (A) CRF-IR varicose fibers in myenteric ganglia and interganglionic connectives. (B) CRF-IR nerve fibers in the tertiary component of the myenteric plexus. (C) CRF-IR fibers in the deep muscular plexus, with a predominant orientation in the circumferential direction in the circular muscle coat. (D) CRF-IR nerve fibers accompanying small blood vessels in the submucosal plexus. Scale bars = 20 μm.
We examined the distribution of CRF-IR neurons in the myenteric and submucosal plexuses in the various regions quantitatively by counting the neuronal cell bodies with CRF-IR and anti-Hu-IR in 30 ganglia from each region in whole-mount preparations from 5-8 guinea-pigs. Colchicine-treated preparations were used to maximize somatic immunostaining of CRF. Double labeling with CRF and Anti-Hu permitted evaluation of the proportions of CRF-IR neurons relative to the total neuronal populations in each region (Fig. 3). Anti-Hu antibody stains essentially all neurons in the ENS and is a convenient neuronal marker (Lin et al., 2002).
Fig. 3.
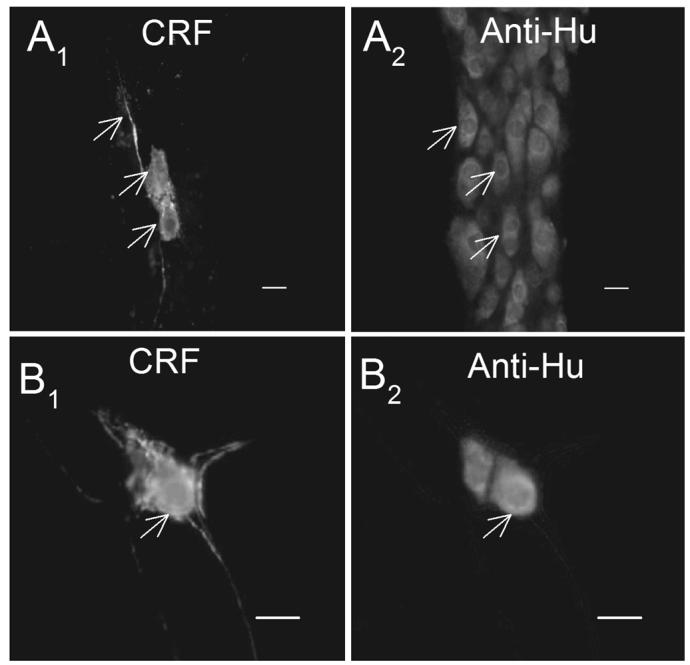
The proportion of neurons expressing CRF IR was determined by double labeling of CRF-IR with anti-Hu-IR. Anti-Hu-IR labeled all neurons and was used to count the total number of neurons. (A1-2) Paired images of myenteric ganglia showing CRF-IR and anti-Hu-IR in whole-mount preparations of guinea-pig ileum. (B1-2) Paired images of submucosal ganglia showing CRF-IR and anti-Hu-IR. Arrows point to CRF-IR neurons. Scale bars = 20 μm.
Myenteric Plexus
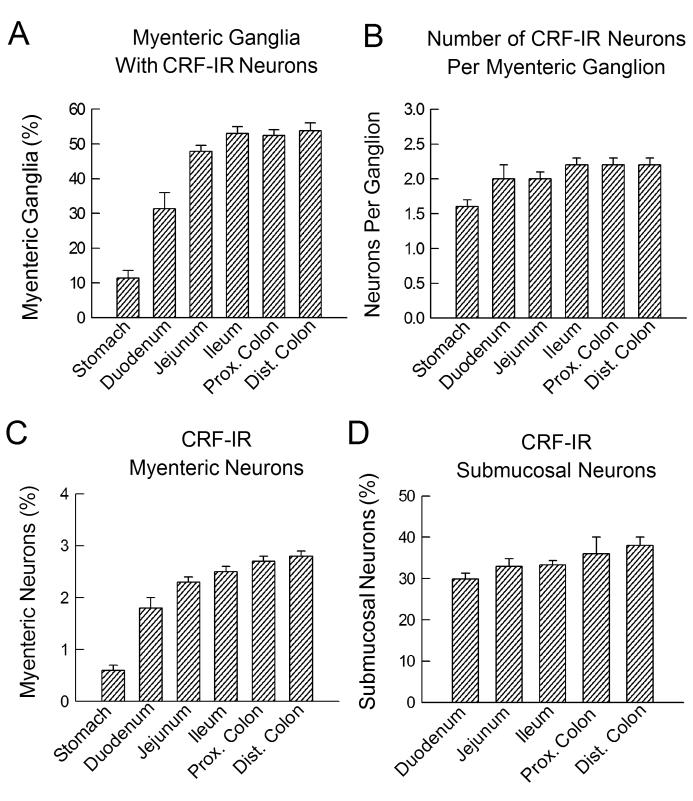
In myenteric plexus preparations, about 50% of the ganglia in the jejunum, ileum, proximal and distal colon contained at least one CRF-IR neuron. A smaller percentage of ganglia in the stomach (11.4%) and duodenum (31.4%) contained CRF-IR neurons (Fig. 4A). A mean of 1.6 neurons per ganglion expressed CRF-IR in the myenteric plexus of the stomach, 2.0 neurons per ganglion in the duodenum and jejunum, and 2.2 neurons per ganglion in the ileum and colon (Fig. 4B). When evaluated as a percentage of the total neuronal population, the proportions of CRF-IR myenteric neurons progressively increased from the stomach (0.6%) to the distal colon (2.8%) (Fig. 4C). The majority of the CRF-IR neurons (95%; 387/409) in the myenteric plexus exhibited Dogiel type I morphology (i.e., an oval or round cell body with a single long process and several short lamellar dendrites). Only a small number of CRF-IR nerve cells (5%; 22 of 409) had Dogiel type II morphology (i.e., a relatively large cell body with two or more long processes) (Fig. 5D). About half of the CRF-IR neurons with Dogiel type I morphology projected their axons in the oral direction, whereas the other half projected their axons in the aboral direction.
Fig. 4.
CRF-IR neurons in the myenteric and submucosal plexuses of the guinea-pig gastrointestinal tract. (A) Data for proportions of myenteric ganglia with at least one CRF-IR neuronal cell body. The bars represent mean for percentages from 5-8 preparations, with 60–170 ganglia counted in each preparation. (B) Numbers of CRF-IR neuronal cell bodies in myenteric ganglia. The bars represent means for 30 ganglia from five preparations in each region. (C) Proportions of neuronal cell bodies with CRF-IR in the myenteric plexus from individual regions of the gastrointestinal tract. The bars represent the means for the percent of the total neuronal population with CRF-IR neuronal cell bodies in 30 ganglia in each of 5 preparations. (D) Proportions of neuronal cell bodies with CRF-IR in the submucosal plexus from individual regions of the intestinal tract. The bars represent the means for the percent of the total neuronal population with CRF-IR neuronal cell bodies in 30 ganglia in each of 5 preparations. Error bars are standard errors of the mean.
Fig. 5.
Co-localization of CRF-IR with chemical codes in the myenteric plexus of the guinea-pig ileum. (A) Co-localization of CRF IR with ChAT-IR. Asterisks are examples of neurons that were co-labeled, thick arrow points to a neuron labeled for ChAT only and the thin arrow points to a neuron labeled for CRF only. (B) Co-localization of CRF-IR with substance P-IR. Asterisks are examples of co-labeled neurons. (C) Co-localization of CRF-IR with NOS-IR. Asterisks are examples of co-labeled neurons, thick arrow points to a neuron labeled for NOS only and the thin arrow points to a neuron labeled for CRF only. (D) Two myenteric ganglion cells with Dogiel type II morphology expressed CRF-IR. None of the CRF-IR neurons expressed calbindin-IR. (E) One myenteric ganglion cell with Dogiel type I morphology expressed CRF-IR and did not express calbindin-IR. (F) CRF-IR did not co-localize with calretinin-IR. (G) CRF-IR did not co-localize with 5-HT-IR. (H) CRF-IR did not co-localize with NPY-IR. (I) CRF-IR did not co-localize with somatostatin-IR. CRF-IR was labeled with fluorescein isothiocyanate (FITC, green) in A-I. Other chemical codes were labeled with indocarbocyanine (Cy3, red). Scale bars = 20 μm.
Submucosal Plexus
The distribution of CRF-IR neurons in the submucosal plexus was evaluated for both the small and large intestine, but not the stomach which is devoid of ganglia in the submucosal plexus. CRF-IR nerve cell bodies in the small and large intestine were more abundant in the submucosal plexus than in the myenteric plexus. Almost all of the submucosal ganglia contained at least one CRF-IR neuron. When evaluated as a percent of the total submucosal neuronal population, similar percentages of CRF-IR neurons were found in the duodenum (29.9%), jejunum (32.9%), ileum (33.3%), proximal colon (36.0%), and distal colon (38.0%) (Fig. 4D). The majority of the CRF-IR neurons were located in the centers of the ganglia. All CRF-IR submucosal neurons expressed Dogiel type I uniaxonal morphology. No CRF-IR was found in Dogiel type II submucosal neurons.
Chemical Coding In the Ileal Myenteric Plexus
Double labeling immunohistochemistry in colchicine-treated preparations was used to identify the chemical codes of subpopulations of neurons, which expressed CRF-IR. The results for the whole-mount preparations appear in Table 2.
TABLE 2.
Distribution of CRF-IR in Relation to Chemical Codes in Myenteric and Submucosal Plexuses of Guinea-Pig Small Intestine
| Region | Chemical Code | Co-Label/Chemical Code | Co-Label/CRF |
|---|---|---|---|
| Myenteric Plexus |
Calbindin | 0% | 0% |
| Calretinin | 0% | 0% | |
| ChAT | 4.5% | 56.9% | |
| CRF1 | 0% | 0% | |
| NOS | 5.0% | 37.9% | |
| NPY | 0% | 0% | |
| Serotonin | 0% | 0% | |
| Substance P | 4.2% | 55.0% | |
| Somatostatin | 0% | 0% | |
| Submucosal Plexus |
Calretinin | 0% | 0% |
| ChAT | 0% | 0% | |
| CRF1 | 0% | 0% | |
| NeuN | 0% | 0% | |
| NPY | 0% | 0% | |
| Substance P | 0% | 0% | |
| VIP | 77.3% | 100% |
ChAT, choline acetyltransferase; CRF1, corticotropin releasing factor type 1 receptor; NeuN, neuronal nuclear protein; NOS, nitric oxide synthase; NPY, neuropeptide Y; VIP, vasoactive intestinal peptide
CRF-IR in the myenteric plexus was detected in 4.5% (202/4448) of ChAT-IR cell bodies, while 57% (202/355) of the CRF-IR neurons expressed ChAT-IR (Fig. 5A). CRF-IR was expressed in 4.2% (208/4953) of the substance P-IR cell bodies, while 55% (208/378) of the CRF-IR neurons expressed substance P-IR (Fig. 5B). Co-localization of CRF-IR and NOS-IR occurred in 5.0% (195/3905) of the NOS-IR myenteric neurons, and 38% (195/515) of the CRF-IR neurons expressed NOS-IR (Fig. 5C). No co-localization of CRF-IR with calbindin-IR, calretinin-IR, NPY-IR, serotonin-IR or somatostatin was detected in the myenteric plexus (Fig. 5D-I).
Chemical Coding In the Ileal Submucosal Plexus
All CRF-IR cell bodies in submucosal ganglia expressed immunoreactivity for VIP (Fig. 6A), which suggests that CRF-IR neurons in the submucosal plexus are exclusively VIPergic secretomotor neurons. The CRF-IR neurons accounted for 77% (1333/1724) of all VIP-IR ganglion cells. Co-localization of CRF-IR with ChAT-IR (Fig. 6B), calretinin-IR (Fig. 6C), NPY-IR (Fig. 6D) or somatostatin-IR (Fig. 6E) was not observed in the submucosal plexus. The absence of co-localization with ChAT-IR suggests that cholinergic secretomotor neurons do not express CRF-IR. Immunoreactivity for neuronal nuclear protein (NeuN), a suggested marker of submucosal Dogiel type II neurons (Poole et al., 2002), was expressed in a population of neurons that did not express CRF-IR. No NeuN-IR neurons showed CRF-IR in 5 submucosal preparations (Fig. 6F). Substance P-IR, which is also a chemical code for submucosal Dogiel type II neurons was not co-expressed with CRF-IR in six submucosal plexus preparations (Fig. 6G).
Fig. 6.
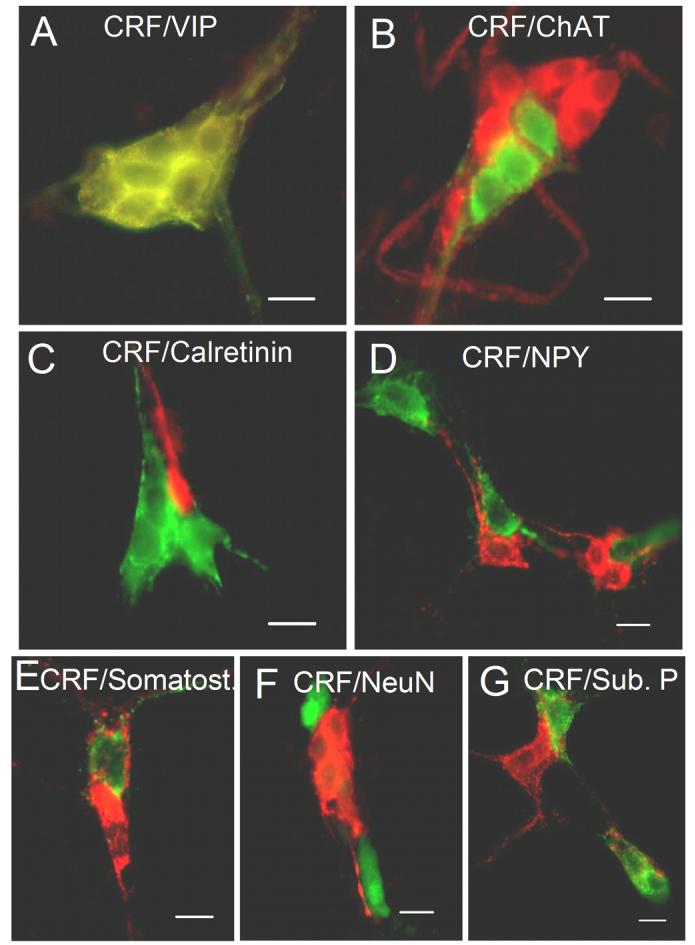
Co-localization of CRF-IR with chemical codes in the submucosal plexus of the guinea-pig ileum. (A) Co-localization of CRF-IR with VIP-IR. In one submucosal ganglion, all neurons that were CRF-IR were also VIP-IR. (B) CRF-IR did not co-localize with ChAT-IR. (C) CRF-IR did not co-localize with calretinin-IR. (D) CRF-IR did not co-localize with NPY-IR. (E) CRF-IR did not co-localize with somatostatin-IR. (F) CRF-IR did not co-localize with neuronal nuclear protein-IR (NeuN). (G) CRF-IR did not co-localize with substance P-IR. CRF was labeled with FITC in A-E and G, and was labeled with Cy3 in F. Other chemical codes were labeled with Cy3 except F where NeuN was labeled with FITC. Scale bars = 20 μm.
Expression of the CRF1 Receptor In Relation To CRF
Expression of the CRF1 receptor was reported recently for both the myenteric and submucosal plexuses of the guinea-pig (Liu et al., 2005). We used double-labeling immunohistochemistry to study the associations of enteric neurons that express CRF-IR with those that express immunoreactivity for the CRF1 receptor (CRF1-IR). CRF1-IR was found in neurons that were neighbors to the neurons with CRF-IR in both the myenteric and submucosal plexuses. However, CRF-IR was never found in neurons that expressed CRF1-IR (Fig. 7), which suggests that one subset of neurons expresses the signal substance while a second subset possesses the receptor for the signal substance.
Fig. 7.
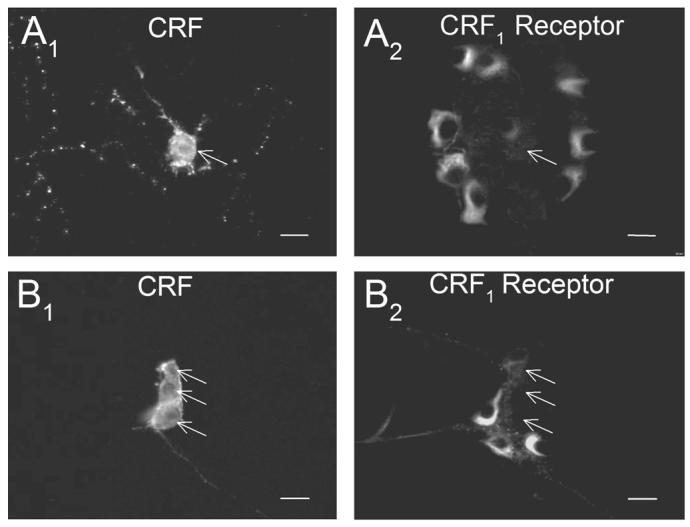
CRF-IR neurons did not co-express CRF1-IR and CRF1-IR neurons did not co-express CRF in the myenteric or submucosal plexuses of the ileum. (A1) CRF-IR in the myenteric plexus. (A2) CRF1-IR in the same microscopic field as A1. (B1) CRF-IR in the submucosal plexus of the ileum. (B2) CRF1-IR in the same microscopic field as B1. Arrows point to the CRF-IR neurons in both sets of paired photomicrographs. Scale bars = 20 μm.
Origins of CRF-IR Nerve Fibers
CRF-IR nerve fibers were apparent throughout the myenteric and submucosal plexuses in all regions of the gut. To test the hypothesis that CRF-IR nerve fibers were intrinsic to the gut, both the myenteric and submucosal plexus preparations were maintained in organotypic culture for 7 days to allow degeneration of extrinsic nerve fibers. Seven days in culture was assumed to be sufficient time for degeneration of the extrinsic sympathetic and sensory nerve fibers in the preparations, based on reports by Mawe and Kennedy (1999) and Hu et al. (2002). Most of the CRF-IR nerve fibers remained in the myenteric and submucosal plexuses after 7 days in culture (Fig. 8A). Moreover, CRF-IR nerve fibers did not overlap with fibers that expressed tyrosine hydroxylase-IR (Fig. 8B) or calcitonin gene-related peptide-IR (Fig. 8C). Fibers that were immunoreactive for tyrosine hydroxylase were assumed to be postganglionic sympathetic nerve fibers that entered the intestine from prevertebral sympathetic ganglia. The nerve fibers that were immunoreactive for calcitonin gene-related peptide and substance P were assumed to be sensory afferent fibers that entered the intestine from dorsal root spinal ganglia.
Fig. 8.
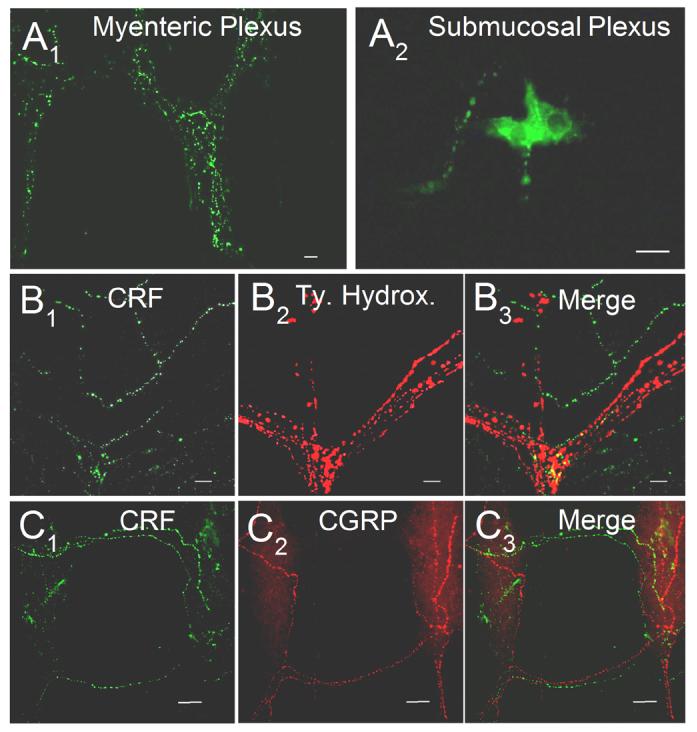
Origin of CRF-IR nerve fibers in the guinea-pig ENS. (A1-2) Seven days in organotypic culture had no effect on the distribution of CRF-IR nerve fibers in either the myenteric (A1) or submucosal (A2) plexus. (B1-3) Double labeling for CRF IR and tyrosine hydroxylase IR, a marker for extrinsic sympathetic nerve fibers, revealed that CRF IR was not co-localized with tyrosine hydroxylase IR in guinea-pig myenteric plexus. (C1-3) Double labeling for CRF IR and CGRP IR, a marker for extrinsic sensory nerve fibers, revealed that CRF IR was not co-localized with CGRP IR in guinea-pig myenteric plexus. Scale bars = 20 μm.
DISCUSSION
The present study compliments the earlier findings of others that intraperitoneal administration of CRF evokes stress-like responses in the gastrointestinal tract, which include: 1) inhibition of gastric emptying (Asakawa et al., 1999; Martinez et al., 1999; Nozu et al., 1999; Wang et al., 2001); 2) inhibition of small intestinal transit (Williams et al., 1987); 3) stimulation of colonic transit and fecal pellet expulsion (Williams et al., 1987; Maillot et al., 2000; Martinez et al., 2002); 4) stimulation of ion secretion in the colon (Santos et al., 1999). The earlier work suggests that these effects of peripherally administered CRF at the organ system level might reflect actions in the ENS. This suggestion is supported by findings that peripherally administered CRF stimulates c-fos expression in enteric neurons (Miampamba et al., 2002).
Aside from the neurally-mediated actions found at the organ level, details of the neurobiology of CRF signaling in the ENS were unclear when we initiated the present study. An earlier suggestion of a signaling mechanism emerged from reports that CRF, which does not enter the brain from the blood, is actively transported out of the brain into the systemic circulation (Martins et al., 1996; 1997). This suggested that CRF, which is released in the brain during stress, might enter and be carried by the blood to the gastrointestinal tract where it might produce its actions by binding to CRF receptors on enteric neurons. Nevertheless, other lines of evidence suggest that CRF is expressed in organs outside the brain and might be released to act locally. CRF-IR was reported to be expressed in the digestive system (Petrusz et al., 1984; Suda et al., 1984; Wolter, 1984; Kawai et al., 1985; Kawahito et al., 1994), in endocrine organs (Petrusz et al., 1983; Suda et al., 1984), and by immune/inflammatory cells (Karalis et al., 1997).
We found that CRF-IR neurons did not express IR for the CRF1 receptor subtype, which is the functional receptor for CRF in the ENS (Liu et al, 2005). CRF1-IR was expressed in subgroups of neurons, which expressed chemical codes that were different from the codes for CRF-IR neurons. This was the case for both the myenteric and submucosal plexuses.
Neurons, which were identified in the submucosal plexus as uniaxonal VIPergic secretomotor neurons, expressed CRF, but not the CRF1 receptor. CRF1 receptors were expressed exclusively by neuronal neighbors of secretomotor neurons, which expressed a different set of chemical codes and different morphology. The neighboring neurons with CRF1 receptors expressed ChAT, NPY, calbindin, and substance P-IR (Liu et al., 2005). Co-expression of NPY and ChAT is a chemical code for uniaxonal cholinergic secretomotor neurons (Furness, 2000; Brookes, 2001). Co-expression of calbindin and substance P is a code for submucosal neurons with Dogiel II morphology (Furness, 2000).
Binding of CRF to CRF1 receptors evokes electrophysiological excitation, which mimics slow synaptic excitation in ENS neurons (Liu et al., 2005). This suggests that firing in a network of CRF-IR neurons might release CRF as a neurotransmitter or neuromodulator, which would then act to elevate excitability in neighboring neurons of a different type and function. The mechanisms by which changes of this nature at the microcircuit level of ENS organization underlie changes in behavior at the level of the integrated organ system is poorly understood and persists as one of the frontiers of enteric neurobiology.
Myenteric Plexus
Based on their chemical coding, morphology and projection patterns, most of the CRF-IR myenteric neurons appeared to be excitatory motor neurons. More than half of the CRF-IR myenteric neurons expressed ChAT and substance P and corresponded to a subset of ChAT-/SPIR neurons with uniaxonal morphology, which are identified as excitatory motor neurons to the circular muscle coat (Furness, 2000; Brookes, 2001). These CRF-IR cell bodies were presumably the source of some of the CRF-IR nerve fibers that were found running parallel to the muscle fibers in the circular muscle layer of the small and large intestine. This suggests that CRF might be co-released with acetylcholine and substance P from a subgroup of excitatory motor neurons and work in parallel with acetylcholine and substance P somehow to influence motility. Evidence for such an influence on motility comes from studies in which peripheral administration of CRF stimulated propulsive motility in the colon as reflected by elevated contractile activity, accelerated transit, and ejection of fecal pellets in rats (Casagliuolo et al., 1996; Maillot et al., 2000).
A smaller proportion (i.e., 38%) of CRF-IR myenteric neurons was immunoreactive for NOS. NOS is a chemical code for inhibitory motor neurons with descending projections to the musculature and descending interneurons that mediate local reflexes (Furness 2000; Brookes, 2001). Co-localization with NOS suggests that the CRF-/NOS-IR myenteric neurons with aboral projections might belong to the population of inhibitory motor neurons to the circular muscle coat and/or descending interneurons that mediate local reflexes. Co-release of CRF from inhibitory motor neurons might be an explanation for the direct action of CRF to relax contractile tension in smooth fibers of the guinea-pig ileum and caecum (Iwakiri et al., 1996; Duridanova et al., 1999) and to suppress small intestinal transit (Williams et al., 1987).
Small calretinin-IR uniaxonal neurons comprise 17-24% of the neurons in the myenteric plexus of guinea-pig small intestine (Brookes et al., 1992; Costa et al., 1996). These neurons also express ChAT-IR and are classified as excitatory motor neurons, which innervate the longitudinal muscle layer or as ascending interneurons (Furness, 2000; Brookes, 2001). Our finding of lack of expression of CRF expression by calretinin-IR myenteric neurons suggests that CRF might not be involved in neural influence on the longitudinal muscle coat.
Calbindin-IR is a chemical code for neurons with multipolar Dogiel type II morphology and AH-type electrophysiological behavior in the ENS (Furness et al., 1990; Wood, 1994; Furness, 2000; Brookes, 2001). Somatostatin-IR identifies a group of descending interneurons in the guinea-pig myenteric plexus (Furness, 2000) and serotonin is expressed by another population of descending interneurons (Furness, 2000; Brookes, 2001). Finding that CRF-IR did not co-localize with calbindin-, somatostatin-, or serotonin-IR might indicate that CRF is not a neurotransmitter in interneuronal microcircuits in the myenteric plexus.
A small group of CRF-IR myenteric neurons had Dogiel type II morphology and expressed ChAT-IR, but not calbindin-IR. Calbindin-IR is expressed by the majority of myenteric neurons with AH-type electrophysiological behavior and Dogiel II morphology (i.e., 81-84%); nevertheless, a small subclass of these neurons does not express calbindin-IR (Iyer et al., 1988; Song et al., 1994). Our results suggest that CRF-IR might be another code for the small calbindin-negative population of Dogiel type II myenteric neurons.
CRF-IR nerve fibers were apparent throughout the myenteric plexus in all regions. Most of these CRF-IR fibers remained after 7 days in organotypic culture. Neither postganglionic sympathetic nerve fibers nor spinal afferent fibers expressed CRF-IR in the myenteric plexus, which suggests that the CRF-IR nerve fibers originated from myenteric neurons and are intrinsic to the ENS. CRF-IR fibers were unlikely to be projections of AH/Dogiel Type II interneurons because CRF-IR was not co-localized with calbindin, which is a chemical code for myenteric neurons with AH electrophysiological behavior and Dogiel II morphology (Iyer etal., 1988; Furness. 2000). Nevertheless, a small proportion of myenteric neurons with CRF-IR (5%) had Dogiel Type II multipolar morphology. Most of the CRF-IR myenteric neurons (95%) had uniaxonal morphology and expressed IR for ChAT, substance P or NOS, which suggests that these CRF-IR neurons might be interneurons or motor neurons to the musculature.
Submucosal Plexus
CRF-IR nerve cell bodies were relatively more abundant in the submucosal plexus than in the myenteric plexus. All of the CRF-IR submucosal neurons had Dogiel type I morphology and expressed VIP-IR. VIP is an established neurotransmitter released by non-cholinergic secretomotor neurons and vasodilator neurons in the submucosal plexus (Vanner and Surprenant, 1991). Co-localization with VIP suggests that CRF might be released at the same time as VIP when secretomotor neurons fire and be involved in the neural coordination of intestinal secretion and submucosal blood flow. Intravenous or intraperitoneal administration of CRF stimulates mucosal secretion of water, electrolytes and mucus and evokes watery diarrhea with rapid onset in a dose-dependent manner in conscious rats (Castagliuolo et al., 1996; Santos et al., 1999; Saunders et al., 2002a,b). This form of neurogenic secretory diarrhea is reminiscent of stress-evoked diarrhea (Saunders et al., 2002a,b).
NPY-IR is identified as a chemical code for cholinergic secretomotor neurons and calretinin-IR is a code for cholinergic secretomotor/vasodilator neurons in the submucosal plexus (Furness, 2000; Brookes, 2001). Substance P-IR and NeuN-IR are chemical codes for submucosal Dogiel type II neurons (Poole et al., 2002). None of these neuronal populations expressed CRF-IR. On the other hand the NPY-, NeuN- and substance P-IR submucosal neurons express the CRF1 receptor subtype and activation of the receptor by CRF evokes excitatory responses reminiscent of slow synaptic excitation in these neurons (Liu et al., 2005). The combined evidence suggests that VIPergic secretomotor/vasodilator neurons, which do not express CRF receptors themselves, might synthesize and release CRF as a neurotransmitter to stimulate excitability in neighboring populations of cholinergic secretomotor neurons.
The numbers of CRF-IR nerve fibers in submucosal ganglia were considerably smaller than in the myenteric ganglia. No CRF-IR fibers were present in more than half of the ganglia. Scarcity of CRF-IR nerve fibers in the submucosal ganglia is consistent with the report of Furness et al. (2003) that VIP neurons have few intra-ganglionic terminals (Furness et al., 2003). On the other hand, CRF-IR fibers in the interganlionic connectives were common. Arterioles outside the ganglia were accompanied by CRF-IR nerve fibers. Usually, a single IR fiber was seen on each side of a vessel in whole-mounts. This suggests that the innervation of the blood vessels might have been derived from submucosal VIPergic secretomotor/vasodilator neurons. Presumably, CRF-IR fibers in the submucosal plexus were projections from CRF-IR/VIP-IR cell bodies in the submucosal ganglia because these fibers persisted in isolated submucosal preparations in organotypic culture. This observation that most of the CRF-IR fibers in the submucosal preparations do not degenerate in organotypic culture suggests that few, if any, CRF-IR neurons in the myenteric plexus project to the submucosal regions.
Acknowledgments
Grant sponsor: National Institutes of Health R01 DK 37238 (J.D. Wood), R01 DK 57075 (J. D. Wood), KO8 DK 060468 (Yun Xia) and Pharmaceutical Manufactures of American Foundation Postdoctoral Fellowship (Sumei Liu).
Our thanks to Dr. Wylie W. Vale for providing the rabbit anti-CRF antiserum.
LITERATURE CITED
- Asakawa A, Inui A, Ueno N, Makino S, Fujino MA, Kasuga M. Urocortin reduces food intake and gastric emptying in lean and ob/ob obese mice. Gastroenterology. 1999;116:1287–1292. doi: 10.1016/s0016-5085(99)70491-9. [DOI] [PubMed] [Google Scholar]
- Brookes SJH. Classes of enteric nerve cells in the guinea-pig small intestine. Anat Rec. 2001;262:58–70. doi: 10.1002/1097-0185(20010101)262:1<58::AID-AR1011>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- Brookes SJH, Song ZM, Steel PA, Costa M. Identification of motor neurons to the longitudinal muscle of the guinea-pig ileum. Gastroenterology. 1992;103:961–973. doi: 10.1016/0016-5085(92)90030-3. [DOI] [PubMed] [Google Scholar]
- Castagliuolo I, Lamont JT, Qiu B, Fleming SM, Bhaskar KR, Nikulasson ST, Kornetsky C, Pothoulakis C. Acute stress causes mucin release from rat colon: role of corticotropin releasing factor and mast cells. Am J Physiol. 1996;271:G884–G892. doi: 10.1152/ajpgi.1996.271.5.G884. [DOI] [PubMed] [Google Scholar]
- Chatzaki E, Crowe PD, Wang L, Million M, Tache Y, Grigoriadis DE. CRF receptor type 1 and 2 expression and anatomical distribution in the rat colon. J Neurochem. 2004;90:309–316. doi: 10.1111/j.1471-4159.2004.02490.x. [DOI] [PubMed] [Google Scholar]
- Chen CY, Million M, Adelson DW, Martinez V, Rivier J, Tache Y. Intracisternal urocortin inhibits vagally stimulated gastric motility in rats: role of CRF(2) Br J Pharmacol. 2002;136:237–247. doi: 10.1038/sj.bjp.0704713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa M, Brookes SJ, Steele PA, Gibbins I, Burcher E, Kandiah CJ. Neurochemical classification of myenteric neurons in the guinea-pig ileum. Neuroscience. 1996;75:949–967. doi: 10.1016/0306-4522(96)00275-8. [DOI] [PubMed] [Google Scholar]
- Drossman DA. Psychosocial factors and the disorders of GI function: what is the link? Am J Gastroenterol. 2004;99:358–360. doi: 10.1111/j.1572-0241.2004.04076.x. [DOI] [PubMed] [Google Scholar]
- Duffy LC, Zielezny MA, Marshall JR, Byers TE, Weiser MM, Phillips JF, Calkins BM, Ogra PL, Graham S. Relevance of major stress events as an indicator of disease activity prevalence in inflammatory bowel disease. Behav Med. 1991;17:101–110. doi: 10.1080/08964289.1991.9937553. [DOI] [PubMed] [Google Scholar]
- Duridanova DB, Petkova-Kirova PS, Lubomirov LT, Gagov H, Boev K. Corticotropin-releasing hormone acts on guinea pig ileal smooth muscle via protein kinase A. Pflügers Arch. 1999;438:205–212. doi: 10.1007/s004240050899. [DOI] [PubMed] [Google Scholar]
- Fone DR, Horowitz M, Maddox A, Akkermans LM, Read NW, Dent J. Gastroduodenal motility during the delayed gastric emptying induced by cold stress. Gastroenterology. 1990;98:1155–1161. doi: 10.1016/0016-5085(90)90328-x. [DOI] [PubMed] [Google Scholar]
- Furness JB. Types of neurons in the enteric nervous system. J Auton Nerv Syst. 2000;81:87–96. doi: 10.1016/s0165-1838(00)00127-2. [DOI] [PubMed] [Google Scholar]
- Furness JB, Trussell DC, Pompolo S, Bornstein JC, Smith TK. Calbindin neurons of the guinea-pig small intestine: quantitative analysis of their numbers and projections. Cell Tissue Res. 1990;260:261–272. doi: 10.1007/BF00318629. [DOI] [PubMed] [Google Scholar]
- Greene BR, Blanchard EB, Wan CK. Long-term monitoring of psychosocial stress and symptomatology in inflammatory bowel disease. Behav Res Ther. 1994;32:217–226. doi: 10.1016/0005-7967(94)90114-7. [DOI] [PubMed] [Google Scholar]
- Hanani M, Wood JD. Corticotropin-releasing hormone excites myenteric neurons in the guinea-pig small intestine. Eur J Pharmacol. 1992;211:23–27. doi: 10.1016/0014-2999(92)90256-4. [DOI] [PubMed] [Google Scholar]
- Hu HZ, Gao N, Lin Z, Gao C, Liu S, Ren J, Xia Y, Wood JD. Chemical coding and electrophysiology of enteric neurons expressing neurofilament 145 in guinea pig gastrointestinal tract. J Comp Neurol. 2002;442:189–203. [PubMed] [Google Scholar]
- Iwakiri Y, Chijiiwa Y, Motomura Y, Akiho H, Osame M, Nawata H. Direct inhibitory effect of corticotropin releasing hormone on isolated caecal circular smooth muscle cells of guinea pig via adenylate cyclase system. Life Sci. 1996;58:2243–2249. doi: 10.1016/0024-3205(96)00219-6. [DOI] [PubMed] [Google Scholar]
- Iyer V, Bornstein JC, Costa M, Furness JB, Takahashi Y, Iwanaga T. Electrophysiology of guinea-pig myenteric neurons correlated with immunoreactivity for calcium binding proteins. J Auton Nerv Syst. 1988;22:141–150. doi: 10.1016/0165-1838(88)90087-2. [DOI] [PubMed] [Google Scholar]
- Karalis K, Muglia LJ, Bae D, Hilderbrand H, Majzoub JA. CRH and the immune system. J Neuroimmunol. 1997;72:131–136. doi: 10.1016/s0165-5728(96)00178-6. [DOI] [PubMed] [Google Scholar]
- Kawahito Y, Sano H, Kawata M, Yuri K, Mukai S, Yamamura Y, Kato H, Chrousos GP, Wilder RL, Kondo M. Local secretion of corticotropin-releasing hormone by enterochromaffin cells in human colon. Gastroenterology. 1994;106:859–865. doi: 10.1016/0016-5085(94)90743-9. [DOI] [PubMed] [Google Scholar]
- Kawai K, Hotate K, Chiba Y, Munekata E, Ohashi S, Wakabayashi I, Yamashita K. The distribution of corticotrophin-releasing factor immunoreactivity in various ovine tissues. Acta Endocrinol (Copenh) 1985;108(4):433–439. doi: 10.1530/acta.0.1080433. [DOI] [PubMed] [Google Scholar]
- Kiliaan AJ, Saunders PR, Bijlsma PB, Berin MC, Taminiau JA, Groot JA, Perdue MH. Stress stimulates transepithelial macromolecular uptake in rat jejunum. Am J Physiol. 1998;275:G1037–G1044. doi: 10.1152/ajpgi.1998.275.5.G1037. [DOI] [PubMed] [Google Scholar]
- Lazar Z, Benko R, Bolcskei K, Rumbus Z, Wolf M, Holzer P, Maggi CA, Bartho L. Actions of endothelin and corticotropin releasing factor in the guinea-pig ileum: no evidence for an interaction with capsaicin-sensitive neurons. Neuropeptides. 2003;37:220–232. doi: 10.1016/s0143-4179(03)00048-9. [DOI] [PubMed] [Google Scholar]
- Lenz HJ, Burlage M, Raedler A, Greten H. Central nervous system effects of corticotropin-releasing factor on gastrointestinal transit in the rat. Gastroenterology. 1988;94:598–602. doi: 10.1016/0016-5085(88)90229-6. [DOI] [PubMed] [Google Scholar]
- Levenstein S, Prantera C, Varvo V, Scribano ML, Berto E, Andreoli A, Luzi C. Psychological stress and disease activity in ulcerative colitis: a multidimensional cross-sectional study. Am J Gastroenterol. 1994;89:1219–1225. [PubMed] [Google Scholar]
- Lin Z, Gao N, Hu H-Z, Liu S, Gao C, Kim G, Ren J, Xia Y, Peck OC, Wood JD. Immunoreactivity of Hu proteins facilitates identification of myenteric neurones in guinea-pig small intestine. Neurogastroenterol Motil. 2002;14:197–204. doi: 10.1046/j.1365-2982.2002.00317.x. [DOI] [PubMed] [Google Scholar]
- Liu S, Gao X, Gao N, Wang X, Fang X, Hu HZ, Wang G, Xia Y, Wood JD. Expression of type 1 corticotropin releasing factor receptor in the guinea-pig enteric nervous system. J Comp Neurol. 2005;481:284–298. doi: 10.1002/cne.20370. [DOI] [PubMed] [Google Scholar]
- Maillot C, Million M, Wei JR, Gauthier A, Taché Y. Peripheral corticotropin-releasing factor and stress-stimulated colonic motor activity involve type 1 receptor in rats. Gastroenterology. 2000;119:1569–1579. doi: 10.1053/gast.2000.20251. [DOI] [PubMed] [Google Scholar]
- Mancinelli R, Azzena GB, Diana M, Forgione A, Fratta W. In vitro excitatory actions of corticotropin-releasing factor on rat colonic motility. J Auto Pharmacol. 1998;18:319–324. doi: 10.1046/j.1365-2680.1998.1860319.x. [DOI] [PubMed] [Google Scholar]
- Martinez V, Rivier J, Tach Y. Peripheral injection of a new corticotropin-releasing factor (CRF) antagonist, astressin, blocks peripheral CRF- and abdominal surgery-induced delayed gastric emptying in rats. J Pharmacol Exp Ther. 1999;290:629–634. [PubMed] [Google Scholar]
- Martinez V, Rivier J, Wang L, Tache Y. Central injection of a new corticotropin-releasing factor (CRF) antagonist, astressin, blocks CRF- and stress-related alterations of gastric and colonic motor function. J Pharmacol Exp Ther. 1997;280:754–760. [PubMed] [Google Scholar]
- Martinez V, Wang L, Rivier J, Grigoriadis D, Tache Y. Central CRF, urocortins and stress increase colonic transit via CRF1 receptors while activation of CRF2 receptors delays gastric transit in mice. J Physiol. 2004;556:221–234. doi: 10.1113/jphysiol.2003.059659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez V, Wang L, Rivier JE, Vale WW, Tache Y. Differential actions of peripheral corticotropin-releasing factor (CRF), urocortin II, and urocortin III on gastric emptying and colonic transit in mice: role of CRF receptor subtypes 1 and 2. J Pharmacol Exp Ther. 2002;301:611–617. doi: 10.1124/jpet.301.2.611. [DOI] [PubMed] [Google Scholar]
- Martins JM, Banks WA, Kastin AJ. Acute modulation of active carrier-mediated brain-to-blood transport of corticotropin-releasing hormone. Am J Physiol. 1997;272:E312–319. doi: 10.1152/ajpendo.1997.272.2.E312. [DOI] [PubMed] [Google Scholar]
- Martins JM, Kastin AJ, Banks WA. Unidirectional specific and modulated brain to blood transport of corticotropin-releasing hormone. Neuroendocrinology. 1996;63:338–348. doi: 10.1159/000126974. [DOI] [PubMed] [Google Scholar]
- Mawe GM, Kennedy AL. Duodenal neurons provide nicotinic fast synaptic input to sphincter of Oddi neurons in guinea pig. Am J Physiol. 1999;277:G226–G234. doi: 10.1152/ajpgi.1999.277.1.G226. [DOI] [PubMed] [Google Scholar]
- Mayer EA. The neurobiology of stress and gastrointestinal disease. Gut. 2000;47:861–869. doi: 10.1136/gut.47.6.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miampamba M, Maillot C, Million M, Tache Y. Peripheral CRF activates myenteric neurons in the proximal colon through CRF(1) receptor in conscious rats. Am J Physiol. 2002;282:G857–G865. doi: 10.1152/ajpgi.00434.2001. [DOI] [PubMed] [Google Scholar]
- Million M, Grigoriadis DE, Sullivan S, Crowe PD, McRoberts JA, Zhou H, Saunders PR, Maillot C, Mayer EA, Tache Y. A novel water-soluble selective CRF1 receptor antagonist, NBI 35965, blunts stress-induced visceral hyperalgesia and colonic motor function in rats. Brain Res. 2003;985:32–42. doi: 10.1016/s0006-8993(03)03027-0. [DOI] [PubMed] [Google Scholar]
- Million M, Maillot C, Saunders P, Rivier J, Vale WW, Tache Y. Human urocortin II, a new CRF-related peptide, displays selective CRF2-mediated action on gastric transit in rats. Am J Physiol. 2002;282:G34–G40. doi: 10.1152/ajpgi.00283.2001. [DOI] [PubMed] [Google Scholar]
- Monnikes H, Schmidt BG, Raybould HE, Tache Y. CRF in the paraventricular nucleus mediates gastric and colonic motor response to restraint stress. Am J Physiol. 1992;262:G137–G143. doi: 10.1152/ajpgi.1992.262.1.G137. [DOI] [PubMed] [Google Scholar]
- Nozu T, Martinez V, Rivier J, Tache Y. Peripheral urocortin delays gastric emptying: role of CRF receptor 2. Am J Physiol. 1999;276:G867–G874. doi: 10.1152/ajpgi.1999.276.4.G867. [DOI] [PubMed] [Google Scholar]
- Pappas T, Debas H, Tache Y. Corticotropin-releasing factor inhibits gastric emptying in dogs. Regul Pept. 1985;11:193–199. doi: 10.1016/0167-0115(85)90050-3. [DOI] [PubMed] [Google Scholar]
- Peck OC, Wood JD. Brain-gut interactions in ulcerative colitis. Gastroenterology. 2000;118:807–808. doi: 10.1016/s0016-5085(00)70157-0. [DOI] [PubMed] [Google Scholar]
- Petrusz P, Merchenthaler I, Maderdrut JL, Vigh S, Schally AV. Corticotropin-releasing factor (CRF)-like immunoreactivity in the vertebrate endocrine pancreas. Proc Natl Acad Sci USA. 1983;80:1721–1725. doi: 10.1073/pnas.80.6.1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrusz P, Merchenthaler I, Ordronneau P, Maderdrut JL, Vigh S, Schally AV. Corticotropin-releasing factor (CRF)-like immunoreactivity in the gastro-entero-pancreatic endocrine system. Peptides. 1984;5(Suppl 1):71–78. doi: 10.1016/0196-9781(84)90266-3. [DOI] [PubMed] [Google Scholar]
- Poole DP, Castelucci P, Robbins HL, Chiocchetti R, Furness JB. The distribution of P2X3 purine receptor subunits in the guinea pig enteric nervous system. Auton Neurosci. 2002;101:39–47. doi: 10.1016/s1566-0702(02)00179-0. [DOI] [PubMed] [Google Scholar]
- Santos J, Saunders PR, Hanssen NPM, Yang PC, Yates D, Groot JA, Perdue MH. Corticotropin-releasing hormone mimics stress-induced rat colonic epithelial pathophysiology in the rat. Am J Physiol. 1999;277:G391–399. doi: 10.1152/ajpgi.1999.277.2.G391. [DOI] [PubMed] [Google Scholar]
- Saunders PR, Hanssen NP, Perdue MH. Cholinergic nerves mediate stress-induced intestinal transport abnormalities in Wistar-Kyoto rats. Am J Physiol. 1997;273:G486–490. doi: 10.1152/ajpgi.1997.273.2.G486. [DOI] [PubMed] [Google Scholar]
- Saunders PR, Kosecka U, McKay DM, Perdue MH. Acute stressors stimulate ion secretion and increase epithelial permeability in rat intestine. Am J Physiol. 1994;267:G794–799. doi: 10.1152/ajpgi.1994.267.5.G794. [DOI] [PubMed] [Google Scholar]
- Saunders PR, Maillot C, Million M, Tache Y. Peripheral corticotropin-releasing factor induces diarrhea in rats: role of CRF1 receptor in fecal watery excretion. Eur J Pharmacol. 2002a;435:231–235. doi: 10.1016/s0014-2999(01)01574-6. [DOI] [PubMed] [Google Scholar]
- Saunders PR, Santos J, Hanssen NP, Yates D, Groot JA, Perdue MH. Physical and psychological stress in rats enhances colonic epithelial permeability via peripheral CRH. Dig Dis Sci. 2002b;47:208–215. doi: 10.1023/a:1013204612762. [DOI] [PubMed] [Google Scholar]
- Song ZM, Brookes SJ, Costa M. All calbindin-immunoreactive myenteric neurons project to the mucosa of the guinea-pig small intestine. Neurosci Lett. 1994;180:219–222. doi: 10.1016/0304-3940(94)90524-x. [DOI] [PubMed] [Google Scholar]
- Suda T, Tomori N, Tozawa F, Mouri T, Demura H, Shizume K. Distribution and characterization of immunoreactive corticotropin-releasing factor in human tissues. J Clin Endocrinol Metab. 1984;59:861–866. doi: 10.1210/jcem-59-5-861. [DOI] [PubMed] [Google Scholar]
- Tache Y, Perdue MH. Role of peripheral CRF signaling pathways in stress-related alterations of gut motility and mucosal function. Neurogastroenterol Motil. 2004;16(Suppl 1):137–142. doi: 10.1111/j.1743-3150.2004.00490.x. [DOI] [PubMed] [Google Scholar]
- Tache Y, Martinez V, Million M, Rivier J. Corticotropin-releasing factor and the brain-gut motor response to stress. Can J Gastroenterol. 1999;13(Suppl A):18A–25A. doi: 10.1155/1999/375916. [DOI] [PubMed] [Google Scholar]
- Tache Y, Martinez V, Million M, Wang L. Stress and the gastrointestinal tract III. Stress-related alterations of gut motor function: role of brain corticotropin-releasing factor receptors. Am J Physiol Gastrointest Liver Physiol. 2001;280:G173–G177. doi: 10.1152/ajpgi.2001.280.2.G173. [DOI] [PubMed] [Google Scholar]
- Tache Y, Martinez V, Wang L, Million M. CRF1 receptor signaling pathways are involved in stress-related alterations of colonic function and viscerosensitivity: implications for irritable bowel syndrome. Br J Pharmacol. 2004;141:1321–1330. doi: 10.1038/sj.bjp.0705760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanner S, Surprenant A. Cholinergic and noncholinergic submucosal neurons dilate arterioles in guinea pig colon. Am J Physiol. 1991;261:G136–144. doi: 10.1152/ajpgi.1991.261.1.G136. [DOI] [PubMed] [Google Scholar]
- Wang L, Martinez V, Rivier JE, Taché Y. Peripheral urocortin inhibits gastric emptying and food intake in mice: differential role of CRF receptor 2. Am J Physiol Regulatory Integrative Comp Physiol. 2001;281:R1401–R1410. doi: 10.1152/ajpregu.2001.281.5.R1401. [DOI] [PubMed] [Google Scholar]
- Williams CL, Peterson JM, Villar RG, Burks TF. Corticotropin-releasing factor directly mediates colonic responses to stress. Am J Physiol. 1987;253:G582–586. doi: 10.1152/ajpgi.1987.253.4.G582. [DOI] [PubMed] [Google Scholar]
- Wolter HJ. Corticotropin-releasing factor is contained within perikarya and nerve fibres of rat duodenum. Biochem Biophys Res Commun. 1984;122:381–387. doi: 10.1016/0006-291x(84)90486-8. [DOI] [PubMed] [Google Scholar]
- Wood JD. Physiology of the enteric nervous system. In: Johnson LR, Alpers DH, Christensen J, Jacobson ED, Walsh JH, editors. Physiology of the Gastrointestinal Tract. 3rd Raven Press; New York: 1994. pp. 423–482. [Google Scholar]
- Wood JD. Neuropathophysiology of irritable bowel syndrome. J Clin Gastroenterol. 2002;35:S11–22. doi: 10.1097/00004836-200207001-00004. [DOI] [PubMed] [Google Scholar]
- Wood JD, Peck OC, Tefend KS, Stonerook MJ, Caniano DA, Mutabagani KH, Lhotak S, Sharma HM. Evidence that colitis is initiated by environmental stress and sustained by fecal factors in the cotton-top tamarin (Saguinus oedipus) Dig Dis Sci. 2000;45:385–393. doi: 10.1023/a:1005485215128. [DOI] [PubMed] [Google Scholar]