Abstract
We have isolated extraretinal and retinal components of firing during smooth pursuit eye movements in the medial-superior-temporal area (MST) in the extrastriate visual cortex. Awake macaque monkeys tracked spots in total darkness to eliminate image motion inputs from the background. For 300 ms during sustained tracking at different speeds, the target was stabilized on the moving eye, practically eliminating image motion inputs from the tracking target. The extraretinal component of firing rate during image stabilization was direction selective and related to eye speed but sometimes showed a different preferred speed from the retinal component of the same neuron's responses. The highly variable firing rate of individual MST neurons allowed an ideal observer to predict target speed correctly on 25% of trials. Pooling the data from 71 MST neurons improved the correct response rate to 50%. Behavioral experiments imposed brief perturbations of target velocity to assess the gain of visual-motor transmission for pursuit. The average response to perturbations increased as a function of target speed. However, the size of the responses to individual perturbations allowed an ideal observer to predict target speed correctly on only 35% of the trials. The imprecision of MST responses argues that the output of MST may be a poor candidate to drive eye velocity and so may instead regulate another component of pursuit. The good agreement between the eye velocity precision of the behavioral responses to perturbations of target motion and the firing of MST neurons raises regulation of the visual-motor gain of pursuit as one candidate component.
INTRODUCTION
Smooth-pursuit eye movements are controlled by the image motion that is created on the retina when objects move with respect to the stationary or moving eye. The visual motion inputs for pursuit arise in the middle temporal area (MT) of the extrastriate visual cortex and are transmitted through the medial superior temporal area (MST) to the frontal pursuit area (FPA) in the arcuate sulcus. All three of these areas provide outputs to subcortical components of the neural circuit for pursuit (Boussaoud et al. 1992; Glickstein et al. 1980). In MT, the signals recorded during pursuit appear to be related mainly to the visual inputs caused by image motion. Even during maintained pursuit, when image motion is small, momentarily extinguishing the target causes the responses of MT neurons to cease (Newsome et al. 1988). Thus we say that the signals emanating from MT are “retinal.” In contrast, many neurons in MST continue to discharge during momentary extinction of a pursuit target and are therefore considered to provide outputs based on “extraretinal” as well as retinal signals (Ilg and Thier 2003; Newsome et al. 1988).
The existence of extraretinal signals in MST raises two sets of related questions that are addressed in the present paper. First, what is the nature of the extraretinal signals? What is the relationship between firing rate and eye speed? How are extraretinal signals related to the retinal signals on the same neurons? Second, what is the function of the extraretinal signals? How might they contribute to the generation of pursuit eye movements?
Prior physiological data have demonstrated that the pursuit responses of MST neurons depend on the direction of smooth eye motion (Erickson and Thier 1991; Newsome et al. 1988; Squatrito and Maioli 1997). However, considerably less is known about the relationship between the firing rate of MST neurons and the magnitude of eye velocity during pursuit. One study varied speed in the context of other experiments but only over a narrow range (Shenoy et al. 2002). The relationship between the speed tuning of retinal versus extraretinal responses also has yet to be explored.
Two features of pursuit provide a conceptual structure to help to understand possible roles for the extraretinal signals in MST. First, pursuit shows “velocity memory”: subjects continue to pursue at the preexisting eye velocity even after image motion from a target has been eliminated by stabilizing the target relative to the smoothly moving eye (Morris and Lisberger 1987). Newsome et al. (1988) suggested that the extraretinal output of MST could contribute to velocity memory, whereas Lisberger and Fuchs (1978) have suggested that velocity memory would be a function for positive feedback of eye-velocity signals through the floccular complex of the cerebellum. Second, the strength of visual-motor transmission for pursuit is subject to “gain control”: in both humans and monkeys, the response to a brief perturbation of image motion is small during fixation and grows as a function of ongoing eye velocity during pursuit (Churchland and Lisberger 2002; Schwartz and Lisberger 1994). Microstimulation experiments have shown that the output from the FPA is able to control the internal gain of pursuit (Tanaka and Lisberger 2002). Because MST neurons project to the FPA, the extraretinal responses in MST could contribute to regulation of gain control by the FPA.
In the present paper, we have used target stabilization to study the extraretinal component of MST neuron responses in isolation from the retinal components. We have found that the firing rate caused by the extraretinal inputs is related to the speed of pursuit. Comparison of the eye velocity precision of the extraretinal signals in MST and the setting of the pursuit gain control implies that the extraretinal signals in MST may be suited best to contribute to regulation of the internal gain of pursuit.
METHODS
Eye movement and neural recordings were obtained from two adult male rhesus monkeys (Macaca mulatta) that had been trained to fixate and pursue visual targets for fluid reward. Monkeys were implanted with a stainless steel socket for head restraint and a scleral search coil for measuring eye position, using methods described in detail elsewhere (Churchland and Lisberger 2000). After initial training, monkeys were implanted with stainless steel or cilux cylinders (Crist Instruments, Hagerstown, MD) to allow access to MST for neural recordings. Surgeries were conducted using sterile technique with the monkeys under isofluorane anesthesia, and analgesics were given under the supervision of veterinarians and veterinary nurses during the recovery after each surgical procedure. For each experimental session, the monkey sat in a primate chair and the implanted socket was used to affix the head to the ceiling of the chair. A tube was positioned at the monkey's mouth for dispensing fluid rewards. All procedures were approved in advance by the Institutional Animal Care and Use Committee at the University of California, San Francisco and were in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Stimulus presentation
Visual stimuli were displayed on a 12-in diagonal analog oscilloscope. The display was positioned 30 cm from the monkey and subtended horizontal and vertical angles of 50 and 40°. Stimuli consisted of either single spots or patches of moving dots. The aperture size for patches of dots varied from 10 × 10° to 30 × 30° depending on exact experiment being conducted and the preferences of the neuron under study. Different trials were customized to analyze visual responses during fixation or extraretinal responses during pursuit.
To analyze visual responses, patches of moving dots were presented while the monkey fixated a stationary spot. Each trial began with the appearance of a fixation point, followed 600 ms later by the appearance of a stationary patch. Two hundred milliseconds later, dots in the patch began to move coherently behind a stationary virtual window, and continued to move for 500 ms. The patch then was extinguished and the fixation light remained on for 200 ms. Analysis of the monkey's eye velocity during presentation of the moving stimulus revealed that fixation was excellent: eye velocity was transiently 0.76°/s for the highest speed tested (128°/s) and was smaller at lower speeds. The small drifts caused by the moving stimulus were insufficiently large or sustained to evoke a substantial extraretinal response or to have a material effect on the speed of image motion across the retina.
To analyze pursuit, trials began with the appearance of a fixation point that was between 2 and 10° eccentric from the center of the screen along the preferred-null axis for the neuron under study. The fixation position was chosen to be progressively more eccentric for faster target speeds so that the 300-ms interval used for data analysis always took place when the eyes were as close as possible to the center of the orbit. Monkeys were required to maintain fixation for a time that varied randomly between 1,000 and 1,200 ms. The target then underwent “step-ramp” target motion (Rashbass 1961). It stepped away from and immediately began to move back toward the position of fixation at one of six speeds: 2, 5, 10, 15, 20, and 30°/s. Target motion continued for 1,000-1,200 ms. The monkey was required to maintain eye position within 3° of target motion throughout the trial or the trial was terminated and the data excluded from analysis.
Three precautions were taken to minimize the presence of visual motion inputs from the stationary background during ongoing pursuit. First, responses were recorded in complete darkness except for the target. Darkness was assessed both by measuring the ambient illumination in the room (0.0 cd/m2) and by dark-adapting experimenters in the room for >20 min to ensure that they could not detect any light sources that had been overlooked. Second, the analog oscilloscope we used was selected because it provides less ambient illumination than traditional video displays or video projection systems. Pursuit targets were extremely small and dim (∼0.2° across, 1.6 cd/m2) so they caused no increase in the ambient illumination in the room. The specifications of the display oscilloscopes indicate that the phosphor decayed to 10% of its maximal level in 10µs to 1 ms. Third, data collection was routinely stopped for ∼30 s every 10 min so that the monkeys could be presented with normal levels of ambient illumination to prevent full dark adaptation.
To study extraretinal signals in isolation, we included trials containing target stabilization. After the monkey had achieved accurate steady-state tracking, the target was driven for 300 ms by a properly scaled and offset version of the eye position voltage, so that the target remained exactly in front of the moving eye (Morris and Lisberger 1987). As a result, target velocity was driven by eye velocity (Fig. 1A, gray trace) so that the target could caused little or no image motion on the retina. Stabilization was imposed 340 ms after the onset of target motion at a time when pursuit eye velocity had already reached a steady-state close to target velocity. After the end of the stabilization period, the target continued to move for a variable amount of time that depended on speed and was randomized within each speed so that the monkey could not predict when the trial would end. The gray trace in Fig. 1B shows an example of the eye velocity evoked during target stabilization. Trials with and without a period of target stabilization were of the same duration and were presented in equal numbers to help prevent the monkey from detecting and possibly anticipating the period of target stabilization.
FIG. 1.
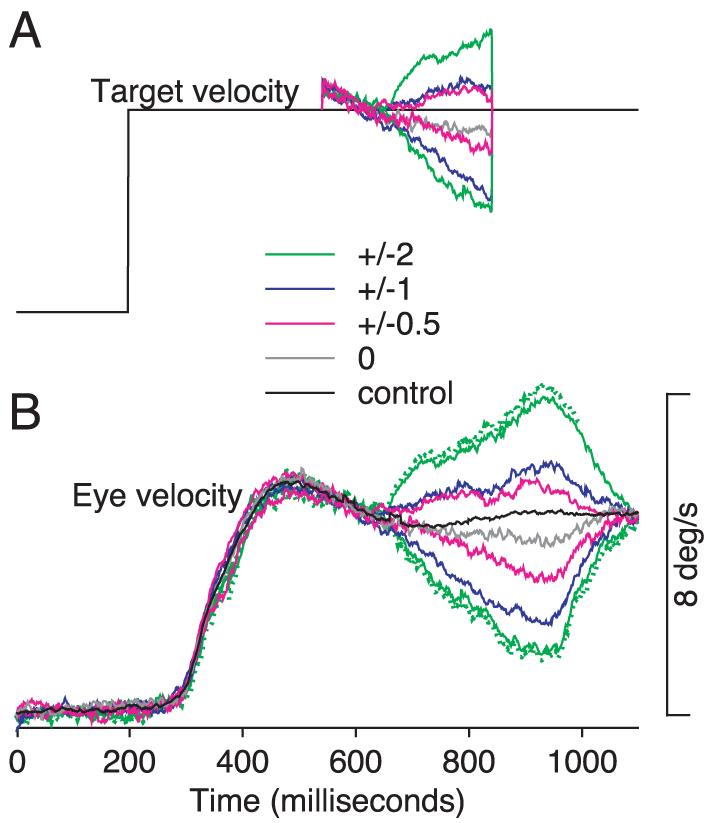
Target- and eye-velocity traces demonstrating target stabilization with respect to the moving eye. A: target velocity at 5°/s except for a 300-ms interval when it could continue uninterrupted, be stabilized on the moving eye, or be stabilized with the addition of constant image motion. Different colors show different conditions of target stabilization: black, control target motion; gray, stabilization without added image motion; red, blue, and green, ±0.5, ±1, or ±2°/s image motion added on top of stabilization. The small step in target velocity at the onset of stabilization indicates that eye velocity was slightly greater than target velocity at this moment. B: eye velocity in response to the different target velocities in A. Dotted lines next to the green traces show 1 SD of eye velocity.
In separate experiments that did not incorporate image stabilization, brief perturbations of target velocity were presented either during fixation or 300 ms after the target began to move in one of two directions (left and right) at six different target speeds (2, 5, 10, 15, 20, and 30°/s), after pursuit had been initiated successfully so that eye velocity was close to target velocity. Perturbations consisted of modulation of target velocity by a single cycle of a sine wave with a frequency of 5 or 10 Hz and a peak-to-peak amplitude of 4 or 8°/s. Perturbations presented during pursuit always caused target speed to first increase and then decrease (“peak-first” orientation) because responses are easier to interpret for peak-first than for trough-first perturbations (Churchland and Lisberger 2002). Perturbations presented during fixation caused the target to move first to either the left or the right. Trials were not used for analysis if the monkey did not complete the trial correctly by maintaining eye position within the 2.5° of target position. The position excursion of the perturbations was too small to disrupt the monkey's ability to fulfill the fixation requirements and complete the trial.
Eye movement and neural recordings
Signals related to horizontal eye velocity and eye position were digitized at a sampling rate of 1,000 Hz for each channel. The eye-position signal was low-pass filtered with a cutoff at 300 Hz. Voltages proportional to eye velocity were obtained by processing the eye-position signals with an analog circuit that differentiated signal content at frequencies ≤25 Hz and filtered signal content of higher frequencies (−20 dB per decade). Data analysis was performed after the experiment.
Image stabilization can come close to eliminating image motion only if the eye coil is properly calibrated. Therefore we used very small fixation targets to calibrate the eye coil, took great care to get the calibration right, and always re-checked the calibration when a new neuron was encountered. Two observations give us some confidence in the quality of the calibration. First, eye velocity responses in stabilized and control trials were very similar during periods where the target was stabilized (gray and black traces are within 1°/s of each other in Fig. 1B). Large differences in responses to the two types of trials would indicate that stabilizing the target was interrupting the monkey's pursuit perhaps because the calibration was poor and the target was moving with respect to the eye when the computer's command was for stabilization. Eye-velocity responses measured during recordings from eight neurons showed larger differences between the control and stabilized trials: to be conservative, these were excluded from further analysis. Second, imposing small amounts of image motion during the stabilized period (Fig. 1A, red, blue, and green traces) had the expected effects on eye velocity (Morris and Lisberger 1987). Figure 1B shows data from an experiment in which target velocity during the stabilized period equaled the monkey's eye velocity plus or minus 0, 0.5, 1, or 2°/s of imposed image motion (gray, red, blue, and green traces in Fig. 1). That we could detect the effect of as little as 0.5°/s of image velocity on eye velocity (red traces) gave us confidence that we could use the comparison of control and stabilized eye velocities to detect instances of poor calibration.
Extracellular action potentials were recorded from single units in area MST using sharp, 1- to 3-MΩ tungsten microelectrodes (Frederick Haer, Bowdoinham, ME). The voltage recorded by the electrode was amplified conventionally (Dagan, Minneapolis, MN), band-pass filtered from 100 Hz to 5 or 10 kHz, and viewed on an analog oscilloscope. The location of each electrode penetration was selected by inserting a guide tube into the chosen hole in a plastic grid (Crist Instruments) that was positioned in the implanted recording cylinder. Electrodes were introduced approximately perpendicular to the cortical surface and penetrations usually went sequentially through MST, the lumen of the superior temporal sulcus, and MT. Neurons were identified as being within of MST if they were dorsal to the lumen and had large receptive fields (Saito et al. 1986; Tanaka et al. 1986). Receptive fields frequently included the fovea and large parts of the ipsilateral visual field. Neurons with smaller receptive fields were categorized as MST neurons if they were close to neurons that were clearly MST neurons or if they were definitely dorsal to the lumen of the superior temporal sulcus. Based on electrophysiological landmarks and the locations of our electrode penetrations, we are confident that our sample of neurons was recorded in area MST. However, to be conservative in our identification of MST neurons, we excluded neurons on the basis that they could have been MT neurons if they had small, purely contralateral receptive fields and were recorded more ventrally without subsequent observation of a clear pass through the lumen of the STS. For this reason, our sample from MST is likely to include more neurons from MSTd than MSTl (Komatsu and Wurtz 1988; Tanaka et al. 1986). Histology is not available because one monkey is currently being used in other experiments and the other has been retired to a sanctuary.
Experimental protocol
When a neuron was first isolated, a series of tests was conducted to determine the optimal size, receptive field placement, and direction of motion for the patch of dots used as a visual stimulus. Because most neurons had large receptive fields that included at least the contralateral hemifield, a 30 × 30° patch that occupied most of the contralateral field was most frequently used. The patch was placed in the ipsilateral portion of the visual field if receptive field mapping indicated that visual stimuli were more effective when placed there. The 30 × 30° patch contained 500 dots (∼ 0.2°/dot) that were moved in each of eight directions on separate trials to determine the neuron's preferred direction. Subsequent trials then presented motion in the direction that was most effective in driving the cell and (for some neurons) the opposite direction. Next we estimated the neuron's preferred direction for pursuit by having the monkey track target motion in each of eight directions. Neurons were selected for further study if they showed above baseline responses to pursuit across a dark background in at least one of eight directions. For many cells, we also assessed pursuit direction tuning during target stabilization for motion at 20°/s in eight directions. This allowed us to confirm that the direction used for target motion at different speeds was indeed the best direction for extraretinal signals. We never encountered a neuron where our initial estimate of preferred direction was very far off. To test whether responses of the neurons we encountered were modulated in relation to eye position rather than eye velocity (Bremmer et al. 1997; de Oliveira et al. 1997; Thiele et al. 1997), responses were recorded during a center-out saccade task. Ten neurons were not studied further because they exhibited responses that depended more strongly on the eye position during eccentric fixation after the saccade than on eye velocity during pursuit.
After culling our sample to include only neurons that responded selectively during pursuit and were directional for pursuit eye movements, we tested the relationship between firing rate and eye speed by presenting pursuit targets that moved in the preferred and null directions at speeds ranging from 0 to 30°/s. We collected sufficient responses to amass ≥10 stabilized and 10 control trials that lacked saccades during the stabilization period at each of six speeds in two directions as well as for a stationary target. Finally, we studied the relationship between firing rate and the speed of visual motion by delivering from 4 to 10 repetitions of patches of dots that moved at speeds ranging from 2 to 128°/s while the monkey fixated a stationary spot.
Data analysis
For analysis of data obtained during pursuit, we defined a 300-ms-long analysis interval that started 100 ms after the onset of stabilization and ended 100 ms after the end of stabilization. The same interval was used for all pursuit trials whether or not the target had been stabilized. We measured the mean eye velocity and the mean firing rate in the analysis interval for each trial. Trials that contained saccades within the analysis interval during target stabilization or the equivalent time period in control trials were excluded from analysis. For analysis of data obtained with visual motion during fixation, we measured the firing rate during the last 400 ms of stimulus motion. For analysis of the eye-velocity responses to perturbations of target velocity, we measured the peak-to-trough eye-velocity deviation in the response to the perturbation in individual trials. Trials that contained saccades near or during the peak or trough of the response to the perturbation were excluded from analysis.
To assess the directionality of neuron responses, firing rates were averaged for all trials in each direction, resulting in eight two-dimensional vectors: the direction of each vector corresponded to the direction of target motion and the length was related to the firing rate of the neuron under study. The eight vectors were summed and the resulting direction was taken as the neuron's preferred direction. The opposite direction was taken as the null direction. We observed preferred directions that spanned 360° but with a bias toward ipsiversive pursuit that is in good agreement with data reported by others (Shenoy et al. 2002; Squatrito and Maioli 1997).
To evaluate the relationship between firing rate and eye speed during pursuit (or image speed during fixation), we first assessed whether firing rates varied significantly as a function of speed. Responses were subjected to a one-way ANOVA using speed as the main factor and a criterion of P = 0.01. Six neurons were excluded from further analysis because their responses failed to exhibit a statistically significant relationship to speed during the interval of target stabilization. Responses to eye and image motion then were fitted with separate cubic smoothing spline functions (Shikin and Plis 1995). For data obtained during pursuit, the knots of the spline functions were the eye velocities generated in response to target motion ranging from −30 to 30°/s (note that during steady-state pursuit, eye and target velocity are nearly identical). For data obtained with image motion during fixation, the knots were the stimulus velocities. The smoothness of the spline was set so that the fitted curves agreed well with visual inspection, and the same smoothness was used for all cells. The value of stimulus speed at the peak of the spline function was taken as an estimate of the neuron's best speed and the value of the spline at that speed was taken as the maximum firing rate. Preferred eye or image speed was always determined from responses to motion in the neuron's preferred direction of eye or image motion even when the preferred directions for eye and image motion differed. For responses to image motion during fixation, preferred speed was determined twice, once for the range of speeds present in the eye velocities during pursuit and once for the full range of image speeds delivered in the experiments.
Our methods for determining significance of the relationship between firing rate and speed and ascertaining preferred speed have been used by others to analyze the speed tuning of visual responses in area MT (Liu and Newsome 2003). More traditional functions for fitting the relationship between firing rate and image or eye speed, such as skewed Gaussian functions (Priebe and Lisberger 2002), were inappropriate for our dataset because firing rate simply increased monotonically with eye velocity for many MST neurons.
Ideal-observer analysis of relationship between behavioral and neural responses
Ideal-observer analysis was used to ask how well the firing rate of a neuron or the amplitude of a perturbation response could be used to estimate the corresponding ongoing eye velocity. The same basic analysis was applied first for the firing rate of MST neurons measured in 20-ms bins as a function of time from the onset of target motion and then for the responses to brief perturbations of target motion during steady-state pursuit. As a control to help interpret the ideal observer performance, we also estimated the precision of the eye-velocity signal itself. This control differs from the other two analyses in that the ideal observer's goal was to use eye velocity at each time point to estimate target velocity.
For neural data, measurements made from individual trials were grouped according to the ongoing eye velocity, providing seven distributions of firing rates, one for each speed including 0°/s. For behavioral perturbation data, trials were again grouped according to ongoing eye velocity, but this time yielding distributions of eye-velocity perturbation responses rather than of firing rates. For the eye-velocity control, distributions consisted of eye-velocity responses. For all data sets, one trial was removed from each of the seven distributions, yielding seven excluded responses. The remaining responses in each distribution were fitted with a normal probability density function. We then used the fitted functions to determine the distribution from which each of the excluded trials was maximally likely to have been drawn, and we scored the prediction as “correct” if the maximally likely distribution corresponded to the true target/eye velocity for that trial, and “incorrect” otherwise. The analysis was repeated by drawing seven different excluded trials until each of the 10 trials in each dataset had served as an excluded trial. The results of the 10 repetitions then were used to compute the percentage of correct assignments for the trials drawn from each of the original seven distributions. To provide shuffled data for statistical controls, the full set of responses was shuffled, each response was reassigned randomly to one of the seven target/eye speeds tested, and the analysis was repeated.
To ask whether performance might be improved by averaging responses across the population, we performed the ideal observer analysis on 10 population averages for each target/eye speed. Each population average was created by averaging the responses to each of the 10 trials in our dataset across all the neurons, yielding 10 population average responses for each target speed instead of 10 individual responses for each neuron. We then performed the ideal observer analysis as before. None of the results from ideal-observer analysis depended on the choice of the normal probability density function to fit the distributions: almost identical results were obtained using a Poisson probability density function to fit the response distributions, except that the percentage of correct assignments for both actual and shuffled data were ∼2% higher than when the normal probability density function was used. Finally, we used two methods to decode the population response in MST. For both methods, we first averaged the firing rates from 9 of 10 trials at each speed for each neuron to create mean response templates for each speed of target motion. The remaining trial from each neuron and each target speed served as “test responses.” Next, we asked which of our seven mean response templates was most similar to the test responses, as a function of time. For the maximum likelihood estimator (Pouget et al. 1998, 2000), we computed the distance between each speed template and the test response. The speed of the response template corresponding to the smallest distance was taken as the population maximum likelihood estimate of speed. For the population mean estimator, we computed the mean of the test responses for each template and the difference between the population mean and each of the response templates. The speed of the response template corresponding to the smallest difference was taken as the estimate of speed. For both methods, the analysis was repeated 10 times using each individual trial as a test response, and the estimates across repetitions of the analysis were assembled to determine the percentage of correct estimates of target speed.
RESULTS
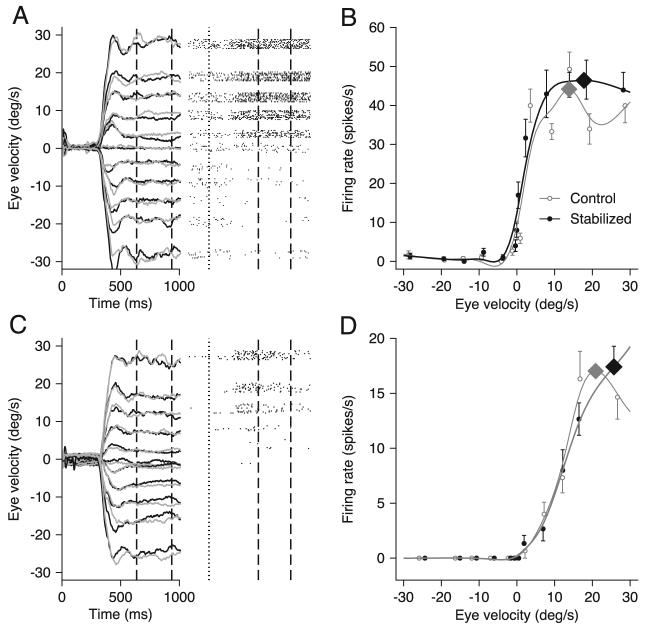
In two monkeys, we recorded from 71 MST neurons that showed a statistically significant relationship between firing rate and eye speed during pursuit with stabilized targets. An additional 24 neurons were excluded from our sample for reasons outlined in METHODS. The responses during pursuit are shown in Fig. 2, A and C, for two example MST neurons. Inspection of the rasters (right) reveals that both neurons showed directionally selective responses with increased firing for rightward pursuit, indicated by upward deflections of the traces. The responses began somewhat after the onset of pursuit (vertical dotted lines) and persisted during the interval when the target was stabilized with respect to the moving eye (between the 2 vertical dashed lines). This particular neuron was among the minority that had a weak sensitivity to eye position and therefore appears to be weakly speed tuned before the target started to move. Eye position before the onset of pursuit varied as a function of target speed because the initial position of the target varied to cause the time of target stabilization to occur as the target crossed straight-ahead gaze for all target speeds.
FIG. 2.
Example eye velocities and neuronal responses to pursuit at different speeds for 2 neurons. A and C, left: the gray and black traces show averages of eye velocity from trials that presented stabilized and control target motions at 0, 5 10, 15, 20, and 30°/s. Responses to 2°/s were not included due to space constraints. Dashed black vertical lines indicate the analysis interval from 100 ms after the onset to 100 ms after the offset of the stabilization period. Right: the rasters indicate neural response during trials that imposed target stabilization. Dotted lines indicate the onset of target motion and dashed vertical lines indicate the analysis interval. B and D: plots of neural response during the analysis interval as a function of eye speed. Symbols show actual responses and curves show the best fitting cubic spline function. Black and gray indicate responses taken from trials in which the targets were or were not stabilized, respectively. Diamonds on each trace indicate the neuron's preferred speed as determined by the highest point of the spline fit.
Plots of the average firing rate during the analysis interval (Fig. 2, B and D) show that firing rate increased as a function of eye speed in the preferred direction. Further, firing rate during target stabilization (black filled symbols and curves) was similar to that in the same interval for control trials without stabilization (gray open symbols and curves). Comparison of the average eye velocities elicited by target motion with (black traces in Fig. 2, A and C) and without (gray traces) target stabilization revealed that pursuit was similar in the two conditions of target motion. Thus the small differences between the gray and black firing rate traces could reflect the presence and absence of image motion from the tracking target during control and stabilized-target trials, respectively.
For both example neurons in Fig. 2, the peak of the curve relating firing rate to eye velocity (Fig. 2, B and D, large diamonds) was similar in the presence or absence of target stabilization. Analysis of our full sample of MST neurons revealed that neurons with similar preferred speeds for stabilized and nonstabilized trials were in the majority: when we plotted the best speed computed from responses during control trials as a function of those during target stabilization (Fig. 3A), many neurons fell near the unity line. At the same time, a number of neurons had lower values of preferred speed during target stabilization than during control trials. The scatter in Fig. 3A (r = 0.673, P < 0.01) emphasizes that visual inputs from the target can affect responses in MST and underscores the need to use stabilized targets to assess most accurately the relationship between firing rate and eye speed for the extraretinal response in MST.
FIG. 3.
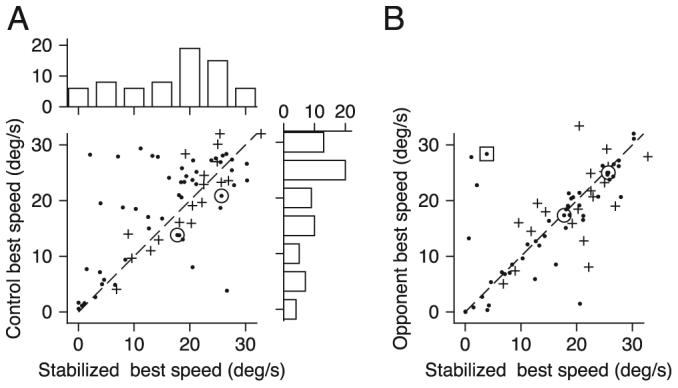
Quantitative analysis of the effect of target stabilization on neural responses. A: scatter plot comparing preferred speed in control trials and trials with stabilized targets. The marginal histograms show the distribution of preferred speeds for each condition. B: scatter plot comparing preferred speed for the opponent response (preferred response minus null response) and the preferred-direction only response. In both cases, trials from the stabilized condition were used. In A and B, ● and +, data from each of the 2 monkeys, ☉, points correspond to the examples from Fig. 2. - - -, slope of 1.
The analysis in Figs. 2 and 3 plots firing rate as a function of eye speed rather than target speed. Because eye speed can be somewhat less than target speed when the latter is 30°/s, this choice blurs the important point that many MST neurons emitted their largest responses for the highest target speed we used to study pursuit. Of the 71 neurons we studied in MST, 30% gave the largest response for the largest target/eye speed during stabilized pursuit.
We next tested whether the neurons' best speeds during target stabilization changed when we considered the opponent motion response instead of only the response in the preferred direction. We estimated each neuron's opponent response for each speed of pursuit by subtracting the response to motion in the neuron's null direction from that for motion in the preferred direction, both recording with stabilized targets. For the example neurons in Fig. 2, there was very little response during pursuit in the null direction, and the best speed computed using the opponent response differed little from that computed using responses in the preferred direction only. Analysis of our full sample of MST neurons revealed that almost all neurons showed the same preferred speed based on the opponent and preferred-direction responses (Fig. 3B). A few outliers did have different preferred speeds for opponent verses preferred-only responses because they were not very directional.
Temporal evolution of pursuit responses
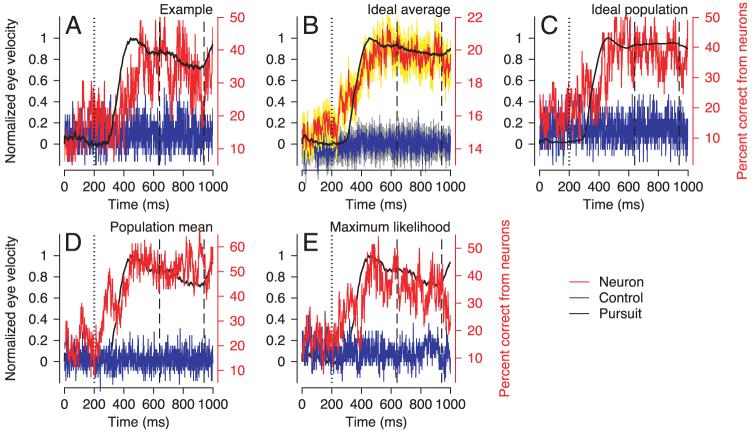
During the initiation of pursuit, the firing rate of MST neurons follows a trajectory similar to that of eye velocity. To compare the firing rate and eye velocity directly, we analyzed firing rate in 20-ms bins and eye velocity on a 1-ms time scale, normalized both traces so they had maximal values of one, and plotted them together. For both the individual neuron illustrated in Fig. 4A and the grand average from all 71 MST neurons in Fig. 4B, firing rate during the initiation of pursuit rose more slowly than did eye velocity, causing the appearance of a time lag of ∼60 ms. However, the average MST response did rise from baseline in advance of the initiation of pursuit (Fig. 4B). We attribute this early response to a small subset of neurons with short-latency responses during the initiation of pursuit, as also was reported by Newsome et al. (1988); in agreement with their report, most MST neurons started to respond somewhat after the initiation of pursuit. Both the neural and the eye-velocity responses had reached a plateau by the time the period of target stabilization began (vertical dashed lines), and neither response changed dramatically over the 300-ms stabilization period.
FIG. 4.
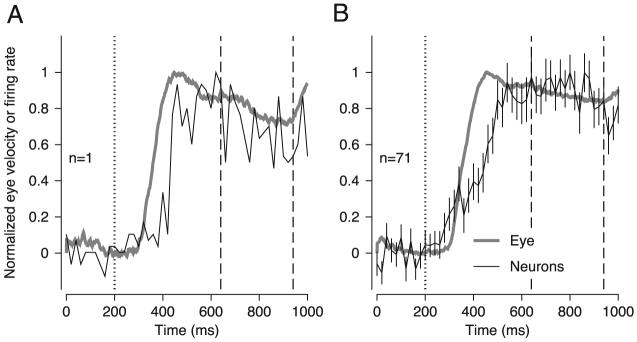
Evolution of eye velocity and extraretinal responses of medial-superior-temporal area (MST) neurons over time. A: an example neuron. B: averages across 71 MST neurons. Thick gray traces indicate average eye velocity in response to 10°/s target motion; black trace indicates the average firing rate. Dotted vertical trace indicates onset of target motion. Dashed vertical lines indicate the onset and offset of the stabilization period delayed by 100 ms. Firing rate and eye velocity magnitudes have been normalized to make them directly comparable. Error bars in B indicate SE. The firing rate traces consisted of only 50 points because firing rate was computed in 20-ms bins. Target speed was 10°/s.
The analysis in Fig. 4 asks how well the average firing rate of MST neurons tracks eye velocity. We next turn this question around and ask how well an ideal observer would be able to guess eye speed in the preferred direction by looking at individual neural responses. For each individual neuron, we computed the time course of the performance of an ideal observer whose goal was to determine the eye speed that generated an individual neural response (details in METHODS). We express the result as percentage correct for the ideal observer, where random behavior would be 14% correct, given that there were seven target speeds (including 0). Analysis of the individual neuron that gave the best performance (Fig. 5A) shows that the ideal observer performance lags eye speed slightly. Although performance was considerably better than for shuffled data (blue trace), it was almost never better than 50%: an ideal observer mostly guessed eye speed incorrectly. Over the period of target stabilization, the time course of ideal observer performance based on the example neuron in Fig. 5A fluctuated between 30 and 50% (mean: 40%). When averaged across all 71 MST neurons (Fig. 5B), the time course of ideal observer performance is not as good as in the example neuron. Over the period of target stabilization, it fluctuated from 23.9 to 25.9% (mean: 25.0%). To improve performance, we then generated population averages for each trial by averaging the firing rates of all the neurons (details in METHODS). Ideal observer performance was somewhat improved (Fig. 5C): over the period of target stabilization, it fluctuated between 22.5 and 53.1% (mean: 40.1%). The values shown represent percent correct averaged over all the eye speeds tested, but note that these estimates did not depend strongly on eye speed, and were best for eye speeds of 5°/s. Further, they improved by only 1-2% if we included analyses done only at each neuron's preferred speed. Our finding of relatively poor prediction of eye speed from the firing rate of MST neurons contrasts with the performance of an ideal observer attempting to predict target velocity from the eye velocity in individual trials (eye velocity control, see METHODS). During the stabilization, interval, target velocity was predicted correctly from eye velocity on 77.4% of trials.
FIG. 5.
Ideal observer performance in attempting to predict eye velocity from the responses of MST neurons. A: individual neuron that had the best performance in the analysis interval. B: averaged ideal observer performance across 71 neurons. The yellow and gray areas show error bars computed at each time point and indicating standard errors of the mean. C: ideal observer performance based on averages of firing rate across neurons. No error bars are present in C because the ideal observer analysis was performed only once on values averaged across neurons. D: percentage correct based on the mean method for decoding the population response in MST. E: percentage correct based on a maximum likelihood decoding of the population response in MST. In each panel, the black trace shows normalized eye velocity aligned with the values of the left-hand y axes. Red and blue traces show ideal observer performance for real vs. shuffled neural responses, aligned on the right-hand, red y axes. Dotted vertical trace indicates onset of target motion. Dashed black vertical lines indicate the onset and offset of the stabilization period delayed by 100 ms.
We also tested two methods of decoding the population response in MST to see whether they could improve ideal observer performance at estimating target velocity. When we decoded the population response by averaging single responses drawn from each neuron in our population of MST neurons, target velocity was predicted correctly ∼50% of the time. When we used a maximum-likelihood estimator based on single responses drawn from each neuron in our population of MST neurons, target velocity was predicted correctly ∼40% of the time. These latter two methods were chosen because they emulate the situation faced by the brain, where an estimate of target velocity must be formed on the basis of one response for each of the neurons in the population. Although it is not possible to test all plausible methods for decoding the population response in MST, we think that the high level of variation in the responses of MST neurons underlies the failure of the methods we have tested to estimate target velocity reliably. Thus we anticipate that other methods for decoding the population responses also would prove unreliable. We also are aware that the estimates might be improved if we had recorded a larger sample of neurons. However, prior analyses (Shadlen et al. 1996) have indicated that the improvement asymptotes at a population of ∼100 neurons, only slightly larger than our sample, when the variation in neural responses is correlated, as it is in many cortical areas.
Although MST firing did not support very accurate performance in predicting target speed, it did start to predict correctly above-chance levels before the initiation of pursuit (Fig. 5, B-E) as might be expected from the observation that the average firing rate of MST neurons also started to rise before the initiation of pursuit (Fig. 4B).
Ideal-observer analysis of pursuit gain control
To provide a context for interpretation of the ideal-observer performance of our neurons, we performed the same analysis on a behavioral response that, like the neuronal responses, depends on eye velocity. We used the techniques of prior studies (Churchland and Lisberger 2002; Schwartz and Lisberger 1994) to measure the behavioral response to brief perturbations of target motion delivered during pursuit over the same range of seven speeds used for recordings in MST. For example, Fig. 6A shows examples of the average time course of eye velocity for perturbations delivered during pursuit at 2 and 10°/s. Figure 6B verifies the previous observation that the response to the perturbation is a function of the speed of pursuit at the time of the perturbation with larger responses for faster ongoing speeds (Churchland and Lisberger 2002; Schwartz and Lisberger 1994).
FIG. 6.
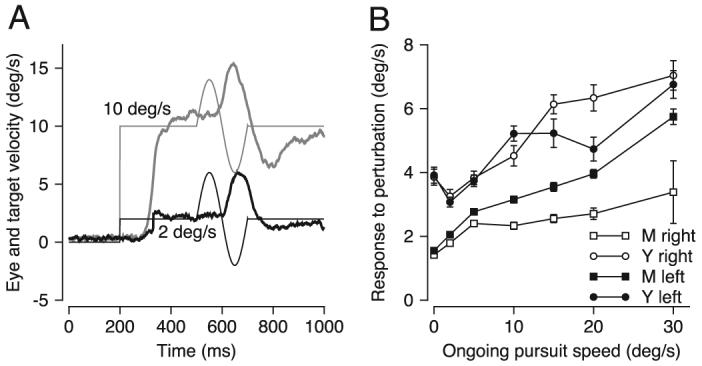
Response of the pursuit system to brief perturbations of target motion presented under different initial conditions. A: fine and bold traces show target and eye velocity, black and gray indicate responses to target motion at 2 and 10°/s. B: average amplitude of the response to perturbations is plotted as a function of ongoing pursuit speed at the time of the perturbation. Different symbols show responses of 2 monkeys during rightward and leftward pursuit. Error bars indicate standard error of the mean. The perturbations used to generate these data comprised single cycles of a 5-Hz sine wave with an amplitude of ±4°/s.
We configured the ideal-observer analysis to ask how well an ideal observer could predict ongoing eye velocity if the observer was given the amplitude of the behavioral response to the perturbation in an individual trial. Performance ranged from 32% correct to 49% correct (Table 1) and averaged 35% over all experiments with perturbations that consisted of a single cycle of a 5-Hz, ±8°/s sine wave. Performance was considerably better for the actual data than for shuffled data (Table 1). Ideal-observer performance did not depend systematically on the perturbation parameters tested.
TABLE 1.
Predictions of target velocity made by an ideal observer based on distributions of the amplitudes of eye-velocity responses to brief perturbations of target velocity
| 5 Hz, 4°/s | 10 Hz, 8°/s | 5 Hz, 8°/s | ||||
|---|---|---|---|---|---|---|
| Right, % | Left, % | Right, % | Left, % | Right, % | Left, % | |
| Each cell in the table shows the percentage of correct decisions. Given 7 target velocities, random performance would yield 14% correct. The three major columns show predictions for three different perturbations (5- or 10-Hz sine wave, 4 or 8°/s), with the minor columns summarizing data for perturbations delivered during rightward and leftward pursuit. For each monkey, the two rows show the predictions based on actual versus shuffled data. | ||||||
| Monkey 1 | ||||||
| Actual data | 37.7 | 34.5 | 31.8 | 35.3 | 30 | 35.7 |
| Shuffled data | 23 | 12.1 | 10.6 | 8.8 | 17.1 | 15.7 |
| Monkey 2 | ||||||
| Actual data | 34.8 | 48.6 | 35.3 | 49 | 37.9 | 35.7 |
| Shuffled data | 12.1 | 12.9 | 18.8 | 11.2 | 10.6 | 18.6 |
The need to present perturbations during accurate tracking precludes assessment of how the ideal observer's estimate of eye velocity would evolve over time. It is not practical to present perturbations during the initiation of pursuit because the large amounts of image velocity present at that time make the responses too small and variable (Churchland and Lisberger 2001). However, similar intervals were used for analysis of the responses to perturbations and the firing rate during target stabilization, allowing direct comparison of these two estimates even though they were obtained in different experiments. Over the 300-ms analysis interval, ideal-observer performance predicted target velocity correctly 40% of the time on the basis of the average firing rate across our sample of 71 MST neurons and 35% of the time on the basis of the amplitude of behavioral responses to brief perturbations of target motion.
Relationship between firing rate and speed for retinal and extra-retinal signals
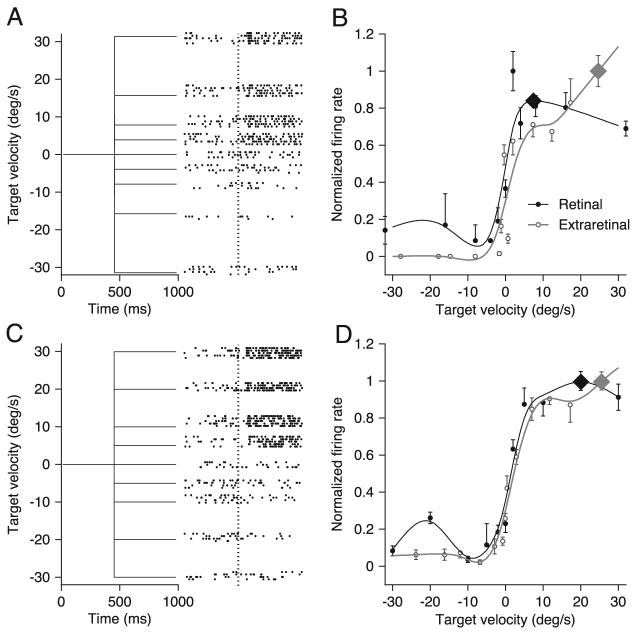
Figure 7 compares the retinal and extraretinal signals of individual neurons in MST by plotting the relationship between the firing rate of MST neurons and the speed of image motion during fixation and the relationship between firing rate and eye velocity during pursuit of stabilized targets. For the two example neurons illustrated in Fig. 7, A and C, the onset of visual stimulus motion during fixation (vertical dotted line in the rasters) caused an initial transient response after a latency of ∼80 ms followed by a sustained response that continued through the duration of stimulus motion. We measured the responses of MST neurons for image motion at speeds ≤128°/s, but we have shown only those for speeds ≤30°/s to allow a fair comparison with the responses of the same neurons during pursuit of target motion at speeds ≤30°/s. The preferred directions for image and eye motion were the same for the example neurons in Fig. 7, but this was not routinely the case. For each neuron, the preferred speed has been derived for image and eye motion in their respective preferred directions. It was necessary to test responses to image motion using a dot texture rather than a spot target for two reasons. First, most MST neurons responded poorly to the motion of spot targets during fixation; second, the use of a dot texture that moved within a virtual stationary aperture prevented the stimulus from quickly reaching the edge of the screen for high speeds.
FIG. 7.
Responses to motion of a large visual stimulus during fixation for 2 representative neurons. A and C, left: target velocity from trials that presented stimulus motion at between −32 and 32°/s. Right: rasters to show neural responses to the stimulus motion. The vertical dotted lines indicate the onset of target motion. B and D: graphs of averaged firing rate as a function of the speed of stimulus motion. Symbols show averages taken from the data and curves show the best fitting cubic spline function. Black points and curves indicate responses to image motion during fixation. Gray points and curves indicate responses taken from the stabilized period of pursuit trials. The large diamonds on each trace indicate the neuron's preferred speed as determined by the highest point of the spline fit, within the range of stimulus speeds presented during the experiment.
To exclude the transient responses from analysis of the responses to image motion during fixation, we computed the average firing rate in the 400-ms interval from 150 ms after motion onset to the end of stimulus motion. We also normalized each curve so that the maximal responses had values of one in each case. As before, the relationships were fitted with smoothing cubic spline functions to determine the speed of image or eye motion that provided the largest neural response. In different neurons, the best speeds for retinal (black filled symbols and curves) and extraretinal inputs (gray open symbols and curves) could be the same (Fig. 7D) or different (Fig. 7B).
Across the full sample of MST neurons, we found little correlation between speeds of image and eye motion that caused the largest responses for retinal and extraretinal inputs. In the scatter plots of Fig. 8, A and B, each point plots the response of one neuron and shows the best speed for extraretinal inputs as a function of that for retinal inputs. To ensure that our conclusions were not affected by the different range of speeds we could test for pursuit and for visual motion presented during fixation, the analysis in Fig. 8A considered only each neuron's responses to image motion ≤30°/s, whereas that in B considered each neuron's responses to the full range of image speeds we presented. The graphs contain different numbers of points because only 42 neurons showed a statistically significant relationship between firing rate and image speed for image speeds ≤30°/s, whereas 52 showed significant relationships when image speeds covered the full range ≤128°/s. In Fig. 8A, regression analysis yielded a statistically insignificant correlation coefficient of 0.03; in Fig. 8B, the correlation coefficient of −0.3 was statistically significant (P = 0.03). Note that our sample of responses to image motion during fixation may have been biased by our search procedure, which selected for further study only MST neurons that were clearly responsive during pursuit.
FIG. 8.
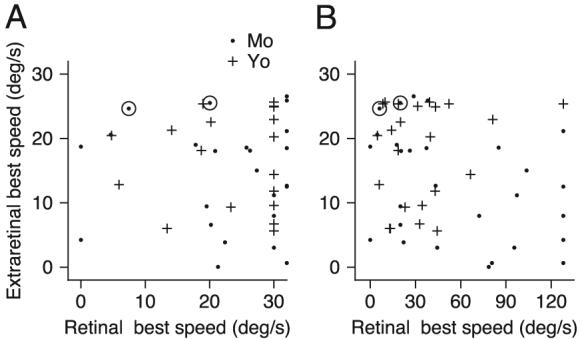
A comparison of the preferred speeds of the visual response during fixation and the extraretinal response during the period of target stabilization. + and ●, response of 1 neuron recorded in the 2 monkeys. A: preferred speeds were generated from responses to visual motion excluding speeds higher than those used to examine pursuit. B: preferred speeds were generated from responses to visual motion at all speeds tested, including 64 and 128°/s.
As illustrated in Fig. 9, the peak response magnitudes for image motion during fixation and for pursuit were weakly correlated (Spearman's rank correlation, r = 0.50 P < 0.01). On average, the maximum responses during image motion were slightly lower than during pursuit (image motion: 20.4 spikes/s; pursuit: 29.4 spikes/s) and this difference was significant (P < 0.05).
FIG. 9.
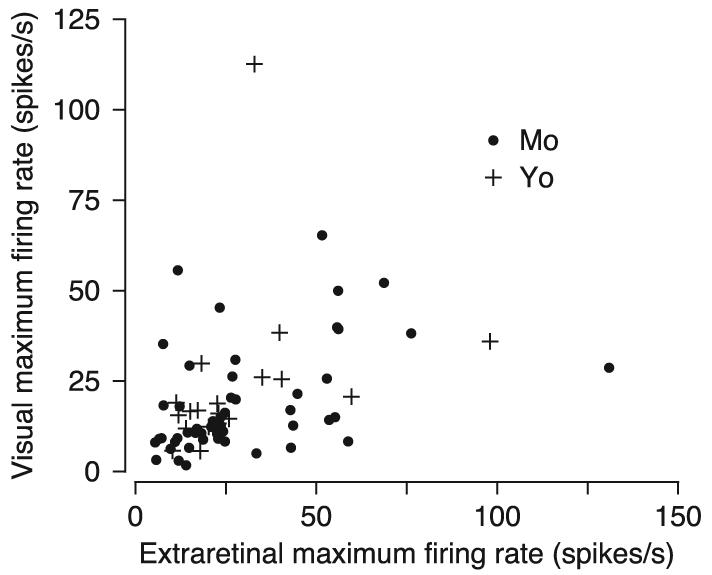
Quantitative comparison of the amplitudes of the largest responses for image motion during fixation and pursuit with target stabilization. + and ●, data, each from a different neuron, recorded in the 2 monkeys.
DISCUSSION
We have used target stabilization in a dark room to eliminate image motion from both the background and the tracking target for a brief interval during sustained smooth-pursuit eye movements at different speeds. For the selected group of MST neurons that are direction selective during pursuit, our measurements of their responses during target stabilization provide a direct assessment of the relationship between firing rate and eye speed for the extraretinal signal. We found that extraretinal responses in area MST are related to eye speed. Our data agree with prior studies based on smaller samples and fewer speeds (Kawano et al. 1984; Shenoy et al. 2002) and demonstrate that measurements of MST responses during sustained pursuit without target stabilization provide an estimate of the extraretinal signal that is only somewhat imperfect.
The fact that lesions of MST cause deficits in both the initiation and maintenance of pursuit (Dursteler and Wurtz 1988) implies that the extraretinal and retinal components of MST neurons' responses play important roles in pursuit. We will consider possible roles as: eye-movement command signals to drive the initiation of pursuit; target-velocity signals to support the maintenance of pursuit; and control signals for regulating the gain of visual-motor transformation in pursuit.
Initiation of pursuit
Analysis of their latency and time course suggests that the pursuit responses of MST neurons are not entirely suitable to drive the initiation of pursuit. In agreement with prior reports, we found that the average pursuit response of MST neurons appears to begin before the onset of pursuit eye velocity, but that the rising phase of average firing rate lags the rising phase of pursuit eye velocity. The activity that precedes the onset of smooth eye velocity would be suitable to contribute to the initiation of pursuit, but the later activity lags eye velocity by too much to be a driving signal. The population average from our sample is consistent with the observation of Newsome et al. (1988) that most MST responses lagged the initiation of pursuit by ≥50 ms, while a small number of MST neurons began firing as much as 100 ms before pursuit initiation. In agreement with our data, Ilg and Thier (2003) also reported that the mean latency of MST pursuit responses was 50 ms shorter than the latency of the eye movements.
Our use of stabilized targets characterizes extraretinal signals only during sustained pursuit because we were not able to stabilize targets during pursuit initiation. Even though the dim, small pursuit targets used in our study were poor stimuli for driving visual responses in area MST, visual responses still may have contributed to the earliest part of the pursuit response. Ilg and Thier (2003) used a different stimulus to measure the extraretinal response during pursuit initiation: they employed an “imaginary” target that provided image motion only in peripheral parts of the visual field, avoiding foveal stimulation. While their “imaginary” target does eliminate foveal image motion during pursuit initiation, it provides image motion on the peripheral retina that comprises the large receptive fields of most MST neurons. Thus it seems possible in their study as well that visual inputs contributed to the early parts of MST responses.
Maintenance of pursuit
A number of reports have suggested that the combination of retinal and extraretinal components of MST neurons may provide a signal related to target velocity (Newsome et al. 1988; Pack et al. 2001) that operates as a major control signal for pursuit (Robinson 1976). According to the model suggested by these prior studies, neurons with retinal and extraretinal components of the same preferred direction would provide retinal inputs at target speed until eye velocity was initiated. As eye velocity increased and reached target velocity, the extraretinal signal would grow as the retinal signal shrank. As a consequence, the discharge of MST neurons would persist at the same level even when the eye is tracking the target and retinal inputs are small or absent. On the one hand, our data would be compatible with the idea that MST drives pursuit eye velocity: the size of the extraretinal component of MST responses often increases monotonically as a function of the eye speed during sustained pursuit. On the other hand, our data contradict the same idea at the level of single neurons by showing that the retinal and extraretinal components of individual MST neurons have different preferred speeds and different sensitivities to image and eye speed. Many individual MST neurons would respond quite differently to image motion at 30°/s versus accurate pursuit at the same speed, even though target velocity is unchanged. However, we concede that this deficit could be obviated at the level of the population of MST neurons.
A greater concern is our finding that single neurons might be ill-suited to provide a signal related to target velocity simply because their responses are too variable. An ideal observer would only be able to discern target velocity correctly from the firing rate of an average neuron ∼25% of the time. Ideal observer performance increases only from 25 to 50% when the population response is decoded. It is possible that more optimal decoding methods could improve ideal observer performance further particularly because the maximum likelihood decoder is more precise when larger numbers of neurons provide a better estimate of firing rate covariance (this explains why the maximum likelihood estimate in Fig. 5E is worse than the population estimate in D). Combining neural responses using an optimized weight for each neuron might also have yielded an improved population estimate, but we were reluctant to add so many free parameters when trying to estimate a relatively small number of eye velocities. However, it seems difficult to bridge the large gap between the 50% correct based on population averages and ≥80% correct that would be needed to drive eye velocity accurately. Therefore poor ideal-observer performance for single neurons at least raises the possibility that MST neurons are too imprecise to provide a coherent signal for driving eye velocity.
It is now generally accepted that velocity memory in the form of positive feedback of eye velocity supports the maintenance of pursuit even in the absence of any image motion during target stabilization (Lisberger et al. 1987). If the extraretinal component of MST responses arose via feedback to the cortex from the cerebellum, then MST could be part of the positive feedback pathway that supports pursuit maintenance. During pursuit across a structured scene, the function of a positive feedback pathway through MST might be enhanced by the retinal inputs to MST neurons, which often show the opposite direction selectivity relative to the extraretinal signals (Komatsu and Wurtz 1988; Shenoy et al. 2002). Again, however, our finding that the firing of MST neurons reveals eye velocity only imprecisely implies that the output of MST might be better suited to a component of pursuit that predicted eye velocity relatively poorly when subjected to the ideal observer analysis. Even if the extraretinal signal in MST were used for other purposes, excellent steady-state tracking during pursuit maintenance still could be mediated by eye velocity positive feedback through the cerebellum (Lisberger and Fuchs 1978; Lisberger and Miles 1980).
Regulation of visual-motor gain
Recent analyses of the pursuit system have uncovered an internal gain control that changes as a function of eye speed. The setting of the gain control under different conditions can be probed by recording the eye velocity evoked by brief perturbations of target motion. In both monkeys and humans, the setting of the gain control increases as a function of the steady tracking eye velocity at the time of the perturbation (Churchland and Lisberger 2002; Schwartz and Lisberger 1994). By subjecting the behavioral responses to the same ideal observer analysis used for the neuronal responses in MST, we were able to compare the resolution of visual-motor gain with the resolution of MST eye-velocity signals. We used the size of the eye velocity responses to brief perturbations of target velocity as estimates of the setting of the gain control. Our analysis revealed that an ideal observer would predict eye velocity about as well from the size of the responses to perturbations as from the responses of MST neurons. Thus the extraretinal signal in MST is suitable in two ways for regulating visual-motor gain: it often increases monotonically as a function of eye velocity (i.e., Fig. 2D) as does the visual-motor gain (Schwartz and Lisberger 1994), and it has a suitable eye velocity resolution. It would interesting to examine firing rates in MST neurons during trials that presented perturbations during ongoing pursuit; however, results would be difficult to interpret for two reasons. First, both eye and image motion are present during perturbations and a change in neural response could reflect either. Second, a change in neural response during perturbations could reflect a change in visual-motor gain but would be expected no matter what role MST plays in pursuit because neural responses depend on eye velocity.
A cause-and-effect relationship between activity in the frontal pursuit area (FPA) and gain control has been demonstrated by applying micro-stimulation in the FPA during brief perturbations of target motion (Tanaka and Lisberger 2002). In the FPA, microstimulation gives rise to enhanced responses to brief target perturbations, implying that activity in the FPA controls the internal gain of visual-motor transmission for pursuit. The same idea has not been tested directly using micro-stimulation in MST, although the results of prior experiments using microstimulation in MST indicate that it may override visual inputs for pursuit rather than enhancing them (Komatsu and Wurtz 1989). Still, anatomical evidence indicates that MST projects to FPA (Tian and Lynch 1996), leaving open the possibility that the extra-retinal component of the output of MST provides inputs to the FPA that ultimately influence the visual-motor gain for pursuit.
ACKNOWLEDGMENTS
We thank K. MacLeod and E. Montgomery for assistance with animal care, S. Ruffner for computer programming, and S. Tokiyama for many forms of technical assistance. We also thank A. Pouget for advice on population decoding.
GRANTS This research was supported by the Howard Hughes Medical Institute and National Eye Institute Grant EY-03878.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- Boussaoud D, Desimone R, Ungerleider LG. Subcortical connections of visual areas MST and FST in macaques. Vis Neurosci. 1992;9:291–302. doi: 10.1017/s0952523800010701. [DOI] [PubMed] [Google Scholar]
- Bremmer F, Ilg UJ, Thiele A, Distler C, Hoffmann KP. Eye position effects in monkey cortex. I. Visual and pursuit-related activity in extrastriate areas MT and MST. J Neurophysiol. 1997;77:944–961. doi: 10.1152/jn.1997.77.2.944. [DOI] [PubMed] [Google Scholar]
- Britten KH, Newsome WT, Shadlen MN, Celebrini S, Movshon JA. A relationship between behavioral choice and the visual responses of neurons in macaque MT. Vis Neurosci. 1996;13:87–100. doi: 10.1017/s095252380000715x. [DOI] [PubMed] [Google Scholar]
- Churchland AK, Lisberger SG. Gain control in human smooth-pursuit eye movements. J Neurophysiol. 2002;87:2936–2945. doi: 10.1152/jn.2002.87.6.2936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchland MM, Lisberger SG. Apparent motion produces multiple deficits in visually guided smooth pursuit eye movements of monkeys. J Neurophysiol. 2000;84:216–235. doi: 10.1152/jn.2000.84.1.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchland MM, Lisberger SG. Experimental and computational analysis of monkey smooth pursuit eye movements. J Neurophysiol. 2001;86:741–759. doi: 10.1152/jn.2001.86.2.741. [DOI] [PubMed] [Google Scholar]
- de Oliveira SC, Thiele A, Hoffmann KP. Synchronization of neuronal activity during stimulus expectation in a direction discrimination task. J Neurosci. 1997;17:9248–9260. doi: 10.1523/JNEUROSCI.17-23-09248.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dursteler MR, Wurtz RH. Pursuit and optokinetic deficits following chemical lesions of cortical areas MT and MST. J Neurophysiol. 1988;60:940–965. doi: 10.1152/jn.1988.60.3.940. [DOI] [PubMed] [Google Scholar]
- Erickson RG, Thier P. A neuronal correlate of spatial stability during periods of self-induced visual motion. Exp Brain Res. 1991;86:608–616. doi: 10.1007/BF00230534. [DOI] [PubMed] [Google Scholar]
- Glickstein M, Cohen JL, Dixon B, Gibson A, Hollins M, Labossiere E, Robinson F. Corticopontine visual projections in macaque monkeys. J Comp Neurol. 1980;190:209–229. doi: 10.1002/cne.901900202. [DOI] [PubMed] [Google Scholar]
- Ilg UJ, Thier P. Visual tracking neurons in primate area MST are activated by smooth pursuit eye movements of an “imaginary” target. J Neurophysiol. 2003;90:1489–1502. doi: 10.1152/jn.00272.2003. [DOI] [PubMed] [Google Scholar]
- Kawano K, Shidara M, Watanabe Y, Yamane S. Neural activity in cortical area MST of alert monkey during ocular following responses. J Neurophysiol. 1994;71:2305–2324. doi: 10.1152/jn.1994.71.6.2305. [DOI] [PubMed] [Google Scholar]
- Komatsu H, Wurtz RH. Relation of cortical areas MT and MST to pursuit eye movements. I. Localization and visual properties of neurons. J Neurophysiol. 1988;60:580–603. doi: 10.1152/jn.1988.60.2.580. [DOI] [PubMed] [Google Scholar]
- Komatsu H, Wurtz RH. Modulation of pursuit eye movements by stimulation of cortical areas MT and MST. J Neurophysiol. 1989;62:31–47. doi: 10.1152/jn.1989.62.1.31. [DOI] [PubMed] [Google Scholar]
- Lisberger SG, Fuchs AF. Role of primate flocculus during rapid behavioral modification of vestibuloocular reflex. I. Purkinje cell activity during visually guided horizontal smooth-pursuit eye movements and passive head rotation. J Neurophysiol. 1978;41:733–763. doi: 10.1152/jn.1978.41.3.733. [DOI] [PubMed] [Google Scholar]
- Lisberger SG, Miles FA. Role of primate medial vestibular nucleus in long-term adaptive plasticity of vestibuloocular reflex. J Neurophysiol. 1980;43:1725–1745. doi: 10.1152/jn.1980.43.6.1725. [DOI] [PubMed] [Google Scholar]
- Lisberger SG, Morris EJ, Tychsen L. Visual motion processing and sensory-motor integration for smooth pursuit eye movements. Annu Rev Neurosci. 1987;10:97–129. doi: 10.1146/annurev.ne.10.030187.000525. [DOI] [PubMed] [Google Scholar]
- Liu J, Newsome WT. Functional organization of speed tuned neurons in visual area MT. J Neurophysiol. 2003;89:246–256. doi: 10.1152/jn.00097.2002. [DOI] [PubMed] [Google Scholar]
- Morris EJ, Lisberger SG. Different responses to small visual errors during initiation and maintenance of smooth-pursuit eye movements in monkeys. J Neurophysiol. 1987;58:1351–1369. doi: 10.1152/jn.1987.58.6.1351. [DOI] [PubMed] [Google Scholar]
- Newsome WT, Wurtz RH, Komatsu H. Relation of cortical areas MT and MST to pursuit eye movements. II. Differentiation of retinal from extraretinal inputs. J Neurophysiol. 1988;60:604–620. doi: 10.1152/jn.1988.60.2.604. [DOI] [PubMed] [Google Scholar]
- Pack C, Grossberg S, Mingolla E. A neural model of smooth pursuit control and motion perception by cortical area MST. J Cogn Neurosci. 2001;13:102–120. doi: 10.1162/089892901564207. [DOI] [PubMed] [Google Scholar]
- Pouget A, Dayan P, Zemel R. Information processing with population codes. Nat Rev Neurosci. 2000;1:125–132. doi: 10.1038/35039062. [DOI] [PubMed] [Google Scholar]
- Pouget A, Zhang K, Deneve S, Latham PE. Statistically efficient estimation using population coding. Neural Comput. 1998;10:373–401. doi: 10.1162/089976698300017809. [DOI] [PubMed] [Google Scholar]
- Priebe NJ, Lisberger SG. Constraints on the source of short-term motion adaptation in macaque area MT. II. tuning of neural circuit mechanisms. J Neurophysiol. 2002;88:370–382. doi: 10.1152/jn.2002.88.1.370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rashbass C. The relationship between saccadic and smooth tracking eye movements. J Physiol. 1961;159:326–338. doi: 10.1113/jphysiol.1961.sp006811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson DA. Adaptive gain control of vestibuloocular reflex by the cerebellum. J Neurophysiol. 1976;39:954–969. doi: 10.1152/jn.1976.39.5.954. [DOI] [PubMed] [Google Scholar]
- Saito H, Yukie M, Tanaka K, Hikosaka K, Fukada Y, Iwai E. Integration of direction signals of image motion in the superior temporal sulcus of the macaque monkey. J Neurosci. 1986;6:145–157. doi: 10.1523/JNEUROSCI.06-01-00145.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz JD, Lisberger SG. Initial tracking conditions modulate the gain of visuo-motor transmission for smooth pursuit eye movements in monkeys. Vis Neurosci. 1994;11:411–424. doi: 10.1017/s0952523800002352. [DOI] [PubMed] [Google Scholar]
- Shadlen MN, Britten KH, Newsome WT, Movshon JA. A computational analysis of the relationship between neuronal and behavioral responses to visual motion. J Neurosci. 1996;16:1486–1510. doi: 10.1523/JNEUROSCI.16-04-01486.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenoy KV, Crowell JA, Andersen RA. Pursuit speed compensation in cortical area MSTd. J Neurophysiol. 2002;88:2630–2647. doi: 10.1152/jn.00002.2001. [DOI] [PubMed] [Google Scholar]
- Shikin EV, Plis AI. Handbook on Splines for the User. CRC; Boca Raton, FL: 1995. [Google Scholar]
- Squatrito S, Maioli MG. Encoding of smooth pursuit direction and eye position by neurons of area MSTd of macaque monkey. J Neurosci. 1997;17:3847–3860. doi: 10.1523/JNEUROSCI.17-10-03847.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka K, Hikosaka K, Saito H, Yukie M, Fukada Y, Iwai E. Analysis of local and wide-field movements in the superior temporal visual areas of the macaque monkey. J Neurosci. 1986;6:134–144. doi: 10.1523/JNEUROSCI.06-01-00134.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka M, Lisberger SG. Enhancement of multiple components of pursuit eye movement by microstimulation in the arcuate frontal pursuit area in monkeys. J Neurophysiol. 2002;87:802–818. doi: 10.1152/jn.00409.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiele A, Bremmer F, Ilg UJ, Hoffmann KP. Visual responses of neurons from areas V1 and MT in a monkey with late onset strabismus: a case study. Vision Res. 1997;37:853–863. doi: 10.1016/s0042-6989(96)00256-8. [DOI] [PubMed] [Google Scholar]
- Tian JR, Lynch JC. Corticocortical input to the smooth and saccadic eye movement subregions of the frontal eye field in Cebus monkeys. J Neurophysiol. 1996;76:2754–2771. doi: 10.1152/jn.1996.76.4.2754. [DOI] [PubMed] [Google Scholar]