Abstract
Coral reef communities are in a state of change throughout their geographical range. Factors contributing to this change include bleaching (the loss of algal symbionts), storm damage, disease, and increasing abundance of macroalgae. An additional factor for Caribbean reefs is the aftereffects of the epizootic that reduced the abundance of the herbivorous sea urchin, Diadema antillarum. Although coral reef communities have undergone phase shifts, there are few studies that document the details of such transitions. We report the results of a 40-month study that documents changes in a Caribbean reef community affected by bleaching, hurricane damage, and an increasing abundance of macroalgae. The study site was in a relatively pristine area of the reef surrounding the island of San Salvador in the Bahamas. Ten transects were sampled every 3–9 months from November 1994 to February 1998. During this period, the corals experienced a massive bleaching event resulting in a significant decline in coral abundance. Algae, especially macroalgae, increased in abundance until they effectively dominated the substrate. The direct impact of Hurricane Lili in October 1996 did not alter the developing community structure and may have facilitated increasing algal abundance. The results of this study document the rapid transition of this reef community from one in which corals and algae were codominant to a community dominated by macroalgae. The relatively brief time period required for this transition illustrates the dynamic nature of reef communities.
It has become an accepted principle of ecology that communities may exist in alternative states that have varying degrees of stability (1, 2). Much of the theoretical work on this subject carries with it the implication that the transition between one state and another may be rapid (2). Factors responsible for causing these transitions may include the removal of a Keystone Species (3, 4) or a change in the frequency and intensity of some form of disturbance so that it no longer has the effect postulated under the Intermediate Disturbance hypothesis (5). Empirical studies of shifts in community structure have emphasized either the effect of species removal (3, 4) or the combined effects of natural and anthropogenic disturbance (6). However, it is rare that shifts in community structure are observed as they happen and documented quantitatively.
Coral reef ecosystems worldwide are undergoing changes in community structure because of a number of natural and anthropogenic processes. Overfishing and nutrient loading have altered interactions among macroalgae and their herbivores, leading to significant increases in macroalgal cover (7–9). In the Caribbean, the 1983 epizootic that effectively removed from most areas the herbivorous urchin, Diadema antillarum (10), and significantly altered the previous balance between macroalgae and a major echinoid herbivore (11). The increased abundance of macroalgae negatively affects coral growth and recruitment, and this has long-term consequences on the physical structure of the reef (9, 12).
Another factor affecting coral communities is the widespread, periodic bleaching of coral colonies (13–16). Bleaching involves the loss or reduction of the symbiotic zooxanthellae. Bleaching reduces the growth, calcification, and survival rates of the affected corals. Although many instances of bleaching are correlated with increased temperature (13, 15, 17), the causes of bleaching appear to be complex (18), and the phenomenon may be a syndrome associated with a variety of environmental effects (16). In some instances, bleaching events may affect only isolated colonies; in other cases, mass bleaching events occur such that >25% of the colonies in a given area are affected (19).
An abiotic element in the dynamics of coral reef communities is the effect of disturbance. The primary cause of physical disturbance for both Caribbean and Indo-Pacific reefs is the effect of severe storms (hurricanes and cyclones or typhoons, respectively). The hydrodynamic forces and scouring effects of suspended sediment associated with these storms can cause significant mortality among reef species. The effects of these storms, and subsequent recovery, vary with the morphology and life history patterns of the individual species (6, 20, 21). However, the effects on overall community structure may occur with a frequency and intensity sufficient to represent an intermediate level of disturbance that contributes to the maintenance of species diversity within the community (5).
There have been a number of reports indicating that coral reefs throughout the Caribbean are making the transition from communities dominated by a living coral substrate to areas with an extensive coverage of macroalgae (6, 22, 23). Previous reports of phase shifts (24) in coral reef communities have effectively emphasized some combination of the factors discussed above, but few studies have had the opportunity to assess the interaction of these factors within a narrow time frame. Here we report the results of a 40-month study of a Caribbean reef community that was initiated more than a decade after the Diadema epizootic, and our observations include both a mass bleaching event and the direct impact of a hurricane. During this time, the reef community made the transition from a state where corals and algae were codominant to a community dominated by algae. The speed with which this transition occurred illustrates the dynamic nature of reef communities.
Study Area and Methods
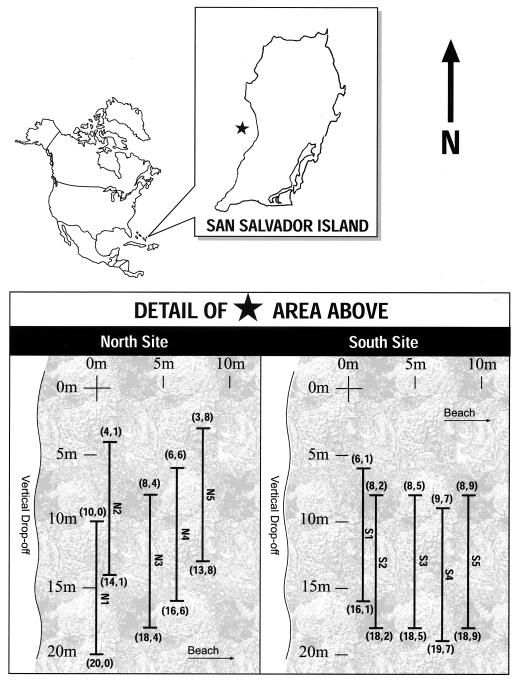
The island of San Salvador (latitude 24o 2′ north, longitude 74o 31′ west) is one of the outermost islands of the Bahamas (Fig. 1). The reef community is geologically young and is situated on an isolated seamount (25). The study site was established as part of the CARICOMP (Caribbean Coastal Marine Productivity) survey program. In accordance with CARICOMP protocols (26), the specific location of the site was chosen to minimize anthropogenic effects. The resident human population of the island does not engage in significant commercial fishing. In addition, the site was not near any point sources of pollution and it was comparatively inaccessible by recreational scuba divers. The characteristics of the site, given the remote location of the island, may make it one of the most pristine reef communities in the western Caribbean.
Figure 1.
Map of the Caribbean showing the location of the island of San Salvador. (Inset) The location of the transect sites. The north and south sites were 40 m apart. The coordinates refer to the grid system used to ensure random placement of the transects.
Ten permanent transect locations were established 1.5 km offshore of the island (Fig. 1) by placing two stainless steel pins 10 m apart for each transect. The location of each transect was determined by using a random number generator to determine its end points within an artificial coordinate system that had been superimposed over a map of the area. The depth at which the transects were placed ranged from ≈15 to 18 m.
The conditions of the survey process did not focus on the identification of individual species. Instead, several major categories were established to assess space utilization (Table 1). These categories were based on taxon (e.g., corals, algae, sponges), growth form (e.g., erect, encrusting), or physical features (e.g., sand, rubble). However, it is well established that sessile reef taxa (e.g., corals, algae) form natural functional groups based on growth form and that these groups show differences in susceptibility to storm damage, and, in the case of algae, susceptibility to damage by herbivores (27–29).
Table 1.
Categories used during transect sampling
| Category | Type |
|---|---|
| Algae | Calacareous algae |
| Encrusting algae | |
| Microalgae (fleshy algae) | |
| Turf algae | |
| Corals | Branching coral |
| Dead or bleached coral | |
| Encrusting coral | |
| Foliaceous coral | |
| Massive corals | |
| Miscellaneous taxa | Gorgonians |
| Millepora (fire corals) | |
| Miscellaneous species (anemone, hermit crab) | |
| Sponges | Encrusting sponges |
| Erect sponges | |
| Substrate | Boulders |
| Unoccupied gaps, holes, or overhangs | |
| Rock | |
| Coral rubble | |
| Exposed sand |
The first step in the sampling process was the placement of a taught nylon line between the transect end points. This ensured proper alignment of the transect chain along the sampling substrate. Quantitative sampling was performed by carefully laying a brass chain over every object directly below the transect line. This method allowed the accurate assessment of each major category of organism or physical structure that could use space along the transect. In contrast to the quadrat method, which assesses space utilization in a two-dimensional manner through estimates of percent cover (e.g., ref. 30), this method reflects the rugosity of the transect. An appreciation of the vertical structure of the community was necessary because most species will use any available substrate, regardless of its profile.
Space utilization by each category (Table 1) was measured by recording the number of chain links (1 link = 1.42 cm) needed to cover each successive feature along the transect. A program of training dives assured inter-observer reliability and indicated that variability of chain placement and category identification was less than 5%. Furthermore, only two members of the survey team (G.K.O. and K.M.A.) collected >98% of the data in the course of the study.
Because sea urchins have been a significant factor in reef communities, a parallel effort was made to assess their population densities. This was accomplished by a systematic visual inspection of an area within 1 m to either side of the transect line. Particular care was taken to examine all crevices among and below the coral and substrate for smaller urchins. On average, 10–15 min was spent looking for urchins on each transect. The total number of urchins, identified to species, was scored for each transect. By using this method we were able to observe urchins as small as 2–3 cm in total diameter. Thus, it is likely that only a small number of urchins either <2 cm in total diameter or those far back in coral or substrate recesses would have escaped our detection.
Beginning in November 1994, the transects were surveyed an average of every 6.5 months (range 3–9 months between surveys) until February 1998. In some instances, surveys were conducted specifically after a critical event had been reported (e.g., bleaching, hurricane damage).
Results
An indication of the rugosity of the transects is that it required from 16.3 to 20.4 m of chain to cover the features along each 10-m transect. Space utilization by each recognized category was converted to a proportion of the total chain length used, which quantifies the relative contribution of the categories to the overall structure of each transect.
Although the general trends in community structure are clear (Fig. 2), the proportional data for major functional groups (e.g., all corals combined, macroalgae, encrusting algae) were subjected to an arcsine transformation and analyzed by using a repeated measures ANOVA. The CARICOMP sampling protocol and its statistical analysis have been critiqued (31). Our results, based on 10 transects and 7 sampling dates, offer sufficient power to assess major changes in community structure, while minimizing the probability of misinterpreting random variability among transects and sampling dates.
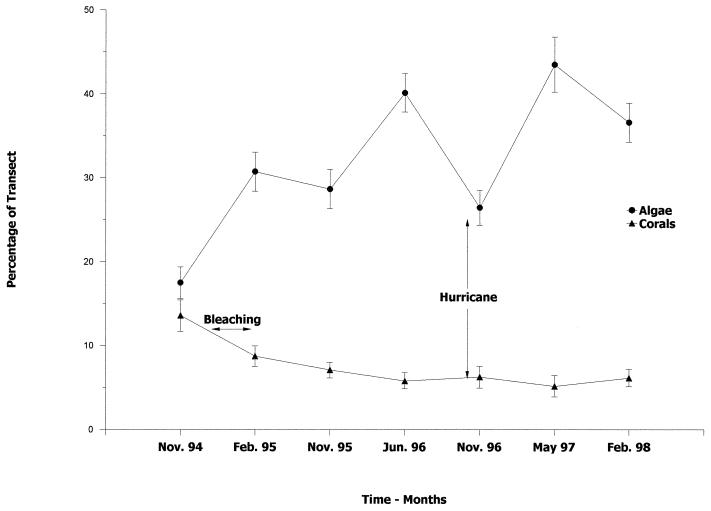
Figure 2.
Mean percentage of combined hard corals and algae categories expressed as a function of total transect length over time. The vertical lines represent 1 SE.
The survey program was initiated in November of 1994, a few months before the widespread bleaching event reported in January 1995. Subsequent surveys showed a significant (F6,54 = 8.3436, P < 0.0001) decline in corals of all growth forms from an average of 13.6% of the transect length in November 1994, to a low of 5.2% in May 1997 (Fig. 2). Algal coverage, in contrast, increased significantly (F6,54 = 19.9829, P < 0.0001) from an average of 17.5% at the beginning of the program to as much as 45% in November 1995. The impact of Hurricane Lili in October 1996 was short lived, as algal coverage declined from 40.1% in June 1996 to 26.4% in November 1996, but had rebounded to 43.5% in May 1997 (Fig. 2).
Sea urchins were rare within the boundaries of the survey. The only two species seen were D. antillarum and Echinodermata lucunter. The total number of urchins, combined for all 10 transects, never exceeded 30 (in February 1998), which represents an average of 3/transect, or <1 urchin/5 m3 of substrate.
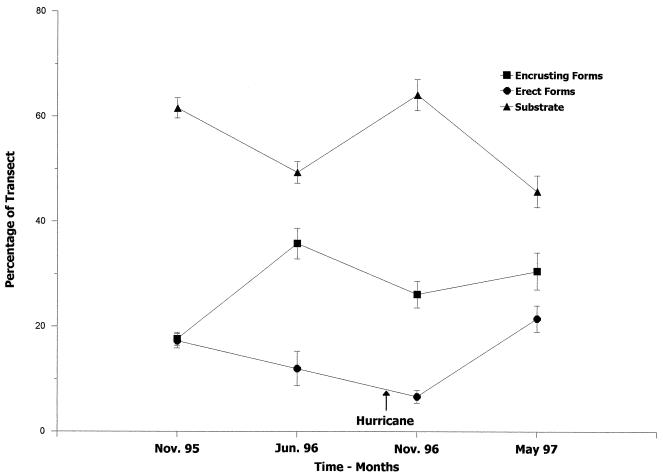
The effects of Hurricane Lili are summarized in Fig. 3. The transect categories were arranged in three groups for purposes of this analysis. The scouring effect of the storm are shown by the increase in unoccupied portions of the transect (Substrate in Fig. 3) compared with the amount of unoccupied space (boulders, gaps, rock, rubble, and sand, combined) in June 1996 (49.3 vs. 64.1%). The amount of unoccupied space is not significantly different (t9 = 1.1727; P > 0.05) from that in November of 1995 (61.5%), which indicates that there may also be a seasonal effect.
Figure 3.
The impact of Hurricane Lili, which struck San Salvador in October 1996. Major transect categories (mean percentage ± 1 SE) are organized into unoccupied substrate, organisms with an erect growth form, and encrusting/low profile species. The two sampling dates before and after the hurricane are plotted for comparison.
The growth forms of the taxa affected their susceptibility to storm damage. Macroalgae and sponges with an erect form (Erect Forms in Fig. 3) are combined because of their higher profile above the substrate. The macroalgae, despite their flexibility, were removed by wave surge and scouring, declining from a mean of 14.5% of the transect length in June 1996 to 5.1% in November of the same year. The macroalgae regenerated or recruited quickly and made the greatest contribution to algal recovery after the hurricane with macroalgal coverage reaching a mean of 34.1%. Their rapid recovery shows the opportunistic nature of these species. The hurricane may have had a beneficial effect by opening additional space for colonization, and deep mixing of the water column may have increased nutrient levels. The sponges with an erect growth form showed major losses from the storm (from 1.1% in June to 0.15% in November), and some of the larger individuals may have been decades old.
Species with a lower morphological profile included calcareous algae, encrusting algae, encrusting corals, encrusting sponges, and turf algae (Encrusting Forms in Fig. 3). This group was also reduced by the storm (from 35.8 to 26.1%, respectively), but their coverage was still significantly greater (t9 = 3.975, P < 0.001) than it had been in November 1995 (17.6%).
Finally, it should be noted that the corals, which had been significantly reduced by the bleaching event, were relatively unaffected by the storm (Fig. 2). However, they showed no signs of opportunistic growth and recruitment.
Discussion
Reef communities have a long evolutionary history, with the systems dominated by scleractinian, hermatypic corals being the most recent version (32). Although modern coral reefs cover at most only 0.2% of ocean area [or less (33)], they represent highly productive communities in otherwise low productivity tropical and subtropical areas, and the deposition of CaCO3 by reef species is a significant component of the global carbon cycle. As a result, the long-term stability of coral reef communities is a matter of considerable importance.
The coral reef community of San Salvador, by 1994, had apparently reached some balance between corals and macroalgae following the Diadema epizootic 12 years earlier. Corals and algae were almost equally frequent when the study began. The bleaching event, however, marked the beginning of a rapid decline in coral abundance and a significant increase in macroalgal dominance. Hurricane Lili did not disturb the community in such a way that it reduced algal dominance, and offered the corals an opportunity to recolonize. Instead, the hurricane appears to have facilitated algal dominance. It is too early to determine whether this is a transient event, but the record of recovery (defined in terms of a return to coral dominance) among Atlantic coral reefs is not good (34).
The long-term structure of any community is a function of the frequency and severity of disturbance, the life history patterns of the component species, and the direct and indirect effects of those species on each other. In the case of Caribbean coral reefs, the epizootic that reduced the importance of D. antillarum for the last 16 years has likely altered the relationship between corals and algae. Macroalgae can rapidly colonize a substrate as long as herbivores, nutrient limitation, and physical factors do not inhibit them. Corals, in turn, cannot successfully colonize a substrate dominated by algae. The removal of a major herbivore would obviously lead to a significant change in the interaction between corals and algae. There are isolated reports of cases where echinoid herbivores are reducing macroalgal cover (35, 36), but herbivores do not appear to be a significant factor in San Salvador.
It has been hypothesized that corals may have a competitive advantage in oligotrophic, tropical oceans (9, 12) because of a combination of factors, including low nutrient levels and high herbivore pressure, which tend to restrict algal growth. This competitive advantage is lost quickly if any factor changes such that conditions become detrimental to corals and/or beneficial for algae. The prevalent phenomenon of bleaching (15, 16), which clearly affected San Salvador, increasing reports of coral diseases (37), and the potential long-term decline in calcification rates because of increasing carbon dioxide levels (38) represent additional factors that may affect corals globally.
Storm damage is another factor that has shown to be of importance in coral reef community structure (20, 21). It can have positive effects on species diversity within a component of the community (the Intermediate Disturbance model; refs. 2 and 5). The effect of Hurricane Lili on San Salvador appears to have been transient and may have actually benefited the macroalgae. Unfortunately, we do not have the data necessary to perform a comparative analysis of the degree of exposure to hurricane damage similar to that reported for terrestrial communities affected by Hurricane Lili (39).
The Intermediate Disturbance hypothesis, which relates the frequency and intensity of disturbance to species diversity, had its major presentation (5) by using coral reefs as one of the supporting examples. The hypothesis, however, implicitly assumes that there is a comparatively equal chance for postdisturbance recovery, constrained only by the respective life history patterns of the affected species. The consequences when communities are affected by multiple disturbances (6, 40, 41), which may not affect all species equally, are understandably harder to predict.
The study site at San Salvador was affected by the bleaching episode of 1995. The mass bleaching observed in the study area may have been part of a larger bleaching event that included the shallow water patch reefs of San Salvador (42). The subsequent survey was conducted for a period and at a resolution (1 transect link = 1.42 cm) sufficient to detect even small levels of coral recruitment and recovery. However, there were no indications of recruitment, and coral coverage remained at significantly lower levels than when the survey began. This is in sharp contrast to the high level of recovery reported for the patch reefs (42).
Algal coverage within our study area increased significantly during the course of our study and recovered quickly following storm damage. The causes of such algal blooms are complex and may be caused by a combination of increased nutrient levels and decreased herbivory (43–45). The relative importance of invertebrate and vertebrate herbivores can also vary with depth (46). It should be noted, however, that we have no evidence of either a change in nutrient levels or herbivore activity over the course of our survey. The causes of the increase in algal cover remain unknown.
The reef community surveyed at San Salvador is as pristine as can be expected for a site in the contemporary Caribbean (8). Despite the negligible anthropogenic effects on the study site, the corals have declined to a low level of abundance, whereas algae, particularly the macroalgae, have become the dominant taxa of the community. The increasing dominance of macroalgae in reef areas of the Caribbean has become a common observation (e.g., refs. 6, 22, and 23). Our data are sufficient to indicate that this is not a transient phenomenon and that Bahamian reef communities are undergoing a substantial change in structure. Furthermore, our results indicate that this transition can be rapid and can take place without detectable changes in herbivore activity or nutrient availability.
Several studies (30, 47) have demonstrated that a full understanding of reef community dynamics requires long-term monitoring. Long-term studies have documented transitions in reef community structure (6, 23) to a state where macroalgae are dominant, but the data are largely comparative over time scales measured in years and do not indicate the actual time scale of the transition. We have shown that transitions in reef community structure can be rapid and such changes may have long term consequences.
Coral reefs represent a dynamic balance among the processes of calcification, bioerosion, and physical dissolution. Although coralline algae and other calcifying species play a role, it is the corals, especially those with symbiotic zooxanthellae, which are largely responsible for reef structure (48). As a result, any reduction in the coral component of the reef community is a threat to the physical structure of the entire reef ecosystem. Although we do not have estimates of calcification rates within the study area, it is obvious that they have been reduced significantly during the course of this study. If the prediction of a global reduction in calcification is accurate (38), then the long-term future of this reef ecosystem is in doubt.
Our study has two implications for the continued monitoring of coral reef communities. First, transitions in coral reef community structure may occur rapidly over comparatively short periods of time. This means that long-term studies must be coupled with intensive observations over shorter time scales. Second, we need to recognize that the factors affecting reef community structure are extremely complex. There has been a tendency to identify either anthropogenic factors or major physical disturbances as the causes of community transitions. The fact that the pristine community of San Salvador changed so quickly, and for no obvious external reason, is likely due to the subtle interaction of a number of factors.
Acknowledgments
The technical assistance of Scott Shaw, Chris Ostrander, Sandy Vogeli, Bill Meehan, Bill Hawkins, David Geter, John Harris, Smith Holt, Debbie Ostrander, James Blair, and Dan Suchy with various aspects of the field studies is gratefully acknowledged. We thank James Ebert, Robert Ginsburg, Robert Buddemeier, and Garriet W. Smith for their critical reading of early versions of this manuscript. These studies have been funded in part by the National Science Foundation, the Bahamian Field Station, Oklahoma State University, and the National Aquarium in Baltimore.
Abbreviations
- CARICOMP
Caribbean Coastal Marine Productivity
Footnotes
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.090104897.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.090104897
References
- 1.Sutherland J P. Am Nat. 1974;108:859–873. [Google Scholar]
- 2.Knowlton N. Am Zool. 1992;32:674–682. [Google Scholar]
- 3.Paine R T. Am Nat. 1966;100:65–75. [Google Scholar]
- 4.Power M E, Tilman D, Estes J A, Menge B A, Bond W J, Miles L S, Daily G, Castilla J C, Lubechenco J, Paine R T. Bioscience. 1996;46:609–620. [Google Scholar]
- 5.Connell J H. Science. 1978;199:1302–1310. doi: 10.1126/science.199.4335.1302. [DOI] [PubMed] [Google Scholar]
- 6.Hughes T P. Science. 1994;265:1547–1551. doi: 10.1126/science.265.5178.1547. [DOI] [PubMed] [Google Scholar]
- 7.Hatcher B G. Trends Ecol Evol. 1990;5:149–155. doi: 10.1016/0169-5347(90)90221-X. [DOI] [PubMed] [Google Scholar]
- 8.Jackson J B C. Coral Reefs. 1997;16,(Suppl.):23–32. [Google Scholar]
- 9.Done T J. Am Zool. 1999;39:66–79. [Google Scholar]
- 10.Lessios H. Annu Rev Ecol Syst. 1988;19:371–373. [Google Scholar]
- 11.Williams S L, Carpenter R C. Mar Ecol Prog Ser. 1988;47:145–152. [Google Scholar]
- 12.Miller M W. Oceanogr Mar Biol Annu Rev. 1998;36:65–96. [Google Scholar]
- 13.Glynn P W. Trends Ecol Evol. 1991;6:175–179. doi: 10.1016/0169-5347(91)90208-F. [DOI] [PubMed] [Google Scholar]
- 14.Glynn P W. Coral Reefs. 1993;12:1–17. [Google Scholar]
- 15.Brown B. Coral Reefs. 1997;16,(Suppl.):129–138. [Google Scholar]
- 16.Meehan W J, Ostrander G K. J Toxicol Environ Health. 1997;50:529–552. doi: 10.1080/15287399709532053. [DOI] [PubMed] [Google Scholar]
- 17.Warner M E, Fitt W K, Schmidt G W. Proc Natl Acad Sci USA. 1999;96:8007–8012. doi: 10.1073/pnas.96.14.8007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fagonee I, Wilson H B, Hassell M P, Turner J R. Science. 1999;283:843–845. doi: 10.1126/science.283.5403.843. [DOI] [PubMed] [Google Scholar]
- 19.McField M D. Bull Mar Sci. 1999;64:155–172. [Google Scholar]
- 20.Porter J W, Woodley J D, Smith G J, Neigel J E, Battey J F, Dallmeyer D G. Nature (London) 1981;294:249–250. [Google Scholar]
- 21.Woodley J D, Chornesky E A, Clifford P A, Jackson J B C, Kaufman L S, Knowlton N, Lang J C, Pearson M P, Porter J W, Rooney M C, et al. Science. 1981;214:749–755. doi: 10.1126/science.214.4522.749. [DOI] [PubMed] [Google Scholar]
- 22.McClanahan T R, Muthiga N. Environ Conserv. 1998;25:122–130. [Google Scholar]
- 23.McClanahan T R, Aronson R B, Precht W F, Muthiga N A. Coral Reefs. 1999;18:61–62. [Google Scholar]
- 24.Done T J. Hydrobiologia. 1992;247:121–132. [Google Scholar]
- 25.McNeill D F, Ginsburg R N, Chang S R, Kirschvink J L. Geology. 1988;16:8–12. [Google Scholar]
- 26.Woodley J D, Smith S R, Garzon-Ferreira J, Koltes K, Alcolado P, Bonair K, Bush P, De Meyer K, Garcia J R, Garcia-Parrado P, et al. Proc 8th Int Coral Reef Symp. 1997;1:651–656. [Google Scholar]
- 27.Chappell J. Nature (London) 1980;286:249–252. [Google Scholar]
- 28.Grause R R, Macintyre I G, Herchenroder B E. Coral Reefs. 1984;3:59–68. [Google Scholar]
- 29.Paul V J, Hay M E. Mar Ecol Prog Ser. 1986;33:255–264. [Google Scholar]
- 30.Hughes T P. Ecology. 1996;77:2256–2260. [Google Scholar]
- 31.Green R H, Smith S R. Proc 8th Int Coral Reef Symp. 1997;2:1459–1464. [Google Scholar]
- 32.Wood R. Annu Rev Ecol Syst. 1998;29:179–206. [Google Scholar]
- 33.Spalding M D, Grenfell A M. Coral Reefs. 1997;16:225–230. [Google Scholar]
- 34.Connell J H. Coral Reefs. 1997;16,(Suppl.):101–114. [Google Scholar]
- 35.Woodley J D. Coral Reefs. 1999;18:192. [Google Scholar]
- 36.Woodley J D, Gayle P M H, Judd N. Coral Reefs. 1999;18:193. [Google Scholar]
- 37.Richardson L L. Trends Ecol Evol. 1998;13:438–443. doi: 10.1016/s0169-5347(98)01460-8. [DOI] [PubMed] [Google Scholar]
- 38.Kleypas J A, Buddemeier R W, Archer D, Gattuso J-P, Langdon C, Opdyke B N. Science. 1999;284:118–120. doi: 10.1126/science.284.5411.118. [DOI] [PubMed] [Google Scholar]
- 39.Spiller D A, Losos J B, Schoener T W. Science. 1998;281:695–697. doi: 10.1126/science.281.5377.695. [DOI] [PubMed] [Google Scholar]
- 40.Hughes T P, Connell J H. Limnol Oceanogr. 1999;44:932–940. [Google Scholar]
- 41.Porter J W, Lewis S K, Porter K G. Limnol Oceanogr. 1999;44:941–949. [Google Scholar]
- 42.McGrath T A, Smith G W. Rev Biol Trop. 1998;46,(Suppl. 5):91–99. [PubMed] [Google Scholar]
- 43.Lapointe B. Limnol Oceanogr. 1997;42:1119–1131. [Google Scholar]
- 44.Hughes T, Szmant A M, Steneck R, Carpenter R, Miller S. Limnol Oceanogr. 1999;44:1583–1586. [Google Scholar]
- 45.Lapointe B. Limnol Oceanogr. 1999;44:1586–1592. [Google Scholar]
- 46.Morrison D. Ecology. 1988;69:1367–1382. [Google Scholar]
- 47.Connell J H, Hughes T P, Wallace C C. Ecol Monog. 1997;67:461–488. [Google Scholar]
- 48.Barnes D J, Chalker B E. In: Ecosystems of the World, Vol. 25: Coral Reefs. Dubinsky Z, editor. New York: Elsevier; 1990. pp. 109–131. [Google Scholar]