Abstract
Five experiments assessed associative symmetry in pigeons. In Experiments 1A, 1B and 2, pigeons learned two-alternative symbolic matching with identical sample- and comparison-response requirements and with matching stimuli appearing in all possible locations. Despite controlling for the nature of the functional stimuli and insuring all requisite discriminations, there was little or no evidence for symmetry. By contrast, Experiment 3 demonstrated symmetry in successive (go/no-go) matching, replicating the findings of Frank and Wasserman (2005). In view of these results, I propose that in successive matching, (1) the functional stimuli are stimulus–temporal location compounds, (2) continual nonreinforcement of some sample–comparison combinations juxtaposed with reinforcement of other combinations throughout training facilitates stimulus class formation, (3) classes consist of the elements of the reinforced combinations, and (4) common elements produce class merger. The theory predicts that particular sets of training relations should yield “antisymmetry”: Pigeons should respond more to a reversal of the nonreinforced symbolic baseline relations than to a reversal of the reinforced relations. Experiment 4 confirmed this counterintuitive prediction. These results and other theoretical implications support the idea that equivalence relations are a natural consequence of reinforcement contingencies.
Keywords: associative symmetry, antisymmetry, stimulus classes, equivalence relations, successive matching, pigeons, key peck
The topic of equivalence-class formation has been an especially vibrant area of research and theory in behavior analysis since the seminal papers by Sidman and his colleagues (e.g., Sidman, 1971; Sidman & Cresson, 1973; Sidman & Tailby, 1982). The excitement generated by this topic arises in part from the demonstration that learning a relatively small number of conditional discriminations can immediately lead to many other conditional discrimination performances never before taught. These derived or emergent performances are not simply examples of familiar principles such as primary stimulus generalization (e.g., Honig & Urcuioli, 1981) but, rather, are instances of what Hull (1939) called secondary generalization (see also Hall, Mitchell, Graham, & Lavis, 2003; Jenkins, 1963; Jenkins & Palermo, 1964). They are by no means secondary, however, in practical or theoretical significance. To the contrary, the ability to account for novel behavior in terms of reinforcement history greatly expands the scope of learning principles and strengthens the argument that the experimental analysis of behavior can provide insights into many aspects of psychological science.
Stimulus equivalence, as defined by Sidman (1990; see also Sidman & Tailby, 1982), involves three types of relations (reflexivity, symmetry, and transitivity) between stimuli involved in other explicitly trained operant relations. Specifically, following reinforcement for choosing B after A and C after B (A–B and B–C matching, respectively), subjects will often match each stimulus to itself (reflexivity; e.g., A–A matching), do the reverse of what was explicitly learned (symmetry; e.g., choosing A after B or B–A matching), and match C to A (transitivity: A–C matching). These emergent relations have been repeatedly demonstrated with humans (Sidman, 1994) but have been only rarely shown in nonhuman animals (e.g., D'Amato, Salmon, Loukas, & Tomie, 1985; Schusterman & Kastak, 1993; Tomonaga, Matsuzawa, Fujita, & Yamamoto, 1991). Moreover, some apparent instances of equivalence relations in animals (e.g., McIntire, Cleary, & Thompson, 1987; Manabe, Kawashima, & Staddon, 1995) are explicable without recourse to equivalence (see, for example, Hayes, 1989; Urcuioli & Vasconcelos, 2008b).
The paucity of convincing evidence of equivalence relations in animals other than humans has raised questions about their origins (e.g., Dube, McIlvane, Callahan, & Stoddard, 1993; K. J. Saunders, Williams, & Spradlin, 1996; Sidman, 1990). On the one hand, the human versus animal discrepancies suggest that human language, or at least language capabilities, might be necessary for the emergent relations that define stimulus equivalence (Horne & Lowe, 1996; although see Carr, Wilkinson, Blackman, & McIlvane, 2000). On the other hand, Sidman (1994, 2000) has maintained that reinforcement contingencies per se are responsible for equivalence; in other words, it is not derived from other processes. Given the prominent role of language in human behavior, the strongest argument in favor of his position would be to show that equivalence relations are demonstrable in nonhuman animals. The present paper provides evidence for one aspect of equivalence in pigeons—namely, symmetry (also known as associative symmetry; Frank & Wasserman, 2005)—and proposes a theory of pigeons' equivalence-class formation to explain why this phenomenon has been difficult to demonstrate and the conditions which should foster its appearance.
The focus on symmetry as opposed to reflexivity and transitivity was deliberate because this emergent relation has been the most elusive of the three equivalence relations in nonhuman animals (D'Amato et al., 1985; Dugdale & Lowe, 2000; Hogan & Zentall, 1977; Lionello-DeNolf & Urcuioli, 2002; Lipkens, Kop, & Matthijs, 1988; Sidman, Rauzin, Lazar, Cunningham, Tailby, & Carrigan, 1982). Consequently, demonstrations of it (e.g., Frank & Wasserman, 2005; García & Benjumea, 2006) are especially eye-catching. For example, Frank and Wasserman found that pigeons responded more to the reverse of the reinforced symbolic (A-B) relations than to the reverse of the nonreinforced relations in successive matching, providing that they also received concurrent training on identity matching using the stimuli serving as samples (A) and comparisons (B) in the symbolic task. These results stand in stark contrast to those of Lionello-DeNolf and Urcuioli (2002) who did not find evidence for symmetry despite training pigeons on symbolic and identity matching in a two-alternative task.
Unlike successive matching, the samples and comparisons in two-alternative matching appear in different spatial locations. Consequently, when their roles are reversed during symmetry tests, their spatial locations change vis-à-vis training and this can preclude symmetry if location is a component of the functional matching stimuli (Lionello & Urcuioli 1998). In other words, if a red stimulus appearing on the center key is functionally different than a red stimulus appearing on a side key (and vice versa), then swapping its location as required in a symmetry test using this procedure generates test relations which are not symmetrical versions of the training relations. However, Lionello-DeNolf and Urcuioli (2002, Experiment 2) went to some length to train pigeons to ignore location by arranging that the samples in symbolic and identity matching sometimes appear on the left response key and sometimes on the right response key (with the comparisons at the remaining two locations). This multiple-location training was successful in its purpose: When later confronted with samples on the center key (and comparisons on the adjacent side keys), pigeons continued to match accurately on their baseline symbolic (A–B) relations. Nevertheless, they were still unable to match accurately on the symmetrical (B–A) relations.
The contrast between these results and those of Frank and Wasserman (2005) is all the more intriguing because each study trained similar sets of baseline relations prior to symmetry testing. Still, there were residual concerns about the functional matching stimuli in Lionello-DeNolf and Urcuioli (2002), so an attempt to resolve these served as the starting point for the present series of experiments. Experiments 1A, 1B, and 2 searched (albeit unsuccessfully) for symmetry in two-alternative matching with procedural variations to redress these other potential problems. Experiment 3 then switched from two-alternative to successive matching to confirm the Frank and Wasserman findings and to see if this procedural switch would, in fact, matter. It did; Experiment 3 replicated the Frank and Wasserman finding of associative symmetry in pigeons. Why such a seemingly minor difference in training procedure should produce very different results prompted the development of a theory of pigeons' equivalence-class formation to explain the “success” of successive matching vis-à-vis two-alternative matching in producing symmetry. Experiment 4 was then designed as an independent test of the theory—in particular, its prediction of antisymmetry following a slightly different set of successive matching training relations.
Experiment 1a
Although Lionello-DeNolf and Urcuioli (2002) failed to find symmetry in two-alternative symbolic matching despite pigeons' familiarity with each matching stimulus at its symmetry-test location, their procedures used different sample- and comparison-response requirements (viz., 10 pecks vs. 1 peck). Consequently, if the number of times pigeons pecked at the samples and at the comparisons was a component of the functional matching stimuli (cf. McIlvane, Serna, Dube, & Stromer, 2000; see also ,Urcuioli & Vasconcelos, 2008a), the “symmetry” test did not actually test for associative symmetry. For instance, if pigeons learned in training that one peck to a vertical line was reinforced after 10 pecks to a red hue, a [red–10 pecks → vertical–1 peck] sample–comparison relation, then reversing the roles of vertical and red while maintaining the sample- and comparison-response requirements evaluates a [vertical–10 pecks → red–1 peck] emergent relation. But the latter is not the symmetrical version of the former. Moreover, the change in the hypothesized functional compounds from training to testing ought to be disruptive, yielding relatively poor accuracies just as Lionello-DeNolf and Urcuioli (2002) reported.
A single-peck requirement for both samples and comparisons would avoid this problem. Its disadvantage is that it entails very short sample-observation periods which can slow the rate of acquisition and yield relatively low asymptotic levels of matching accuracy (Sacks, Kamil, & Mack, 1972; see also Eckerman, Lanson, & Cumming, 1968). Thus, the alternative solution was adopted: Require pigeons to peck the reinforced comparison the same, multiple number of times that they peck each sample stimulus (cf. Kuno, Kitadate, & Iwamoto, 1994).
Experiment 1A used this modified matching procedure. Furthermore, throughout symbolic matching training, the samples appeared equally often on the center, left, and right keys (with the comparison stimuli appearing at the alternative two locations) to insure the pigeons were familiar with each matching stimulus at its to-be-tested location(s).
Method
Subjects
Four White Carneau pigeons from the Palmetto Pigeon Plant (Sumter, SC) participated. All had previous experience in a Simon discrimination task (Urcuioli, Vu, & Proctor, 2005). Each pigeon was individually housed in a stainless-steel, wire-mesh cage in a colony room on a 14 h/10 h light-dark cycle with lights on at 07:00. Throughout the experiment, they were maintained at 80% of their free-feeding body weights by restricting feeding to the experimental sessions, except for the one day per week the experiment was not run. Grit and water were freely available in the home cage.
Apparatus
A single BRS/LVE (Laurel, MD) pigeon chamber (Model PIP-016 three-key response panel inside a Model SEC-002 enclosure) was used. The 2.5-cm-diameter response keys were spaced 5.7 cm apart, center to center, and were horizontally aligned in a row 7.5 cm from the top of the panel. Behind each key was an inline projector (BRS/LVE Model IC-901-IDD) equipped with films and filters for displaying red and green hues, and three white vertical and three white horizontal lines on black backgrounds (BRS/LVE Pattern No. 692). A 5.8-cm-square opening located 13 cm below the center key permitted access to a rear-mounted food hopper containing Purina ProGrains. Raising the food hopper was accompanied by lighting a small miniature bulb (ESB-28) in the metal housing surrounding the hopper. Chamber illumination was provided by a partially shielded GE #1829 bulb located 7.6 cm above the center key whose light was directed toward the ceiling. A constantly running blower fan attached to the chamber provided ventilation and masking noise. All experimental events were controlled and recorded by an IBM-compatible computer.
Procedure
Preliminary Training
Prior to symbolic matching training, pigeons learned to peck multiple times to each stimulus that would later serve as a sample or as a comparison stimulus. These 60-trial sessions were divided equally between red and green hues, or vertical and horizontal lines, with each stimulus appearing equally often on the left, center, and right keys. Each trial began with the appearance of one of the two scheduled stimuli on one of the three keys. Completing the scheduled fixed-ratio (FR) requirement for pecking the lit key turned the stimulus off and produced food. This requirement was gradually raised from 1 to 10 over the 6–9 sessions of training with each pair of stimuli. Pecking an unlit key had no programmed consequences. A 10-s intertrial interval (ITI) separated successive trials. The house light was off for the first 9 s of the ITI; it was turned on for the last 1 s and remained on through the next reinforcement cycle. Food reinforcement duration was constant within a session but varied between 2–6 s across sessions to maintain each pigeon's body weight as close to 80% of free feeding as possible. Preliminary training with red and green was conducted first, followed by training with vertical and horizontal lines.
Symbolic Matching Training
Next, pigeons learned to match red and green samples to vertical and horizontal comparisons. Each 96-trial session consisted of an equal number of each of the 12 possible trial types: two samples × three sample locations (left, center, right) × two sample-specific locations of the comparison stimuli (e.g., center and right, and vice versa, for left-key samples). Each appeared in random order with the constraint that none occur more than twice in a row.
Each matching trial began with the red or green sample on one of the three keys. Pecking the sample 10 times turned it off and produced vertical- and horizontal-line comparison stimuli on the remaining two keys. A single peck to either comparison immediately turned off the other after which nine additional pecks to the remaining comparison either produced food (if the initially pecked comparison was “correct”) or an equivalent timeout period with the house light off (if the initially pecked comparison was “incorrect”). If the incorrect comparison was pecked first, it went off automatically after 5 s if pigeons did not complete the additional nine pecks to it. Completion of the FR 10 requirement to the correct comparison, however, was necessary to produce food. Following reinforcement or timeout, a 10-s ITI structured in the same way as previously described ensued.
Training for each pigeon continued until its overall accuracy was 90% correct or higher for 5 of 6 consecutive sessions and 85% correct or higher for each sample location for those sessions. One pigeon (SN 2) showed little signs of acquisition after 20 sessions so a correction procedure was implemented (i.e., any incorrect choice repeated that trial after the usual ITI) until the performance criteria were met, at which point the correction contingencies were removed and criterion levels of accuracy were reestablished.
Symmetry Test
After meeting criterion, each pigeon received a single 96-trial test session in which the vertical and horizontal lines appeared as sample stimuli on any of the three keys and the red and green hues appeared as the comparison alternatives on the remaining keys. The 12 possible test trials occurred equally often and in pseudorandom order in the session, and the FR 10 sample- and comparison-response requirements remained in effect. For 2 pigeons, the reinforced line–hue relations in testing were symmetrical versions of the reinforced hue–line relations in training. For instance, if pecking the vertical lines had been reinforced after the red sample in training, then pecking red was now reinforced after the vertical-line sample in testing. For the remaining 2 pigeons, the reinforced relations in testing were the opposite of those in training. For instance, if pecking the vertical lines had been reinforced after the red sample in training, then pecking green was now reinforced after the vertical-line sample in testing. All other procedural details were identical to those for training.
All statistical decisions reported here and in subsequent experiments referenced the tabled F values provided by Rodger (1975) to control Type I error rate on a per-decision basis. Type I error rate was set at .05.
Results and Discussion
The pigeons (SP 1, SP 2, and SN 1) not requiring a correction procedure completed symbolic matching training in 20, 11, and 13 sessions, respectively. The other (SN 2) needed a total of 54 sessions to meet the performance criteria (including correction sessions). For the last 3 baseline sessions, average matching accuracies were uniformly high for left-, center-, and right-key samples: 95.8%, 94.8%, and 94.8% correct, respectively, F(2, 6) = 0.22.
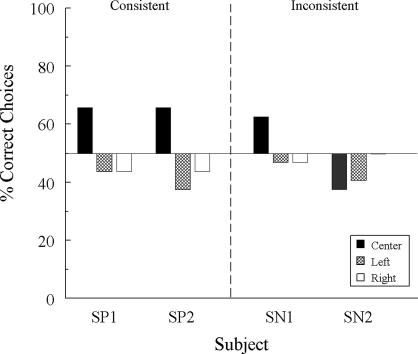
Figure 1 shows individual matching accuracies by sample location when the reinforced test relations were consistent with the symmetrical versions of the training relations or were inconsistent with (i.e., the opposite of) them. The predictions are that the 2 pigeons whose reinforced test relations were consistent with symmetry should exhibit above-chance levels of accuracy and the 2 pigeons whose reinforced test relations were inconsistent with symmetry should exhibit below-chance levels of accuracy. On center-sample test trials, the two “consistent” pigeons matched at 65.6% accuracy, whereas one “inconsistent” pigeon (SN 2) matched at only 37.5% accuracy, in line with the predictions. However, the remaining “inconsistent” pigeon (SN 1) matched at 62.5% accuracy on the center-sample test trials. Furthermore, all 4 pigeons were at or below chance levels of accuracy on test trials in which the sample appeared on the left or right side key. Overall, then, there is little compelling evidence for associative symmetry in these data. Indeed, when averaged over sample locations, accuracies were comparable in the two test conditions: 51.0% and 49.0% for the two consistent pigeons versus 52.1% and 42.7% correct for the two inconsistent pigeons.
Fig 1.
Percentage of correct choices for individual subjects by sample location on the reinforced symmetry test in Experiment 1A. The reinforced test relations were either symmetrical versions of the symbolic training relations (“Consistent”) or the opposite of them (“Inconsistent”).
Clearly, having identical sample- and comparison-response requirements does not yield associative symmetry in two-alternative symbolic matching with pigeons. This is a significant finding because even if the functional samples and comparisons in training were compounds consisting of each matching stimulus plus the number of times pigeons pecked at it, those same compounds appeared in testing. Thus, the lack of evidence for symmetry cannot be attributed to changing those compounds in the shift from training to testing.
But this experiment may still have been procedurally “deficient”. In order to have demonstrated accurate symmetry-test performances (which they did not), pigeons must have successively discriminated between the vertical and horizontal lines when they appeared singly as sample stimuli (cf. Carter & Eckerman, 1975). Multiple-location symbolic matching training, however, did not insure this requisite discrimination (R. R. Saunders & Green, 1999). Likewise, symmetry required that pigeons could simultaneously discriminate between red and green when they appeared as comparisons in testing. This, too, was not guaranteed by their training despite having successively discriminated between red and green as samples. Experiment 1B was designed to avoid these problems.
Experiment 1b
In this experiment, the pigeons from Experiment 1A received two-alternative matching training on hue-identity and line-identity matching. Together with continued refresher training on their already learned (hue–line) symbolic matching task, the two identity tasks required the successive line-sample and the simultaneous hue-comparison discriminations necessary for accurate symmetry (line–hue) test performances
Method
Subjects and Apparatus
The pigeons and apparatus from Experiment 1A were used here.
Procedure
Training
After reestablishing the symbolic matching baseline, pigeons learned to match red and green samples to red and green comparisons, respectively. Each 96-trial hue-identity training session was structured identically to the corresponding symbolic matching sessions described in Experiment 1A, including the FR 10 sample- and comparison-response requirements. Pigeons were trained to the same performance criterion as in Experiment 1A. Next, they learned to match vertical and horizontal samples to vertical and horizontal comparisons, respectively. Except for the change in matching stimuli, these line-identity sessions were the same as the hue-identity sessions, and were run to the same criterion levels of performance.
Finally, all three tasks—symbolic, hue-identity, and line-identity matching—were rotated individually or in blocks of 2–4 sessions across days to insure that accuracy on each task was at or above criterion levels immediately prior to testing.
Symmetry Testing
Twenty reinforced symmetry test sessions followed the last symbolic matching refresher session. The line–hue test contingencies for each pigeon were identical to those during its single symmetry test in Experiment 1A.
Results and Discussion
The 4 pigeons (SP 1 & 2, and SN 1 & 2) needed 9, 8, 9, and 17 training sessions, respectively, to meet criterion on hue-identity matching, and 21, 21, 13, and 35 sessions, respectively, on line-identity matching. The slower acquisition of line-identity matching is typical for pigeons (Carter & Eckerman, 1975; Urcuioli & Zentall, 1986).
Average accuracies collapsed across sample location for the last refresher session on each task were 97.4%, 96.4%, and 94.3% correct, respectively, for symbolic, hue-identity, and line-identity matching. Although line-identity accuracy was significantly lower than accuracies for the other two tasks, F(2, 6) = 6.27, it was nonetheless very high and indicative of an excellent successive line discrimination. In large part, the difference reflected the fact that pigeons were significantly less accurate for left-key line samples (90.6% correct) than for center- and right-key line samples (96.1% and 96.1% correct, respectively), F(2, 6) = 5.45. By contrast, accuracies did not differ significantly across sample location for symbolic and hue-identity matching, Fs (2, 6) < 2.85.
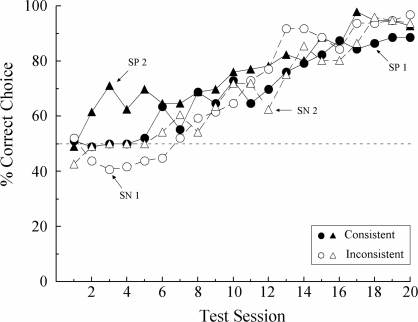
Figure 2 shows each pigeon's performance on the 20 symmetry test sessions. Accuracies have been averaged across sample location because the critical comparisons were not affected by this variable. The results confirm what was found in Experiment 1A: Pigeons tested with reinforced line–hue relations that were symmetrical versions of their reinforced baseline relations (consistent condition) performed no more accurately than pigeons tested with reinforced relations that were the opposite of the symmetrical versions of the baseline relations (inconsistent condition).
Fig 2.
Percentage of correct choices for individual subjects averaged over sample location on the reinforced symmetry tests in Experiment 1B. The reinforced test relations were either symmetrical versions of the symbolic training relations (“Consistent”) or the opposite of them (“Inconsistent”).
In short, even with assurances that pigeons discriminated each line sample from the other and each hue comparison from the other at the time of testing, they showed no evidence of symmetry despite identical sample- and comparison-response requirements and familiarity with each matching stimulus at each key location.
Experiment 2
The three matching tasks used in Experiment 1B were trained separately. By contrast, in their report of associative symmetry, Frank and Wasserman (2005, Experiment 1) trained symbolic and identity successive matching concurrently. Experiment 2, then, was designed to assess associative symmetry in two-alternative matching after concurrent symbolic and identity training.
Method
Subjects and Apparatus
Four experimentally naïve White Carneau pigeons obtained from the Palmetto Pigeon Plant were used. Upon arrival in the laboratory, free-feeding body weights were established via unrestricted access to Purina ProGrains for a period of 5–10 days. Pigeons were then gradually reduced to, and maintained at, 80% of their free-feeding weights by restricted feeding. All other housing details were identical to those described in Experiment 1A. The same apparatus was also used, although for this experiment the matching stimuli were red and yellow hues, a solid white triangle and three vertically aligned white dots on black backgrounds (BRS/LVE Pattern No. 692)1. A homogeneous white field was used for initial shaping and preliminary training.
One pigeon died during training and was not replaced.
Procedure
Preliminary Training
Each pigeon was first taught to eat quickly and reliably from a periodically raised and lit food hopper, after which the key peck response to a white center-key stimulus was shaped by the method of successive approximations. This was followed by two 60-trial sessions during which single pecks to a center-key triangle and dots, and to center-key red and yellow hues, were reinforced. The stimuli for each session appeared equally often and in random order, with successive trials separated by a 10-s ITI.
Next, pigeons learned to peck multiple times to white on the left, center, and right keys to obtain food reinforcement. Over the course of 10 sessions, the FR requirement was raised from 1 to 20.2 Afterwards, pigeons learned to peck multiple times to the triangle and dot stimuli on each key for food, and then to the red and yellow stimuli. Four sessions were run using each stimulus set. The FR requirement was set at 3 for the first session, 5 for the second session, and 10 for the third and fourth sessions. All other details of these sessions were identical to those described for the corresponding sessions in Experiment 1A.
Two-Alternative Matching Training
Next, pigeons learned to match red and yellow sample hues to triangle and dot comparison forms (symbolic matching), each hue sample to its corresponding hue comparison (hue identity matching) and each form sample to its corresponding form comparison (form identity matching). The reinforced symbolic sample-comparison relations were counterbalanced across subjects. Each 108-trial session consisted of 36 symbolic matching trials, 36 hue-identity matching trials, and 36 form-identity matching trials. The 12 possible sample-comparison configurations for each task (two samples × three sample locations × two alternative locations of the comparisons) occurred equally often in each session, and the 36 different trial types were randomized with the constraint that none occur more than twice in succession. An FR 10 requirement was in effect for both sample and comparison responding. All other procedural details were identical to those previously described.
Each pigeon was trained until (a) its matching accuracy was 90% correct overall for 5 of 6 consecutive sessions, (b) average accuracies for all three matching tasks (symbolic, hue identity, and form identity) were at least 87.5% correct for the last 5 criterion sessions, and (c) for each task, accuracy was at least 83.3% correct for each sample location. After meeting these criteria, 10 additional overtraining sessions were given. A correction procedure was begun for one pigeon (SYMM2) after 50 sessions and remained in place until the performance criteria were met, at which point noncorrection training resumed until its performances again met the criteria.
Symmetry Test 1
Ten symmetry test sessions were run after the completion of training. Each session contained 108 baseline training trials (as previously described) plus 12 test trials with triangle and form samples appearing on the center key, red and yellow comparisons appearing on the left and right side keys, and FR 10 response requirements. Each test-trial choice ended in reinforcement (i.e., test-trial choices were nondifferentially reinforced).
Baseline Retraining
After the initial 10 tests returned ambiguous to null results (see Results and Discussion), each pigeon was returned to its three concurrent baseline tasks until it again met the performance criteria in preparation for a second test similar to those used in Experiments 1A and 1B.
Symmetry Test 2
In this second test, 30 additional sessions were run during which test-trial choices were differentially reinforced. For 2 pigeons, their reinforced comparison choices were consistent with the symmetrical versions of the symbolic matching training relations; for the other pigeon, its reinforced choices were inconsistent with the symmetrical versions of the training relations. All other details were identical to Symmetry Test 1.
Results and Discussion
Two-Alternative Matching Training
Pigeons SYMM 2, SYMM 3, and SYMM 4 needed 134, 91, and 117 sessions, respectively, to reach criterion levels of accuracy in training. For the last 5 sessions preceding testing, form-identity accuracy (88.8% correct) was significantly lower than hue identity and symbolic matching accuracies (97.0% and 95.9% correct, respectively), F(2, 4) = 37.99, which did not differ from one another, F(2, 4) = 0.59. Although not ideal, the difference was expected (cf. Carter & Eckerman, 1975; Urcuioli & Zentall, 1986) and form-identity matching accuracy was nonetheless high. Performances were comparable across sample locations: Accuracies for left-, center-, and right-key samples averaged 93.9%, 94.4%, and 93.5% correct, respectively, over the last 5 training sessions, F(2, 4) = 0.31.
Symmetry Test 1
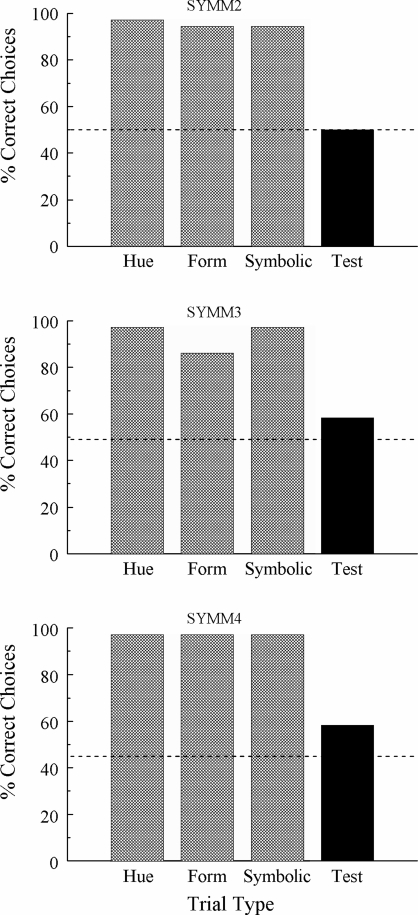
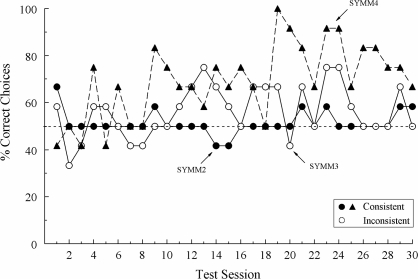
Figure 3 shows each pigeon's first-session accuracies on each differentially reinforced baseline task (shaded bars) and the nondifferentially reinforced symmetry probes (solid bar). Although baseline accuracies remained uniformly high, the percentages of test-trial choices consistent with associative symmetry were at, or only slightly above, chance: 50%, 58.3%, and 58.3% correct for SYMM2, SYMM3, and SYMM4, respectively.
Fig 3.
Percentage of correct choices for individual subjects on the first symmetry test session in Experiment 2. Hue = hue-identity baseline trials; Form = form-identity baseline trials; Symbolic = symbolic matching baseline trials; Test = nondifferentially reinforced symmetry probe trials.
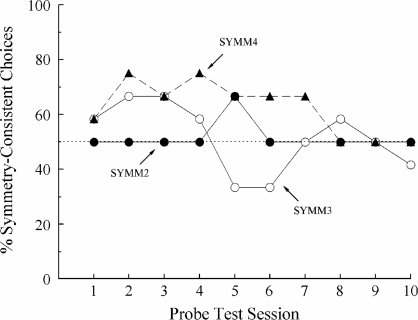
Figure 4 shows the percentages of symmetry-consistent choices for each bird across all 10 test sessions. For Pigeon SYMM2, these percentages were mostly at 50% throughout testing. For Pigeon SYMM3, the percentages were above 50% for the first 4 sessions but dropped below 50% for 2 sessions before stabilizing near 50%. For Pigeon SYMM4, its percentages were well above 50% for Test Sessions 2–7 and then dropped to 50% for the last 3 sessions.
Fig 4.
Percentage of choices consistent with symmetry for individual subjects on the nondifferentially reinforced probe trials in each session of the first symmetry test in Experiment 2.
Baseline Retraining
Average accuracies for hue identity, form identity, and symbolic matching over the last five retraining sessions were 97.4%, 92.0%, and 97.6% correct, respectively. Once again, form identity accuracy was significantly lower than hue identity and symbolic accuracies, F(2, 4) = 24.46, which did not differ from one another, F(2, 4) = 0.02. Performances across sample locations, however, were again comparable: 97.0%, 95.6%, and 94.6% correct for left-, center-, and right-key samples, respectively, F(2, 4) = 2.27, n.s.
Symmetry Test 2
Figure 5 plots each pigeon's accuracy on the differentially reinforced symmetry probe trials for each of the 30 test sessions. There was considerable session-to-session variability in probe-trial accuracy for 2 of the 3 pigeons (SYMM3 and SYMM 4). More importantly, there was no systematic trend(s) across consistent versus inconsistent test conditions indicative of an emergent symmetry effect. Although pigeon SYMM4 (whose reinforced line–hue choices were symmetrical versions of the baseline symbolic matching relations) routinely matched above chance levels of accuracy after 8 sessions, even reaching 90% and higher on 2 sessions, accuracy for the other pigeon in the consistent test condition (SYMM2) was mostly at or around 50% correct throughout testing. Indeed, pigeon SYMM2's accuracy was frequently lower than that of the one inconsistent pigeon (SYMM3) whose reinforced line–hue choices were the opposite of the symmetrical versions of the baseline symbolic relations. Although the sensitivity of this second, differentially reinforced symmetry test might be questioned given that every probe-trial choice had been nondifferentially reinforced in the first test, that initial test experience would be more likely to delay (rather than negate) the appearance of a subsequent between-condition difference (e.g., Hall & Channell, 1980; Newlin & Thomas, 1978)
Fig 5.
Percentage of correct choices for individual subjects on differentially reinforced symmetry probes of the second symmetry test in Experiment 2. The reinforced test relations were either symmetrical versions of the symbolic training relations (“Consistent”) or the opposite of them (“Inconsistent”).
In their entirety, then, the test results from this experiment provide no compelling evidence of associative symmetry in pigeons. This occurred despite (a) concurrent training on multiple-location symbolic and identity matching (to insure experience with each matching stimulus at each location) and (b) identical sample- and comparison-response requirements (to avoid another potential stimulus-definition problem). Together with other nonhuman animal data (e.g., Dugdale & Lowe, 2000; Hogan & Zentall, 1977; Lionello-DeNolf & Urcuioli, 2002; Lipkens, et al., 1988; Sidman et al. 1982), two-alternative matching does not appear to be conducive to associative symmetry.
Experiment 3
In contrast to the results from two-alternative matching, Frank and Wasserman (2005) found evidence for associative symmetry in pigeons using a go/no-go (successive) matching procedure (Nelson & Wasserman, 1978; Urcuioli & Zentall, 1990; Wasserman, 1976). In this procedure, samples and comparisons appear singly on the same response key with certain sample–comparison sequences ending in reinforcement and other sequences ending in extinction. Accurate performances are evident when subjects respond frequently to the comparisons on the reinforced trials and little, if at all, to the comparisons on the nonreinforced trials.
Using color clip-art stimuli, Frank and Wasserman (2005, Experiment 1) trained 2 pigeons concurrently on symbolic successive matching and on two identity tasks using the stimuli appearing in the symbolic task. Following acquisition, nonreinforced symmetry probe trials were interspersed among the various baseline trials. Frank and Wasserman found that both pigeons responded frequently to the comparisons on probes that were symmetrical versions of the reinforced symbolic baseline relations, and infrequently to the comparisons on probes that were symmetrical versions of the nonreinforced symbolic baseline relations.
Successive matching possesses a number of features that may be conducive to obtaining such results. First, samples and comparisons always appear at one spatial location avoiding any problems if location is part of the functional stimuli (cf. Lionello & Urcuioli, 1998). Second, using equal sample and comparison durations as Frank and Wasserman (2005) did also avoids any problems if duration is a feature of the functional stimuli. Third, training on identity matching as well as symbolic matching insures that all requisite discriminations necessary for symmetrical responding are learned prior to testing. Fourth, throughout training, one half of all sample–comparison combinations end in extinction independently of the accuracy of pigeons' performances. The latter may be the most influential feature, and I will return to it later.
At this point, it seemed wise to attempt a replication of the Frank and Wasserman (2005) results (cf. Vasconcelos, Urcuioli, & Lionello-DeNolf, 2007) with stimuli of the sort used in Experiments 1A, 1B, and 2. Experiment 3, then, modeled their procedure with hue and form stimuli.
Method
Subjects and Apparatus
Eight experimentally naïve White Carneau pigeons from the Palmetto Pigeon Plant (Sumter, SC) served in the experiment. After establishing their free-feeding body weights, they were food-deprived to 80% of those weights and maintained at that level throughout the experiment. Housing and other maintenance conditions were identical to those previously described.
Pigeons were run in one of two experimental chambers, each identical to the one used in Experiment 1A. Only the center response key of each chamber was used. The projector behind it could present white, red, and green homogenous fields, an inverted white triangle on a black background, and three horizontal white lines also on a black background (BRS/LVE Pattern No. 692).
Procedure
Preliminary Training
After training to eat out of a raised and lit food hopper and to peck white on the center key, pigeons learned to peck red and green center-key hues, and the center-key triangle and horizontal lines, for food in separate 60-trial sessions. Three sessions were given with each stimulus set, alternated across days. Over the next eight 60-trial sessions (four with red and green and four with the triangle and horizontal lines, alternated across days), pecking was reinforced on fixed-interval (FI) schedules. Each session had equal numbers of center-key presentations of the two hues or the two forms in random order. The first peck to the stimulus appearing on each trial initiated the FI; the first peck after the interval elapsed immediately turned the stimulus off and produced food. Successive trials were separated by a 15-s ITI, the first 14 s of which was spent in darkness. The house light came on for the last 1 s of the ITI and remained on until the end of the next trial. The FI parameter was initially set at 2 s and was gradually raised over sessions to 5 s. All other procedural details were identical to those for FR preliminary training in Experiment 1A.
Successive Matching Acquisition
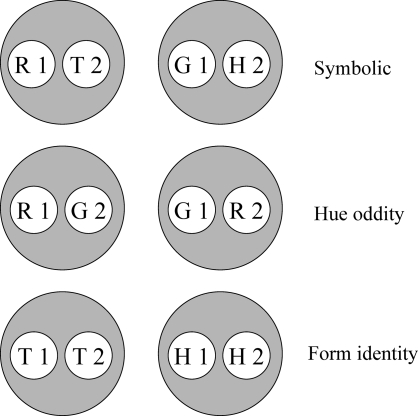
Next, pigeons began concurrent training on symbolic (hue–form), hue-identity, and form-identity successive matching. Each matching trial began with the onset of the sample stimulus on the center key (see Table 1). The first sample key peck initiated a FI 5-s schedule ending in the offset of the sample, a 500-ms blank interval, and the onset of the comparison stimulus on the center key. For reinforced sample–comparison combinations, the first comparison key peck after 5 s turned off the comparison and produced food. For nonreinforced sample–comparison combinations, the comparison stimulus and the house light went off automatically after 5 s. For all pigeons, pecking the red comparison after a red sample and the green comparison after a green sample was reinforced (hue identity), as was pecking the triangle comparison after a triangle sample and the horizontal comparison after a horizontal sample (form identity). Pecking the mismatching hue comparison or the mismatching form comparison was nonreinforced. On symbolic matching trials, pecking the triangle comparison after the red sample and the horizontal comparison after the green sample produced food after 5 s for half of the pigeons, whereas pecking the triangle comparison after the green sample and the horizontal comparison after the red sample were nonreinforced. For the remaining pigeons, the opposite contingencies were in effect.
Table 1.
Successive Matching Training Contingencies in Experiment 3.
| R → T • FI 5″ | |
| R → H • EXT | |
| G → T • EXT | Symbolic |
| G → H • FI 5″ | |
| R → R • FI 5″ | |
| R → G • EXT | |
| G → R • EXT | Hue identity |
| G → G • FI 5″ | |
| T → T • FI 5″ | |
| T → H • EXT | |
| H → T • EXT | Form identity |
| H → H • FI 5″ |
Training sessions contained 32 trials each of symbolic, hue identity, and form identity successive matching, with the four possible combinations of samples and comparisons for each task occurring equally often in each session. The 12 total trial types (four sample–comparison combinations × three matching tasks) were presented in random order with the constraint that none occur more than twice in a row.
For each task, a discrimination ratio (DR) was computed by dividing the total number of comparison pecks on reinforced trials by the total number of comparison pecks on both reinforced and nonreinforced trials. Only key pecks occurring within the first 5 s of comparison onset were recorded. A DR of approximately .50 indicates no discrimination between reinforced and nonreinforced sample–comparison combinations, and it approaches 1.00 as the discrimination is learned. Each pigeon was trained until it achieved a DR of 0.80 or higher on each matching task for 5 of 6 consecutive training sessions. It then received 10 additional overtraining sessions. One pigeon failed to meet criterion after 250 training sessions and was dropped from the experiment.
Symmetry Testing
Eight symmetry test sessions, run in two-session blocks separated by at least five baseline training sessions, were run after successive matching acquisition. Each test session contained 96 reinforced trials divided equally among the three baseline tasks and 8 nonreinforced symmetry test trials. Test trials consisted of a triangle or horizontal sample followed by a red or green comparison. The four possible combinations of the form samples with the hue comparisons occurred equally often in each session. On all test trials, the comparison stimulus (and house light) went off automatically after 5 s, independently of responding. The FI 5-s sample-response requirement remained in effect. Successive test trials were separated by at least 6 baseline trials, and the first test trial in a session did not occur until at least one of each of the 12 possible baseline trials had been presented. All other procedural details were identical to those for successive matching acquisition.
Results and Discussion
Acquisition and Baseline Performances
The average numbers of training sessions to reach criterion levels of performance on symbolic, hue-identity, and form-identity successive matching were 29.9, 28.6, and 40.7, respectively. Post-hoc contrasts (Rodger, 1975) on these data showed that form identity was acquired more slowly than symbolic and hue-identity matching, F(2, 6) = 4.92, which did not differ from one another, F(2, 6) = 0.05. By the end of training, however, the three successive matching performances were comparable. For the five sessions preceding the first symmetry test, the average DRs for symbolic, hue-identity and form-identity matching were 0.91, 0.91, and 0.88, F(2, 6) = 1.62, n.s.
Accurate baseline performances were also maintained during testing with no significant between-task differences. The average DRs during testing for the symbolic, hue-identity, and form-identity matching were 0.91, 0.93, and 0.87, respectively, F(2, 6) = 1.88, n.s.
Symmetry Testing
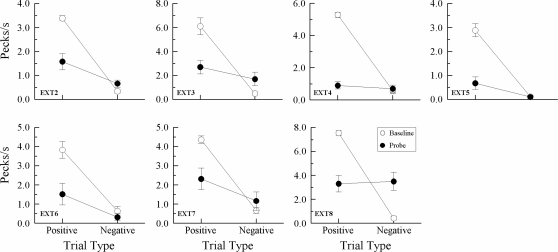
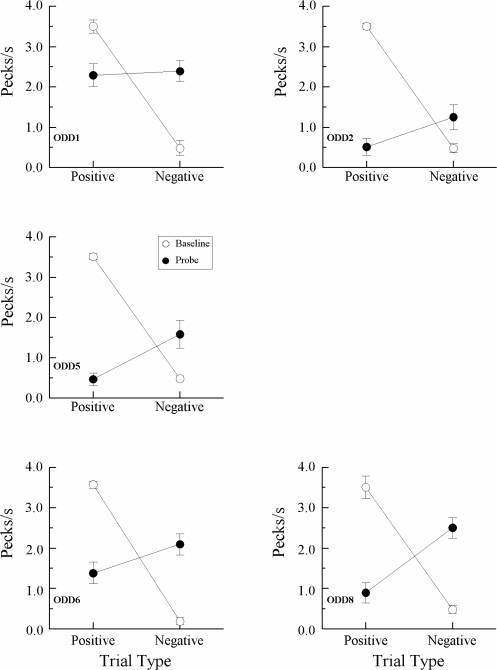
Figure 6 presents individual-subject data averaged over the first four symmetry test sessions. These particular data were chosen for display because as testing progressed, pigeons discriminated that the symmetry probe trials always ended in nonreinforcement, resulting in increasingly lower overall responding on these trials. Using the same presentation format as Frank and Wasserman (2005), solid circles plot the average number of comparison pecks per second on symmetry probes that were the reverse of the reinforced symbolic baseline trials (“positive”) and the reverse of the nonreinforced symbolic baseline trials (“negative”). Open circles plot comparison-response rates on the reinforced (positive) and nonreinforced (negative) symbolic baseline trials themselves. The baseline data are averaged over all symbolic matching trials in these test sessions.
Fig 6.
Comparison responses/sec (± 1 SEM) on symbolic matching baseline trials (open circles) and nonreinforced symmetry probe trials (filled circles) averaged over the first four symmetry tests for each pigeon in Experiment 3. Positive = reinforced baseline relations and their symmetrical test relations. Negative = nonreinforced baseline relations and their symmetrical test relations. Note the different ranges of comparison-response rates.
First, the open-symbol functions clearly show that pigeons continued to respond appropriately on their baseline symbolic matching task, pecking rapidly to the comparisons on reinforced (positive) trials and very little to the comparisons on the nonreinforced (negative) trials. These results simply confirm what the baseline DRs mentioned above already indicated. Second, and of greater interest, was the finding that the rate of comparison responding by 5 of the 7 pigeons (EXT 2, EXT 3, EXT 5, EXT 6, and EXT 7) was higher on symmetry probes that were the reverse of the reinforced (positive) baseline symbolic relations than on probes that were the reverse of the nonreinforced (negative) baseline symbolic relations. The other 2 pigeons (EXT 4 and EXT 8) responded nondifferentially on the symmetry probes. Finally, overall comparison response rates on the probes were lower than on the baseline trials, no doubt reflecting in part the pigeons' ability to discriminate that the former always ended in nonreinforcement.
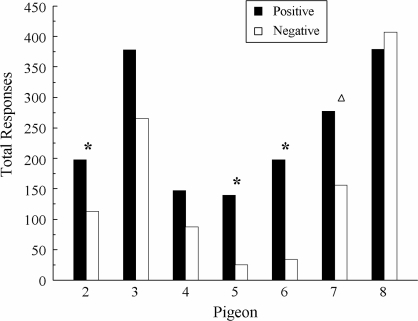
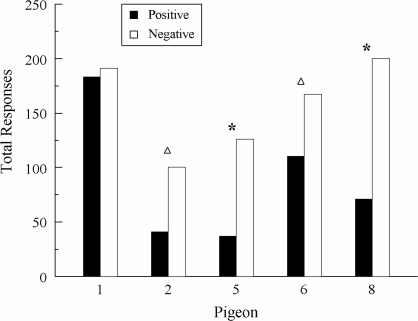
Figure 7 shows the total number of comparison responses on positive versus negative symmetry-probe trials for each pigeon summed over its first four test sessions. A higher number of comparison responses on positive than on negative probes is indicative of associative symmetry. The stars indicate the 3 pigeons (EXT 2, EXT 5, and EXT 6) for which this difference was statistically significant, Fs(1, 30) = 6.11, 4.75, and 7.29, respectively. The difference was not significant for EXT 3 because despite appearances, it responded equally often on both types of probes during the first two test sessions. Although the difference was also not significant for EXT 7, this pigeon pecked significantly more often to the comparisons on positive than on negative probes on its first two test sessions: 106 versus 27, respectively, F(1, 14) = 5.04. This difference was not maintained during its subsequent test sessions.
Fig 7.
Total number of responses by subject to the comparisons on the symmetry probe trials in Experiment 3 summed over the first four test sessions. Positive = test relations that were symmetrical versions of the reinforced symbolic matching baseline relations. Negative = test relations that were symmetrical versions of the nonreinforced symbolic matching baseline relations. Stars indicate statistically significant differences. Triangle indicates a statistical significant difference for the first two test sessions.
Table 2 provides another measure of differential responding on the symmetry probe trials: the percentage of the positive and negative probes on which pigeons made one or no pecks to the comparison stimulus over the first four test sessions and over all eight sessions. A larger percentage of trials with one or no comparison responses on the negative than on the positive probes would be indicative of symmetry. Probe trials with one comparison response were part of this count because pigeons sometimes pecked through the short interstimulus interval separating sample from comparison stimulus, thus pecking the comparison at the moment it appeared. As the table shows, every pigeon pecked once or not at all to the comparison on a larger percentage of the negative probe trials, and this was true for both the initial four and all eight test sessions. The average differences between positive and negative probes for the first four and all eight tests were statistically significant, Fs (1, 6) = 18.10 and 15.72, respectively.
Table 2.
Percentage of positive and negative symmetry probe trials with 1 or no comparison pecks for each pigeon over its first four and all eight test sessions in Experiment 3.
| Pigeon | First Four Tests |
All Eight Tests |
||
| Positive | Negative | Positive | Negative | |
| EXT 2 | 12.5 | 25.0 | 15.6 | 21.9 |
| EXT 3 | 18.8 | 25.0 | 12.5 | 34.4 |
| EXT 4 | 37.5 | 50.0 | 50.0 | 53.1 |
| EXT 5 | 68.8 | 87.5 | 50.0 | 87.5 |
| EXT 6 | 37.5 | 81.2 | 53.1 | 84.4 |
| EXT 7 | 18.8 | 43.8 | 34.4 | 50.0 |
| EXT 8 | 12.5 | 31.2 | 15.6 | 31.2 |
| Mean | 29.5 | 49.1 | 33.0 | 51.8 |
Overall, the combined test results show evidence for associative symmetry in 3 pigeons (EXT 2, EXT 5, and EXT 6) and, arguably, a 4th (EXT 7). For them, higher rates of comparison responding on the reinforced than on nonreinforced symbolic baseline trials were reproduced when the roles of the samples and comparisons were reversed. This replicates the findings of Frank and Wasserman (2005, Experiment 1) and places those findings on a firmer footing. Furthermore, these pigeons also refrained from pecking the comparison stimulus on negative symmetry probe trials more often than they refrained from pecking the comparison stimulus on positive symmetry probe trials, lending additional support to the claim of emergent symmetry. This, too, is noteworthy in view of the important theoretical implications of Frank and Wasserman's seminal findings (Hayes, 1991; Horne & Lowe, 1996; Sidman, 2000).
Nonetheless, there are some notable differences between their results and mine. First, the comparison response rates on the symmetry probes in Frank and Wasserman (2005, Experiment 1) were similar to the rates observed on the symbolic baseline trials. Here, comparison response rates were considerably lower on the positive symmetry probes than on the positive (reinforced) symbolic baseline trials. The across-experiment difference is due to the positive baseline response rates in Frank and Wasserman (2005): Their pigeons pecked at a much lower rate (viz., mostly around 1 peck/s; see their Table 2) than the pigeons in the present experiment (see Figure 6). The longer sample and comparison durations used by Frank and Wasserman (10 vs. 5 s), their use of random ITIs (mine were constant), the matching stimuli themselves, the recording apparatus (touch screen vs. standard pecking keys), or any combination thereof could be responsible for the difference.
Second, both pigeons in Frank and Wasserman (2005, Experiment 1) showed associative symmetry. In the present experiment, only about half of the pigeons did. Again, the reason(s) for the difference is (are) unclear. Although not every pigeon in the present experiment showed associative symmetry, not every human does either in studies of equivalence-class formation (e.g., Eikeseth & Smith, 1992; see also Devany, Hayes, & Nelson, 1986, p. 252). Besides, the fact that some pigeons did exhibit symmetry in successive matching contrasts sharply with repeated failures to find evidence for associative symmetry in two-alternative matching (e.g., see Experiments 1A, 1B, and 2). The next section offers a theory of pigeons' equivalence-class formation to account for the success of concurrent symbolic and identity successive matching in producing associative symmetry and, by implication, for the failure of the corresponding two-alternative matching procedure to do the same.
A Theory of Pigeons' Equivalence-Class Formation
Prior to presenting the theory, it might be helpful to reiterate some of the likely consequential advantages of successive matching over two-alternative matching in attempts to demonstrate associative symmetry. First, samples and comparisons appear at a single spatial location, thus avoiding the functional stimulus problem inherent in two-alternative matching (cf. Lionello & Urcuioli, 1998). Second, each matching stimulus appears by itself so all requisite discriminations are successive ones in both training and testing (R. R. Saunders & Green, 1999; see also K. J. Saunders & Spradlin, 1989). Third, intermixing identity training with symbolic matching training insures that all requisite discriminations are in place at the time of testing and that each matching stimulus has appeared at each temporal location (viz., first when appearing as a sample and second when appearing as a comparison). Fourth, half of all matching trials always end in nonreinforcement. In other words, even after pigeons have learned all baseline tasks to high levels of discrimination accuracy, they still frequently experience nonreinforcement following certain sample–comparison combinations. By contrast, pigeons rarely experience nonreinforcement once they achieve high levels of accuracy in two-alternative matching.
I propose that the reinforcement versus nonreinforcement continually encountered throughout successive matching training yields stimulus classes containing the stimuli comprising each reinforced sample–comparison combination. One might view this difference as further evidence for the facilitating effect of differential outcomes on class formation (Sidman, 1994, 2000; see also Dube & McIlvane, 1995; Dube, McIlvane, Mackay, & Stoddard, 1987; Joseph, Overmier, & Thompson, 1997). However, the term “differential outcomes” as used here is atypical both in its reference to trial outcomes (reinforcement vs. nonreinforcement; although see Urcuioli & Zentall, 1990) and because all reinforced sample–comparison combinations end with the same reinforcer (Urcuioli, 2005). Nevertheless, the dramatic difference in trial outcomes for the positive versus negative baseline trials (viz., reinforcement vs. nonreinforcement) is postulated as a critical feature in the eventual segregation of class elements.
I also propose that those elements—the functional stimuli in successive matching—are the nominal stimuli plus their temporal location. Thus, red seen first in a trial (viz., as a sample) is a functionally different stimulus for pigeons than red seen second in a trial (viz., as a comparison). In other words, I assume that pigeons do not ignore temporal location even when each matching stimulus appears at each temporal location during training, as happens when identity matching is intermixed with symbolic matching. The hypothesized coding of each stimulus in terms of what it is and when it appears is consistent with other views emphasizing the importance of temporal factors in animal learning (see, for example, Miller & Barnet, 1993; Weisman, Wasserman, Dodd, & Larew, 1980).
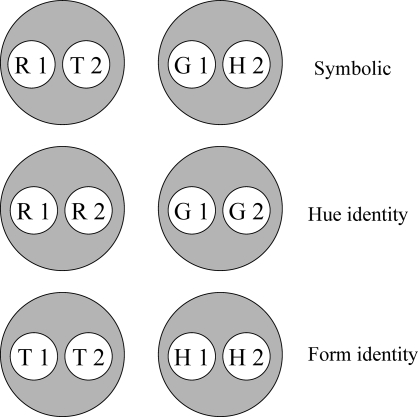
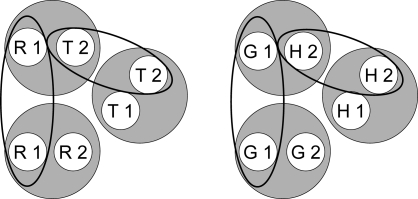
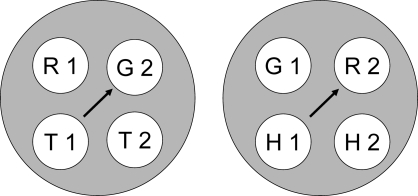
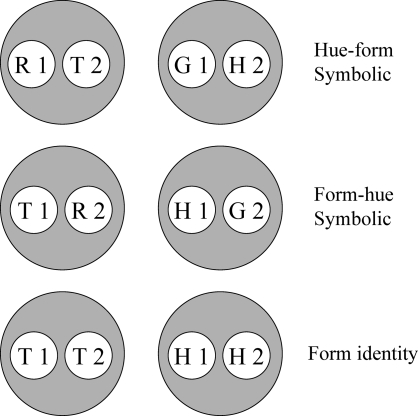
Given these assumptions, successive symbolic matching training should yield two stimulus classes; hue-identity training should yield two additional classes; and form-identity training two more. Figure 8 shows the resulting six hypothesized classes. Note that the stimuli in each class are not simply red (R), green (G), etc. but rather red in the first temporal position (R1), red in the second temporal position (R2), green in the first temporal position (G1), etc. This assumption is essential to the theory's predictions.
Fig 8.
Hypothesized stimulus classes arising from the reinforced sample–comparison combinations in symbolic, hue-identity, and form-identity successive matching. R = red, G = green, T = triangle, H = horizontal, 1 = first temporal position within a matching trial, 2 = second temporal position within a matching trial.
Given the training contingencies in Experiment 3 (see Table 1), R1 and T2 become members of one class because responding to the triangle comparison (T2) was reinforced after the red sample (R1) but not after the green sample (G1). Likewise, G1 and H2 become members of another class because responding to the horizontal comparison (H2) was reinforced after the green sample (G1) but not after the red sample (R1). Because the other sample–comparison combinations in the symbolic matching task (R1 and H2, and G1 and T2) were nonreinforced (see Table 1), their component elements are in different classes (i.e., nonreinforcement of these combinations helps keep the reinforced classes apart). The same logic applies to the two identity tasks, creating a red sample–red comparison (R1 and R2) class, a triangle sample–triangle comparison (T1 and T2) class, etc.
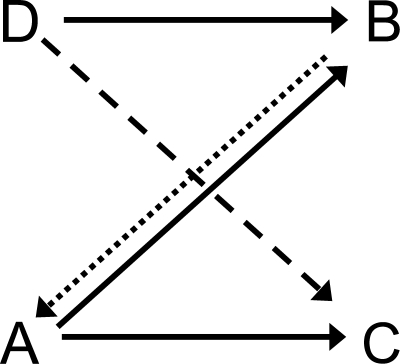
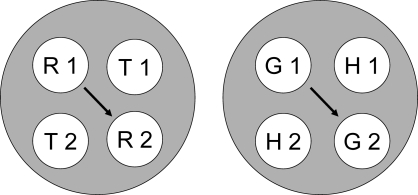
Note, too, that some classes have elements in common—e.g., the red sample (R1) from symbolic and hue-identity training, and the horizontal comparison (H2) from symbolic and form-identity training. These common elements are highlighted by the ellipses in Figure 9. If common elements cause their respective classes to merge (Sidman & Tailby, 1982; Sidman, Kirk, & Willson-Morris, 1985; see also Dube, McIlvane, Maguire, Mackay, & Stoddard, 1989), then the result is two 4-member classes as shown in Figure 10: one containing the red sample and comparison (R1 and R2) and the triangle sample and comparison (T1 and T2) and the other containing the green sample and comparison (G1 and G2) and the horizontal sample and comparison (H1 and H2).
Fig 9.
The six stimulus classes shown in Figure 8 rearranged to show common class members (highlighted by the ellipses). R = red, G = green, T = triangle, H = horizontal, 1 = first temporal position within a matching trial, 2 = second temporal position within a matching trial.
Fig 10.
Two 4-member stimulus classes hypothesized to arise from the merger of the stimulus classes in Figure 9 via their common elements. R = red, G = green, T = triangle, H = horizontal, 1 = first temporal position within a matching trial, 2 = second temporal position within a matching trial. Arrows indicate sample–comparison combinations to which the pigeons should preferentially respond in a symmetry test.
If pigeons respond to comparisons appearing after samples in the same reinforced class, then in testing they should preferentially respond to the red comparison (R2) after the triangle sample (T1) and to the green comparison (G2) after the horizontal sample (H1). These combinations (denoted by the arrows in Figure 10) represent associative symmetry given that the reinforced symbolic baseline combinations were the triangle comparison after the red sample (T2 after R1) and the horizontal comparison after the green sample (H2 after G1). Indeed, the results of Experiments 3 support this theoretical prediction.
The theory also makes many other testable predictions. One unusual, and perhaps counterintuitive, prediction is that if hue oddity rather than hue identity is part of concurrent successive matching training, pigeons should subsequently respond more to the reverse of the nonreinforced symbolic training relations than to the reverse of the reinforced symbolic training relations. This antisymmetry prediction is explained more fully and was tested in the next experiment.
Experiment 4
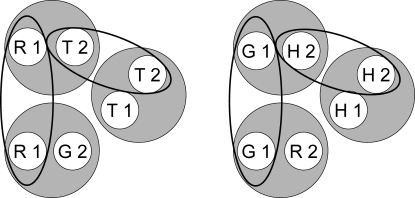
Experiment 4 was identical in design to Experiment 3 except that hue oddity (rather than hue identity) was one of the three baseline matching tasks (see Table 3). Given the assumptions of the theory, Figure 11 shows the six stimulus classes that should develop during training. Note that one class now contains the red sample and the green comparison (R1 and G2) and another now contains the green sample and the red comparison (G1 and R2) because these are the reinforced combinations for hue oddity. Figure 12 rearranges the six classes so that their common elements can be easily connected; Figure 13 shows the two 4-member classes resulting from class merger.
Table 3.
Successive Matching Training Contingencies in Experiment 4.
| R → T • FI 5″ | |
| R → H • EXT | |
| G → T • EXT | Symbolic |
| G → H • FI 5″ | |
| R → G • FI 5″ | |
| R → R • EXT | |
| G → G • EXT | Hue oddity |
| G → R • FI 5″ | |
| T → T • FI 5″ | |
| T → H • EXT | |
| H → T • EXT | Form identity |
| H → H • FI 5″ |
Fig 11.
Hypothesized stimulus classes arising from the reinforced sample–comparison combinations in symbolic, hue-oddity, and form-identity successive matching. R = red, G = green, T = triangle, H = horizontal, 1 = first temporal position within a matching trial, 2 = second temporal position within a matching trial.
Fig 12.
The six stimulus classes shown in Figure 11 rearranged to show common class members (highlighted by the ellipses). R = red, G = green, T = triangle, H = horizontal, 1 = first temporal position within a matching trial, 2 = second temporal position within a matching trial.
Fig 13.
Two 4-member stimulus classes hypothesized to arise from the merger of the stimulus classes in Figure 12 via their common elements. R = red, G = green, T = triangle, H = horizontal, 1 = first temporal position within a matching trial, 2 = second temporal position within a matching trial. Arrows indicate sample–comparison combinations to which pigeons should preferentially respond in a symmetry test.
Again, if pigeons respond more to a comparison that is a member of the same reinforced class as the sample that precedes it, then in testing they should preferentially peck the green comparison (G2) after the triangle sample (T1) and the red comparison (R2) after the horizontal sample (H1) even though in training the triangle comparison (T2) was reinforced after the red sample (R1) and the horizontal comparison (H2) was reinforced after the green sample (G1). The arrows in Figure 13 illustrate this antisymmetry prediction. Note that the mere experience of seeing each matching stimulus in each temporal location during successive matching training (cf. Frank & Wasserman, 2005) does not make this prediction. Indeed, such a familiarity account would predict associative symmetry—in other words, exactly the same pattern of positive versus negative probe responding like that observed in Experiment 3.
Method
Subjects and Apparatus
Eight experimentally naïve White Carneau retired breeders from the Palmetto Pigeon Plant (Sumter, SC) began the experiment. One died during training and was not replaced; 2 others were dropped from the experiment when they failed to achieve criterion levels of performance after 150 and 200 acquisition sessions, respectively. Housing, maintenance conditions, and the two experimental chambers were identical to those previously described.
Procedure
Preliminary training was the same as in Experiment 3. So, too, was successive matching training except that one of the three concurrent tasks was hue oddity rather than hue identity. In other words, on trials involving red and green samples and comparisons, the first comparison peck after 5 s produced food if that comparison did not match the preceding sample (i.e., green after red, and red after green), whereas the comparison went off response-independently after 5 s if the comparison matched the preceding sample (i.e., red after red, and green after green). All other procedural details for these sessions, including the acquisition criteria and overtraining, were the same as for successive matching training in Experiment 3.
The eight probe test sessions that followed acquisition were again run in blocks of two successive test sessions separated by at least five baseline sessions in which pigeons' performances on each matching task—symbolic, hue oddity, and form identity—were at or above a DR of .80. Details of these probe sessions were the same as in Experiment 3.
Results and Discussion
Acquisition and Baseline Performances
The average numbers of training sessions to reach criterion levels of performance on the symbolic, hue-oddity, and form-identity successive tasks for the 5 pigeons completing the experiment were 42.8, 44.0, and 55.2, respectively, F(2, 4) = 0.78. For the five sessions preceding the first symmetry test, the average DRs for these three tasks were 0.90, 0.91, and 0.86, which did not differ significantly, F(2, 4) = 5.17. Baseline performances remained accurate during testing: The corresponding DRs were 0.91, 0.93, and 0.88, respectively, F(2, 4) = 3.26, n.s.
Symmetry Testing
Figure 14 shows individual-subject data averaged over the first four symmetry test sessions. The open circles plot performances on the baseline symbolic matching task; the solid circles plot performances on the symmetry test trials.
Fig 14.
Comparison responses/sec (± 1 SEM) on symbolic matching baseline trials (open circles) and nonreinforced symmetry probe trials (filled circles) averaged over the first four symmetry tests for each pigeon in Experiment 4. Positive = reinforced baseline relations and their symmetrical test relations. Negative = nonreinforced baseline relations and their symmetrical test relations.
The baseline symbolic matching discrimination was well maintained in testing: All pigeons pecked rapidly to the comparisons on reinforced (positive) trials and very little to the comparisons on the nonreinforced (negative) trials. On the symmetry test trials, every pigeon except one responded differentially to positive versus negative probes. However, the pattern of differential responding on the probe trials was noteworthy: Pigeons responded more to the reverse of the nonreinforced (negative) baseline symbolic relations than to the reverse of the reinforced (positive) baseline symbolic relations—antisymmetry.
Figure 15 shows the total number of comparison responses on positive versus negative test trials for each pigeon over its first four test sessions. A greater number of responses on negative than on positive probes illustrates antisymmetry. This difference was clearly apparent and was significant for 2 pigeons (ODD 5 and ODD 8), Fs(1, 30) = 8.66 and 19.90, respectively, and it approached significance for 2 others (ODD 2 and ODD 6), Fs(1, 30) = 3.97 and 3.68, p s = .055 and .064, respectively. For Pigeon ODD 2, the difference was significant over its first two test sessions, F(1, 14) = 4.96 (data not shown).
Fig 15.
Total number of responses by subject to the comparisons on the symmetry probe trials in Experiment 4 summed over the first four test sessions. Positive = test relations that were symmetrical versions of the reinforced symbolic matching baseline relations. Negative = test relations that were symmetrical versions of the nonreinforced symbolic matching baseline relations. Stars indicate statistically significant differences. Triangles indicate differences approaching statistical significance.
Table 4 shows the percentage of the positive and negative probes on which pigeons made one or no pecks to the comparison stimulus over the first four test sessions and over all eight sessions. A larger percentage of trials with one or no comparison responses on the positive than on the negative probes would be consistent with an antisymmetry effect. Indeed, 4 of the 5 pigeons showed this pattern of results for the first four test sessions, and all did over all eight test sessions. The average difference between positive and negative probes for all eight sessions was statistically significant, F(1, 4) = 8.54, and it approached significance for the first four sessions, F(1, 4) = 6.43, p = .06.
Table 4.
Percentage of positive and negative symmetry probe trials with one or no comparison pecks for each pigeon over its first four and all eight test sessions in Experiment 4.
| Pigeon | First Four Tests |
All Eight Tests |
||
| Positive | Negative | Positive | Negative | |
| ODD 1 | 6.2 | 12.5 | 28.1 | 21.9 |
| ODD 2 | 68.8 | 43.8 | 78.1 | 65.6 |
| ODD 5 | 68.8 | 31.2 | 78.1 | 37.5 |
| ODD 6 | 25.0 | 12.5 | 34.4 | 25.0 |
| ODD 8 | 31.2 | 6.2 | 34.4 | 12.5 |
| Mean | 40.0 | 21.2 | 50.6 | 32.5 |
These results confirm the prediction derived from the proposed theory of pigeons' equivalence-class formation. Although training hue–form symbolic successive matching concurrently with hue oddity and form identity gives pigeons experience with each matching stimulus in each temporal location, this concurrent training regimen does not yield associative symmetry like that observed in Experiment 3. To the contrary, it yields results which are the exact opposite of the earlier ones: a higher rate of comparison responding on probe trials that are the reverse of the nonreinforced (negative) symbolic baseline relations than on probe trials that are the reverse of the reinforced (positive) symbolic baseline relations. These results support the theoretical proposal that, for pigeons, the functional matching stimuli in successive matching are compounds of the nominal stimuli and their temporal location within a trial and that class formation results from the reinforcement versus nonreinforcement of particular sequential sample–comparison combinations.
General Discussion
In conjunction with previous findings from this lab and others (e.g., Hogan & Zentall, 1977; Lionello-DeNolf & Urcuioli, 2002; Lipkens, et al., 1988; see also Sidman et al. 1982), the present findings make a strong case that two-alternative matching is not conducive to the development of the emergent relations that define stimulus equivalence, at least in pigeons. Despite controlling for the location at which the matching stimuli appear, the requisite discriminations for symmetry, and the number of times pigeons responded to the samples and comparisons, the first three experiments found no evidence of symmetry. It would seem, then, that a lack of congruence between the experimentally defined stimuli and the functional matching stimuli (McIlvane et al., 2000) is not at fault here. This is not to say that congruence is irrelevant but, rather, that congruence alone in conditional discrimination learning does not guarantee that those conditional relations will be equivalence relations.
After all, Experiments 1A, 1B and 2 found no evidence of associative symmetry whereas Experiments 3 and 4 did. The difference between these two sets of experiments was the switch to successive matching in Experiments 3 and 4. Interestingly, Lipkens et al. (1988) anticipated the success of successive matching (see also Debert, Matos, & McIlvane, 2007) in these types of investigations:
“…it might be better to use a matching-to-sample procedure with one single key, on which the sample is presented first and thereafter the correct or the incorrect comparison. With such a procedure it may be easier to ensure that the stimuli maintain their functional identity. In addition, the contingencies require the subject to learn not only the positive relation between the sample and correct comparison (if A1, then peck B1), but also the negative relation between the sample and the incorrect comparison (if A1, then do not peck B2). Perhaps the explicit establishment of a negative controlling relation between the sample and the negative comparison facilitates the development of stimulus equivalence.” (p. 407)
Indeed, it does appear to be the case that explicit establishment of negative controlling relations (Johnson & Sidman, 1993) facilitates pigeons' equivalence-class formation, as evidenced by the emergent effects observed in Experiments 3 and 4. I would also argue that negative controlling relations should not only be established but that they should be maintained throughout training by explicit nonreinforcement. After all, even in two-alternative matching, negative controlling relations between samples and incorrect comparisons are established during training: Not pecking the incorrect comparison after viewing a sample stimulus is an integral part of pigeons' ability to perform accurately. In my estimation, the substantive procedural difference is that pigeons performing accurately on two-alternative matching rarely encounter nonreinforcement because seeing the incorrect comparison has become a cue to switch to the alternative (correct) comparison which then yields reinforcement when it is pecked (see Wright & Sands, 1981). By contrast, there is (loosely speaking) no escape from nonreinforcement of the negative sample–comparison combinations in successive matching.
Is stimulus class formation in successive matching facilitated by the reinforcement versus nonreinforcement associated with different sample–comparison combinations or by responding versus not-responding, respectively, to the comparisons on the positive versus negative trials? The two are clearly correlated in procedures like those used in Experiments 3 and 4, so no definitive answer to this question can be given at this point. One way to distinguish between them would be to arrange successive matching contingencies like those depicted in Table 1 but with a differential-reinforcement-of-other-behavior (DRO) reinforcement schedule instead of extinction on the negative baseline trials. With this modification, all successive matching trials would end in reinforcement, albeit contingent upon responding to the comparison stimulus on half of the trials and upon not-responding to the comparison stimulus on the other half (see, for example, Urcuioli & Zentall, 1990). If different trial outcomes are necessary for class formation, then this modified procedure will not yield symmetry. On the other hand, if differential responding to the comparisons is sufficient for class formation, then the symbolic baseline relations should be symmetrical.
As an original part of Experiment 3, a separate group of pigeons was trained with just such contingencies but, unfortunately, their baseline behavior was not conducive to assessing symmetry (or, for that matter, baseline discriminative performances). These DRO pigeons simply learned to wait 5 s when the comparison stimulus appeared on every matching trial and if reinforcement was not forthcoming, to then peck that comparison. In view of this highly appropriate but experimentally uncooperative behavior, other means will be necessary to evaluate the relative roles of different trial outcomes (reinforcement vs. nonreinforcement) and differential comparison responding in class formation. One possibility is to require a left versus right choice following each center-key sample–comparison sequence. This pair comparison procedure (Shimp & Moffitt, 1977; Pontecorvo, 1985; Richards, 1988, Experiment 3; Urcuioli & DeMarse, 1997) is a hybrid of successive matching and two-alternative matching: Samples and comparisons are presented sequentially on a single key in all possible combinations, but each combination is followed by a two-alternative choice. Because all correct choices are reinforced, the view that class formation hinges upon reinforcement versus nonreinforcement of particular sample–comparison combinations predicts that the symbolic matching combinations would not be symmetrical. Alternatively, assuming that left versus right key pecks are different responses, a differential response view predicts that those combinations would be symmetrical. Obtaining the latter finding would be particularly interesting given that different choice responses in standard two-alternative tasks are not sufficient to promote symmetry (see Experiments 1A, 1B, and 2).
The proposed theory of pigeons' equivalence-class formation gets its predictive power in large part because of the assumption that the functional stimuli for pigeons performing successive matching are [stimulus–temporal location] compounds. Given this, one could reasonably object to the characterization of the test results in Experiment 3 as “symmetry” (and, similarly, to the antisymmetry characterization of the test results in Experiment 4). Closer examination of the two 4-member classes depicted in Figure 10 shows why. In training, responding to T2 (the triangle comparison) was reinforced after R1 (the red sample) as was responding to H2 (the horizontal comparison) after G1 (the green sample). But responding to R1 after T2 and to G1 after H2 (the technically symmetrical relations) are impossible because a stimulus in the first temporal position (“1”) can never come after a stimulus in the second temporal position (“2”). However, what an observer sees are pigeons responding more frequently to the reverse of reinforced symbolic-matching training relations than to the reverse of nonreinforced symbolic-matching training relations. In other words, from an observational standpoint (see Figures 6 and 7), symmetry emerges from the baseline training relations. From a theoretical standpoint, this simply reflects the proper combination of first and second temporal-location stimuli in a class (see Figure 10). Although technically not symmetry from the latter standpoint, what is just as important, if not more so, is the theoretical statement that these stimulus classes develop in the first place and that the results are emergent relations that cannot be explained by unidirectional mediational processes (Hull, 1939; K. J. Saunders et al., 1996; Urcuioli & Lionello-DeNolf, 2001).
If the same stimulus presented in different temporal positions constitutes functionally different stimuli, as my theory assumes, an argument could be made that the form–hue probe trials in Experiments 3 and 4 tested for transitivity rather than symmetry. This argument becomes clearer in Figure 16 by representing the training and test relations using the following notation: hue–form (symbolic) matching = AB, form-form (identity) matching = DB, hue–hue (identity or oddity) matching = AC, and the form–hue probes = DC. Each letter indicates particular stimuli (hues or forms) and their temporal position within a trial. Thus, D represents forms when they appear as sample stimuli (first temporal position), whereas B represents those same stimuli when they appear as comparison stimuli (second temporal position). Likewise, A represents hues as samples (first temporal position) and C represents hues as comparisons (second temporal position). Baseline training, then, consists of learning [DB + AB + AC] conditional relations. Note that the D and A stimuli have one node in common (viz., B) and the B and C stimuli have another node in common (viz., A; Fields, Adams, & Verhave, 1993). The DC probes are, thus, two-node transitivity test trials assuming that the AB relation is reversible (dotted line in Figure 16): DB + BA + AC = DC.
Fig 16.
An alternative characterization of the explicitly trained relations (solid lines) and emergent relations (dashed and dotted lines) in Experiments 3 and 4. A = hue samples, B = form comparisons, C = hue comparisons, and D = form samples.
But this characterization begs the question: It presupposes as one of its premises the very phenomenon that is sought—namely, associative symmetry. In other words, two-node transitivity can produce the observed results only to the extent that the AB training relation is reversible (symmetrical). But that is precisely the phenomenon in question. Moreover, symmetry following AB training has been routinely absent in prior pigeon studies including those using the successive matching paradigm (Richards, 1988).
Another way to appeal to two-node transitivity is to assume that baseline training produced functional or acquired equivalence between the hue and form samples (e.g., Urcuioli, 2006; Wasserman, deVolder, & Coppage, 1992) which, combined with concurrent reassignment training, yielded transfer effects on the probe trials that look like associative symmetry (or antisymmetry). Using standard equivalence notation as above, pigeons learned to match two sets of samples to a common set of comparisons during training (viz., AB and DB). These many-to-one conditional relations (Urcuioli, Zentall, & DeMarse, 1995) should yield acquired equivalence between the A and D samples. Pigeons also learned AC matching during training which can be thought of as reassignment of one set of equivalent samples to “new” comparisons. Given that functionally equivalent samples are interchangeable with one another in new contexts (Goldiamond, 1962), the D samples should readily substitute for the A samples in successive matching with the C comparisons, yielding the observed DC test results. The problem with this analysis is that in both Experiments 3 and 4, pigeons did not learn the two components of the many-to-one relations (AB and DB) prior to learning the “reassignment” (AC) relations. All were trained concurrently. Moreover, pigeons reached criterion levels of performance sooner on AB and AC matching than on DB matching. Thus, it would be more accurate to characterize their acquisition as the learning of one-to-many relations followed by reassignment. For pigeons performing two-alternative matching, this training regimen does not yield transfer effects indicative of acquired equivalence in contrast to the transfer effects routinely observed following many-to-one matching (Urcuioli et al., 1995).
In short, the theoretical account I have proposed seems better suited to explaining the symmetry and antisymmetry effects demonstrated here and is more parsimonious as well. It also correctly predicts which successive-matching training combinations will not produce symmetry. For example, if pigeons are concurrently trained on AB, BC, and DA successive matching (where each letter now refers to the same set of stimuli independent of temporal location), they should not, and do not (Frank, 2007), exhibit BA symmetry despite the fact that in training, pigeons are familiar with the A and B stimuli in each temporal location and learn the requisite successive discriminations between the stimuli in each set. The results obtained by Frank (2007) indicate that concurrent identity training was necessary for obtaining the symmetry results reported by Frank and Wasserman (2005). The theory proposed here would qualify this by stating that it is not identity training per se that is crucial but, rather, identity training with the same stimuli that appear in the symbolic successive matching task. This, too, was confirmed by Frank (2007).
The theory also predicts other emergent relations that define stimulus equivalence—for example, reflexivity. Figure 17 illustrates the six stimulus classes that should yield reflexivity after class merger. Two of the successive matching baseline tasks are the same as in Experiment 3: hue–form symbolic matching and form–identity matching. The other, however, is form–hue symbolic matching (rather than hue identity). Class merger via the common elements across classes should yield two 4-member classes as shown in Figure 18. The arrows indicate the emergent (reflexive) relations: Pigeons should respond more to red after red (R2 after R1) and green after green (G2 after G1) than to the mismatching sample–comparison combinations. This predicted result is not simply the result of a transitive combination of the hue–form and form–hue baseline relations. Instead, the theory states that it can only occur if pigeons concurrently learn form-identity matching during training because the T1, T2, H1, and H2 stimuli from that task are necessary for class merger.
Fig 17.
Hypothesized stimulus classes arising from the reinforced sample-comparison combinations in hue-form symbolic, form-hue symbolic, and form-identity successive matching. R = red, G = green, T = triangle, H = horizontal, 1 = first temporal position within a matching trial, 2 = second temporal position within a matching trial.
Fig 18.
Two 4-member stimulus classes hypothesized to arise from the merger of the stimulus classes in Figure 17 via their common elements. R = red, G = green, T = triangle, H = horizontal, 1 = first temporal position within a matching trial, 2 = second temporal position within a matching trial. Arrows indicate sample–comparison combinations to which pigeons should preferentially respond in a reflexivity test.
In summary, the theory of pigeons' equivalence-class formation proposed here along with the demonstrations of emergent symmetry and antisymmetry from particular sets of conditional relations in successive matching indicate that equivalence relations are not beyond the capabilities of nonhuman animals. To the extent that other theoretical predictions are confirmed, the results provide additional support for the argument that equivalence relations are a natural consequence of reinforcement contingencies.
Acknowledgments
This research was supported by NIMH Grant MH 66915. The data and theory presented here were reported at the 24th Annual Meeting of the Southeastern Association for Behavior Analysis, Athens, GA, October 2007. The author expresses his appreciation to Sarah Michalek, Maggie Sweeney, and Marco Vasconcelos for their assistance in conducting this research, and to Karen Lionello-DeNolf whose dissertation inspired this work.
Footnotes
The change in the matching stimuli vis-à-vis Experiments 1A and 1B was done in order to accommodate other on-going research that used the same apparatus at the time Experiment 2 was being run.
The decision to initially train FR responding to a stimulus that did not subsequently appear as one of the matching stimuli was made to avoid the otherwise prolonged non-differential FR response training with the matching stimuli themselves. It was thought that such prolonged training might diminish the possibility that the different samples and their associated reinforced comparisons would become members of different stimulus classes.
References
- Carr D, Wilkinson K.M, Blackman D, McIlvane W.J. Equivalence classes in individuals with minimal verbal repertoires. Journal of the Experimental Analysis of Behavior. 2000;74:101–114. doi: 10.1901/jeab.2000.74-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter D.E, Eckerman D.A. Symbolic matching by pigeons: Rate of learning complex discriminations predicted from simple discriminations. Science. 1975;187:662–664. doi: 10.1126/science.1114318. [DOI] [PubMed] [Google Scholar]
- D'Amato M.R, Salmon D.P, Loukas E, Tomie A. Symmetry and transitivity in the conditional relations in monkeys (Cebus apella) and pigeons (Columba livia) Journal of the Experimental Analysis of Behavior. 1985;44:35–47. doi: 10.1901/jeab.1985.44-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debert P, Matos M.A, McIlvane W. Conditional relations with compound abstract stimuli using a go/no-go procedure. Journal of the Experimental Analysis of Behavior. 2007;87:89–96. doi: 10.1901/jeab.2007.46-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devany J.M, Hayes S.C, Nelson R.O. Equivalence class formation in language-able and language-disabled children. Journal of the Experimental Analysis of Behavior. 1986;46:243–257. doi: 10.1901/jeab.1986.46-243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dube W.V, McIlvane W.J. Stimulus-reinforcer relations and emergent matching to sample. The Psychological Record. 1995;45:591–612. [Google Scholar]
- Dube W.V, McIlvane W.J, Callahan T.D, Stoddard L.T. The search for stimulus equivalence in nonverbal organisms. The Psychological Record. 1993;43:761–778. [Google Scholar]
- Dube W.V, McIlvane W.J, Maguire R.W, Mackay H.A, Stoddard L.T. Stimulus class formation and stimulus-reinforcer relations. Journal of the Experimental Analysis of Behavior. 1989;51:65–76. doi: 10.1901/jeab.1989.51-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dube W.V, McIlvane W.J, Mackay H.A, Stoddard L.T. Stimulus class membership established by stimulus-reinforcer relations. Journal of the Experimental Analysis of Behavior. 1987;47:159–175. doi: 10.1901/jeab.1987.47-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dugdale N, Lowe C.F. Testing for symmetry in the conditional discriminations of language-trained chimpanzees. Journal of the Experimental Analysis of Behavior. 2000;73:5–22. doi: 10.1901/jeab.2000.73-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckerman D.A, Lanson R.N, Cumming W.W. Acquisition and maintenance of matching without a required observing response. Journal of the Experimental Analysis of Behavior. 1968;11:435–441. doi: 10.1901/jeab.1968.11-435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eikeseth S, Smith T. The development of functional and equivalence classes in high-functioning autistic children: The role of naming. Journal of the Experimental Analysis of Behavior. 1992;58:123–133. doi: 10.1901/jeab.1992.58-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields L, Adams B.J, Verhave T. The effects of equivalence class structure on test performances. The Psychological Record. 1993;43:697–712. [Google Scholar]
- Frank A.J. An examination of the temporal and spatial stimulus control in emergent symmetry in pigeons. 2007. Unpublished doctoral dissertation, University of Iowa.
- Frank A.J, Wasserman E.A. Associative symmetry in the pigeon after successive matching-to-sample training. Journal of the Experimental Analysis of Behavior. 2005;84:147–165. doi: 10.1901/jeab.2005.115-04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García A, Benjumea S. The emergence of symmetry in a conditional discrimination task using different responses as proprioceptive samples in pigeons. Journal of the Experimental Analysis of Behavior. 2006;86:65–80. doi: 10.1901/jeab.2006.67-04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldiamond I. Perception. In: Bachrach A.J, editor. Experimental foundations of clinical psychology. NY: Basic Books; 1962. pp. 280–340. [Google Scholar]
- Hall G, Channell S. A search for perceptual differentiation by nondifferential reinforcement. Quarterly Journal of Experimental Psychology. 1980;32:185–195. doi: 10.1080/14640748008401156. [DOI] [PubMed] [Google Scholar]
- Hall G, Mitchell C, Graham S, Lavis Y. Acquired equivalence and distinctiveness in human discrimination learning: Evidence for associative mediation. Journal of Experimental Psychology: General. 2003;132:266–276. doi: 10.1037/0096-3445.132.2.266. [DOI] [PubMed] [Google Scholar]
- Hayes S.C. Nonhumans have not yet shown stimulus equivalence. Journal of the Experimental Analysis of Behavior. 1989;51:385–392. doi: 10.1901/jeab.1989.51-385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes S.C. Relational frame theory: A functional approach to verbal events. In: Hayes S.C, Hayes L.J, Sato M, Ono K, editors. Behavior analysis of language and cognition. Reno, NV: Context Press; 1991. pp. 9–30. [Google Scholar]
- Hogan D.E, Zentall T.R. Backward association in the pigeon. American Journal of Psychology. 1977;90:3–15. [Google Scholar]
- Honig W.K, Urcuioli P.J. The legacy of Guttman and Kalish (1956): 25 years of research on stimulus generalization. Journal of the Experimental Analysis of Behavior. 1981;36:405–445. doi: 10.1901/jeab.1981.36-405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horne P.J, Lowe C.F. On the origins of naming and other symbolic behavior. Journal of the Experimental Analysis of Behavior. 1996;65:185–241. doi: 10.1901/jeab.1996.65-185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hull C.L. The problem of stimulus equivalence in behavior theory. Psychological Review. 1939;46:9–30. [Google Scholar]
- Jenkins J.J. Mediated associations: Paradigms and situations. In: Cofer C.N, Musgrave B.S, editors. Verbal behavior and learning: Problems and processes. NY: McGraw-Hill; 1963. pp. 210–245. [Google Scholar]
- Jenkins J.J, Palermo D.S. Mediation processes and the acquisition of linguistic structure. Bellugi U, Brown R, editors. The acquisition of language: Monographs of the Society for Research in Child Development. 1964;29:141–191. In. [PubMed] [Google Scholar]
- Johnson C, Sidman M. Conditional discrimination and equivalence relations: Control by negative stimuli. Journal of the Experimental Analysis of Behavior. 1993;59:333–347. doi: 10.1901/jeab.1993.59-333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph B, Overmier J.B, Thompson T. Food- and nonfood-related differential outcomes in equivalence learning by adults with Prader-Willi syndrome. American Journal on Mental Retardation. 1997;101:374–386. [PubMed] [Google Scholar]
- Kuno H, Kitadate T, Iwamoto T. Formation of transitivity in conditional matching to sample by pigeons. Journal of the Experimental Analysis of Behavior. 1994;62:399–408. doi: 10.1901/jeab.1994.62-399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lionello K.M, Urcuioli P.J. Control by sample location in pigeons' matching to sample. Journal of the Experimental Analysis of Behavior. 1998;70:235–251. doi: 10.1901/jeab.1998.70-235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lionello-DeNolf K.M, Urcuioli P.J. Stimulus control topographies and tests of symmetry in pigeons. Journal of the Experimental Analysis of Behavior. 2002;78:467–495. doi: 10.1901/jeab.2002.78-467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipkens R, Kop P.F.M, Matthijs W. A test of symmetry and transitivity in the conditional discrimination performances of pigeons. Journal of the Experimental Analysis of Behavior. 1988;49:395–409. doi: 10.1901/jeab.1988.49-395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manabe K, Kawashima T, Staddon J.E.R. Differential vocalization in budgerigars: Towards and experimental analysis of naming. Journal of the Experimental Analysis of Behavior. 1995;63:111–126. doi: 10.1901/jeab.1995.63-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIlvane W.J, Serna R.W, Dube W.V, Stromer R. Stimulus control topography coherence and stimulus equivalence: Reconciling test outcomes with theory. In: Leslie J, Blackman D.E, editors. Experimental and applied analysis of human behavior. Reno, NV: Context Press; 2000. pp. 85–110. [Google Scholar]
- McIntire K.D, Cleary J, Thompson T. Conditional relations by monkeys: Reflexivity, symmetry, and transitivity. Journal of the Experimental Analysis of Behavior. 1987;47:279–285. doi: 10.1901/jeab.1987.47-279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller R.R, Barnet R.C. The role of time in elementary associations. Current Directions in Psychological Science. 1993;2:106–111. [Google Scholar]
- Nelson K.R, Wasserman E.A. Temporal factors influencing the pigeon's successive matching-to-sample performance: Sample duration, intertrial interval, and retention interval. Journal of the Experimental Analysis of Behavior. 1978;30:153–162. doi: 10.1901/jeab.1978.30-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newlin R.J, Thomas D.R. Nondifferential training of pigeons retards acquisition of subsequent discriminations involving other stimuli. Animal Learning & Behavior. 1978;6:385–390. [Google Scholar]
- Pontecorvo M.J. Memory for a stimulus versus anticipation of a response: Contrasting effects of proactive interference in delayed comparison tasks. Animal Learning & Behavior. 1985;13:335–364. [Google Scholar]
- Richards R.W. The question of bidirectional associations in pigeons' learning of conditional discrimination tasks. Bulletin of the Psychonomic Society. 1988;26:577–579. [Google Scholar]
- Rodger R.S. The number of non-zero, post hoc contrasts from ANOVA and error rate. I. British Journal of Mathematical and Statistical Psychology. 1975;28:71–78. [Google Scholar]
- Sacks R.A, Kamil A.C, Mack R. The effects of fixed-ratio sample requirements on matching to sample in the pigeon. Psychonomic Science. 1972;36:291–293. [Google Scholar]
- Saunders K.J, Spradlin J.E. Conditional discrimination in mentally retarded adults: The effect of training the component simple discriminations. Journal of the Experimental Analysis of Behavior. 1989;52:1–12. doi: 10.1901/jeab.1989.52-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders K.J, Williams D.C, Spradlin J.E. Derived stimulus control: Are there differences among procedures and processes. In: Zentall T.R, Smeets P.M, editors. Stimulus class formation in humans and animals. NY: Elsevier; 1996. pp. 93–109. [Google Scholar]
- Saunders R.R, Green G. A discrimination analysis of training-structure effects on stimulus equivalence outcomes. Journal of the Experimental Analysis of Behavior. 1999;72:117–137. doi: 10.1901/jeab.1999.72-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schusterman R.J, Kastak D. A California sea lion (Zalophus Californianus) is capable of forming equivalence relations. Psychological Record. 1993;43:823–839. [Google Scholar]
- Shimp C.P, Moffitt M. Short-term memory in the pigeon: Delayed-pair-comparison procedures and some results. Journal of the Experimental Analysis of Behavior. 1977;28:13–25. doi: 10.1901/jeab.1977.28-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidman M. Reading and auditory-visual equivalences. Journal of Speech and Hearing Research. 1971;14:5–13. doi: 10.1044/jshr.1401.05. [DOI] [PubMed] [Google Scholar]
- Sidman M. Equivalence relations: Where do they come from. In: Lejeune H, Blackman D, editors. Behavior analysis in theory and practice: Contributions and controversies. Hillsdale, NJ: Erlbaum; 1990. pp. 93–114. [Google Scholar]
- Sidman M. Equivalence relations and behavior: A research story. Boston: Authors Cooperative; 1994. [Google Scholar]
- Sidman M. Equivalence relations and the reinforcement contingency. Journal of the Experimental Analysis of Behavior. 2000;74:127–146. doi: 10.1901/jeab.2000.74-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidman M, Cresson O., Jr. Reading and crossmodal transfer of stimulus equivalences in severe retardation. Journal of Mental Deficiency. 1973;77:515–523. [PubMed] [Google Scholar]
- Sidman M, Kirk B, Willson-Morris M. Six-member stimulus classes generated by conditional-discrimination procedures. Journal of the Experimental Analysis of Behavior. 1985;43:21–42. doi: 10.1901/jeab.1985.43-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidman M, Rauzin R, Lazar R, Cunningham S, Tailby W, Carrigan P. A search for symmetry in the conditional discrimination of rhesus monkeys, baboons, and children. Journal of the Experimental Analysis of Behavior. 1982;37:23–44. doi: 10.1901/jeab.1982.37-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidman M, Tailby W. Conditional discrimination vs. matching-to-sample: An expansion of the testing paradigm. Journal of the Experimental Analysis of Behavior. 1982;37:5–22. doi: 10.1901/jeab.1982.37-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomonaga M, Matsuzawa T, Fujita K, Yamamoto J. Emergence of symmetry in a visual discrimination by chimpanzees (Pan troglodytes) Psychological Reports. 1991;68:51–60. doi: 10.2466/pr0.1991.68.1.51. [DOI] [PubMed] [Google Scholar]
- Urcuioli P.J. Behavioral and associative effects of differential outcomes in discrimination learning. Learning & Behavior. 2005;33:1–21. doi: 10.3758/bf03196047. [DOI] [PubMed] [Google Scholar]
- Urcuioli P.J. Responses and acquired equivalence classes. In: Wasserman E.A, Zentall T.R, editors. Comparative cognition: Experimental explorations of animal intelligence. NY: Oxford University Press; 2006. pp. 405–421. [Google Scholar]
- Urcuioli P.J, DeMarse T.B. Memory processes in delayed spatial discriminations: Response intentions or response mediation. Journal of the Experimental Analysis of Behavior. 1997;67:323–336. doi: 10.1901/jeab.1997.67-323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urcuioli P.J, Lionello-DeNolf K.M. Some tests of the anticipatory mediated generalization model of acquired sample equivalence in pigeons' many-to-one matching. Animal Learning & Behavior. 2001;29:265–280. [Google Scholar]
- Urcuioli P.J, Vasconcelos M. Effects of within-class differences in sample responding on acquired sample equivalence. Journal of the Experimental Analysis of Behavior. 2008a;89:341–358. doi: 10.1901/jeab.2008-89-341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urcuioli P.J, Vasconcelos M. On the origins of emergent differential sample behavior. Journal of the Experimental Analysis of Behavior. 2008b;90:61–80. doi: 10.1901/jeab.2008.90-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urcuioli P.J, Vu K-P.L, Proctor R.W. A Simon effect in pigeons. Journal of Experimental Psychology: General. 2005;134:93–107. doi: 10.1037/0096-3445.134.1.93. [DOI] [PubMed] [Google Scholar]
- Urcuioli P.J, Zentall T.R. Retrospective coding in pigeons' delayed matching-to-sample. Journal of Experimental Psychology: Animal Behavior Processes. 1986;12:69–77. [PubMed] [Google Scholar]
- Urcuioli P.J, Zentall T.R. On the role of trial outcomes in delayed discriminations. Animal Learning & Behavior. 1990;18:141–150. [Google Scholar]
- Urcuioli P.J, Zentall T.R, DeMarse T. Transfer to derived sample-comparison relations by pigeons following many-to-one and one-to-many matching with identical training relations. Quarterly Journal of Experimental Psychology. 1995;48B:158–178. [Google Scholar]
- Vasconcelos M, Urcuioli P.J, Lionello-DeNolf K.M. Failure to replicate the “work ethic” effect in pigeons. Journal of the Experimental Analysis of Behavior. 2007;87:383–399. doi: 10.1901/jeab.2007.68-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasserman E.A. Successive matching-to-sample in the pigeon: Variation on a theme by Konorski. Behavior Research Methods & Instrumentation. 1976;8:278–282. [Google Scholar]
- Wasserman E.A, deVolder C.L, Coppage D.J. Nonsimilarity-based conceptualization in pigeons. Psychological Science. 1992;3:374–379. [Google Scholar]
- Weisman R.G, Wasserman E.A, Dodd P.W.D, Larew M.B. Representation and retention of two-event sequences in pigeons. Journal of Experimental Psychology: Animal Behavior Processes. 1980;6:312–325. [Google Scholar]
- Wright A.A, Sands S.F. A model of detection and decision processes during matching-to-sample by pigeons: Performance with 88 different wavelengths in delayed and simultaneous matching tasks. Journal of Experimental Psychology: Animal Behavior Processes. 1981;7:191–216. [Google Scholar]