Abstract
Previous research has shown that Lewis rats make more impulsive choices than Fischer 344 rats. Such strain-related differences in choice are important as they may provide an avenue for exploring genetic and neurochemical contributions to impulsive choice. The present systematic replication was designed to determine if these findings could be reproduced using a procedure less susceptible to within- or between-session carry-over effects that may have affected previous findings. Specifically, delays to the larger–later food reinforcer were manipulated between conditions following steady-state assessments of choice, and the order of delays across conditions was mixed. The results confirmed previous findings that Lewis rats made significantly more impulsive choices than Fischer 344 rats. Fischer 344 rats' preference for the larger–later reinforcer, on the other hand, was less extreme than reported in prior research, which may be due to carry-over effects inherent to the commonly used technique of systematically increasing delays within session. Previously reported across-strain motor differences were reproduced as Lewis rats had shorter latencies than Fischer 344 rats, although these latencies were not correlated with impulsive choice. Parallels between reduced dopamine function in Lewis rats and clinical reports of impulse-control disorders following treatment of Parkinson patients with selective D2/D3 dopamine agonists are discussed.
Keywords: Fischer 344 rats, Lewis rats, choice, impulsivity, delay-discounting, rat, lever press
Self-control has been defined in operant preparations as the choice of a larger, more delayed reinforcer over a smaller, more immediate one, and impulsivity has been defined as the opposite choice (Ainslie, 1975; Rachlin & Green, 1972). The outcome selected serves as a metric of the subjective, discounted value of the larger–later (LL) reinforcer (e.g., Mazur, 1987; Rachlin, Raineri, & Cross, 1991). For example, choosing the smaller–sooner (SS) reinforcer indicates that its value exceeds the discounted value of the LL reinforcer. When an organism chooses the two reinforcers equally often (indifference), the discounted value of the LL reinforcer may be quantified in terms of the magnitude of the SS reinforcer.
The rate at which delayed consequences are discounted may underlie socially important human choices. For example, several studies have revealed that problem drug usage is correlated with high rates of delay discounting (e.g., Coffey, Gudleski, Saladin, & Brady, 2003; Madden, Petry, Badger, & Bickel, 1997; Vuchinich & Simpson, 1998) and a similar relation has been reported with pathological gambling (e.g., Alessi & Petry, 2003; Dixon, Marley, & Jacobs, 2003). Although these correlations have been well established, the causal mechanisms underlying them have not. That is, these studies do not reveal whether, for example, chronic drug use increases the degree to which an individual discounts the value of delayed outcomes or, either alternatively or in combination, if genetic predispositions toward greater delay discounting put individuals at risk of substance abuse.
Understanding the mechanisms of causation will require knowledge of ontogenetic and phylogenetic variables; both of which have been shown to affect discounting rates. For example, learning history may alter rates of delay discounting (e.g., Logue & Mazur, 1981; Mazur & Logue, 1978). At the same time, when learning history is held constant, some evidence suggests that high rates of delay discounting may predispose rats toward drug self-administration (Perry, Larson, German, Madden, & Carroll, 2005; Perry, Nelson, & Carroll, 2008; Poulos, Le, & Parker, 1995). Consistent with the latter findings, Anderson and Woolverton (2005) reported that Lewis rats made significantly more impulsive choices than did Fischer 344 (F344) rats given equivalent experimental histories. Moreover, previous studies have shown that Lewis rats more readily consume and/or acquire responding maintained by cocaine (e.g., Kosten et al., 1997), opiates (e.g., Suzuki, Otani, Koike, & Misawa, 1988), nicotine (Brower, Fu, Matta, & Sharp, 2002) and ethanol (Suzuki, George, & Meisch, 1988) than F344 rats (although see Kosten, Zhang, & Haile, 2007).
Anderson and Woolverton (2005) used a frequently employed procedure developed by Evenden and Ryan (1996). In this procedure, sessions begin with a simple choice: more food vs. less food, both available immediately. Across subsequent blocks of trials, the delay to the larger food reinforcer is systematically increased to 60 s (0, 10, 20, 40, 60 s). Forced-choice trials are programmed at the beginning of each block to ensure contact with the changed choice parameters, followed by six free choice trials. Across sessions, animals are exposed repeatedly to the procedure until choice proportions at each delay have stabilized. Thus, the procedure provides a simple means of assessing effects of drug or neurological manipulations on impulsive decision making across a wide range of delays (e.g., Cardinal, Robbins, & Everitt, 2000).
The present experiment was conducted to determine if the difference in impulsive choices between Lewis and F344 rats that was reported by Anderson and Woolverton (2005) would be obtained when delays were manipulated between conditions rather than within sessions. Systematic replication was warranted because some evidence suggests either that rats may be somewhat insensitive to within-session delay manipulations (Cardinal, Daw, Robbins, & Everitt, 2002) or that impulsive choice may be affected by carry-over effects from one delay to the next (Fox, Hand, & Reilly, 2008). Interestingly, Anderson and Woolverton's F344 rats more often selected the larger reinforcer in the first trial block of the Evenden and Ryan (1996) procedure (in which large and small reinforcers are both immediately available) than did their Lewis rats. If this stronger preference for the larger reinforcer carried over into subsequent trial blocks in which the delay to the LL reinforcer was increased, the carry-over effect might be mistaken for a strain difference in impulsive choice. If so, then manipulating delays between conditions (as in the present study) would fail to produce the expected strain difference.
A second reason for systematically replicating the Anderson and Woolverton (2005) study is related to the finding that F344 are generally less active than Lewis rats (e.g., Kosten et al., 2007). In the Anderson and Woolverton study, rats completed one forced-choice trial on the left and right levers before completing six free-choice trials at a particular delay. Because the order of the forced-choice trials was randomized at each delay, a lethargic rat that simply pressed whichever lever was closest (presumably the one pressed on the last forced-choice trial) would be expected to demonstrate indifference between the levers, which might be mistaken for insensitivity to reinforcer delay. Evidence for this comes from the choices made by Anderson and Woolverton's F344 rats at delays ranging from 10 to 40 s. At these delays, the group average choice deviated by less than one standard deviation from indifference.
To address these alternative explanations of the Anderson and Woolverton (2005) findings, the present experiment examined impulsive and self-control choices in Lewis and F344 rats when delays were held constant within sessions and manipulated between conditions after choice had achieved steady state. The chance of within-session carry-over effects contaminating the outcome was decreased further by requiring a response on a center lever before the side (choice) levers were made available. Finally, we reversed the lever to which the reinforcers were assigned at each delay, thereby ruling out the mistaking of a side bias for preference. Under these conditions, reproducing the strain differences reported by Anderson and Woolverton would represent a strong systematic replication (Sidman, 1960).
Method
Subjects
Twenty experimentally naïve male rats (10 Lewis and 10 F344; Harlan Sprague-Dawley, Indianapolis, IN) served as subjects. All rats were approximately 3 months old at the start of the experiment. Rats were weighed daily and maintained at approximately 85% of their free-feeding weights through supplemental, postsession feeding provided 90 min after each session. Between sessions, rats were individually housed in plastic cages within a temperature-controlled colony room providing a 12:12 hr light/dark cycle. Water was continuously available between sessions.
Apparatus
Ten identical operant chambers (Med Associates, St. Albans, VT) were used. Each chamber measured 24.1 cm wide, 30.5 cm long, and 21 cm high. One wall of the chamber was an intelligence panel equipped with a nonretractable center lever (11 cm above the floor) and two retractable side levers (horizontally aligned 11 cm apart and 6.5 cm above the floor). Above each lever was a white, 28-volt DC cue light (2.5 cm in diameter and 6 cm above each lever). A feeder (Coulbourn, Allentown, PA) delivered 45-mg grain-based food pellets (Bioserve, Frenchtown, NJ) into a receptacle (3 cm wide and 4 cm long) equipped with a 2-W light in the center of the intelligence panel (1 cm above the floor and 10 cm below the center lever). Chambers were enclosed within a light- and sound-attenuation cubicle (Med Associates® ENV-018MD) equipped with a ventilation fan and a white noise speaker. A Med Associates® interface system controlled the sessions and collected data.
Procedure
Pretraining
An autoshaping program trained pressing of all three levers; a separate lever was operational in each session. After responding was established, ten 100-trial sessions were conducted in which a response on the lit center lever caused the light to be darkened, the left or right lever to be inserted into the chamber, and the light above the chosen lever to be illuminated. Selection of the side lever followed a strictly alternating pattern. Following a side-lever response, the lever retracted, a single food pellet was delivered, and the light was turned off. This ensured that the rats' recent reinforcement history on the left and right levers was equivalent.
Choice
All subsequent sessions lasted for 42 trials, organized into seven blocks of 6 trials (no programmed stimuli separated trial blocks). Each block consisted of 4 forced-choice trials followed by 2 free-choice trials. Two forced-choice trials were arranged on the left lever and the other 2 on the right (order randomized without replacement within block). The light above the center lever signaled the start of every trial. On forced-choice trials, a response on the center lever extinguished the cue light and caused one side lever to be inserted into the chamber with the light above that lever simultaneously lit. A single response on the side lever retracted the lever, and began the pellet delivery sequence. During this sequence, either the light above the lever was extinguished and one food pellet was delivered immediately (0.01-s delay; the SS reinforcer) or the light flashed in 0.25-s intervals during a delay, after which three pellets were delivered (the LL reinforcer) and the cue light extinguished. A flash of light in the food receptacle accompanied the delivery of each pellet. For half of the rats, the SS reinforcer was initially assigned to the left lever and for the other rats it was assigned to the right.
The last two trials in each six-trial block were free-choice trials. The procedure followed on these trials was identical to forced-choice trials except that both side levers were inserted into the chamber and the cue light above each lever was illuminated. As during forced-choice trials, a single side-lever response initiated the corresponding SS or LL pellet delivery sequence.
Pellet deliveries were followed by an intertrial interval (ITI) during which no stimuli were presented. The duration of the ITI was adjusted to ensure that the start of each trial was separated by exactly 100 s regardless of response latency and delay associated with the lever selected. Within each trial, a 30-s limited hold was in effect: If a center- or side-lever response latency exceeded 30 s following the illumination of the relevant cue light, the trial was terminated, the ITI initiated, and the trial was scored as an omission. Omissions were infrequent and were not used in the calculation of any choice proportions.
Delays to the LL reinforcers were manipulated between conditions after choice was assessed with the LL reinforcer assigned to both the left and right lever. The same sequence of delays (10, 5, 0, and 15 s) was followed by all rats of both strains. In 7 of 80 conditions in which stable choices were assessed, rats developed a persistent bias favoring one lever regardless of the amount and delay of reinforcement arranged. When this happened, the rat completed at least four remedial sessions in which the consequence arranged on the preferred lever was changed in an attempt to eliminate persistent bias (increasing the delay to 30 s or delivering no pellets). After these remedial sessions, the prior condition was reinstated and sessions were continued for at least 10 sessions and until a quantitative stability criterion was met. One Lewis rat (L5) demonstrated an intractable side bias in the final condition (15-s delay) which was unaffected by these remediation procedures. Because this rat's choices otherwise were affected systematically by shorter delays, data from only its 15-s delay condition were excluded from analysis.
Stability Criterion
Each condition lasted for a minimum of 10 sessions and until the mean percentage of LL free choices in the final three sessions deviated from the preceding three-session mean by 8% or less with no visually apparent trend. Due to experimenter error in 4% of the stability assessments, conditions were changed when the mean of the final two sessions deviated from the mean of the preceding two sessions by 5% or less (all other criteria were met). In these instances, only the data from the final four sessions are presented below.
Statistical Analysis
Repeated measures analysis of variance (ANOVA) was used to examine effects of strain and delay on choice and response latencies (SPSS ver. 14.0). Choice percentages were not normally distributed so they were arcsine transformed prior to analysis (Howell, 1992). Response latencies were also not normally distributed so they were reciprocal transformed prior to analysis.
Results
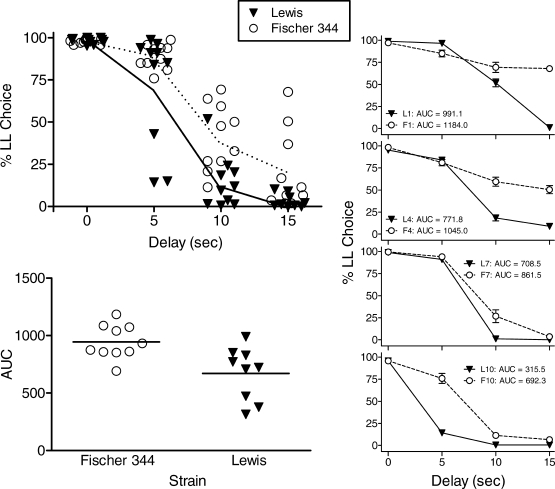
The numbers of sessions completed at each delay are shown for individual rats in Table 1. Because no systematic across-subject differences were observed when the SS reinforcer was assigned to the left or right levers, choice percentages are collapsed across position assignment for all analyses. The upper-left panel of Figure 1 shows the average percentage of LL free choices made by individual rats in their stable sessions at each delay (these means and SEM are provided in Table 1). Solid and dashed lines connect the average choice percentages of the Lewis and F344 strains, respectively. Both strains consistently selected the larger number of pellets when both reinforcers were immediately available. As delays were increased, both strains selected the LL reinforcer less frequently (significant main effect of delay, F(3,51) = 146.3, p < .05). A significant delay x strain interaction reflected the Lewis rats' steeper decline in choosing the LL alternative as delays increased, F(3,51) = 3.4, p < .05.
Table 1.
Number of sessions completed by individual Lewis and F344 rats at each delay. Also included are area under the choice curve (AUC), and average percent choice of the larger–later (LL) reinforcer.
| Rat | AUC | Delay | Sessions | %LL (SEM) | Rat | AUC | Delay | Sessions | %LL (SEM) |
| L1 | 991.1 | 0 | 25 | 98.8 (0.8) | F1 | 1184.0 | 0 | 26 | 97.0 (1.4) |
| 5 | 23 | 96.4 (1.4) | 5 | 29 | 85.0 (3.7) | ||||
| 10 | 49 | 51.8 (4.4) | 10 | 38 | 69.3 (5.7) | ||||
| 15 | 23 | 1.2 (0.8) | 15 | 72 | 67.9 (2.7) | ||||
| L2 | 852.3 | 0 | 23 | 100.0 (0.0) | F2 | 1087.0 | 0 | 22 | 100.0 (0.0) |
| 5 | 41 | 91.1 (2.7) | 5 | 38 | 98.8 (0.8) | ||||
| 10 | 47 | 24.3 (4.5) | 10 | 31 | 50.1 (10.2) | ||||
| 15 | 32 | 10.1 (2.8) | 15 | 74 | 36.9 (7.8) | ||||
| L3 | 829.0 | 0 | 30 | 97.6 (1.0) | F3 | 1073.0 | 0 | 50 | 98.2 (0.9) |
| 5 | 33 | 94.1 (1.5) | 5 | 36 | 80.9 (3.7) | ||||
| 10 | 46 | 20.2 (5.7) | 10 | 33 | 59.4 (5.1) | ||||
| 15 | 27 | 5.4 (1.3) | 15 | 43 | 50.4 (4.6) | ||||
| L4 | 771.8 | 0 | 25 | 95.2 (1.6) | F4 | 1045.0 | 0 | 21 | 98.8 (0.8) |
| 5 | 34 | 83.9 (3.1) | 5 | 31 | 94.8 (2.0) | ||||
| 10 | 42 | 18.4 (3.5) | 10 | 38 | 63.8 (4.3) | ||||
| 15 | 24 | 8.9 (2.3) | 15 | 20 | 1.8 (1.3) | ||||
| L5 | - | 0 | 27 | 99.4 (0.6) | F5 | 931.3 | 0 | 21 | 95.8 (1.1) |
| 5 | 34 | 98.8 (0.8) | 5 | 45 | 87.5 (2.3) | ||||
| 10 | 23 | 3.6 (1.9) | 10 | 50 | 47.6 (3.9) | ||||
| 15 | - | - | 15 | 42 | 6.5 (2.7) | ||||
| L6 | 723.0 | 0 | 24 | 98.8 (0.8) | F6 | 875.8 | 0 | 26 | 97.8 (1.3) |
| 5 | 58 | 85.1 (2.7) | 5 | 37 | 85.1 (4.7) | ||||
| 10 | 54 | 9.5 (2.8) | 10 | 27 | 32.9 (9.2) | ||||
| 15 | 26 | 1.2 (0.8) | 15 | 77 | 16.7 (3.6) | ||||
| L7 | 708.5 | 0 | 27 | 98.8 (0.8) | F7 | 861.5 | 0 | 24 | 99.4 (0.6) |
| 5 | 43 | 91.1 (3.1) | 5 | 28 | 94.0 (1.2) | ||||
| 10 | 20 | 1.2 (0.8) | 10 | 56 | 26.8 (7.3) | ||||
| 15 | 26 | 0.0 (0.0) | 15 | 35 | 3.6 (1.1) | ||||
| L8 | 473.0 | 0 | 22 | 100 (0.0) | F8 | 858.3 | 0 | 21 | 98.8 (0.8) |
| 5 | 38 | 42.9 (13.0) | 5 | 39 | 95.8 (1.4) | ||||
| 10 | 20 | 1.4 (0.9) | 10 | 39 | 20.8 (3.9) | ||||
| 15 | 25 | 0.6 (0.6) | 15 | 44 | 11.3 (3.8) | ||||
| L9 | 378.0 | 0 | 26 | 97.6 (1.0) | F9 | 852.5 | 0 | 20 | 98.8 (0.8) |
| 5 | 41 | 14.9 (2.2) | 5 | 74 | 93.3 (2.4) | ||||
| 10 | 22 | 11.9 (1.8) | 10 | 44 | 26.9 (7.1) | ||||
| 15 | 26 | 0.0 (0.0) | 15 | 32 | 1.8 (1.3) | ||||
| L10 | 315.5 | 0 | 27 | 95.8 (1.4) | F10 | 692.3 | 0 | 26 | 95.8 (1.6) |
| 5 | 43 | 14.3 (2.9) | 5 | 24 | 76.0 (5.7) | ||||
| 10 | 20 | 0.6 (0.6) | 10 | 40 | 11.3 (2.8) | ||||
| 15 | 23 | 0.6 (0.6) | 15 | 25 | 6.5 (2.0) |
Fig 1.
Upper left panel: Individual percent choice of the LL alternative plotted as a function of LL delay value. Mean percentages are connected by solid (Lewis) and dashed (F344) lines. Lower left panel: Area under the curve for individual rats within each strain. Right panels: Average choices of individual rats rank-ordered by AUC value. Top panel shows rats with the highest AUC, and lower panels show rats ranked 4, 7, and 10 (of 10 rats within each strain), respectively. In all panels, Lewis rats are depicted as filled triangles (▾) and F344 as open circles (○).
To compare further strain differences in degree of sensitivity to delay, we calculated the area under individual rats' percent choice curves across all nonzero delays (AUC).1 These values are shown in Table 1; note that no AUC value is reported for rat L5 because this rat's intractable side bias prevented our assessing choice at the 15-s delay. The lower-left panel of Figure 1 shows individual AUC values separated by strain. A t-test revealed the difference between strains was statistically significant, t(17) = 3.1, p <.05, and this outcome is consistent with the main effect of strain detected by the repeated measures ANOVA based on choice percentages at each delay, F(1,17) = 9.7, p < .05. The right column of graphs in Figure 1 shows the average stable choices (and SEM) of four Lewis and four F344 rats. The top graph shows choices of the rat within each strain that was least sensitive to the delay to the LL reinforcer (highest AUC). The remaining graphs show the rats ranked 4, 7, and 10 (most sensitive to delay) for each strain. The graphs illustrate at the individual subject level the orderly decrease in choice of the LL reinforcer as the delay to its delivery was increased.
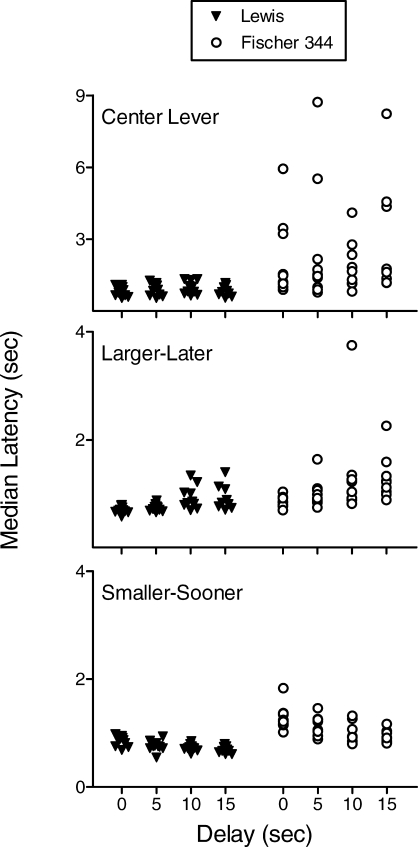
Figure 2 shows, for individual Lewis and F344 rats, median latencies to press the center lever (top panel), the lever on which the LL reinforcer was arranged (middle panel), and the SS lever (bottom panel); note the different y-axis scaling for the top panel. Because no systematic latency differences were observed between forced- and free-choice trials, latencies across these trial types are combined in Figure 2. The top panel of Figure 2 reveals uniformly brief center-lever latencies for Lewis rats and considerably longer latencies for many of the F344 rats, F(1,17) = 12.41, p < .05. A significant delay x strain interaction, F(3,51) = 4.90, p < .05, suggests center-lever latencies were more affected by delay in the F344 than the Lewis strain. Center-lever latencies at the 0-s delay and AUC were not significantly correlated within either strain (p > .83 in both cases) or when the strains were combined (p = .14). The correlation was not improved by collapsing center-lever latencies across delays.
Fig 2.
Median latencies to press the center (top panel), LL (middle panel), and SS (bottom panel) levers. Note the differences in y-axis scaling between the top and lower panels.
The center panel of Figure 2 shows that latencies to press the LL lever increased with delay, F(3,51) = 28.67, p < .05, and that F344 rats tended to pause longer at all delays than Lewis rats, F(1,17) = 13.45, p < .05; no significant interaction was detected. The lower panel of Figure 2 shows that delay to the LL reinforcer had the opposite effect on latencies to press the SS lever, F(3,51) = 40.86, p < .05, and, as with the other latencies, the F344 rats tended to pause longer than the Lewis rats, F(1,17) = 50.03, p < .05; no significant interaction was detected.
Discussion
The present study assessed steady-state differences in selecting a SS over a LL food reinforcer in Lewis and F344 inbred strains of rats. For this assessment, the delay to the LL reinforcer remained constant within session and was manipulated across conditions. For both strains, the percentage of LL choices decreased as a function of increasing delays to the delivery of this reinforcer. Consistent with the findings reported by Anderson and Woolverton (2005), this decrease was significantly more pronounced in Lewis rats compared to F344 rats. This constitutes a strong systematic replication of the Anderson and Woolverton procedures and our findings support their conclusion that Lewis rats are more sensitive than F344 rats to reinforcer delay.
Although a significant strain difference in impulsive choice was observed, readers should note the overlap between strains. At each delay, several F344 rats more frequently chose the SS reinforcer than several Lewis rats (see Figure 1). Thus, it would be a mistake to regard the significant strain difference as indicative of two strains widely and completely separated on degree of delay discounting. Such variability suggests that within-strain individual differences may prove useful in identifying biological variables that play a role in these choice differences in an otherwise homogeneous inbred organism.
Consistent with studies demonstrating motoric differences between Lewis and F344 rats (e.g., Kosten et al., 2007), the latter strain tended to pause longer before pressing all three levers regardless of the consequences of the lever press. The difference was largest for latencies on the center lever which initiated a trial. This may reflect the F344 rats taking longer to consume food pellets or to move out of the pellet receptacle and rear up to the center lever. No significant correlation between center-lever latencies and AUC was detected, which is consistent with other reported failures to observe a simple correlation between activity level and sensitivity to reinforcer delay (e.g., Perry et al., 2005).
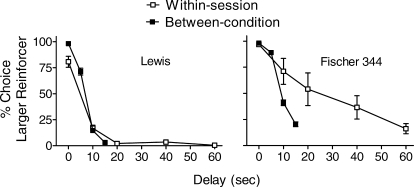
There are two noteworthy differences between the present findings and those reported by Anderson and Woolverton (2005). First, as illustrated by the solid data points in Figure 3, when our rats chose between one and three food pellets, both delivered immediately, all rats (regardless of strain) selected the larger reinforcer on almost every trial. By contrast, when Anderson and Woolverton used the Evenden and Ryan (1996) within-session delay-change procedure to assess choice between these same consequences, Lewis rats chose the larger reinforcer on 80.7% (range 60–100%) of the trials (data supplied by Karen Anderson). This difference proved to be statistically significant, t(15) = 4.0, p < .05. Our finding suggests that Lewis rats are no less sensitive to reinforcer amount than F344 rats.
Fig 3.
Group mean percent LL choice as a function of delays when manipulated within-session (□) by Anderson and Woolverton (2005) and between-condition (▪) in the present experiment. All data are from the stable sessions reported in both studies; error bars show SEM as per Anderson and Woolverton.
Second, our F344 rats appear to have been more sensitive to delays to the LL reinforcer than were Anderson and Woolverton's (2005). In the latter study, F344 rats more frequently selected the LL reinforcer than did our rats at the 10-s delay (see Figure 3; t[10.5] = 2.2, p = .05). Indeed, at the 15-s delay our F344 rats selected the LL reinforcer somewhat less frequently than did Anderson and Woolverton's at a 20-s delay, (t[8.6] = 2.1, p = .07). Between-subjects variability was also more pronounced in Anderson and Woolverton's F344 rats at 10-s and 20-s delays when compared with those at 10 and 15 s in our study.
Attributing these between-experiment differences to a single procedure is difficult because so many procedural differences separate these studies (e.g., our rats were required to press a center lever before making a choice and were exposed to a higher ratio of forced- to free-choice trials). We will suggest, however, that reports of insensitivity to within-session manipulations of the delay to the LL reinforcer (Cardinal et al., 2002) and carry-over effects from one delay to the next (Fox et al., 2008) make within-session vs. between-condition delay manipulations a likely candidate.
With respect to the first between-experiment difference (choices made by Lewis rats at the 0-s delay), under the Evenden and Ryan (1996) procedure, Lewis rats' hypersensitivity to delays of 20 s and greater (i.e., those arranged in the second half of the session) may have carried over to the first trial block of the next session, resulting in diminished preference for three over one food pellet. Such an effect would be consistent with the between-session carry-over effects reported by Fox et al. (2008). These researchers reported that the percentage of impulsive choices made by Wistar and Spontaneously Hypertensive rats was affected by the delay arranged in the previous session. When the delay to the larger food reinforcer was long in the preceding session (e.g., 24 s), rats tended to make more impulsive choices in the subsequent session (at a 12-s delay) than when a 6-s delay was arranged in the preceding session. Such carry-over effects have also been apparent in unpublished data collected in our lab using the Evenden and Ryan procedure: rats that strongly prefer the SS reinforcer at long delays also tend to demonstrate weak preferences for the larger reinforcer in the first trial block of the next session, when both reinforcers are immediately available.
That F344 rats more often selected the LL reinforcer in Anderson and Woolverton (2005) than at comparable delays in our study is consistent with the hypothesis that higher rates of choosing the larger reinforcer in the first (no-delay) trial block of the Evenden and Ryan (1996) procedure carried over into subsequent trial blocks in which delays were increased. Other findings suggest rats may be somewhat insensitive to within-session changes in the delay to the LL reinforcer (Cardinal et al., 2002) and when combined with a low baseline level of sensitivity to delay (F344 rats) would be expected to produce particularly poor discrimination of increasing delays and an under-estimation of sensitivity to delay. The post-hoc nature of these speculations speaks to the need for a follow-up experiment in which sensitivity to within-session and between-condition delay manipulations are compared within-subjects.
Although between-condition delay manipulations yield stable measures of choice which do not appear to be confounded by carry-over effects from previous conditions, this procedure is not well suited to assessing the effects of acute neurochemical manipulations on sensitivity to reinforcer delay. Assessing these effects across multiple delays requires multiple conditions with considerable time and experience between neurochemical manipulations. Measuring sensitivity to multiple delays within a single session has been the strength of the Evenden and Ryan procedure.
Neurochemical Differences Between Lewis and F344 Rats
Having reproduced the strain difference reported by Anderson and Woolverton (2005), we will briefly examine the basal neurochemical differences separating Lewis and F344 rats. As one might expect, there are too many of these differences to list here (see also Cadoni & Di Chiara, 2007). Therefore we will narrow our discussion to differences in serotonin (5-HT) and dopamine levels and function as these have received some attention in the context of operant impulsivity tasks.
Lewis rats have lower levels of 5-HT and fewer 5-HT receptors in the hippocampus and frontal cortex (Burnet, Mefford, Smith, Gold, & Sternberg 1996; Selim & Bradberry, 1996) and this is consistent with the hypothesis that decreased 5-HT function increases the probability of impulsive action (e.g., Soubrié, 1986). The role of baseline 5-HT levels in the discounting of delayed reinforcers is somewhat unclear. Selective lesions of ascending serotonergic pathways (dorsal and median raphe nuclei) have at least temporarily increased operant impulsivity (Bizot, Le Bihan, Puech, Hamon, & Thiébot, 1999; Mobini, Chiang, Ho, Bradshaw, & Szabadi, 2000; Wogar, Bradshaw, & Szabadi, 1993) while intracerebroventicular lesions producing 85% or more depletions of forebrain 5-HT have not (Winstanley, Dalley, Theobald, & Robbins, 2003, 2004). Anderson and Woolverton (2005) found that chlomipramine (3.0 mg/kg), which increases extracellular 5-HT by inhibiting synaptic uptake, had no effect on the choices of either Lewis or F344 rats (see also Charrier & Thiébot, 1996). To muddle things further, Evenden and Ryan (1996) reported that the nonselective 5-HT antagonist metergoline decreased impulsive choices; the opposite of what one would predict if a hypofunctioning 5-HT system played a simple role in Lewis rats' impulsive decision making.
Previous research has suggested that reinforcement processes are dependent on the functioning of the mesolimbic dopamine pathway, especially those connections to the nucleus accumbens (Carelli, 2002; Koob & Kreet, 2007; Wise, 2005). It is therefore interesting that Lewis rats have fewer dopamine D2 receptors in the striatum and nucleus accumbens core, and fewer D3 receptors in the nucleus accumbens shell and olfactory tubercle than F344 rats (Flores, Wood, Barbeau, Quirion, & Srivastava, 1998). Further, Lewis rats have lower levels of dopamine transporter in all of these brain regions (Flores et al., 1998).
Differences in D2 and D3 receptor function in Lewis and F344 rats raise the possibility that dopamine agonists with specific affinity to these receptors may provide clues to the relation between receptor function and impulsivity. To date, only one study has examined the effects of a D2/D3 agonist (pramipexole) on the discounting of delayed rewards. Hamidovic, Kang, and de Wit (2008) found acute 0.25- and 0.5-mg doses of pramipexole produced no statistically significant effect on human participants' choices made in a delay discounting task. The small sample size (n = 10) and a trend toward higher rates of delay discounting at the 0.5-mg dose suggest further research is needed.
Two other findings suggest further research with D2/D3 agonists is warranted. First, clinical-report evidence suggests that Parkinson patients treated with selective D2/D3 dopamine agonists are at greater risk of developing impulse control disorders such as hypersexuality, compulsive shopping, and pathological gambling (Dodd et al., 2005; Lu, Bharmal, & Suchowersky, 2006; Weintraub et al., 2006). In these cases, the impulsive behavior has been reported to dissipate with reductions in agonist dose (Seedat, Kesler, Niehaus, & Stein, 2000). Second, recent evidence suggests D2 and D3 receptor populations in the nucleus accumbens are important in the expression of a second type of impulsive behavior: response-inhibition impulsivity (Dalley et al., 2007). Interestingly, the more impulsive rats in the Dalley et al. study (who had reduced D2/D3 receptor availabilities in the ventromedial striatum—part of the mesolimbic dopamine pathway) also infused more cocaine in a subsequent self-administration paradigm. Similar correlations between D2-receptor availability in the ventral striatum and increased cocaine taking in rhesus monkeys has also been reported (Nader et al., 2006).
The correlation between more extreme discounting of delayed rewards in human drug-addicted individuals when compared to matched controls (Bickel & Marsch, 2001) has raised the possibility that the tendency toward selecting SS reinforcers may put one at risk of substance abuse (see Perry et al., 2005; Poulos et al., 1995). Because Lewis rats are more likely to self-administer a variety of drugs commonly abused by humans than are F344 rats, the hypothesized link between impulsive decision making and substance abuse is seemingly strengthened. Caution is warranted, however, because many of the reported differences in drug self-administration across these strains are limited to acquisition and, when all too infrequently assessed, often dissipate with continued experience in the operant chambers (e.g., Ambrosio, Goldberg, & Elmer, 1995; Martin et al., 1999). Further, because Lewis rats more quickly acquire lever pressing than F344s in an autoshaping procedure where food is the unconditioned stimulus (Kearns, Gomez-Seranno, Weiss, & Riley, 2006) and are more likely to acquire operant lever pressing maintained by nonresetting delayed food reinforcement (Anderson & Elcoro, 2007; exactly the opposite of what one might predict based on Lewis rats' tendency toward greater sensitivity to reinforcer delays) one must be cognizant of the possibility that the strain differences in drug self-administration may have more to do with motoric differences (more active rats are probably more likely to acquire a lever pressing response, see Mitchell, Cunningham, & Mark, 2005) than differential susceptibility to drug reinforcement. Differential sensitivity to initial exposure to drug reinforcers is clearly an important variable in understanding human drug taking, so further research is warranted.
To conclude, our findings are consistent with those of Anderson and Woolverton (2005) and suggest that carry-over effects may contaminate estimates of impulsive choice obtained when using the Evenden and Ryan (1996) procedure. Given that strain-related patterns in choice appear reliable, the stage is set for further examination of the role of strain differences in 5-HT and dopamine neurotransmission that may participate in impulsive decision making. The several clinical reports of enhanced impulsivity and risk taking following treatment with selective D2/D3 agonists suggest further exploration in that domain may prove important in understanding clinically relevant human decision making.
Acknowledgments
This research was supported by a grant from the National Institute on Drug Abuse (DA023564). We thank Adam Pyszczynski and Jeff Stein for their assistance in data collection.
Footnotes
It should be noted that this is a different AUC measure than that suggested by Myerson, Green, and Warusawitharana (2001). Because our AUC values were calculated from the area under percent preference data points rather than indifference points reflecting degree of delay discounting, our AUC values should not be compared with those reported in other studies using the Myerson et al. method.
References
- Ainslie G. Specious reward: A behavioral theory of impulsiveness and impulse control. Psychological Bulletin. 1975;82:463–496. doi: 10.1037/h0076860. [DOI] [PubMed] [Google Scholar]
- Alessi S.M, Petry N.M. Pathological gambling severity is associated with impulsivity in a delay discounting procedure. Behavioural Processes. 2003;64:345–354. doi: 10.1016/s0376-6357(03)00150-5. [DOI] [PubMed] [Google Scholar]
- Ambrosio E, Goldberg S.R, Elmer G.I. Behavior genetic investigation of the relationship between spontaneous locomotor activity and the acquisition of morphine self-administration behavior. Behavioral Pharmacology. 1995;6:229–237. [PubMed] [Google Scholar]
- Anderson K.G, Elcoro M. Response acquisition with delayed reinforcement in Lewis and Fischer 344 rats. Behavioural Processes. 2007;74:311–318. doi: 10.1016/j.beproc.2006.11.006. [DOI] [PubMed] [Google Scholar]
- Anderson K.G, Woolverton W.L. Effects of clomipramine on self-control choice in Lewis and Fischer 344 rats. Pharmacology, Biochemistry, & Behavior. 2005;80:387–393. doi: 10.1016/j.pbb.2004.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickel W.K, Marsch L.A. Toward a behavioral economic understanding of drug dependence: Delay discounting processes. Addiction. 2001;96:73–86. doi: 10.1046/j.1360-0443.2001.961736.x. [DOI] [PubMed] [Google Scholar]
- Bizot J, Le Bihan C, Puech A.J, Hamon M, Thiébot M. Serotonin and tolerance to delay of reward in rats. Psychopharmacology. 1999;146:400412. doi: 10.1007/pl00005485. [DOI] [PubMed] [Google Scholar]
- Brower V.G, Fu Y, Matta S.G, Sharp B.M. Rat strain differences in nicotine self-administration using an unlimited access paradigm. Brain Research. 2002;15:12–20. doi: 10.1016/s0006-8993(01)03375-3. [DOI] [PubMed] [Google Scholar]
- Burnet P.W, Mefford I.N, Smith C.C, Gold P.W, Sternberg E.M. Hippocampal 5-HT1A receptor binding site densities, 5-HT1A receptor messenger riboneucleic acid abundance and serotonin levels parallel the activity of the hypothalamo-pituitary-adrenal axis in rats. Behavioural Brain Research. 1996;73:365–368. doi: 10.1016/0166-4328(96)00116-7. [DOI] [PubMed] [Google Scholar]
- Cadoni C, Di Chiara G. Differences in dopamine responsiveness to drugs of abuse in the nucleus accumbens shell and core of Lewis and Fischer 344 rats. Journal of Neurochemistry. 2007;103:487–499. doi: 10.1111/j.1471-4159.2007.04795.x. [DOI] [PubMed] [Google Scholar]
- Cardinal R.N, Daw N, Robbins T.W, Everitt B.J. Local analysis of behaviour in the adjusting-delay task for assessing choice of delayed reinforcement. Neural Networks. 2002;15:617–634. doi: 10.1016/s0893-6080(02)00053-9. [DOI] [PubMed] [Google Scholar]
- Cardinal R.N, Robbins T.W, Everitt B.J. The effects of d-amphetamine, chlordiazepoxide, α-fluepenthixol, and behavioural manipulations on choice of signalled and unsignalled delayed reinforcement in rats. Psychopharmacology. 2000;152:362–375. doi: 10.1007/s002130000536. [DOI] [PubMed] [Google Scholar]
- Carelli R.M. The nucleus accumbens and reward: Neurophysiological investigations in behaving animals. Behavioral and Cognitive Neuroscience Reviews. 2002;4:281–296. doi: 10.1177/1534582302238338. [DOI] [PubMed] [Google Scholar]
- Charrier D, Thiébot M.H. Effects of psychotropic drugs on rat responding in an operant paradigm involving choice between delayed reinforcers. Pharmacology, Biochemistry, and Behavior. 1996;54:149–157. doi: 10.1016/0091-3057(95)02114-0. [DOI] [PubMed] [Google Scholar]
- Coffey S.F, Gudleski G.D, Saladin M.E, Brady K.T. Impulsivity and rapid discounting of delayed hypothetical rewards in cocaine-dependent individuals. Experimental and Clinical Psychopharmacology. 2003;11:18–25. doi: 10.1037//1064-1297.11.1.18. [DOI] [PubMed] [Google Scholar]
- Dalley J.W, Fryer T.D, Brichard L, Robbinson E.S, Theobald D.E, Lääne K, et al. Nucleus accumbens D2/3 receptors predict trait impulsivity and cocaine reinforcement. Science. 2007;315:1267–1270. doi: 10.1126/science.1137073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon M.R, Marley J, Jacobs E.A. Delay discounting by pathological gamblers. Journal of Applied Behavior Analysis. 2003;36:449–458. doi: 10.1901/jaba.2003.36-449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodd M.L, Klos K.J, Bower J.H, Geda Y.E, Josephs K.A, Ahlskog J.E. Pathological gambling caused by drugs used to treat Parkinson disease. Archives of Neurology. 2005;62:1377–1381. doi: 10.1001/archneur.62.9.noc50009. [DOI] [PubMed] [Google Scholar]
- Evenden J.L, Ryan C.N. The pharmacology of impulsive behaviour in rats: The effects of drugs on response choice with varying delays of reinforcement. Psychopharmacology. 1996;128:161–170. doi: 10.1007/s002130050121. [DOI] [PubMed] [Google Scholar]
- Flores G, Wood G.K, Barbeau D, Quirion R, Srivastava L.K. Lewis and Fischer rats: A comparison of dopamine transporter and receptors levels. Brain Research. 1998;814:34–40. doi: 10.1016/s0006-8993(98)01011-7. [DOI] [PubMed] [Google Scholar]
- Fox A.T, Hand D.J, Reilly M.P. Impulsive choice in a rodent model of attention-deficit/hyperactivity disorder. Behavioural Brain Research. 2008;187:146–152. doi: 10.1016/j.bbr.2007.09.008. [DOI] [PubMed] [Google Scholar]
- Hamidovic A, Kang U.J, de Wit H. Effects of low to moderate acute doses of pramipexole on impulsivity and cognition in healthy volunteers. Journal of Clinical Psychopharmacology. 2008;28:45–51. doi: 10.1097/jcp.0b013e3181602fab. [DOI] [PubMed] [Google Scholar]
- Howell D.C. Statistical methods for psychology (3rd ed.) Belmont, CA: Duxbury Press; 1992. [Google Scholar]
- Kearns D.N, Gomez-Serrano M.A, Weiss S.J, Riley A.L. A comparison of Lewis and Fischer rat strains on autoshaping (sign-tracking), discrimination reversal learning and negative auto-maintenance. Behavioural Brain Research. 2006;169:193–200. doi: 10.1016/j.bbr.2006.01.005. [DOI] [PubMed] [Google Scholar]
- Koob G, Kreet M.G. Stress, dysregulation of drug reward pathways, and the transition to drug dependence. American Journal of Psychiatry. 2007;164:1149–1159. doi: 10.1176/appi.ajp.2007.05030503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosten T.A, Miserendino M.J, Haile C.N, DeCaprio J.L, Jatlow P.I, Nestler E.J. Acquisition and maintenance of intravenous cocaine self-administration in Lewis and Fischer inbred rat strains. Brain Research. 1997;778:418–429. doi: 10.1016/s0006-8993(97)01205-5. [DOI] [PubMed] [Google Scholar]
- Kosten T.A, Zhang X.Y, Haile C.N. Strain differences in maintenance of cocaine self-administration and their relationship to novelty activity responses. Behavioral Neuroscience. 2007;121:380–388. doi: 10.1037/0735-7044.121.2.380. [DOI] [PubMed] [Google Scholar]
- Logue A.W, Mazur J.E. Maintenance of self-control acquired through a fading procedure: Follow-up on Mazur and Logue (1978) Behaviour Analysis Letters. 1981;1:131–137. [Google Scholar]
- Lu C, Bharmal A, Suchowersky O. Gambling and Parkinson disease. Archives of Neurology. 2006;63:298. doi: 10.1001/archneur.63.2.298-a. [DOI] [PubMed] [Google Scholar]
- Madden G.J, Petry N, Badger G.J, Bickel W.K. Impulsive and self-control choices in opiate-dependent patients and non-drug-using control participants: Drug and monetary rewards. Experimental and Clinical Psychopharmacology. 1997;5:256–262. doi: 10.1037//1064-1297.5.3.256. [DOI] [PubMed] [Google Scholar]
- Martin S, Mansanares J, Corchero J, Garcia-Lecumberri C, Crespo J.A, Fuentes J.A, et al. Differential basal proenkephalin gene expression in dorsal striatum and nucleus accumbens, and vulnerability to morphine self-administration in Fischer 344 and Lewis rats. Brain Research. 1999;821:350–355. doi: 10.1016/s0006-8993(99)01122-1. [DOI] [PubMed] [Google Scholar]
- Mazur J.E. An adjusting procedure for studying delayed reinforcement. In: Commons M.L, Mazur J.E, Nevin J.A, Rachlin H, editors. Qualitative analyses of behavior: The effect of delay and of intervening events on reinforcement value. Hillsdale, NJ: Erlbaum; 1987. pp. 55–73. [Google Scholar]
- Mazur J.E, Logue A.W. Choice is a self-control paradigm: Effects of a fading procedure. Journal of the Experimental Analysis of Behavior. 1978;30:11–17. doi: 10.1901/jeab.1978.30-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell J.M, Cunningham C.L, Mark G.P. Locomotor activity predicts acquisition of self-administration behavior but not cocaine intake. Behavioral Neuroscience. 2005;119:464–472. doi: 10.1037/0735-7044.119.2.464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mobini S, Chiang T.J, Ho M.Y, Bradshaw C.M, Szabadi E. Effects of central 5-hydroxytryptamine depletion on sensitivity to delayed and probabilistic reinforcement: A quantitative analysis. Psychopharmacology. 2000;152:390–397. doi: 10.1007/s002130000542. [DOI] [PubMed] [Google Scholar]
- Myerson J, Green L, Warusawitharana M. Area under the curve as a measure of discounting. Journal of the Experimental Analysis of Behavior. 2001;76:235–243. doi: 10.1901/jeab.2001.76-235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nader M.A, Morgan D, Gage H.D, Nader S.H, Callhoun T.L, Buchheimer N, et al. PET imaging of dopamine D2 receptors during chronic cocaine self-administration in monkeys. Nature Neuroscience. 2006;9:1050–1056. doi: 10.1038/nn1737. [DOI] [PubMed] [Google Scholar]
- Perry J.L, Larson E.B, German J.P, Madden G.J, Carroll M.E. Impulsivity (delay discounting) as a predictor of acquisition of i.v. cocaine self-administration in female rats. Psychopharmacology. 2005;178:193–201. doi: 10.1007/s00213-004-1994-4. [DOI] [PubMed] [Google Scholar]
- Perry J.L, Nelson S.E, Carroll M.E. Impulsivity (delay discounting) as a predictor of acquisition and reinstatement of i.v. cocaine self-administration in male (v female) rats. Experimental and Clinical Psychopharmacology. 2008;16:165–177. doi: 10.1037/1064-1297.16.2.165. [DOI] [PubMed] [Google Scholar]
- Poulos C.X, Le A.D, Parker J.L. Impulsivity predicts individual susceptibility to high levels of alcohol self-administration. Behavioural Pharmacology. 1995;6:810–814. [PubMed] [Google Scholar]
- Rachlin H, Green L. Commitment, choice, and self-control. Journal of the Experimental Analysis of Behavior. 1972;17:15–22. doi: 10.1901/jeab.1972.17-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rachlin H, Raineri A, Cross D. Subjective probability and delay. Journal of the Experimental Analysis of Behavior. 1991;55:233–244. doi: 10.1901/jeab.1991.55-233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selim M, Bradberry C.W. Effect of ethanol on extracellular 5-HT and glutamate in the nucleus accumbens and prefrontal cortex: Comparison between the Lewis and Fischer 344 rat strains. Brain Research. 1996;716:157–164. doi: 10.1016/0006-8993(95)01385-7. [DOI] [PubMed] [Google Scholar]
- Seedat S, Kesler S, Niehaus D.J, Stein D.J. Pathological gambling behaviour: Emergence secondary to treatment of Parkinson's disease with dopaminergic agents. Depression & Anxiety. 2000;11:185–186. doi: 10.1002/1520-6394(2000)11:4<185::AID-DA8>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Sidman M. Tactics of Scientific Research. New York: Basic Books; 1960. [Google Scholar]
- Soubrié P. Reconciling the role of central serotonin neurons in human and animal behavior. Behavioral Brain Sciences. 1986;9:319–335. [Google Scholar]
- Suzuki T, George F.R, Meisch R.A. Differential establishment and maintenance of oral ethanol reinforced behavior in Lewis and Fischer 344 inbred rat strains. Journal of Pharmacology and Experimental Therapeutics. 1988;245:164–170. [PubMed] [Google Scholar]
- Suzuki T, Otani K, Koike Y, Misawa M. Genetic differences in preferences for morphine and codeine in Lewis and Fischer 344 inbred rat strains. Japanese Journal of Pharmacology. 1988;47:425–431. doi: 10.1254/jjp.47.425. [DOI] [PubMed] [Google Scholar]
- Vuchinich R.E, Simpson C.A. Hyperbolic temporal discounting in social drinkers and problem drinkers. Experimental and Clinical Psychopharmacology. 1998;6:292–305. doi: 10.1037//1064-1297.6.3.292. [DOI] [PubMed] [Google Scholar]
- Weintraub D, Siderowf A.D, Potenza M.N, Goveas J, Morales K.H, Duda J.E, et al. Association of dopamine agonist use with impulse control disorders in Parkinson disease. Archives of Neurology. 2006;63:969–973. doi: 10.1001/archneur.63.7.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winstanley C.A, Dalley J.W, Theobald D.E, Robbins T.W. Global 5-HT depletion attenuates the ability of amphetamine to decrease impulsive choice in rats. Psychopharmacology. 2003;170:320–331. doi: 10.1007/s00213-003-1546-3. [DOI] [PubMed] [Google Scholar]
- Winstanley C.A, Dalley J.W, Theobald D.E, Robbins T.W. Fractionating impulsivity: Contrasting effects of central 5-HT depletion on different measures of impulsive behavior. Neuropsychopharmacology. 2004;29:1331–1343. doi: 10.1038/sj.npp.1300434. [DOI] [PubMed] [Google Scholar]
- Wise R.A. Forebrain substrates of reward and motivation. Journal of Comparative Neurology. 2005;493:115–121. doi: 10.1002/cne.20689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wogar M.A, Bradshaw C.M, Szabadi E. Effect of lesions of the ascending 5-hydroxytrypraminergic pathways on choice between delayed reinforcers. Psychopharmacology. 1993;111:239–243. doi: 10.1007/BF02245530. [DOI] [PubMed] [Google Scholar]