Abstract
The interresponse-time structures of pigeon key pecking were examined under variable-ratio, variable-interval, and variable-interval plus linear feedback schedules. Whereas the variable-ratio and variable-interval plus linear feedback schedules generally resulted in a distinct group of short interresponse times and a broad distribution of longer interresponse times, the variable-interval schedules generally showed a much more continuous distribution of interresponse times. The results were taken to indicate that a log survivor analysis or double exponential fit of interresponse times may not be universally applicable to the task of demonstrating that operant behavior can be dichotomized into bouts of engagement and periods of disengagement.
Keywords: interresponse time, variable-ratio, variable-interval, yoked schedules, Shull machine, key peck, pigeons
In the past, the conceptualization of behavior as two-state (Gilbert, 1958) has not enjoyed widespread research interest because of the difficulty in validly dichotomizing the behavior stream into visits and nonvisits by simply specifying some specific interresponse time (IRT) duration as a criterion. The split between within-visit IRTs and visit-initiation IRTs is unlikely to be at the same value across individuals, or even within an individual across procedures.
Shull, Gaynor, and Grimes (2001) described one possible solution for resolving the categorization problem. They displayed the total distribution of obtained IRTs as a survivor plot with a logarithmically scaled y axis. In this semilogarithmic plot, the slope between any two points on the x axis is an indicator of the relative decrease in the frequency of the IRTs per opportunity between those points. If a single exponential decay governed the occurrence of all responding, then the IRT distribution would appear as a single straight line. If, on the other hand, behavior occurred in short bouts of responding separated by longer delays, then the short IRTs governed by one exponential decay process would result in an initial steep slope, while the longer delays between response bouts characterized by a different exponential would be spread across a broad range of values and would generate a second shallower slope.
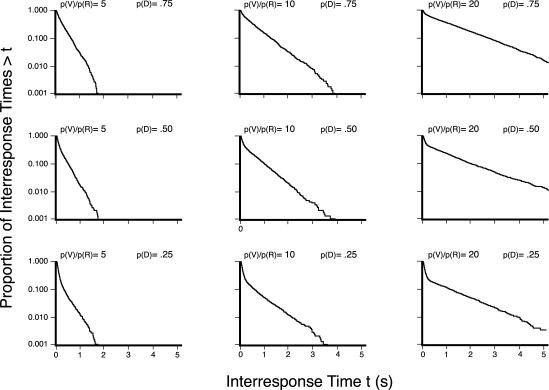
Shull and his colleagues (Shull et al., 2001) found that the log survivor plots of the output of their explicitly two-state emulator had two relatively straight lines intersecting at an angle less than 180°, or had a “broken-stick” appearance, such that the function and therefore the two classes of behavior were easily dichotomized by simple inspection. Figure 1 provides example log survivor plots of the output of Shull's model. The ratio of the within-visit response rate to the between-visit response rate and the probability of disengaging are given above each frame. The columns of frames illustrate how increasing the relative value of the probability of entering the engaged state, p(V), with respect to the probability of a response during a visit, p(R), (i.e., larger ratios of within-visit to the between-visit response rates) makes the broken stick more pronounced by decreasing the slope of the right limb of the log survivor plot. The rows of frames illustrate how changing the probability of disengaging from a visit, p(D), affects the number of within-visit responses, thus changing how far the left limb drops before the break in the stick. As can be seen, the two classes of IRTs are more or less easily identified depending on the amount of responding within a visit, and the difference between the within- and between-visit responding.
Fig 1.
Outputs of the two exponential model as described by Shull et al. (2001), displayed as log survivor functions. The columns of frames illustrate the effects of increasing ratios of the exponentials for p(V) and p(R). The rows of frames illustrate the effect of different values of the probability of disengaging from a visit, p(D).
Shull et al. (2001) then evaluated the validity of their two-state conceptualization of behavior by exposing rats to different schedules of reinforcement in order to compare the form of the log survivor functions for actual behavior with the synthetic behavior of an explicitly two-state machine. It was found that the rat data had the same dual slope appearance as an explicitly two-state machine. This was taken to indicate that the rats' behavior had two states.
The essential point advanced by Shull et al.'s (2001) article was the very plausible view that behavior could best be understood as bouts of engaged terminal behavior (Staddon & Simmelhag, 1971) separated by other behaviors. A secondary point of the article was that log survivor plots or double exponential fits of log survivor functions could be used as convenient analytical techniques to separate those two classes of behavior.
A prerequisite of the general applicability of a log survivor function as an index of the engagement and disengagement in operant behavior is a demonstration that the results found using rats on some variable-interval (VI) schedules will generalize to other organisms, schedules, and parameter values. The present research was an attempt to examine the differences between responding to interval and ratio contingencies in pigeons by using Shull et al.'s (2001) log survivor analysis. It was expected that if the resulting IRTs did appear like a broken stick, then it would add credence to the view that all operant behavior can best be characterized as two-state. This would in turn provide an essential precursor to a deeper understanding of how contingencies alter response rate.
Experiment 1 obtained a broad data set for examination by exposing subjects to a wide range of schedule values for each of three schedules of reinforcement. This allowed a log survivor analysis of not only the differences between schedule types, but also an examination of the differences across schedule values. A principal question would be whether increases in reinforcement rate increase the rate of visit initiation (Shull, Grimes, & Bennett, 2004). A VI-plus-linear feedback schedule (VI+) (McDowell & Wixted, 1986) was included in Experiment 1. That schedule made it possible to evaluate the role of the differential reinforcement of short IRTs within a visit on the obtained log survivor functions.
Experiment 2 yoked the occurrence of reinforcement in VI and variable-ratio (VR) schedules in a multiple schedule format. This data set ensured that any difference in behavior under the VI and VR schedules was the result of the contingency itself rather than a variety of other factors.
General Method
Subjects
Twenty-one adult, experimentally naive pigeons obtained from a local supplier were used. They were housed in individual cages with free access to water. Each pigeon received approximately 50 food presentations during each experimental session. Pigeons requiring supplemental feeding to maintain them at 80% of their free-feeding weights were fed at least 60 min after the experimental session. Layer pellets were used for both maintenance feeding and as the reinforcer.
Apparatus
Eleven experimental chambers were used. The workspace within each was a 30 by 30 by 34 cm high box. An unfinished aluminum panel served as one wall of the box; the other sides were painted white. The aluminum panel had a feeder aperture 5 cm in diameter medially located 10 cm above the grid floor. Three response keys, 2 cm in diameter were located 9 cm apart, 29 cm above the grid floor. They required a force equivalent to 15 g (0.15 N) to operate. The translucent Plexiglas keys could be transilluminated by stimulus projectors containing color filters. Colors included the following Rosco theatrical gels: orange (23), yellow (12), green (91), and turquoise (95). Response keys were transilluminated during all phases of the experiment, except during food presentation when a lamp in the food magazine provided the only illumination. Two houselights directed upward were located on the stimulus panel, 32 cm above the grid floor. Ventilation was provided by an exhaust fan mounted on the outside of the chamber. A white noise generator provided ambient masking noise within the chamber.
Stimulus events were controlled and key pecks were recorded by a computer network composed of a host computer and an independent control computer for each chamber (Palya & Walter, 1993). The host computer archived the time of each stimulus and response event in 1-ms intervals. Subsequent data extraction and analysis routines provided the resulting behavioral indices. Complete raw data event logs of all research are maintained for 10 years and are available for electronic download upon request.
Procedure
All pigeons were trained to approach and eat from a food magazine within 3 s on three consecutive presentations. During magazine training the keys were dark. Each pigeon was exposed to a procedure that began with autoshaping and subsequently brought behavior under the control of an intermittent schedule of reinforcement as will be subsequently detailed. The reinforcement duration was 3 s throughout the shaping procedure and the remainder of both experiments. Each analysis for each pigeon was based on the mean of the obtained data for that pigeon taken across the last five sessions of a phase.
The scheduled interreinforcement intervals (IRIs) for VI schedules and response requirements for VR schedules and their sequential order were determined by a standard sequence (Palya & Allan, 2003), which was constructed as follows: Five sets of 20-element Fleshler-Hoffman (Fleshler & Hoffman, 1962) factors normalized to one were generated. An algorithm, which randomly selected the 100 factors without replacement, was iteratively implemented to produce an ordering of the 100 factors that minimized the sample-to-sample variance when samples contained 12 consecutive elements. An independent random starting point in the resulting ordered set of 100 factors was selected for each procedure for each pigeon for each session. Factors were subsequently drawn in consecutive order from the standard sequence. The actual values for a given schedule were determined by multiplying the consecutive factors by the value which, over repeated factors, would produce a schedule with the specified average requirement.
Experiment 1
Method
Subjects
Twelve naïve pigeons were used.
Apparatus
Eleven experimental chambers were used. Response keys were illuminated green.
Procedure
The procedure is detailed below, but in summary, each pigeon was exposed to each of the following procedures for between 10 and 30 sessions: VR 10, 50, 100, 200, and 400; VI+ 10, 50, 100, 200, and 400 s; and VI′ 10, 50, 100, 200, and 400. Table 1 provides the counterbalanced sequence of schedules used for each pigeon in Experiment 1.
Table 1.
Procedures and Their Sequence for Experiment 1.
| Condition order |
Pigeon |
|||||
| 410 & 481 | 364 & 440 | 365 & 356 | 372 & 373 | 371 & 384 | 370 & 378 | |
| 1 | VR 10 | VR 100 | VI+ 10 | VI+ 200 | VI′ 50 | VI′ 10 |
| 2 | VR 400 | VR 50 | VI+ 50 | VI+ 400 | VI′ 100 | VI′ 400 |
| 3 | VR 50 | VR 10 | VI+ 200 | VI+ 10 | VI′ 400 | VI′ 200 |
| 4 | VR 100 | VR 400 | VI+ 100 | VI+ 100 | VI′ 200 | VI′ 50 |
| 5 | VR 200 | VR 200 | VI+ 400 | VI+ 50 | VI′ 10 | VI′ 100 |
| 6 | VI+ 50 | VI′ 50 | VR 10 | VI′ 200 | VR 200 | VI+ 50 |
| 7 | VI+ 400 | VI′ 400 | VR 200 | VI′ 400 | VR 100 | VI+ 400 |
| 8 | VI+ 10 | VI′ 10 | VR 400 | VI′ 10 | VR 50 | VI+ 100 |
| 9 | VI+ 100 | VI′ 200 | VR 50 | VI′ 100 | VR 400 | VI+ 10 |
| 10 | VI + 200 | VI′ 100 | VR 100 | VI′ 50 | VR 10 | VI+ 200 |
| 11 | VI′ 100 | VI+ 100 | VI′ 400 | VR 10 | VI+ 200 | VR 100 |
| 12 | VI′ 50 | VI+ 10 | VI′ 100 | VR 100 | VI+ 10 | VR 10 |
| 13 | VI′ 10 | VI+ 50 | VI′ 50 | VR 200 | VI+ 400 | VR 200 |
| 14 | VI′ 200 | VI+ 200 | VI′ 10 | VR 400 | VI+ 50 | VR 400 |
| 15 | VI′ 400 | VI + 400 | VI′ 200 | VR 50 | VI+ 100 | VR 50 |
In separate phases, each pigeon was exposed to VI+ schedules yoked to the reinforcement rate obtained under each of its five different VR schedules, or best estimates when pigeons were exposed to the VI+ schedules first (Palya & Walter, 1997). A VI+ schedule is a synthetic schedule that reinforces the first response after a temporal interval that is a function of the average IRT for that IRI (McDowell & Wixted, 1986). For example, a VI+ 100-s schedule provides reinforcement for the first response after, on average, 100 times the average IRT in that IRI. In this way, faster responding results in a higher reinforcement rate, but the probability that a specific IRT will be followed by reinforcement is similar to that in an interval schedule, rather than being based on the response count as it is in a ratio schedule. Because of the way it was scheduled, a pigeon's VI+ schedule necessarily had the same mean reinforcement rate as did the VR to which it was yoked. The schedule value specified for each VI+ schedule indicates to which VR it was yoked.
In separate phases, each pigeon also received a VI′ schedule (as described below) yoked to the reinforcement rate under each of the five different VR schedules, or best estimates when pigeons were exposed to the VI′ schedules first (Palya & Walter, 1997). For ease of designating the appropriate schedule value for comparison, and for ease of labeling the VI values for the 12 pigeons under the 15 conditions, the VI′ schedule values were specified as the VR values to which they were yoked. For example, a VI that was yoked to a VR 100 (and was therefore labeled VI′ 100) had the same IRI as that pigeon's VR 100. Across pigeons, the mean IRI for the VI′ 10, 50, 100, 200 and 400 schedules were 5, 24, 50, 103 and 218 s, respectively. This simple labeling convention eliminated the need to specify 60 different VI schedule values and the tables indicating which VI value corresponded to which VR value for each pigeon.
Each procedure was implemented with each pigeon until responding was judged stable by a time-series analysis on eight consecutive mean daily response rates based on the C statistic (Tryon, 1982; Young, 1941) and the judgment of stability was corroborated by visual inspection.
The session duration for a pigeon on a given day was determined by the number of reinforcers necessary to maintain the pigeon at its 80% body weight or 50 reinforcers (30 when the schedule value was 400), whichever was smaller. The session duration, therefore, varied somewhat day to day and more substantially from schedule to schedule. For example, at the lowest reinforcement rate, sessions were approximately 100 min long, whereas at the highest reinforcement rate, sessions were approximately 4 min long. The two extremes of the reinforcement rate approached the practical limits for daily sessions with pigeons for our laboratory and set the range of schedule values implemented. Across pigeons and variations in the schedules, the IRIs contained from a minimum of 1 response to a maximum of approximately 1600 responses. The VR 400 did not maintain reliable responding in Bird 440.
The present experiment is the reanalysis of data files from a procedure carried out to answer other questions, because these data were well suited to provide information bearing on the present research question. Other aspects of the data have been previously presented (Palya & Walter, 1997).
Results and Discussion
Based on the results obtained by Shull and his colleagues (Shull, et al., 2001; Shull, Grimes & Bennett, 2004), log survivor plots of the IRTs were expected to fall into two distinct functions with the discrepancies between the slopes governed by the schedule value. The expected results were a steep left limb and a less steep right limb whose slope decreased as a function of lower reinforcement rate. Additionally, the y-intercept of the right limb was expected to increase as the reinforcer rate decreased.
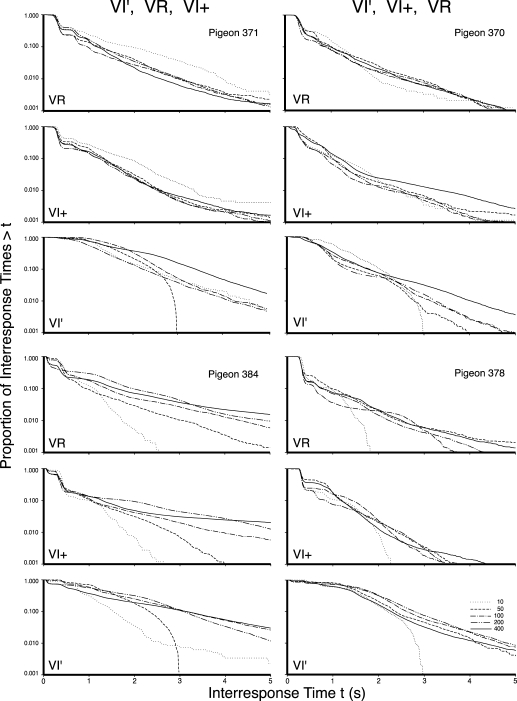
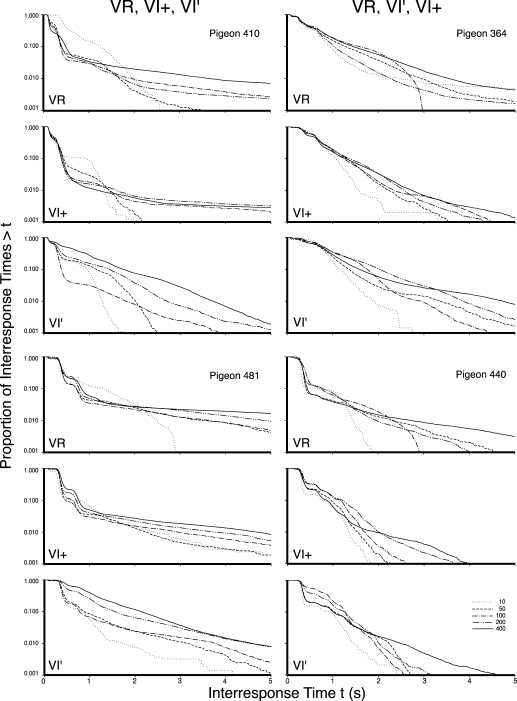
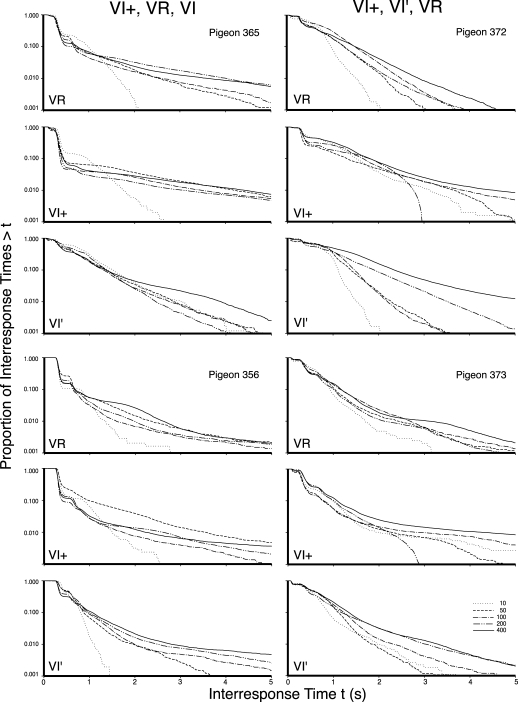
Figures 2, 3, and 4 present the log survivor plots of the behavior for the 12 pigeons under the three types of schedules. The five functions within each frame present the log survivor plots for the indicated pigeon under the five schedule values of the indicated schedule type. The top frame in each set presents the data obtained under the VR schedule, the middle frame presents the data obtained under the VI+ schedule, and the bottom frame provides the data for the VI′ schedule. Differences within each frame indicate schedule parameter effects (e.g., VR 10 vs. VR 400). Differences between frames within each set of three indicate schedule effects (e.g., VR vs. VI). Differences between sets of frames within a column indicate individual differences (e.g., Pigeon 371 vs. Pigeon 384). The six counterbalanced orders are provided in the six columns of frames across the three figures, and are as labeled at the top of each column. Differences between columns indicate order effects (e.g., VR exposure first vs. VI exposure first).
Fig 2.
The log survivor plots for all pigeons in Experiment 1 that began with exposure to VI′ schedules. The left column provides the VI′, VR, and then VI+ exposure sequence, while the right column displays the VI′, VI+, and then VR exposure sequence. The top, middle, and bottom frame for each pigeon depicts the VR, VI+, and VI′, respectively. The five functions within each frame provide the data for the schedule values as indicated in the legend.
Fig 3.
The log survivor plots for all pigeons in Experiment 1 that began with exposure to VR schedules. The left column provides the VR, VI+, and then VI′ exposure sequence, while the right column displays the VR, VI′, and then VI+ exposure sequence. The top, middle, and bottom frame for each pigeon depicts the VR, VI′, and VI+, respectively. The five functions within each frame provide the data for the schedule values as indicated in the legend.
Fig 4.
The log survivor plots for all pigeons in Experiment 1 that began with exposure to VI+ schedules. The left column provides the VI+, VR, and then VI′ exposure sequence, while the right column displays the VI+, VI′, and then VR exposure sequence. The top, middle, and bottom frame for each pigeon depicts the VR, VI+, and VI, respectively. The five functions within each frame provide the data for the schedule values as indicated in the legend.
Many of the functions from all three schedules had multiple inflection points rather than a single change in slope. However, in general, across a very wide range of reinforcement rates, VI schedules generally controlled a more continuous distribution of IRT values, whereas VR schedules and the synthetic VI schedules with VR-like properties (VI+) generally controlled a cluster of short IRTs and a second distribution of longer IRTs. By comparing the behavior maintained by a VR (top frame) with the behavior maintained by a VI (bottom frame) for each pigeon, it is apparent that VR schedules most typically controlled steeply declining initial limbs (leftmost portion of the functions), whereas VI schedules most typically did not. By comparing the output under all three schedules for each pigeon, it can be seen that the VI+ schedules generally controlled behavior comparable to the VR schedules. This similarity indicates that not only do VR schedules result in substantially different log survivor functions than do VI schedules, it indicates that that difference is preserved even when the VI schedule is compared to a VI schedule with added molar feedback. Additionally, for the majority of birds, the functions resulting from higher reinforcement rates tended to have steeper slopes that intercepted the x axis at lower values. Shull and his colleagues (Shull et al., 2001) had interpreted this as a higher rate of initiating visits under schedules with higher reinforcement rates.
Each of the 180 functions obtained in this experiment showed some horizontal portion on the extreme left indicating a lower limit to the emitted IRT values or a refractory period (Killeen, Hall, Reilly, & Kettle, 2002). The extreme right of each function shows to a greater or lesser extent that the very longest IRTs were relatively rare. However, between these two extremes of the IRT distribution, there were various patterns. For example, some functions show a sharp drop followed by a period of little change followed by a second precipitous drop, such as the VR 10 schedule (dashed line) for Pigeon 440. Some showed an initial small drop, then a sharp drop followed by a regular decrement (VI+ 50-s for Pigeon 365—dash dot); some showed multiple sharp drops followed by a regular decrement (VI+ 400-s for Pigeon 481—solid line); while still others exhibited a regular decrement without a sharp drop at all (VI′ 100 for Pigeon 371—dash dot dot).
Finally, there appeared to be instances where the initial schedule to which the pigeons had been exposed had a carryover effect. Pigeons that were exposed to a VR schedule first (364, 410, 440, and 481) were the most likely to exhibit an initial steep drop in the left portion of the function under the VI schedule, whereas the pigeons that were exposed to the VI schedule first (371, 384, 370, and 378) were the most likely to exhibit smoother, continuous functions under the VR. This effect was evaluated in Experiment 2.
In summary, the diversity in the pattern of the obtained IRT distributions suggests that log survivor analysis or double exponential fits may not be an adequate tool to prove that operant behavior is fundamentally two distinct classes of behavior. Nearly half (15 of the 36) of the frames presented in Figures 2, 3, and 4 do not have functions that can be readily dichotomized (approximately one third of the VR and VI+ frames and two thirds of the VI frames). As noted by Kessel and Lucke (2008) and Shull (2004), little variance can be accounted for by double exponential fits in those cases.
Experiment 2 was designed to determine if the relatively continuous log survivor plots obtained under the VI schedules of Experiment 1 were reliable, and to further assess any difference in the log survivor functions resulting from VR and VI behavior. A multiple-schedule comparison was used in order to maximize the ability to detect any schedule effects, and to more closely replicate procedural elements utilized by Shull et al. (2001). A yoking procedure was used to maximize the similarity in the distribution of reinforcers under those two schedules so that differences in the obtained response distributions could not be attributed to differences in reinforcement rates. Additionally, different pretraining groups were compared in order to ensure that whatever carryover effects that had occurred in Experiment 1 were not germane to the overall conclusions drawn.
Experiment 2
Method
Subjects
Nine naïve pigeons were used.
Apparatus
Nine experimental chambers were used. Response keys were illuminated orange, yellow, or turquoise.
Procedure
Each pigeon was exposed to a procedure that began with autoshaping and that subsequently brought behavior under the control of a VR schedule in the case of Group 1, and a VI schedule for both Groups 2 and 3. Each group consisted of 3 pigeons.
Table 2 presents a summary of the conditions, order of presentation, and number of sessions in each condition. As a control, Group 1 was pretrained on a VR schedule, while Groups 2 and 3 were pretrained on VI schedules. These different pretraining groups were implemented because the results of Experiment 1 had suggested that there may be residual effects of an early exposure to a particular contingency. Phase 1 exposed Group 1 to a VR 60 schedule, and Groups 2 and 3 to a VI 60-s schedule. All pigeons were then exposed to a multiple VI 60-s VI 60-s schedule baseline in Phase 2. The first component occurred in the presence of a turquoise light on the center key. The first component was immediately followed by the second component, in the presence of an orange light on the center key. Each component was in effect for five reinforcer presentations. After the second component was completed, the first component began again. This cycle continued until 50 reinforcers had been presented (five occurrences of each component) or that number of reinforcers just sufficient to maintain the pigeon at 80% of its free-feeding weight.
Table 2.
Procedures and Their Sequences for Experiment 2.
| Condition order |
Group One |
Number of Sessions | ||
| 627 | 675 | 710 | ||
| 1 | VR 60 | VR 60 | VR 60 | 30 |
| 2 | VI 60-s VI 60-s | VI 60-s VI 60-s | VI 60-s VI 60-s | 45 |
| 3 | VI 60-s Yoked VR | VI 60-s Yoked VR | VI 60-s Yoked VR | 50 |
| 4 | VI 60-s VI 60-s | VI 60-s VI 60-s | VI 60-s VI 60-s | 30 |
| 5 | VR 87 Yoked VI | VR 70 Yoked VI | VR 35 Yoked VI | 40 |
| 6 | VI 60-s VI 60-s | VI 60-s VI 60-s | VI 60-s VI 60-s | 40 |
| Condition order |
Group Two |
Number of Sessions | ||
| 688 | 752 | 772 | ||
| 1 | VI 60-s | VI 60-s | VI 60-s | 21 |
| 2 | VI 60-s VI 60-s | VI 60-s VI 60-s | VI 60-s VI 60-s | 45 |
| 3 | VI 60-s Yoked VR | VI 60-s Yoked VR | VI 60-s Yoked VR | 30 |
| 4 | VI 60-s VI 60-s | VI 60-s VI 60-s | VI 60-s VI 60-s | 40 |
| 5 | VR 90 Yoked VI | VR 48 Yoked VI | VR 43 Yoked VI | 30 |
| 6 | VI 60-s VI 60-s | VI 60-s VI 60-s | VI 60-s VI 60-s | 30 |
| Condition order |
Group Three |
Number of Sessions | ||
| 694 | 706 | 727 | ||
| 1 | VI 60-s | VI 60-s | VI 60-s | 21 |
| 2 | VI 60-s VI 60-s | VI 60-s VI 60-s | VI 60-s VI 60-s | 45 |
| 3 | VR 82 Yoked VI | VR 23 Yoked VI | VR 53 Yoked VI | 30 |
| 4 | VI 60-s VI 60-s | VI 60-s VI 60-s | VI 60-s VI 60-s | 30 |
| 5 | VI 60-s Yoked VR | VI 60-s Yoked VR | VI 60-s Yoked VR | 30 |
| 6 | VI 60-s VI 60-s | VI 60-s VI 60-s | VI 60-s VI 60-s | 30 |
Groups 1 and 2 were then exposed to a multiple VI 60-s yoked VR schedule. Each VR was yoked by requiring the same number of responses per reinforcer as had been obtained under its corresponding VI schedule in the immediately preceding component of the multiple schedule. Phase 4 was a return to the multiple VI 60-s VI 60-s baseline. Phase 5 was a multiple VR yoked VI schedule in which the intervals required between each reinforcer on the VI schedule were the same as those that had occurred in each of the corresponding VR schedules in the preceding VR component. Phase 6 was a return to the multiple VI 60-s VI 60-s baseline. Group 3 underwent the same procedures as Groups 1 and 2, except that the procedures in Phases 3 and 5 were presented in the opposite order to reveal any sequential effects.
Each phase continued until the pigeon's response rate plotted as a function of session number showed no apparent session-to-session trends over five consecutive sessions in both components of the multiple schedules. After this stability criterion had been met, the phase was then continued until data sufficient for potential analyses had been obtained, and the change could be fitted within the constraints of other laboratory activities. Additionally, the informal lab policy of changing procedures at 5-day boundaries, when convenient, was typically upheld. The intent of this latter, behaviorally arbitrary criterion was to reduce the probability that some atypical local aspect of the data was consistently included in the data representing the effects of a phase (Palya & Allan, 2003).
Results and Discussion
As seen in Experiment 1, the log survivor functions from both schedules in the present experiment had multiple inflection points. Additionally, in general, VI schedules controlled a continuous distribution of IRT values producing a gradual decline in their log survivor functions, whereas the VR schedules often controlled more concentrated bursts of short IRTs. This latter pattern of responding resulted in a noticeable knee in the log survivor plots for VR schedules. These findings systematically replicated the results of Experiment 1 by demonstrating that IRT distributions for VR and VI schedules differ under yoked multiple schedules as well as under schedules presented in isolation.
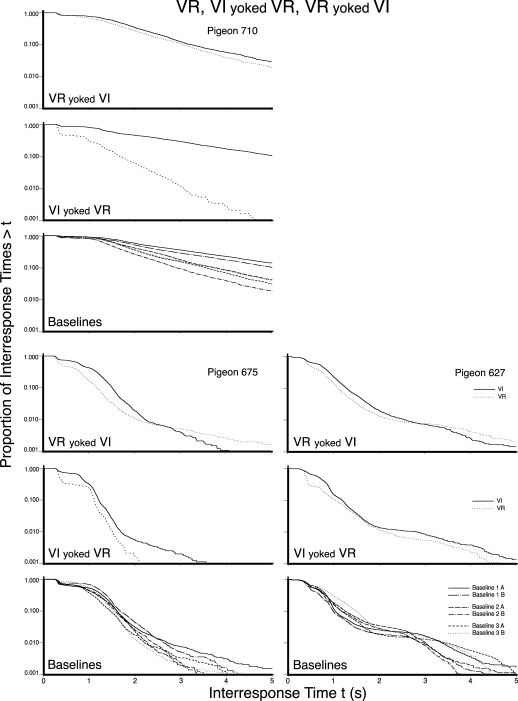
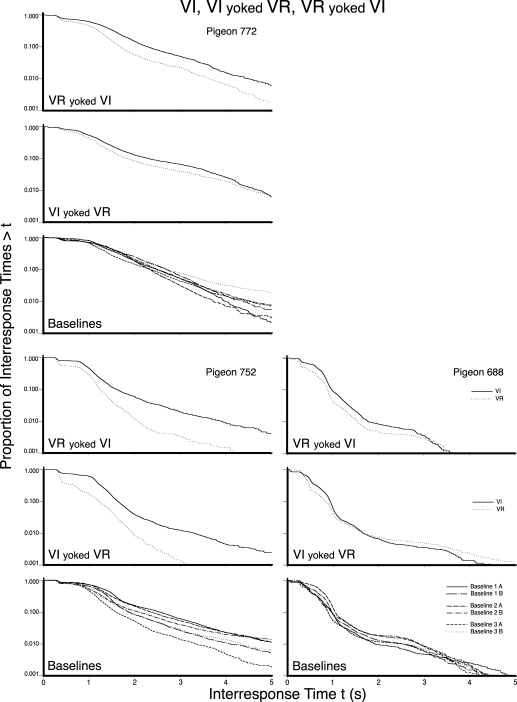
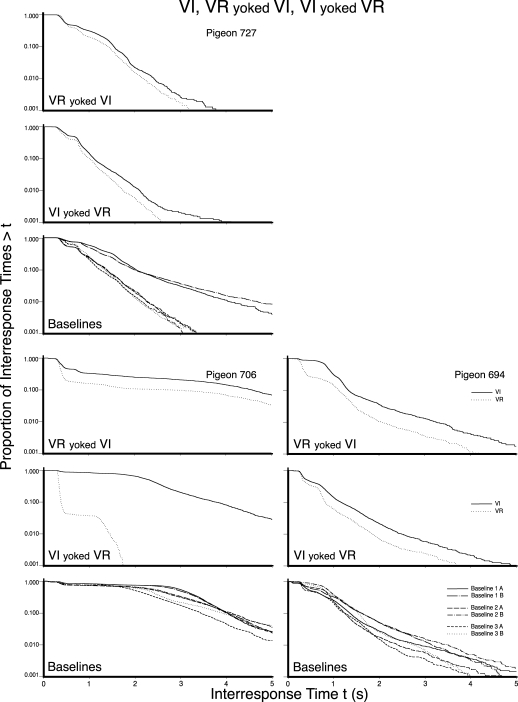
Figures 5, 6, and 7 present the log survivor plots of the behavior for the 9 pigeons under the three schedules of Experiment 2. Each set of three frames presents the data for an individual pigeon. The VR pretraining group and the two counterbalanced presentation order groups (i.e., Groups 1, 2, and 3) are provided in Figures 5, 6, and 7, respectively. The top frame in each set presents the data obtained when the VI schedule was yoked to the VR IRIs (VR yoked VI), while the middle frame presents the data obtained when the VR schedule was yoked to the VI response counts (VI yoked VR). The bottom frame in each set provides the data for the three implementations of the multiple VI 60-s VI 60-s baseline (i.e., six functions, each of which details the behavior under a VI 60-s schedule).
Fig 5.
The log survivor plots for the 3 pigeons in Experiment 2 that received VR pretraining. The top, middle, and bottom frame for each pigeon provides the results of the VR yoked VI, the VI yoked VR condition, and the three implementations of the baseline, respectively. The functions within each frame provide the data for the schedule values as indicated in the legend.
Fig 6.
The log survivor plots for the 3 pigeons in Experiment 2 that received VI pretraining. The top, middle, and bottom frame for each pigeon provides the results of the VR yoked VI, the VI yoked VR condition, and the three implementations of the baseline, respectively. The functions within each frame provide the data for the schedule values as indicated in the legend.
Fig 7.
The log survivor plots for the 3 pigeons in Experiment 2 that received VI pretraining and counterbalanced yoked conditions. The top, middle, and bottom frame for each pigeon provides the results of the VR yoked VI, the VI yoked VR condition, and the three implementations of the baseline, respectively. The functions within each frame provide the data for the schedule values as indicated in the legend.
Differences between the two functions within the upper two frames of each set provide a direct comparison of the log survivor functions obtained under a VI and a VR schedule when those two schedules were yoked. Differences between the upper and middle frame in a set of frames indicate differences in the effect of the yoking direction. Differences between the functions in the bottom frame of each set indicate the variability in the behavior controlled by a VI 60-s schedule across the components of a multiple schedule and across repeated exposures to the same schedule. Differences between sets of frames within a figure indicate individual differences. Differences between figures indicate the effects of different presentation orders.
By comparing the behavior maintained by a VI (solid line) with the behavior maintained by a VR (dotted line) in the upper two frames of each set, it is apparent that VR schedules most typically controlled responding that could be visually identified as two-state, whereas VI schedules most typically did not. There did not appear to be any systematic difference between the two pretraining conditions (VR vs. VI), nor the two presentation orders of the yoking conditions (VI yoked VR first vs. VR yoked VI first), nor yoking direction (VR yoked VI vs. VI yoked VR).
The difference in the appearance of the log survivor functions for the VR and VI schedules resulted from a combination of a change in the slope of the right limb, p(V), as well as a change in the length of the left limb, p(D). The VR schedules typically had a sharper slope, or shorter visit-initiation IRTs (right limb), and a greater proportion of within-visit IRTs as indicated by a longer left limb. When viewed from the perspective of Shull et al.'s (2001) model, the VR schedules typically resulted in both a higher probability of entering a visit and a lower probability of disengaging from a visit as compared to the VI schedules.
The absence of a carryover effect in the present results was not consistent with the carryover effect obtained in Experiment 1. This discrepancy could have resulted from either the difference in the single schedule format in Experiment 1 as opposed to the multiple schedule format in Experiment 2, or the difference in the criterion for changing phases in the two experiments. In either event, the replication of the difference in VR and VI log survivor functions while failing to find a carryover effect in Experiment 2 indicated that the differences in the IRT distributions for VR and VI schedules found in Experiment 1 were not dependent on whatever had caused the carryover effect.
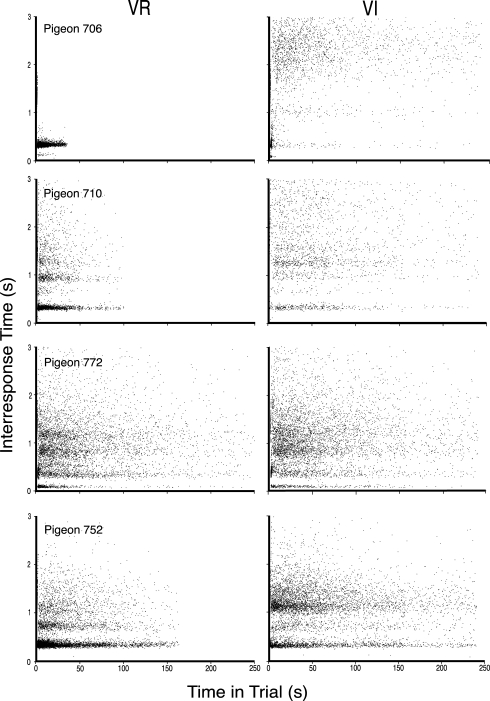
Figure 8 depicts some of the IRT distributions obtained in the present experiment under VI yoked VR schedules as dot plots of IRTs by time in the trials rather than as log survivor plots, in order to better portray the dynamics of key pecking in the 0-s to 3-s regime. These figures reveal the structure underlying the multiple points of inflection in the left portions of log survivor plots. Behavior in this regime often exhibits systematic patterns that can be attributed to the recurrent nature of pecking (Killeen et al., 2002; Palya, 1992). Palya's model suggests that, at times, pigeons peck at a constant rate but that some of those regularly spaced head movements fail to contact or operate the key. This results in IRTs at integer multiples of a base IRT value (e.g., 30, 60, 90 ms). This pattern of behavior can easily be seen when IRTs are plotted as a function of elapsed time in the interfood interval in a scatter plot format as they are in Figure 8. The base IRT and its integer multiples are seen as dense bands of dots parallel to the x axis. Further details of this conceptualization, and of dot plots, are outside the scope of the present article and are available in the original work. Cases for Figure 8 were chosen based on the difference between the two log survivor functions for a bird under a procedure.
Fig 8.
Dot plots of IRTs as a function of time in the trial for four multiple schedule procedures drawn from Experiment 2. The data from the VR component is presented on the left, while the data from the VI component is presented on the right.
The top two frames of Figure 8 present the data for the case with the most discrepant log survivor functions (Pigeon 706). The behavior under the VR schedule is shown in the left frame. It indicates that the vast majority of IRTs less than 3 s fell at virtually the same value (ca. 0.3 s) for this pigeon. There were virtually no IRTs that exceeded 2 s. This can also be seen by the precipitous drop in the log survivor function for this behavior in Figure 7. As can be seen in Figure 8, this pattern of responding resulted in few IRIs lasting more than 35 s. The upper right frame of Figure 8 depicts the data from the VI component of that multiple schedule for this bird. It also shows many IRTs at approximately 0.3 s and its third integer multiple throughout the IRI; however, the bulk of the IRTs were widely dispersed. As can be seen by referring to Figure 7, this pattern of responding resulted in a very smooth log survivor function.
The second row of frames in Figure 8 shows the data for Pigeon 710. In this case, the VR log survivor function showed a clear broken stick appearance while the VI function did not. By examining the two frames in Figure 8, it can be seen that both dot plots for this pigeon show many instances of IRTs of approximately 0.3 s. The left frame depicting the behavior under the VR schedule shows most IRTs at about 0.3 s, with an additional band at 0.9 s, but a wide variety of other values. Few trials exceeded 150 s. The VI frame on the right shows the same main band at 0.3 s and another at about 1.2 s but a majority of IRTs had substantially greater dispersion. As a result, many trials exceeded 250 s.
The third row of frames in Figure 8 provides the data for Pigeon 772 with the data for the VR schedule presented on the left. This case was chosen because the log survivor functions were among the most similar in the present experiment. The dot plots indicate high frequencies of IRTs at about 0.4, 0.8, and 1.2 s, suggesting periods of engagement, but this effect is not discernable in the log survivor functions. In any event, both the dot plots and log survivor functions are virtually identical.
The bottom pair of frames of Figure 8 illustrates the IRT structures for Pigeon 752. The VR schedule is presented on the left. This case was chosen because the log survivor functions suggest different bout lengths or durations of engagement. The left frame of Figure 8 for this bird shows a preponderance of responding at its fundamental pecking frequency, or main band, just above 0.3 s. This frame also shows that there were instances of pecks with resulting IRTs occurring at the second and third multiple. The right frame shows that the VI schedule continued to control many IRTs just above 0.3 s, but that the preponderance of IRTs shifted to the third multiple.
In sum, the results of Experiment 2 replicate Experiment 1 in that the majority of the log survivor functions for VI responding and even some of those for VR responding had no distinct left limb. Additionally, both experiments resulted in many IRT distributions with multiple inflection points attributable to recurrent pecking.
General Discussion
Shull et al. (2001) have assembled substantial evidence supporting a view that operant behavior is organized into a series of bouts of engagement separated by periods of disengagement. The original evidence was based on noting obvious changes in the log survivor plots of the IRTs. Subsequently, Shull and his colleagues changed the operant from a nose poke to a lever press (Shull & Grimes, 2003), and found that most of the IRTs when plotted as log survivor functions still appeared to be two-state, but were smoother than those based on nose poking. Because the somewhat smoother functions made the visual identification of a dichotomization point in the IRT distribution more difficult, the best sum of two exponentials was used to dichotomize behavior into within-visit and disengaged states.
In the present research, the log survivor plots for VR schedules in pigeons typically exhibited a change in slope similar to the results obtained by Shull et al. (2001) with rats. However, the VI schedules typically did not exhibit a clear change in slope. Additionally, log survivor functions resulting from both VR and VI often showed multiple inflection points.
As was illustrated in Figure 1, demonstrating that operant behavior is two-state based on visual inspection of log survivor plots can be problematic in cases where there is no single, readily identifiable change in slope in a log survivor function. Difficulties in dichotomizing behavior have been reported in other research. Podlesnick, Jiminez-Gomez, Ward, and Shahan (2006) found a general lack of IRTs below 0.2 seconds, and some functions that appeared to be best characterized by a single exponential. Similar results were reported in Bennett, Hughes, and Pitts (2007) using pigeons, as well as Kulubekova and McDowell (2007) using a computational model.
The failure to find two readily identifiable states of behavior in pigeons can plausibly be attributed to differences in the species and the experimental situations implemented. Shull (2005) analyzed an extensive data set examining the sensitivity of response rate to changes in reinforcement rate in both rats and pigeons within the context of Herrnstein's hyperbola (Herrnstein, 1970). With reinforcement rates between about 10 and 100 per hour, the behavior of rats was more sensitive to changes in reinforcement rate than the behavior of pigeons. Shull found that although maximum response rates were similar in both species, either the obtained reinforcers were more potent for pigeons, or the design of typical pigeon chambers provided less alternative reinforcers for pigeons. From this view, the uniformly high response rate of pigeons obscures the two-state nature of behavior by diminishing the ratio of within- to between-visit response rates. As a result, the log survivor plots would not show a clearly visible break. Figure 1 illustrates the effect of the ratio of within- to between-visit response rates. When the ratio is approximately 20, as it is with rats, then the change in the slope is obvious. However, when the ratio of within- to between-response rates is 5 or less, as it is with pigeons, then the break is difficult to identify.
Davison (2004) argued that differences in the ease of partitioning IRT distributions of rats and pigeons could be attributed to differences in the interaction of the feeding characteristics of the species and the precise topographical aspects of the operant (Timberlake, Pecoraro, & Tinsley, 2000). The pigeon's key peck may be optimal for examining the temporal structures of behaviors due to its relative frequency and lightness. In contrast, some of the typical operanda used for rats may be more useful for examining the defined response itself. This view is consistent with smoother log survivor functions for lever press than for nose poke responses in rats (Shull & Grimes, 2003).
In either case, the present results are consistent with findings that suggest that Shull et al.'s (2001) analytical techniques may not necessarily be reliably generalized to key pecks in pigeons. Either the two-state model may not be applicable to all operant situations, or the pigeon key peck preparation is suboptimal for the task of understanding the visit/disengagement structure of operant behavior in the absence of a more powerful analytical strategy.
A sophisticated analytical approach to fitting a double exponential to obtained IRT distributions has been proposed by Kessel and Lucke (2008). They reframed Shull's model into a quantitative form in order to enable an objective dichotomization of IRTs into an engaged and a disengaged state, even in the absence of visually apparent changes in the log survivor function. It should be noted, however, that whereas fitting a double exponential to smooth functions establishes the location of the breakpoint, if in fact behavior is correctly characterized as a double exponential, it does not establish that characterization as correct (Shull, 2004).
A second impediment to the straightforward dichotomization of operant behavior was noted by Davison (2004), following his detailed analysis of IRT structures in three concurrent choice procedures. Log survivor functions for some of the conditions analyzed showed a broken-stick appearance, but closer inspection revealed multiple inflections at short IRT values, making both the determination of the correct dichotomization of the behavior and its interpretation problematic. Those seemingly minor wavelets that fell between 0.1 and 1.0 on the logarithmically scaled y axis represented 90% of the obtained IRTs and therefore were a substantial portion of the obtained behavior. Davison suggested that the multiple breakpoints typically found in log survivor functions may imply that behavior is multi-state rather than two-state. However, the multiple points of inflection at the lower IRT values that occurred in Davison's data, as well as in the present research, are consistent with Palya's recurrent pecking model (Killeen, et al., 2002; Palya, 1992) and are not necessarily inconsistent with the view that operant behavior is two-state.
Acknowledgments
The authors gratefully acknowledge the contributions of Robert Kessel whose tireless efforts were essential for the successful completion of the manuscript; Donald Walter and Stephen Pickford for data analysis and discussions; and Elizabeth Palya for contributions in all phases of this research. Contact Donald Walter (walter@jsu.edu) for access to raw data files. An earlier version of this paper was presented at the annual conference of the Association for Behavior Analysis (2003). That version along with additional material not included in the present paper is available at http://sebac.psychology.org /VR-VI-IRT.
References
- Bennett J.A, Hughes C.E, Pitts R.C. Effects of methamphetamine on response rate: A microstructural analysis. Behavioural Processes. 2007;75:199–205. doi: 10.1016/j.beproc.2007.02.013. [DOI] [PubMed] [Google Scholar]
- Davison M. Interresponse times and the structure of choice. Behavioural Processes. 2004;66:173–187. doi: 10.1016/j.beproc.2004.03.003. [DOI] [PubMed] [Google Scholar]
- Fleshler M, Hoffman H.S. A progression for generating variable-interval schedules. Journal of the Experimental Analysis of Behavior. 1962;5:529–530. doi: 10.1901/jeab.1962.5-529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert T.F. Fundamental dimensional properties of the operant. Psychological Review. 1958;65:272–282. doi: 10.1037/h0044071. [DOI] [PubMed] [Google Scholar]
- Herrnstein R.J. On the law of effect. Journal of the Experimental Analysis of Behavior. 1970;13:243–266. doi: 10.1901/jeab.1970.13-243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessel R, Lucke R. An analytic form for the response rate analysis of Shull, Gaynor, and Grimes with applications and extensions. Journal of the Experimental Analysis of Behavior. 2008;90:363–386. doi: 10.1901/jeab.2008.90-363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killeen P.R, Hall S.S, Reilly M.P, Kettle L.C. Molecular analyses of the principal components of response strength. Journal of the Experimental Analysis of Behavior. 2002;78:127–160. doi: 10.1901/jeab.2002.78-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulubekova S, McDowell J.J. A computational model of selection by consequences: Log survivor plots. Behavioural Processes. 2007;78:291–296. doi: 10.1016/j.beproc.2007.12.005. [DOI] [PubMed] [Google Scholar]
- McDowell J.J, Wixted J.T. Variable-ratio schedules as variable-interval schedules with linear feedback loops. Journal of the Experimental Analysis of Behavior. 1986;46:315–329. doi: 10.1901/jeab.1986.46-315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palya W.L. Dynamics in the fine structure of schedule-controlled behavior. Journal of the Experimental Analysis of Behavior. 1992;57:267–287. doi: 10.1901/jeab.1992.57-267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palya W.L, Allan R.W. Dynamical concurrent schedules. Journal of the Experimental Analysis of Behavior. 2003;79:1–20. doi: 10.1901/jeab.2003.79-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palya W.L, Walter D.E. A powerful, inexpensive experiment controller or IBM PC interface and experiment control language. Behavior Research Methods, Instruments, & Computers. 1993;25:127–136. [Google Scholar]
- Palya W.L, Walter D.E. Rate of a maintained operant as a function of temporal position within a session. Animal Learning & Behavior. 1997;25:291–300. [Google Scholar]
- Podlesnick C.A, Jimenez-Gomez C, Ward R.D, Shahan T.A. Resistance to change of responding maintained by unsignaled delays to reinforcement: A response bout analysis. Journal of the Experimental Analysis of Behavior. 2006;85:329–347. doi: 10.1901/jeab.2006.47-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shull R.L. Bouts of responding on variable-interval schedules: Effects of deprivation level. Journal of the Experimental Analysis of Behavior. 2004;81:155–167. doi: 10.1901/jeab.2004.81-155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shull R.L. The sensitivity of response rate to the rate of variable-interval reinforcement for pigeons and rats: A review. Journal of the Experimental Analysis of Behavior. 2005;84:99–110. doi: 10.1901/jeab.2005.03-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shull R.L, Gaynor S.T, Grimes J.A. Response rate viewed as engagement bouts: Effects of relative reinforcement and schedule type. Journal of the Experimental Analysis of Behavior. 2001;75:247–274. doi: 10.1901/jeab.2001.75-247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shull R.L, Grimes J.A. Bouts of responding from variable-interval reinforcement of lever pressing by rats. Journal of the Experimental Analysis of Behavior. 2003;80:159–171. doi: 10.1901/jeab.2003.80-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shull R.L, Grimes J.A, Bennett J.A. Bouts of responding: The relation between bout rate and the rate of variable-interval reinforcement. Journal of the Experimental Analysis of Behavior. 2004;81:65–83. doi: 10.1901/jeab.2004.81-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staddon J.E.R, Simmelhag V.L. The “superstition” experiment: A reexamination of its implications for the principles of adaptive behavior. Psychological Review. 1971;78:3–43. [Google Scholar]
- Timberlake W, Pecoraro N, Tinsley M. An integrative approach to the modeling of behavior. Behavioral and Brain Sciences. 2000;23:268. [Google Scholar]
- Tryon W.W. A simplified time-series analysis for evaluating treatment interventions. Journal of Applied Behavior Analysis. 1982;15:423–429. doi: 10.1901/jaba.1982.15-423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young L.C. On randomness in ordered sequences. Annals of Mathematical Statistics. 1941;12:293–300. [Google Scholar]