Abstract
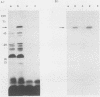
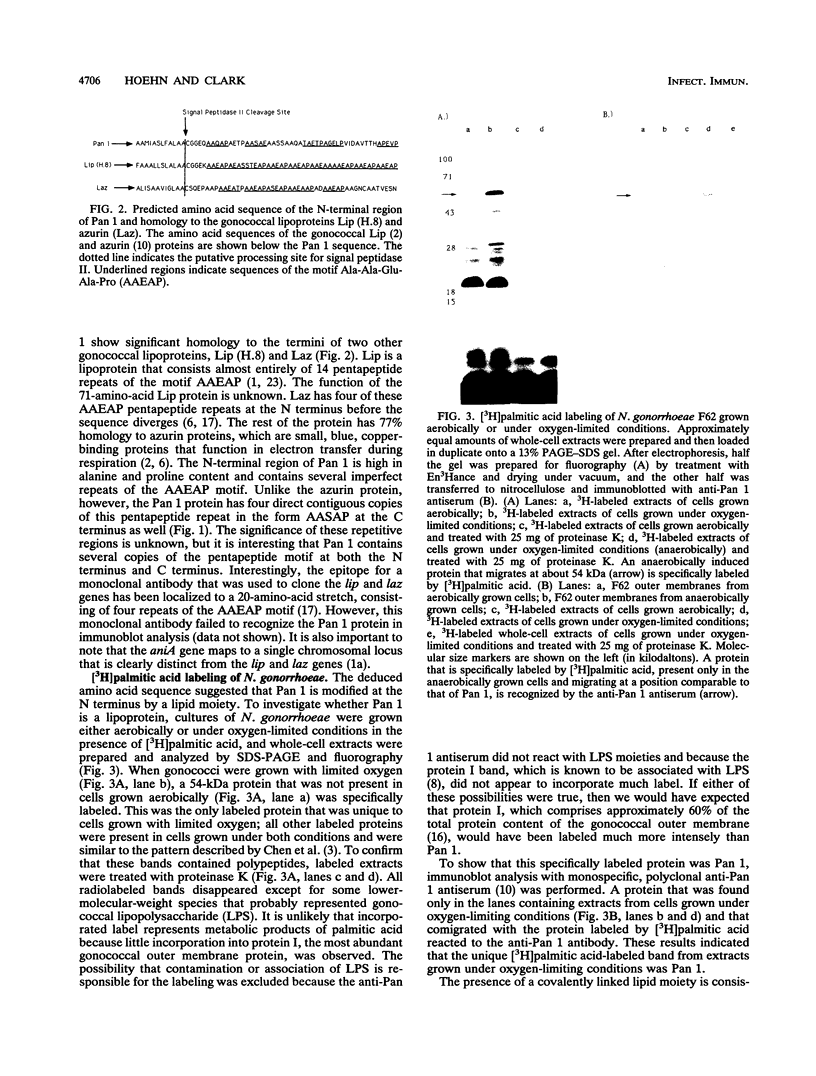
Pan 1 is an acidic outer membrane protein of Neisseria gonorrhoeae that is expressed only when gonococci are grown anaerobically. On silver-stained sodium dodecyl sulfate-polyacrylamide gels, Pan 1 migrates as an intense but diffuse 54-kDa protein. The deduced amino acid sequence of Pan 1 from the aniA (anaerobically induced protein) open reading frame reveals a lipoprotein consensus sequence, Ala-Leu-Ala-Ala-Cys, and a processed molecular mass of 39 kDa. Furthermore, there is strong homology at the N terminus and C terminus of Pan 1 to the termini of the gonococcal outer membrane lipoproteins Lip and Laz. [3H]palmitic acid labeling of gonococci grown under oxygen-limited conditions demonstrated specific incorporation of label into Pan 1, suggesting further that Pan 1 is a lipoprotein.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baehr W., Gotschlich E. C., Hitchcock P. J. The virulence-associated gonococcal H.8 gene encodes 14 tandemly repeated pentapeptides. Mol Microbiol. 1989 Jan;3(1):49–55. doi: 10.1111/j.1365-2958.1989.tb00103.x. [DOI] [PubMed] [Google Scholar]
- Cannon J. G. Conserved lipoproteins of pathogenic Neisseria species bearing the H.8 epitope: lipid-modified azurin and H.8 outer membrane protein. Clin Microbiol Rev. 1989 Apr;2 (Suppl):S1–S4. doi: 10.1128/cmr.2.suppl.s1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark V. L., Campbell L. A., Palermo D. A., Evans T. M., Klimpel K. W. Induction and repression of outer membrane proteins by anaerobic growth of Neisseria gonorrhoeae. Infect Immun. 1987 Jun;55(6):1359–1364. doi: 10.1128/iai.55.6.1359-1364.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark V. L., Knapp J. S., Thompson S., Klimpel K. W. Presence of antibodies to the major anaerobically induced gonococcal outer membrane protein in sera from patients with gonococcal infections. Microb Pathog. 1988 Nov;5(5):381–390. doi: 10.1016/0882-4010(88)90038-1. [DOI] [PubMed] [Google Scholar]
- Hayashi S., Wu H. C. Lipoproteins in bacteria. J Bioenerg Biomembr. 1990 Jun;22(3):451–471. doi: 10.1007/BF00763177. [DOI] [PubMed] [Google Scholar]
- Hitchcock P. J. Analyses of gonococcal lipopolysaccharide in whole-cell lysates by sodium dodecyl sulfate-polyacrylamide gel electrophoresis: stable association of lipopolysaccharide with the major outer membrane protein (protein I) of Neisseria gonorrhoeae. Infect Immun. 1984 Oct;46(1):202–212. doi: 10.1128/iai.46.1.202-212.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hitchcock P. J., Hayes S. F., Mayer L. W., Shafer W. M., Tessier S. L. Analyses of gonococcal H8 antigen. Surface location, inter- and intrastrain electrophoretic heterogeneity, and unusual two-dimensional electrophoretic characteristics. J Exp Med. 1985 Dec 1;162(6):2017–2034. doi: 10.1084/jem.162.6.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoehn G. T., Clark V. L. Distribution of a protein antigenically related to the major anaerobically induced gonococcal outer membrane protein among other Neisseria species. Infect Immun. 1990 Dec;58(12):3929–3933. doi: 10.1128/iai.58.12.3929-3933.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoehn G. T., Clark V. L. Isolation and nucleotide sequence of the gene (aniA) encoding the major anaerobically induced outer membrane protein of Neisseria gonorrhoeae. Infect Immun. 1992 Nov;60(11):4695–4703. doi: 10.1128/iai.60.11.4695-4703.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y. X., Ching G., Inouye M. Comparison of the lipoprotein gene among the enterobacteriaceae. DNA sequence of Morganella morganii lipoprotein gene and its expression in Escherichia coli. J Biol Chem. 1983 Jul 10;258(13):8139–8145. [PubMed] [Google Scholar]
- Hussain M., Ichihara S., Mizushima S. Accumulation of glyceride-containing precursor of the outer membrane lipoprotein in the cytoplasmic membrane of Escherichia coli treated with globomycin. J Biol Chem. 1980 Apr 25;255(8):3707–3712. [PubMed] [Google Scholar]
- Hussain M., Ichihara S., Mizushima S. Mechanism of signal peptide cleavage in the biosynthesis of the major lipoprotein of the Escherichia coli outer membrane. J Biol Chem. 1982 May 10;257(9):5177–5182. [PubMed] [Google Scholar]
- Judd R. C. Protein I: structure, function, and genetics. Clin Microbiol Rev. 1989 Apr;2 (Suppl):S41–S48. doi: 10.1128/cmr.2.suppl.s41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawula T. H., Spinola S. M., Klapper D. G., Cannon J. G. Localization of a conserved epitope and an azurin-like domain in the H.8 protein of pathogenic Neisseria. Mol Microbiol. 1987 Sep;1(2):179–185. doi: 10.1111/j.1365-2958.1987.tb00510.x. [DOI] [PubMed] [Google Scholar]
- Knapp J. S., Clark V. L. Anaerobic growth of Neisseria gonorrhoeae coupled to nitrite reduction. Infect Immun. 1984 Oct;46(1):176–181. doi: 10.1128/iai.46.1.176-181.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller J. F., Mekalanos J. J., Falkow S. Coordinate regulation and sensory transduction in the control of bacterial virulence. Science. 1989 Feb 17;243(4893):916–922. doi: 10.1126/science.2537530. [DOI] [PubMed] [Google Scholar]
- Pugsley A. P., Chapon C., Schwartz M. Extracellular pullulanase of Klebsiella pneumoniae is a lipoprotein. J Bacteriol. 1986 Jun;166(3):1083–1088. doi: 10.1128/jb.166.3.1083-1088.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods J. P., Dempsey J. F., Kawula T. H., Barritt D. S., Cannon J. G. Characterization of the neisserial lipid-modified azurin bearing the H.8 epitope. Mol Microbiol. 1989 May;3(5):583–591. doi: 10.1111/j.1365-2958.1989.tb00205.x. [DOI] [PubMed] [Google Scholar]
- Woods J. P., Spinola S. M., Strobel S. M., Cannon J. G. Conserved lipoprotein H.8 of pathogenic Neisseria consists entirely of pentapeptide repeats. Mol Microbiol. 1989 Jan;3(1):43–48. doi: 10.1111/j.1365-2958.1989.tb00102.x. [DOI] [PubMed] [Google Scholar]