Abstract
Doxorubicin is one of the most effective chemotherapeutic agents; however, it causes dose-dependent cardiomyopathy that may lead to heart failure. Conventional measures of ventricular function, such as fractional shortening, are insensitive in detecting early doxorubicin cardiomyopathy. We tested whether novel 2-dimensional radial strain echocardiography (2DSE) can detect early doxorubicin injury following chronic administration in a rat model. 14 male Sprague Dawley rats (240−260 g) received doxorubicin 2.5 mg/k IV per week for 10 (n=4) or 12 weeks (n=10); 17 controls received saline (10 weeks, n=7 and 12 weeks, n=10). Serial 2DSE from 0−12 weeks was done at the mid left ventricle using Vivid 7 echo (General Electric, Waukesha, WI, USA). With Q analysis software, radial strain was obtained. From the 2D image, anatomical M-mode through the anterior/inferior walls was used to measure fractional shortening. Fibrosis (Masson's trichrome) and caspase-3 activity were measured from excised hearts. Radial strain was lower in the doxorubicin group (12 week: 26.7±3 vs. 38.3±2.6%, p=0.006), with significant difference by 8 weeks whereas fractional shortening was lower with doxorubicin only after 12 weeks (30.2±1.7 vs. 37.6±1.4%, p=0.02). Doxorubicin group had lower cardiac mass (0.85±0.09 vs. 1.14±0.04 g, p=0.001), higher caspase-3 activity (1.95±0.2 fold increase over control, p<0.0001) and fibrosis (3.9±0.7 vs. 0.7±0.1%, p=0.005). Radial strain was related directly to cardiac mass (R=0.61, p=0.0007) and inversely to caspase-3 activity (R=−0.5, p=0.005). 2-dimensional radial strain echocardiography is useful in the early detection of doxorubicin cardiac injury and the reduction in radial strain is associated with histologic markers of doxorubicin cardiomyopathy.
Keywords: echocardiography, cardiomyopathy, heart failure, ventricular function, apoptosis
INTRODUCTION AND LITERATURE
Doxorubicin is one of the most effective chemotherapeutic agents but its use is limited by development of dose-dependent cardiomyopathy involving cardiomyocyte apoptosis and myocardial fibrosis that may lead to heart failure (Minotti, Menna 2004). Doxorubicin causes cardiomyocyte apoptosis by reactive oxygen species-dependent mechanism, a pathway distinct from apoptosis induced by doxorubicin in tumor cells (Wang, Konorev 2004). Histologic changes in the myocardium may occur as early as the second cycle of chemotherapy and is linearly related to dose (Elbl, Hrstkova 2003). The development of cardiomyopathy impacts further chemotherapy and has implications in the medium and long term prognoses of patients. Current practice relies on evaluation of left ventricular systolic function using ejection fraction or fractional shortening by echocardiography or nuclear ventriculography to detect development of cardiomyopathy, but these are insensitive and unreliable markers of early doxorubicin injury (McKillop, Bristow 1983, Tjeerdsma, Meinardi 1999). An easy, rapid, sensitive and reliable method of earlier detection of doxorubicin injury has potential clinical impact in the assessment and therapeutic decision-making in these patients. 2-dimensional strain echocardiography (2DSE) is a novel method of assessment of myocardial function that is based on measurement of myocardial deformation using speckle-tracking from B mode images. It was shown recently that radial strain (RS) measurement derived from 2DSE provided enhanced sensitivity and specificity in detecting myocardial viability and stunning following ischemia-reperfusion in an animal model (Migrino, Zhu 2007), and 2DSE provided incremental benefit to conventional measures of regional function in the assessment of human ischemic heart disease (Becker, Bilke 2006, Reisner, Lysyansky 2004). Our aim is to determine if radial strain (RS) derived from 2DSE can detect doxorubicin-induced cardiomyopathy, and compare the timing of detection of cardiomyopathy with fractional shortening, a conventional measure of ventricular function.
MATERIALS AND METHODS
Animal Subjects
Fourteen male Sprague-Dawley rats (240−260 g) were given intravenous doxorubicin 2.5 mg/kg weekly through the tail vein. Four rats had planned sacrifice at 10 weeks (Figure 1). Of the remaining 10 rats, 2 rats died before final imaging at 12 weeks and 12 week data was available on 8 rats. 17 control rats were given intravenous saline weekly. One rat died at 10 weeks. Six rats had planned sacrifice at 10 weeks. The remaining 10 rats were sacrificed at 12 weeks. The animals were sedated and anesthetized using 0.25 mg/kg of medetomide and 75 mg/kg of ketamine. The protocol was approved by the local Institutional Animal Care Committee. The investigation conforms to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85−23, revised 1996).
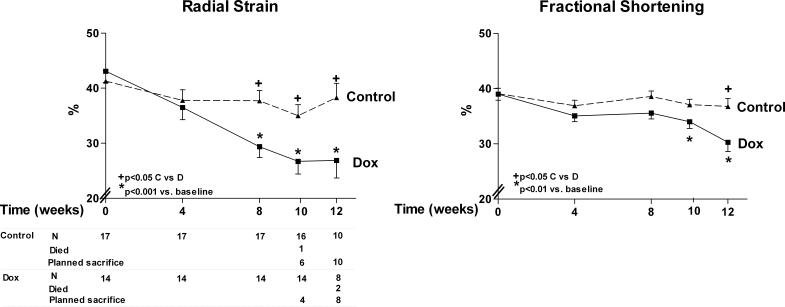
Figure 1.
Radial strain and fractional shortening by time in doxorubicin-treated rats and controls. A. Radial strain is lower in doxorubicin versus control group by 8 weeks. B. Fractional shortening is lower in doxorubicin versus control group by 12 weeks. The number of surviving animals and animals that died or sacrificed at each time point is at the bottom of graph A.
Echocardiography
A Vivid 7 echocardiograph (General Electric, Waukesha, Wisconsin, USA) was used together with M12L high frequency (11 MHz) linear array transducer. The parasternal short axis view at the mid left ventricular level was used, verified by the presence of prominent papillary muscles. Image depth was 2−2.5 cm with 256 frames per second acquisition, second harmonic imaging and with electrocardiographic gating. Images were acquired at baseline, 4, 8, 10 and 12 weeks.
Image analysis
The images were transferred to the EchoPACS workstation with Q analysis software Version 4.0.3 (General Electric, Waukesha, Wisconsin, USA) for processing. Each cardiac cycle was defined from the peak of the R wave to the following R wave. The method has been previously described (Becker, Bilke 2006, Leitman, Lysyansky 2004, Migrino, Zhu 2007, Notomi, Lysyansky 2005, Reisner, Lysyansky 2004). The endocardial border was traced in an endsystolic frame. The software automatically selected six equally-spaced tissue tracking regions of interest in the myocardium between the endocardium and epicardium. The outer border was adjusted to approximate the epicardial region. The software then selects suitable stable acoustic objects for tracking, searches for them in the next frame by using absolute differences algorithm and provides a tracking score that reflects the degree of decorrelation of the block-matching (ranging from 1, excellent to 3, poor). There is an assumption that the natural acoustic markers change position from frame to frame in conjunction with motion of surrounding tissue (Becker, Bilke 2006, Leitman, Lysyansky 2004). The computer provides a profile of RS with time. On parasternal short axis view, RS is deformation towards the left ventricular center. Peak RS for each region was measured and averaged to obtain a global RS that was used for analysis. Fractional shortening (%) was calculated from 2D images in short axis by using anatomical M-mode feature of the Vivid 7 echo. Using this feature, an M-mode display was generated from raw data 2D images with the line selected passing through the anterior and inferior segments. The formula for fractional shortening was: enddiastolic dimension (EDD)-endsystolic dimension (ESD)/EDD × 100%. For every measurement, three consecutive heartbeats were measured and the average used for analyses. The measurements were performed by a cardiologist (RQM) who was blinded to treatment assignment.
Histology
Hearts were explanted at 10 (n=10) or 12 weeks (n=21), weighed and a section of mid ventricle was fixed in zinc-formalin solution for 24 hours. Cardiac tissues were then stained with Masson's trichrome, photographed using Nikon E400 microscope with SPOT Insight (Diagnostic Instrument, Inc., Sterling Heights, Michigan, USA). The area of fibrosis was measured using Metamorph 7.0 software (Universal Imaging, Inc., Downington, Pennsylvania, USA) as a percentage of myocardial area. The remainder of the heart was homogenized in lysate buffer (1% triton buffer). Homogenate was incubated with highly specific caspase-3 substrate Ac (N-acetyl)-DEVD-7-amino-4-trifluoromethylcoumarin (BD Biosciences Pharmingen, San Jose, California, USA) at 37°C for 2h. Liberated 7-amino-4-trifluoromethylcoumarin (AFC) was measured using a fluorescence plate reader (Perkin Elmer Life and Analytical Sciences, Sheldon, Connecticut, USA) with λex = 440nm and λem = 505nm. Liberated fluorescence was normalized to tissue lysate protein measured with bicinchoninic protein assay kit (PierceBiotechnology Inc., Rockford, Illinois, USA). In a separate but related experiment, male Sprague-Dawley rats were sedated and anesthetized in the same manner, given doxorubicin at 2.5 mg/kg per week I.V. and sacrificed at 2, 4, 6, and 8 weeks (n=3 each) with control rats receiving saline and sacrificed at the same time points (n=3 each for 2 and 4 weeks, n=4 for 6 weeks and n=5 for 8 weeks) for evaluation of caspase-3 level at different time points.
Data Analyses and Statistics
Data are expressed as means±standard deviation. Radial strain and fractional shortening were analyzed by two way repeated measures analysis of variance with time and treatment as variables using SigmaStat 3.5 (Systat Software Inc., San Jose, California, USA). Post-hoc analysis was performed using Holm-Sidak method. Left ventricular mass, area of fibrosis and caspase-3 activity were compared using Student's t-test; t- test with Welch's correction was used for unequal variances. Correlation analysis was performed using Pearson's correlation. A significant p value was set at 0.05. In six randomly selected rats, radial strain from different treatment times was measured independently by 2 observers blinded to treatment assignment. The first observer repeated the measurements at a later time point blinded to previous readings. Intraobserver and interobserver variability was calculated as the absolute difference between observations in percent of their mean.
RESULTS
The heart rates of the rats did not differ between control and doxorubicin groups at any time point (baseline control vs. doxorubicin: 247± 9 vs. 246±22; 4 weeks: 232±9 vs. 222±12; 8 weeks: 235±9 vs. 223±22; 10 weeks: 224±9 vs. 209±24 and 12 weeks: 202±12 vs. 184±26 beats per minute, all p=NS by group and by time).
Doxorubicin treated rats had reduced radial strain (12 week: 26.7±3 vs. 38.3±2.6%, p=0.006) (Figures 1 and 2). There was progressive and significant reduction compared with control rats by 8 weeks of treatment (Figure 1). Fractional shortening, on the other hand, showed significant decrease in the doxorubicin group, but after 12 weeks of treatment compared to controls (12 week: 30.2±1.7 vs. 37.6±1.4%, p=0.02). Control rats did not show any significant serial change in both radial strain and fractional shortening from baseline.
Figure 2.
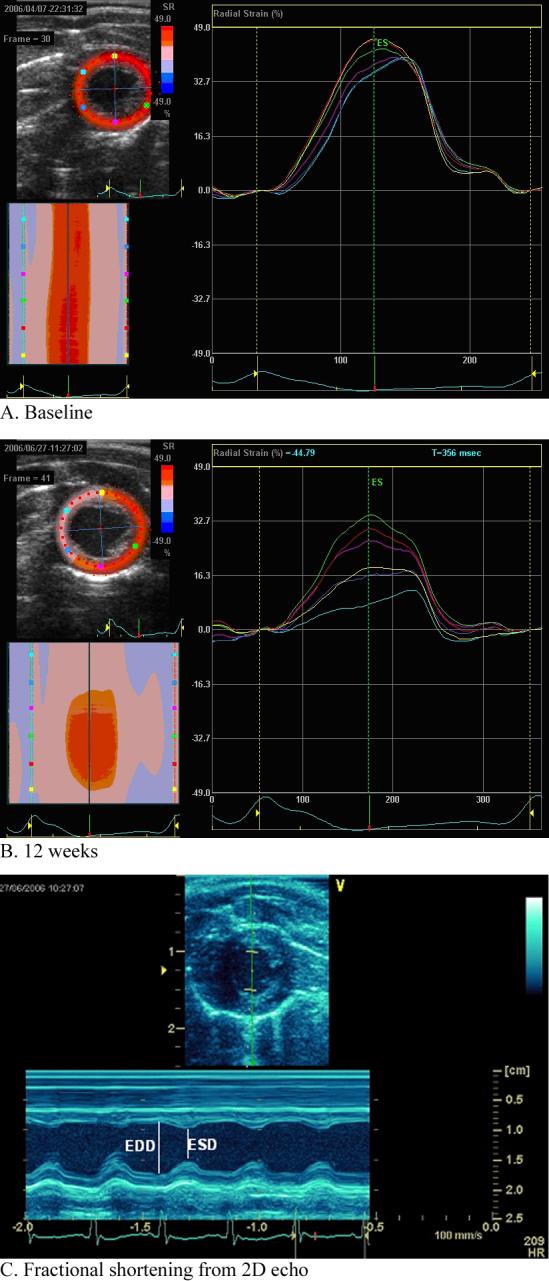
A. 2 dimensional radial strain echo analysis in a doxorubicin treated rat at baseline. Left upper panel shows a parametric image color map of peak radial strain at the midventricular level with scale shown to its immediate right. On the left lower panel, the parametric image is reconstructed to show radial strain by time in the cardiac cycle (x axis) among the different regions (y axis, coded by color). On the right panel is the strain by time in the cardiac cycle in the 6 regions of the myocardium. Global radial strain is calculated as the mean of the regional radial strain. B. 2 dimensional radial strain echo analysis in a doxorubicin treated rat at 12 weeks. Following doxorubicin treatment, there is both a regional and global reduction in radial strain. C. Fractional shortening. Fractional shortening was obtained from short axis 2D images using anatomical M mode through the anterior and inferior segments. Fractional shortening was calculated as: End-diastolic diameter (EDD)-End-systolic diameter (ESD)/EDD × 100%.
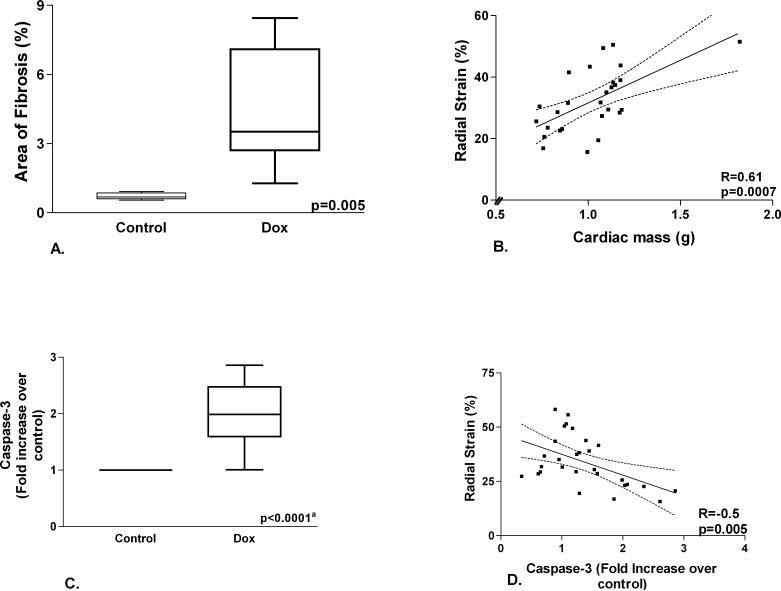
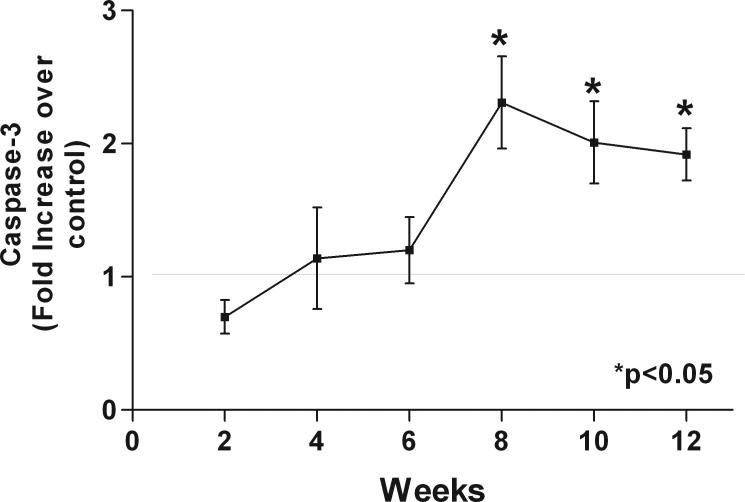
The hearts of doxorubicin treated rats showed increased interstitial and perivascular fibrosis (Figure 3). The area of fibrosis is higher in doxorubicin treated rats versus controls (Figure 4A). Cardiac mass was significantly reduced in the doxorubicin group (0.85±0.09 vs. 1.14±0.04 g, p=0.001). Caspase-3 activity was higher in the doxorubicin group (Figure 4C). Radial strain is directly related to cardiac mass (Figure 4B) and inversely related to caspase-3 (Figure 4D). Caspase-3 activity was significantly higher in the doxorubicin group compared to controls starting at 8 weeks of treatment (Figure 5).
Figure 3.
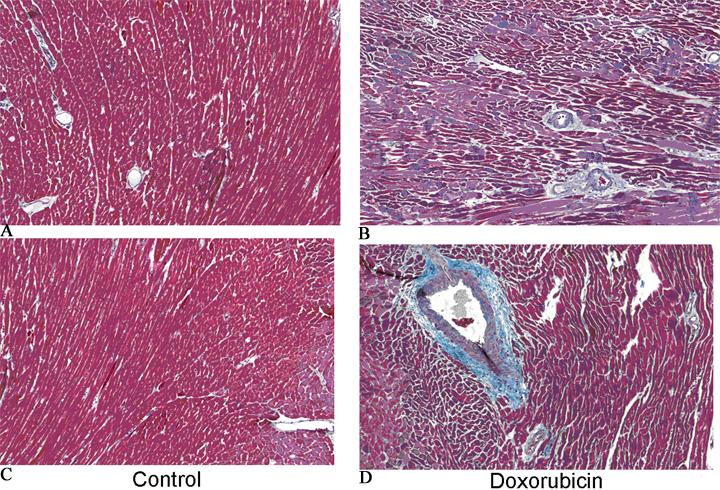
Masson's trichrome stain at the mid ventricular level in a control rat (A and C) and a doxorubicin treated rat (B and D). There is prominent interstitial and perivascular fibrosis (blue stain) in the doxorubicin rat.
Figure 4.
A. Area of fibrosis. There is greater area of myocardial fibrosis in doxorubicin versus control rats. B. Left ventricular mass and radial strain. There is a significant correlation between left ventricular mass and radial strain. C. Caspase-3 level. There is higher caspase-3 level in doxorubicin versus control rats (a-comparison of fluorescence/mg protein between doxorubicin and control rats). D. Caspase-3 level and radial strain. There is an inverse relationship between radial strain and caspase-3 level.
Figure 5.
Caspase-3 level in hearts of doxorubicin treated rats. Caspase-3 level starts to significantly increase over controls following 8 weeks of doxorubicin treatment.
In a randomly selected subset, intraobserver variability was 4.6% (observer 1: 46.7±12.7% versus 47.2±13.6%, R=0.98, p<0.0001) while interobserver variability was 10.3% (observer 2: 44.0±13.4%, versus observer 1 R=0.91, p<0.0001).
DISCUSSION AND SUMMARY
Doxorubicin is one of the most effective chemotherapeutic agents against malignancies such as breast and prostate cancers and childhood leukemia (Iarussi, Indolfi 2005), but its use is associated with dose-dependent development of cardiomyopathy (Elbl, Hrstkova 2003). Myocardial injury is caused by induction of myocyte apoptosis (Kalyanaraman, Joseph 2002, Konorev, Kotamraju 2002, Kotamraju, Kalivendi 2004, Kotamraju, Konorev 2000, Wang, Konorev 2004, Wang, Kotamraju 2002), but other mechanisms play a role such as lipid peroxidation, inhibition of nucleic acid and protein synthesis, release of vasoactive amines, changes in adrenergic function, abnormalities in calcium handling, reduced expression of specific genes, impairment of mitochondrial creatinine kinase and induction of nitric oxide synthase (Minotti, Menna 2004). The development of cardiomyopathy may lead to heart failure and preclude further treatment with doxorubicin. Furthermore, it has been demonstrated that doxorubicin causes myocyte injury early in the course of treatment with the first changes occurring as early as the second cycle of chemotherapy (Elbl, Hrstkova 2003). Thus, even with use of cumulative doses of less than 450 mg/m2, delayed cardiotoxicity manifesting more than a year following termination of treatment is important, especially in the pediatric and young adult population (Elbl, Hrstkova 2003, Giantris, Abdurrahman 1998, Lipshultz, Lipsitz 2005, Steinherz, Steinherz 1991). Subclinical impairment of left ventricular function has been reported in more than 50% of patients in the first decade following chemotherapy with clinical symptoms occurring in 5−19% of patients (Elbl, Hrstkova 2003, Grenier and Lipshultz 1998, Kremer, van der Pal 2002, Nysom, Holm 1998). The early detection of subclinical cardiomyopathy is therefore relevant for chemotherapeutic decisions, prognostication and follow up care.
The most commonly used method of detecting doxorubicin cardiomyopathy in the clinical setting is evaluation of left ventricular systolic function by measurement of ejection fraction or fractional shortening, either by nuclear methods (multigated radionuclide ventriculography or MUGA) or by echocardiography. However, it has been demonstrated that left ventricular ejection fraction and fractional shortening are insensitive to detection of doxorubicin cardiomyopathy (McKillop, Bristow 1983, Tjeerdsma, Meinardi 1999). Other measures were found useful in earlier detection of doxorubicin cardiomyopathy such as indices of diastolic dysfunction (Marchandise, Schroeder 1989, Stoddard, Seeger 1992), but considerable individual variability limits practical applicability in the clinical setting (Bu'Lock, Mott 1999)
2-dimensional strain echocardiography is a rapid, easy and objective way of measuring myocardial function. Strain is a measure of myocardial deformation, which is an intrinsic mechanical property. Myocardial strain derived by tagged magnetic resonance imaging (MRI) (Garot, Lima 2004), by Doppler echocardiography (Hanekom, Jenkins 2005) and by 2DSE (Reisner, Lysyansky 2004) have incremental value to conventional measures of regional myocardial function in ischemic heart disease. Our group recently demonstrated that 2DSE is a sensitive and specific means of detecting myocardial viability and stunning in a rat ischemia-reperfusion model, and was superior to regional thickening by 2D and M-mode echocardiography (Migrino, Zhu 2007). 2DSE is based on tracking of natural acoustic markers formed by speckle patterns arising from interference of ultrasound backscattered from tissue structures (Leitman, Lysyansky 2004, Notomi, Lysyansky 2005, Reisner, Lysyansky 2004). There is an assumption that the motion of these speckles in the image represents the motion of the corresponding tissue (Becker, Bilke 2006, Leitman, Lysyansky 2004). The technique provides high spatial and temporal resolution, ease of use and is not encumbered by angle dependency and translational motion (Becker, Bilke 2006, Leitman, Lysyansky 2004, Notomi, Lysyansky 2005), distinct advantages over Doppler and tagged MRI based strain assessment.
The study points to the potential application of 2DSE for detecting subclinical doxorubicin cardiac injury. Doxorubicin cardiomyopathy is associated with progressive loss of cardiac myocytes and the development of variable degrees of interstitial fibrosis (Billingham, Mason 1978, Bu'Lock, Mott 1999, Isner, Ferrans 1983, Lefrak, Pitha 1973, Mortensen, Olsen 1986). The model used in this study demonstrated the classic histologic findings of doxorubicin injury. A significant increase in apoptosis, measured by caspase-3 level, occurred following 8 weeks of repeated doxorubicin treatment, the same time interval when significant reduction in radial strain was also demonstrated, pointing to the sensitivity of the technique in detecting myocardial dysfunction related to apoptotic injury. At this time point, fractional shortening was still not significantly diminished compared to controls, pointing to the limitation of this conventional method in detecting early disease. Following 10−12 weeks of treatment, the reduction of radial strain correlated with cardiac mass and caspase-3 level, suggesting some of the potential underlying structural basis for the observed reduction in myocardial function.
The incremental value of 2DSE over conventional measures of ventricular function in the assessment of doxorubicin cardiomyopathy needs to be validated in the clinical setting, and the prognostic significance and therapeutic implications of early impairment of radial strain need to be studied in a prospective longitudinal clinical study.
The chronic doxorubicin cardiomyopathy model used in this study was patterned after an established animal model (Schwarz, Pollick 1998), that may not reflect changes associated with chronic therapeutic dosing in humans. 2-dimensional strain echocardiography relies on good quality B-mode images in order for the assumption that speckle tracking reflects underlying tissue motion to be valid. In this regard, the software has a tracking score that measures the degree of decorrelation of the block-matching (ranging from 1, excellent to 3, poor) (Becker, Bilke 2006, Leitman, Lysyansky 2004). All segments were deemed evaluable in this study, with most segments having a score of 1, and no segment with score ≥2.5. The validity of serial measurements also depends on reproducibility of the same heart section on imaging. This was a particular concern for this study, and we were careful in choosing only 1 view for serial measurements. The short axis view at the mid-ventricle demands a circular slice that ensures the plane to be orthogonal to the long axis of the ventricle; in addition, the presence of papillary muscles as well as the position of the anterior and posterior insertion of the right ventricle to the left ventricular septum provides easily reproducible anatomic landmarks. As regards temporal resolution, the use of high frequency probe allowed acquisition frame rates close to the animal's heart rate. Previous studies have validated using magnetic resonance imaging 2-dimensional based speckle tracking analysis of regional ventricular function (Becker, Bilke 2006) and rotational motion (Notomi, Lysyansky 2005) using frame rates close to the subjects' heart rate in patients with ischemic and nonischemic cardiomyopathy. Furthermore, 2D strain measurement using high frame rates (>150 frames per second) has been validated in an animal model using embedded sonomicrometry crystals (Tee, Tee 2006). In a rat ischemia-reperfusion study, there was excellent correlation between strain measured at high (230−258 fps) versus lower frame rates (136−189 and 95−105 fps) (Migrino, Zhu 2007) at heart rates similar to the current study. The intraobserver and interobserver variability reported for this study was comparable to human studies using 2D speckle tracking to assess strain (Becker, Bilke 2006) and torsion (Notomi, Lysyansky 2005).
The study showed that global radial strain derived from 2-dimensional strain echo detected early doxorubicin induced cardiomyopathy, and that the reduction in radial strain is associated with the degree and the onset of histologic indices of myocardial injury. Myocardial dysfunction was detected earlier using radial strain as compared to fractional shortening, a conventional method of assessment of ventricular function.
ACKNOWLEDGEMENTS
This work was supported in part by National Institutes of Health grant RO1 CA 77822 and Advancing a Healthier Wisconsin grant 5520053. The authors would like to acknowledge Glenn Slocum (Department of Physiology, MCW) for his assistance in histology imaging and Dan Eastwood MS (Department of Biostatistics, MCW) for his assistance in biostatistical analysis.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Becker M, Bilke E, Kuhl H, Katoh M, Kramann R, Franke A, Bucker A, Hanrath P, Hoffmann R. Analysis of myocardial deformation based on pixel tracking in two dimensional echocardiographic images enables quantitative assessment of regional left ventricular function. Heart. 2006;92:1102–8. doi: 10.1136/hrt.2005.077107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billingham ME, Mason JW, Bristow MR, Daniels JR. Anthracycline cardiomyopathy monitored by morphologic changes. Cancer Treat Rep. 1978;62:865–72. [PubMed] [Google Scholar]
- Bu'Lock FA, Mott MG, Oakhill A, Martin RP. Left ventricular diastolic filling patterns associated with progressive anthracycline-induced myocardial damage: A prospective study. Pediatr Cardiol. 1999;20:252–63. doi: 10.1007/s002469900459. [DOI] [PubMed] [Google Scholar]
- Elbl L, Hrstkova H, Chaloupka V. The late consequences of anthracycline treatment on left ventricular function after treatment for childhood cancer. Eur J Pediatr. 2003;162:690–6. doi: 10.1007/s00431-003-1275-y. [DOI] [PubMed] [Google Scholar]
- Garot J, Lima JA, Gerber BL, Sampath S, Wu KC, Bluemke DA, Prince JL, Osman NF. Spatially resolved imaging of myocardial function with strain-encoded MR: comparison with delayed contrast-enhanced MR imaging after myocardial infarction. Radiology. 2004;233:596–602. doi: 10.1148/radiol.2332031676. [DOI] [PubMed] [Google Scholar]
- Giantris A, Abdurrahman L, Hinkle A, Asselin B, Lipshultz SE. Anthracycline-induced cardiotoxicity in children and young adults. Crit Rev Oncol Hematol. 1998;27:53–68. doi: 10.1016/s1040-8428(97)10007-5. [DOI] [PubMed] [Google Scholar]
- Grenier MA, Lipshultz SE. Epidemiology of anthracycline cardiotoxicity in children and adults. Semin Oncol. 1998;25:72–85. [PubMed] [Google Scholar]
- Hanekom L, Jenkins C, Jeffries L, Case C, Mundy J, Hawley C, Marwick TH. Incremental value of strain rate analysis as an adjunct to wall-motion scoring for assessment of myocardial viability by dobutamine echocardiography: a follow-up study after revascularization. Circulation. 2005;112:3892–900. doi: 10.1161/CIRCULATIONAHA.104.489310. [DOI] [PubMed] [Google Scholar]
- Iarussi D, Indolfi P, Casale F, Martino V, Di Tullio MT, Calabro R. Anthracycline-induced cardiotoxicity in children with cancer: strategies for prevention and management. Paediatr Drugs. 2005;7:67–76. doi: 10.2165/00148581-200507020-00001. [DOI] [PubMed] [Google Scholar]
- Isner JM, Ferrans VJ, Cohen SR, Witkind BG, Virmani R, Gottdiener JS, Beck JR, Roberts WC. Clinical and morphologic cardiac findings after anthracycline chemotherapy. Analysis of 64 patients studied at necropsy. Am J Cardiol. 1983;51:1167–74. doi: 10.1016/0002-9149(83)90364-8. [DOI] [PubMed] [Google Scholar]
- Kalyanaraman B, Joseph J, Kalivendi S, Wang S, Konorev E, Kotamraju S. Doxorubicin-induced apoptosis: implications in cardiotoxicity. Mol Cell Biochem. 2002;234−235:119–24. [PubMed] [Google Scholar]
- Konorev EA, Kotamraju S, Zhao H, Kalivendi S, Joseph J, Kalyanaraman B. Paradoxical effects of metalloporphyrins on doxorubicin-induced apoptosis: scavenging of reactive oxygen species versus induction of heme oxygenase-1. Free Radic Biol Med. 2002;33:988. doi: 10.1016/s0891-5849(02)00989-9. [DOI] [PubMed] [Google Scholar]
- Kotamraju S, Kalivendi SV, Konorev E, Chitambar CR, Joseph J, Kalyanaraman B. Oxidant-induced iron signaling in Doxorubicin-mediated apoptosis. Methods Enzymol. 2004;378:362–82. doi: 10.1016/S0076-6879(04)78026-X. [DOI] [PubMed] [Google Scholar]
- Kotamraju S, Konorev EA, Joseph J, Kalyanaraman B. Doxorubicin-induced apoptosis in endothelial cells and cardiomyocytes is ameliorated by nitrone spin traps and ebselen. Role of reactive oxygen and nitrogen species. J Biol Chem. 2000;275:33585–92. doi: 10.1074/jbc.M003890200. [DOI] [PubMed] [Google Scholar]
- Kremer LC, van der Pal HJ, Offringa M, van Dalen EC, Voute PA. Frequency and risk factors of subclinical cardiotoxicity after anthracycline therapy in children: a systematic review. Ann Oncol. 2002;13:819–29. doi: 10.1093/annonc/mdf167. [DOI] [PubMed] [Google Scholar]
- Lefrak EA, Pitha J, Rosenheim S, Gottlieb JA. A clinicopathologic analysis of adriamycin cardiotoxicity. Cancer. 1973;32:302–14. doi: 10.1002/1097-0142(197308)32:2<302::aid-cncr2820320205>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Leitman M, Lysyansky P, Sidenko S, Shir V, Peleg E, Binenbaum M, Kaluski E, Krakover R, Vered Z. Two-dimensional strain-a novel software for real-time quantitative echocardiographic assessment of myocardial function. J Am Soc Echocardiogr. 2004;17:1021–9. doi: 10.1016/j.echo.2004.06.019. [DOI] [PubMed] [Google Scholar]
- Lipshultz SE, Lipsitz SR, Sallan SE, Dalton VM, Mone SM, Gelber RD, Colan SD. Chronic progressive cardiac dysfunction years after doxorubicin therapy for childhood acute lymphoblastic leukemia. J Clin Oncol. 2005;23:2629–36. doi: 10.1200/JCO.2005.12.121. [DOI] [PubMed] [Google Scholar]
- Marchandise B, Schroeder E, Bosly A, Doyen C, Weynants P, Kremer R, Pouleur H. Early detection of doxorubicin cardiotoxicity: interest of Doppler echocardiographic analysis of left ventricular filling dynamics. Am Heart J. 1989;118:92–8. doi: 10.1016/0002-8703(89)90077-x. [DOI] [PubMed] [Google Scholar]
- McKillop JH, Bristow MR, Goris ML, Billingham ME, Bockemuehl K. Sensitivity and specificity of radionuclide ejection fractions in doxorubicin cardiotoxicity. Am Heart J. 1983;106:1048–56. doi: 10.1016/0002-8703(83)90651-8. [DOI] [PubMed] [Google Scholar]
- Migrino RQ, Zhu X, Pajewski N, Brahmbhatt T, Hoffmann R, Zhao M. Assessment of segmental myocardial viability using regional 2-dimensional strain echocardiography. J Am Soc Echocardiogr. 2007;20:342–51. doi: 10.1016/j.echo.2006.09.011. [DOI] [PubMed] [Google Scholar]
- Minotti G, Menna P, Salvatorelli E, Cairo G, Gianni L. Anthracyclines: molecular advances and pharmacologic developments in antitumor activity and cardiotoxicity. Pharmacol Rev. 2004;56:185–229. doi: 10.1124/pr.56.2.6. [DOI] [PubMed] [Google Scholar]
- Mortensen SA, Olsen HS, Baandrup U. Chronic anthracycline cardiotoxicity: haemodynamic and histopathological manifestations suggesting a restrictive endomyocardial disease. Br Heart J. 1986;55:274–82. doi: 10.1136/hrt.55.3.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Notomi Y, Lysyansky P, Setser RM, Shiota T, Popovic ZB, Martin-Miklovic MG, Weaver JA, Oryszak SJ, Greenberg NL, White RD, Thomas JD. Measurement of ventricular torsion by two-dimensional ultrasound speckle tracking imaging. J Am Coll Cardiol. 2005;45:2034–41. doi: 10.1016/j.jacc.2005.02.082. [DOI] [PubMed] [Google Scholar]
- Nysom K, Holm K, Lipsitz SR, Mone SM, Colan SD, Orav EJ, Sallan SE, Olsen JH, Hertz H, Jacobsen JR, Lipshultz SE. Relationship between cumulative anthracycline dose and late cardiotoxicity in childhood acute lymphoblastic leukemia. J Clin Oncol. 1998;16:545–50. doi: 10.1200/JCO.1998.16.2.545. [DOI] [PubMed] [Google Scholar]
- Reisner SA, Lysyansky P, Agmon Y, Mutlak D, Lessick J, Friedman Z. Global longitudinal strain: a novel index of left ventricular systolic function. J Am Soc Echocardiogr. 2004;17:630–3. doi: 10.1016/j.echo.2004.02.011. [DOI] [PubMed] [Google Scholar]
- Schwarz ER, Pollick C, Dow J, Patterson M, Birnbaum Y, Kloner RA. A small animal model of non-ischemic cardiomyopathy and its evaluation by transthoracic echocardiography. Cardiovasc Res. 1998;39:216–23. doi: 10.1016/s0008-6363(98)00009-1. [DOI] [PubMed] [Google Scholar]
- Steinherz LJ, Steinherz PG, Tan CT, Heller G, Murphy ML. Cardiac toxicity 4 to 20 years after completing anthracycline therapy. Jama. 1991;266:1672–7. [PubMed] [Google Scholar]
- Stoddard MF, Seeger J, Liddell NE, Hadley TJ, Sullivan DM, Kupersmith J. Prolongation of isovolumetric relaxation time as assessed by Doppler echocardiography predicts doxorubicin-induced systolic dysfunction in humans. J Am Coll Cardiol. 1992;20:62–9. doi: 10.1016/0735-1097(92)90138-d. [DOI] [PubMed] [Google Scholar]
- Tee MW, Tee TL, Ashraf M, Lee G, Pulvers E, Ren B, Burns R. Comparison of tissue Doppler and speckle tracking based strain methods in a physiological beating and twisting porcine heart: an in vitro model study. J Am Coll Cardiol. 2006;47:102A. [Google Scholar]
- Tjeerdsma G, Meinardi MT, van Der Graaf WT, van Den Berg MP, Mulder NH, Crijns HJ, de Vries EG, van Veldhuisen DJ. Early detection of anthracycline induced cardiotoxicity in asymptomatic patients with normal left ventricular systolic function: autonomic versus echocardiographic variables. Heart. 1999;81:419–23. doi: 10.1136/hrt.81.4.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Konorev EA, Kotamraju S, Joseph J, Kalivendi S, Kalyanaraman B. Doxorubicin induces apoptosis in normal and tumor cells via distinctly different mechanisms. intermediacy of H(2)O(2)- and p53-dependent pathways. J Biol Chem. 2004;279:25535–43. doi: 10.1074/jbc.M400944200. [DOI] [PubMed] [Google Scholar]
- Wang S, Kotamraju S, Konorev E, Kalivendi S, Joseph J, Kalyanaraman B. Activation of nuclear factor-kappaB during doxorubicin-induced apoptosis in endothelial cells and myocytes is pro-apoptotic: the role of hydrogen peroxide. Biochem J. 2002;367:729–40. doi: 10.1042/BJ20020752. [DOI] [PMC free article] [PubMed] [Google Scholar]