Abstract
The present experiments determined the effects of bupropion on the motivational (aversive and rewarding) and locomotor properties of nicotine in CD-1 mice. Preliminary experiments determined effective nicotine doses (0.1 – 2.0 mg/kg) to produce a conditioned taste aversion (CTA) or conditioned place preference (CPP; Experiments 1a and 2a, respectively). Mice were administered vehicle or bupropion (1 – 20 mg/kg) followed by vehicle or nicotine after drinking saccharin during CTA training (Experiment 1b). Mice were administered vehicle or bupropion (1 – 20 mg/kg) 15 (Experiment 2b) or 30 (Experiment 2c) minutes prior to vehicle or nicotine during CPP training. The two highest nicotine doses produced CTAs and a moderate nicotine dose (0.4 mg/kg) produced a CPP. Bupropion dose-dependently blocked nicotine CTA. For the 15-minute pretreatment interval, bupropion dose-dependently increased locomotor activity and produced CPPs when administered alone; whereas for the 30-minute pretreatment interval, only the highest bupropion dose increased locomotor activity and produced a CPP. However, bupropion failed to alter nicotine CPP and the co-administration of bupropion and nicotine did not increase locomotor activity more so than when bupropion was administered alone regardless as to the pretreatment interval. Thus, bupropion selectively altered the aversive properties of nicotine in CD-1 mice.
Keywords: Bupropion, Conditioned Place Preference, Conditioned Taste Aversion, Locomotor Activity, Motivation, Mouse, Nicotine, Reward
1. Introduction
In the United States, tobacco smoking is the number one preventable cause of disease, disability and death (United States Department of Health and Human Services, 2004). While the harmful effects of tobacco smoking are widely known, 80% of smokers who attempt to quit without a smoking cessation aid relapse within the first year (Balfour and Fagerstrom, 1996). Recently, several double blind, placebo-controlled studies have shown the atypical antidepressant, bupropion, to be an efficacious smoking-cessation agent (Hays et al., 2001; Hurt et al., 1997; Jorenby et al., 1999; for review, Richmond and Zwar, 2003). The mechanism of action of bupropion as a smoking-cessation agent has not been elucidated. Numerous studies, however, have examined the effects of bupropion on the motivational (aversive and reinforcing/rewarding) properties of nicotine in dependent and non-dependent subjects (for a recent review, Dwoskin et al., 2006), as these properties of nicotine are thought to contribute to its addictive potential (Kumar et al., 1983; Picciotto and Corrigall, 2002).
Human and non-human animal studies have shown that acute or chronic bupropion pretreatment attenuates the expression of somatic (Cryan et al., 2003; Malin et al., 2006) and affective (Cryan et al., 2003; Hurt et al., 1997; Jorenby et al., 1999; Malin et al., 2006; Shiffman et al., 2000) symptoms associated with nicotine withdrawal in nicotine-dependent subjects. For example, acute bupropion pretreatment attenuated the increase in brain stimulation reward thresholds occurring during nicotine withdrawal in nicotine-dependent rats (Cryan et al., 2003). Furthermore, chronic bupropion pretreatment attenuated the acquisition of mecamylamine-precipitated conditioned place aversion in nicotine-dependent rats (Malin et al., 2006). The effects of bupropion on craving following a period of withdrawal are unclear presently, as bupropion has been reported to either reduce craving (Brody et al., 2004; Hurt et al., 1997; Jorenby et al., 1999) or to have no effect on craving (Cousins et al., 2001; Shiffman et al., 2000) in human smokers. On balance, this collection of studies suggests that bupropion attenuates many of the aversive effects of nicotine during periods of withdrawal.
Animal studies also have examined the effect of bupropion on the aversive properties of nicotine in non-dependent subjects. For example, using a two-bottle choice task, Shoaib et al. (2003) examined the ability of bupropion to attenuate the acquisition of nicotine-conditioned taste aversion (CTA) in rats. Shoaib et al. (2003) found that bupropion did not alter the acquisition of nicotine CTA, suggesting that bupropion does not alter the aversive properties of nicotine. However, bupropion was administered 30 minutes in advance of the nicotine during the conditioning sessions. Indeed, Dwoskin et al. (2006) found that bupropion (5 mg/kg) co-administered with nicotine (0.8 mg/kg), facilitated the acquisition of nicotine CTA in rats. This latter experiment suggests that the temporal parameter of bupropion administration is an important factor in determining its effect on nicotine CTA.
Animal studies examining the effects of bupropion on the reinforcing/rewarding properties of nicotine have produced equivocal results. Several studies have found that acute or chronic bupropion pretreatment increases nicotine self-administration in rats (Rauhut et al., 2003; Shoaib et al., 2002). Furthermore, acute bupropion pretreatment has been found to facilitate acquisition of nicotine conditioned place preference (CPP) in rats (Dwoskin et al., 2006). Collectively, these studies suggest that bupropion enhances the reinforcing/rewarding effects of nicotine during periods of nicotine exposure/maintenance. Other studies, however, have found that acute bupropion pretreatment, or chronic pretreatment with a high bupropion dose (70 mg/kg), attenuates nicotine self-administration in rats (Bruijnzeel and Markou, 2003; Glick et al., 2002; Maisonneuve and Glick, 2003; Rauhut et al., 2005). Acute bupropion pretreatment also has been found to dose-dependently attenuate nicotine-induced decreases in brain stimulation reward thresholds in non-dependent rats (Cryan et al., 2003). Taken together, these latter studies suggest that acute bupropion pretreatment, or chronic pretreatment with a high bupropion dose, attenuates the reinforcing efficacy of nicotine. It has been suggested that rat strain differences may account for the between-studies differences with respect to the effects of bupropion on the reinforcing properties of nicotine (Bruijnzeel and Markou, 2003).
In the Dwoskin et al. (2006) study, only a single, low bupropion dose (5 mg/kg) was tested for its ability to alter nicotine CTA or CPP. While this single bupropion dose was found to enhance nicotine CTA or CPP, the study was limited due to its single-dose nature. Furthermore, all of the studies to date examining the effects of bupropion on the motivational properties of nicotine have used rats as experimental subjects. Such studies have been important in the understanding of bupropion’s therapeutic mechanism of action as a smoking-cessation agent, as the motivational properties of nicotine are thought to contribute to its addictive potential (Kumar et al., 1983; Picciotto and Corrigall, 2002). However, rats and humans metabolize bupropion differently (Suckow et al., 1986), thus limiting rats as an optimal animal model for understanding the effects of bupropion. A better animal model for understanding the effects of bupropion is mice, as mice have been found to metabolize bupropion similar to humans (Suckow et al., 1986). Thus, given the single-dose nature of the Dwoskin et al. (2006) study, and the similarity of bupropion metabolism by mice and humans, it is important to determine the effects of bupropion, across a wide dose-response range, on the motivational properties of nicotine in mice.
To this end, the effects of bupropion (1 – 20 mg/kg) on the aversive or rewarding properties of nicotine in CD-1 mice were determined in the present experiments. Preliminary experiments examined several nicotine doses (0.1 – 2.0 mg/kg) to determine an effective nicotine dose to produce a CTA or CPP and to characterize nicotine CTA or CPP in CD-1 mice (Experiment 1a or 2a, respectively). Nicotine-induced changes in locomotor activity also were examined and characterized in CD-1 mice in the CPP experiment. Once an effective nicotine dose was found and characterized, the effects of bupropion on nicotine CTA (Experiment 1b) or nicotine CPP (Experiments 2b and 2c) were determined. The effects of bupropion on potential nicotine-induced changes in locomotor activity were determined simultaneously in the CPP experiments, as the locomotor effects of nicotine also have been suggested to be related to its addictive potential (Di Chiara, 2000).
2. Methods
2.1 Animals
Male, experimentally-naïve CD-1 mice, obtained from Charles River Laboratories (Raleigh, NC), were used in all experiments. The mice ranged in weight from 16 – 18 g and were approximately 21 – 28 days of age (adolescence) at the start of the experiment. Adolescent mice were selected to optimize the robustness of the CPP, as a number of recent reports have shown that adolescent rodents are more sensitive to the rewarding properties of nicotine compared to adult rodents (Belluzzi et al., 2004; Kota et al., 2007; Vastola et al., 2002). Except where noted, ad libitum access to food and water was available for the duration of the experiment. For the CTA or CPP experiments, mice were housed individually in 9 3/8 × 5 7/16 × 5 1/8 in or 8 × 12 × 6 in (L × W × H) plastic tubs with wire tops, respectively. The room containing the mice was kept at 68 – 70°F and was on a 12:12 light/dark cycle. Experiments were conducted during the light portion of the cycle. Upon arrival, the mice were acclimated to the animal colony for at least a 7-day period. In all experiments, mice were handled daily for 1 min during the acclimation period. The Institutional Animal Care and Use Committee of Dickinson College approved the experiments described in this paper. The experiments conform to the guidelines established by the NIH Guide for the Care and Use of Laboratory Animals (1996 Edition).
2.2 Apparatus
For the CPP experiments, 8 open-field activity chambers (ENV-OFA-510; Med-associates, Burlington, VT) with CPP inserts (ENV-512; Med-associates, Burlington, VT) were used. The walls of the compartments were constructed of Plexiglas and the overall inside dimensions were 27.9 × 27.9 cm (L × W). The CPP inserts divided the chamber into 2 equally-sized compartments. One compartment had white construction paper line the outer walls and contained a wire-mesh floor. The other compartment had black construction paper line the outer walls and contained a rod floor. Three, 16-beam I/R arrays recorded locomotor activity and determined place preference. A white-noise generator, located in the room, produced an ambient background noise of ~ 70 dB. All data were recorded by a personal computer (MED-PC Open-Field Activity Software) located in an adjacent control room.
2.3 Materials
In the CTA experiments, 50-ml polypropylene graduated centrifuge tubes (VWR International, Baltimore, MD) were used. The graduated centrifuge tubes were demarcated in 1 ml intervals and fluid levels were recorded to the nearest 1 ml.
2.4 Drugs
S(-)-Nicotine di-tartrate (Sigma, St. Louis, MO) was prepared in physiological saline, to which NaOH was added to obtain a pH of 7.4. Nicotine doses were injected subcutaneously (s.c.) in a volume of 10 ml/kg (body weight) and were expressed as the freebase weight. (±)-Bupropion HCl (Sigma, St. Louis, MO) was prepared in physiological saline, injected (s.c.) in a volume of 10 ml/kg (body weight), and doses were expressed as the salt weight.
2.5 Procedure
2.5.1. Conditioned Taste Aversion Experiments
Experiment 1a
Following the 7-day acclimation period, 4 phases occurred: initial water restriction, pretest, conditioning and test. During the first phase, which lasted 1 session, mice were restricted from water for 24 hours (h), and then were allowed 20 minutes (min) access to tap water in their home cages (initial water restriction). Twenty four h following this initial water-restriction phase, mice were allowed 20 min access to saccharin in their home cages (pretest). Mice were required to begin drinking within 2 s in order to advance to the next phase. In addition, mice were assigned to groups based on their saccharin consumption at the time of pretest in order to match the groups prior to the conditioning phase. Next, an 8-session conditioning phase occurred. This phase consisted of 4 alternating drug and non-drug sessions (counterbalanced). On drug sessions, mice (n = 7–8/dose) were injected with vehicle (physiological saline) or nicotine (0.2, 0.4, 0.8 or 2.0 mg/kg) approximately 5 min after a 60-min period of drinking saccharin (0.15%). On the intervening non-drug sessions, mice were allowed 60 min access to tap water for rehydration purposes. The final phase occurred 48 h after the last drug session and consisted of 2 sessions (test). On the first session, half of the mice received 60 min access to the saccharin solution whereas the other half received 60 min access to tap water in the absence of any injections. On the second session, the other half of the mice received 60 min access to the saccharin solution and the mice that received saccharin the previous day received 60 min access to tap water. For all phases, the volume (ml) of fluid (saccharin or water) consumed by the mice during the drinking period was recorded.
Experiment 1b
This experiment resembled Experiment 1a except that mice (n = 6–7/group) were injected with vehicle or bupropion (1, 5, 10 or 20 mg/kg) 5 min before an injection of vehicle or a single nicotine dose (0.8 mg/kg) following the 60-min saccharin drinking period on drug sessions. A 5-min pretreatment interval was selected, as this bupropion pretreatment interval has been shown to alter the behavioral effects of nicotine in mice (Slemmer et al., 2000). Moreover, it has been shown that co-administration of bupropion and nicotine alters nicotine CTA in rats (Dwoskin et al., 2006). The amount of water consumed by mice on non-drug sessions was not recorded.
2.5.2. Locomotor Activity and Conditioned Place Preference Experiments
Experiment 2a
This experiment consisted of 3 phases: pretest, conditioning, and test. Twenty-four h after the 7-day acclimation period, mice received an injection of the vehicle (physiological saline) and were placed immediately into either the white or black compartment (counterbalanced) and permitted to explore both compartments for a 15-min period (pretest). This session determined a mouse’s non-preferred compartment. Moreover, mice were assigned to groups in order to match all groups in terms of their initial preference ratios. Twenty-four h after the pretest session, the conditioning phase began and consisted of 4 alternating drug and non-drug sessions (counterbalanced). On drug sessions, mice (n = 18/dose) were injected with vehicle (physiological saline) or nicotine (0.1, 0.2, 0.4 or 0.8 mg/kg) and immediately confined to the non-preferred compartment for a 30-min period (i.e., a “biased” CPP procedure). A biased CPP procedure was selected in order to enhance the likelihood of observing a nicotine CPP in CD-1 mice, as reports suggest that a biased procedure is more effective in producing a CPP (Calcagnetti and Schechter, 1994; for review, Tzschentke, 1998) and “more suitable” for assessing the rewarding properties of nicotine compared to an unbiased procedure (Le Foll and Goldberg, 2005). On the intervening non-drug sessions, mice received an injection of vehicle and were immediately confined to the preferred compartment for a 30-min period. Twenty-four h after the last session of the conditioning phase, mice were given an injection of vehicle, placed in either the white or black compartment (counterbalanced) and permitted to explore both compartments for a 15-min period (test).
Experiments 2b and 2c
These experiments resembled Experiment 2a except that mice (n = 16 or 24/group) were injected with vehicle (physiological saline) or bupropion (5, 10 or 20 mg/kg) 15 min (Experiment 2b) before an injection of vehicle (physiological saline) or a single nicotine dose (0.4 mg/kg) and confined to the non-preferred compartment for a 30-min period on drug sessions. A 15-min bupropion pretreatment interval was selected, as this bupropion pretreatment interval has been shown to alter the reinforcing efficacy of nicotine in rats (Rauhut et al., 2003), a process related to, though different from, the rewarding properties of nicotine. Because bupropion has been shown to produce a CPP in rats (Ortmann, 1985), an additional CPP experiment was conducted in which the bupropion pretreatment interval was extended. In this experiment (Experiment 2c), mice (n = 11–12/group) were injected with vehicle (physiological saline) or bupropion (1, 5, 10 or 20 mg/kg) 30 min before an injection of vehicle or nicotine and confined to the non-preferred compartment on the drug sessions. In both experiments, all mice received an injection of vehicle followed 15 (Experiment 2b) or 30 (Experiment 2c) min later by a second injection of vehicle and then confined to the preferred compartment on the non-drug sessions.
2.5.3. Data Analysis
CPP Experiments
For each mouse, preference ratios were calculated on the pretest and test sessions. The preference ratios were defined according to the following formula: (time spent in initially non-preferred compartment) / (time spent in initially non-preferred compartment + time spent in initially preferred compartment). A preference ratio of 0.5 indicates no compartment preference. However, because a biased CPP procedure was used, nicotine was administered to nicotine-treated mice in the initially non-preferred compartment. Thus, by definition, the preference ratios were less than 0.5 prior to the conditioning phase. Evidence for a CPP was shown by significantly higher preference ratios for nicotine-treated mice compared to vehicle-treated control mice on the test session.
For the preliminary CTA and CPP experiments, the data were subjected to one- or two-way analyses of variances (ANOVAs; SPSS 14.0 for Windows). Dose was a between-subjects factor and Session was a within-subjects factor. In the bupropion experiments, the data were subjected to two- or three-way ANOVAs. Pretreatment with Bupropion (Bupropion vs. Vehicle) and Pretreatment with Nicotine (Nicotine vs. Vehicle) were between-subject factors and Session was a within-subjects factor. Significant main effects or interactions of interest motivated additional one-way ANOVAs on specific sessions followed by post hoc contrasts involving Tukey’s HSD tests. All statistical decisions were made at α = 0.05.
3. Results
3.1. Conditioned Taste Aversion Experiments
Experiment 1a
A one-way ANOVA conducted on the pretest data revealed no significant dose differences at the time of pretest, F < 1.0 (Figure 1, top panel).
Figure 1. (Experiment 1a, CTA).
Saccharin (top panel) or water (bottom panel) consumption (mean ± SEM) on the pretest, drug and test sessions in mice that received vehicle (physiological saline) or nicotine (0.2, 0.4, 0.8 or 2.0 mg/kg) during the conditioning phase. The "* " and "#" denotes a significant difference from vehicle control mice for 0.8 and 2.0 mg/kg nicotine mice, respectively, ps < 0.05. n = 7–8 mice/dose.
A two-way (Dose × Session) ANOVA conducted on the saccharin consumption on the drug sessions and test session revealed a significant main effect of Session, F (4, 136) = 2.6, p < 0.05, Dose, F (4, 34) = 5.9, p = 0.001, and Dose × Session interaction, F (16, 136) = 2.3, p < 0.01. Subsequent post hoc tests comparing nicotine doses on the individual drug sessions and test session revealed that 0.8 and 2.0 mg/kg nicotine mice drank less than vehicle control mice on the test session only, ps < 0.05.
A two-way (Dose × Session) ANOVA conducted on the water consumption on the non-drug and test sessions revealed only a significant main effect of Session, F (4, 136) = 2.4, p = 0.05 (Figure 1, bottom panel). Neither the main effect of Dose nor the Dose × Session interaction was significant.
Separate two-way (Dose × Session) ANOVAs, similar to those conducted on the consumption data, were conducted on the mice weights during the drug, non-drug and test sessions. These ANOVAs revealed only significant main effects of Session, Fs (4, 136) > 182.0, ps < 0.001 (data not shown), indicating that mice gained weight during the course of the experiment. No significant main effect of Dose or Dose × Session interactions were detected, indicating that group differences in consumption observed during the drug sessions were not complicated by group differences in weight.
Experiment 1b
A one-way ANOVA conducted on the pretest data revealed no significant group differences at the time of pretest, F < 1.0 (Table 1).
Table 1.
Experiment 1b – Saccharin Consumption (Mean ± SEM) in milliliters on Pretest and Drug Sessions
| Drug Session | ||||||
|---|---|---|---|---|---|---|
| Pretrmt With Bupropion | Pretrmnt With Nicotine | Pretest | 1 | 2 | 3 | 4 |
| Vehicle | Vehicle | 5.4 (± 0.30) | 6.7 (± 0.36) | 6.6 (± 0.30) | 6.6 (± 0.20) | 7.1 (± 0.40) |
| Nicotine | 6.0 (± 0.31) | 6.7 (± 0.18) | 5.6 (± 0.30) | 5.3 (± 0.18) * | 5.4 (± 0.20) * | |
| 1 Bup | Vehicle | 6.0 (± 0.26) | 6.3 (± 0.33) | 7.5 (± 0.22) | 7.0 (± 0.26) | 7.2 (± 0.31) |
| Nicotine | 5.9 (± 0.26) | 6.4 (± 0.20) | 5.4 (± 0.20) | 5.6 (± 0.20) * | 5.6 (± 0.30) * | |
| 5 Bup | Vehicle | 5.8 (± 0.31) | 7.0 (± 0.58) | 6.5 (± 0.34) | 6.7 (± 0.21) | 6.2 (± 0.31) |
| Nicotine | 6.0 (± 0.22) | 6.6 (± 0.37) | 6.1 (± 0.26) | 6.0 (± 0.22) | 6.3 (± 0.18) | |
| 10 Bup | Vehicle | 5.8 (± 0.17) | 6.8 (± 0.48) | 6.5 (± 0.34) | 6.8 (± 0.17) | 6.7 (± 0.21) |
| Nicotine | 5.6 (± 0.48) | 6.7 (± 0.29) | 6.1 (± 0.34) | 6.6 (± 0.20) + | 6.3 (± 0.29) | |
| 20 Bup | Vehicle | 5.8 (± 0.31) | 6.3 (± 0.21) | 6.5 (± 0.22) | 6.2 (± 0.17) | 6.3 (± 0.21) |
| Nicotine | 5.9 (± 0.14) | 6.6 (± 0.30) | 6.6 (± 0.20) | 6.7 (± 0.29) + | 6.6 (± 0.20) | |
The “*” and “+” symbols denote a significant difference from Veh+Veh and Veh+Nic control mice, respectively, on a specific session, ps < 0.05.
A three-way (Pretreatment with Bupropion × Pretreatment with Nicotine × Session) repeated-measures ANOVA conducted on the saccharin consumption of the drug sessions and test session revealed significant main effects of Session, F (4, 224) = 2.4, p < 0.05, and Pretreatment with Nicotine, F (1, 56) = 23.9, p < 0.001. The Pretreatment with Nicotine × Session, F (16, 224) = 3.6, p < 0.01, Pretreatment with Bupropion × Pretreatment with Nicotine, F (4, 56) = 6.7, p < 0.001, and Pretreatment with Bupropion × Pretreatment with Nicotine × Session, F (16, 224) = 1.9, p < 0.05, interactions also were significant. The main effect of Pretreatment with Bupropion and the Pretreatment with Bupropion × Session interaction were not significant. Follow-up post hoc tests comparing groups on the individual drug sessions and test session revealed that Veh+Nic and 1 Bup+Nic mice drank less than Veh+Veh control mice on Drug Sessions 3 and 4 (Table 1) and the test session (Figure 2), ps < 0.05 (i.e., CTAs). Moreover, on all drug sessions, 1 Bup+Nic mice did not differ from Veh+Nic mice, suggesting that the lowest bupropion dose did not alter nicotine CTA. On all drug sessions and the test session, 5 Bup+Nic mice did not differ from Veh+Veh control mice or Veh+Nic mice, suggesting that a moderate bupropion dose attenuated the nicotine CTA. However, on Drug Session 3 and the test session, 10 Bup+Nic and 20 Bup+Nic mice differed from Veh+Nic mice, ps < 0.05. Moreover, on all drug sessions and the test session, 10 Bup+Nic and 20 Bup+Nic mice did not differ from Veh+Veh control mice. Taken together, these results suggest that the highest bupropion doses completely blocked nicotine CTA on certain sessions. No bupropion dose administered alone decreased saccharin consumption relative to the Veh+Veh control mice, indicating that bupropion administered alone did not produce a CTA.
Figure 2. (Experiment 1b, CTA Test).
Saccharin consumption (mean ± SEM) on the test session in mice that received Pretreatment with Bupropion (Vehicle or Bupropion) and Pretreatment with Nicotine (Vehicle or Nicotine) on drug sessions of the conditioning phase. The "*" and “+” symbols denote a significant difference from Veh+Veh and Veh+Nic control mice on the test session, respectively, ps < 0.05. n = 6–7 mice/group.
A three-way (Pretreatment with Bupropion × Pretreatment with Nicotine × Session) repeated-measures ANOVA conducted on the mice weights during the drug sessions and test session revealed a significant main effect of Session, F (4, 224) = 222.5, p < 0.001 (data not shown), indicating that groups gained weight during the course of the experiment. A significant Pretreatment with Bupropion × Pretreatment with Nicotine × Session also was detected, F (16, 224) = 2.5, p < 0.01. An inspection of the data suggests that 1 Bup+Nic mice tended to weigh less than Veh+Veh control mice, perhaps accounting for the significant three-way interaction. However, post hoc tests, comparing 1 Bup+Nic and Veh+Veh control mice, failed to detect any reliable differences between the groups. The failure to detect reliable group differences with respect to weight suggests that group differences in saccharin consumption were not complicated by group differences in weight.
3.2. Locomotor Activity and Conditioned Place Preference Experiments
In the CPP experiments, mice > 2 SDs from the group mean at the time of the CPP test were deemed “aberrant” and excluded for all analyses. This criterion resulted in the elimination of 4, 12, and 8 mice in Experiments 2a, 2b, and 2c, respectively. The elimination of mice from Experiments 2a and 2b resulted in new sample sizes of 16–18 mice/dose and 15 or 19 mice/group, respectively, and 9–12 mice/group for Experiment 2c.
Experiment 2a (Locomotor Activity)
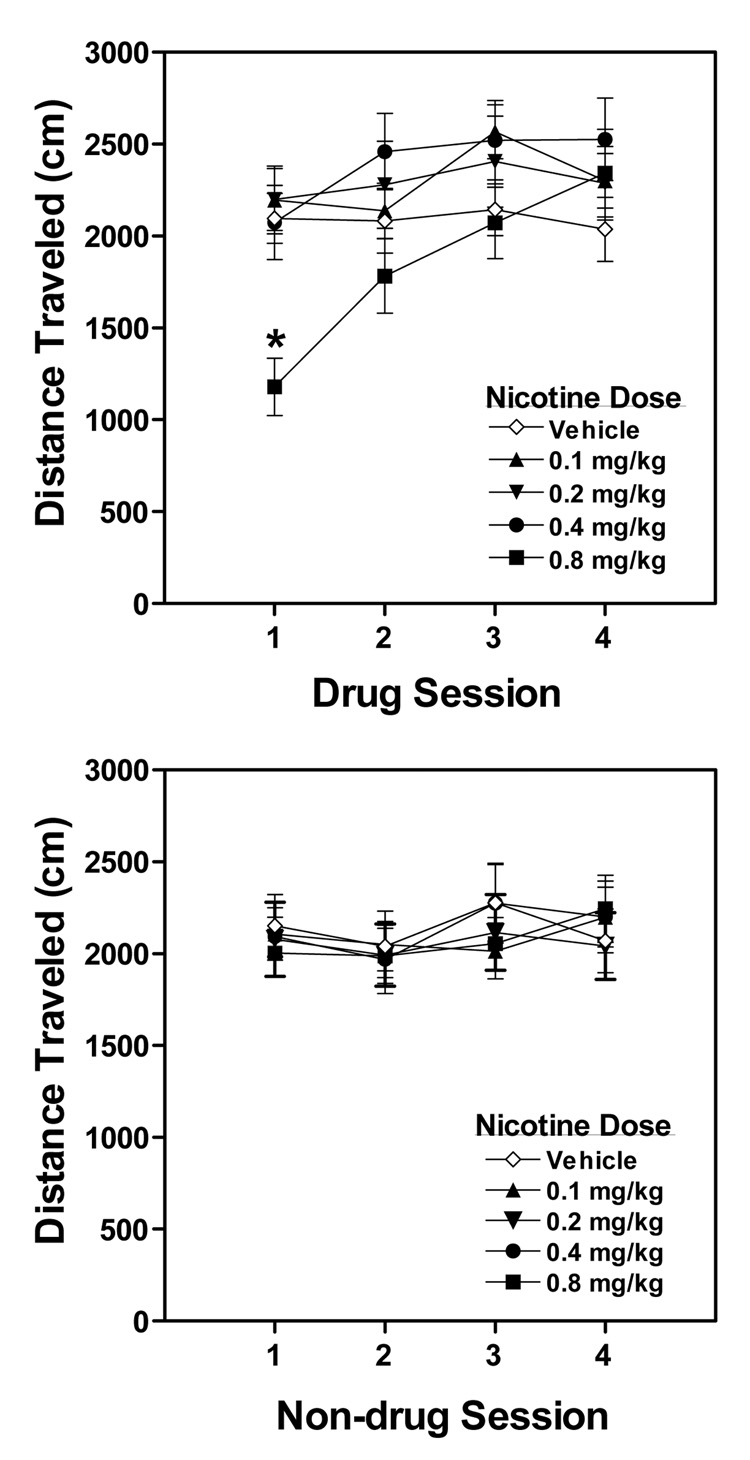
A two-way (Dose × Session) ANOVA conducted on the locomotor activity data of the drug sessions revealed a significant main effect of Session, F (3, 243) = 16.9, p < 0.001, and Dose × Session interaction, F (12, 243) = 4.9, p < 0.001 (Figure 3, top panel). The main effect of Dose was not significant. Subsequent post hoc tests conducted on individual drug sessions found that 0.8 mg/kg nicotine mice were less active than vehicle control mice on Drug Session 1, p < 0.01, suggesting that this nicotine dose produced hypoactivity. No other group differences were noted on any other drug sessions, indicating that the 0.8 mg/kg nicotine mice developed tolerance to the hypoactive effects of nicotine with repeated administration.
Figure 3. (Experiment 2a; Locomotor Activity).
Distance traveled (mean ± SEM) on the drug (top panel) and non-drug (bottom panel) sessions in mice that received vehicle or nicotine (0.1, 0.2, 0.4 or 0.8 mg/kg) during the conditioning phase of the CPP experiment. The "*" denotes a significant difference from vehicle control mice, p < 0.05. n = 16–18 mice/dose.
A two-way (Dose × Session) ANOVA conducted on the locomotor activity data of the non-drug sessions revealed only a significant main effect of Session, F (3, 243) = 3.2, p < 0.05 (Figure 3, bottom panel). No main effect of Dose or Dose × Session interaction was detected, indicating that group differences in locomotor activity on drug sessions were not complicated by general, non-specific group differences. Moreover, the failure to detect group differences on the non-drug sessions, suggests no carry-over effects of nicotine from the drug sessions.
Experiment 2a (CPP)
A one-way ANOVA conducted on the preference ratios revealed no significant dose differences at the time of pretest, F < 1.0 (Figure 4, top panel), indicating that groups did not differ prior to the conditioning phase.
Figure 4. (Experiment 2a; CPP Pretest and Test).
Preference ratios (mean + SEM) on the pretest (top panel) or test (bottom panel) sessions in mice that received vehicle or nicotine (0.1, 0.2, 0.4 or 0.8 mg/kg) during the conditioning phase of the CPP experiment. The "*" denotes a significant difference from vehicle control mice, p < 0.05. n = 16–18 mice/dose.
A one-way ANOVA conducted on the preferences ratios of the test session revealed a significant effect, F (4, 81) = 3.2, p < 0.05 (Figure 4, bottom panel). Follow-up post hoc tests found that 0.4 mg/kg nicotine mice had higher preference ratios than vehicle control mice, p < 0.01 (i.e., a CPP). No other nicotine doses differed from the vehicle control condition.
Experiment 2b (Locomotor Activity – 15 Min Pretreatment Interval)
A three-way (Pretreatment with Bupropion × Pretreatment with Nicotine × Session) repeated-measures ANOVA conducted on the locomotor activity data of the drug sessions revealed significant main effects of Session, F (3, 348) = 2.6, p = 0.05, Pretreatment with Bupropion, F (3, 116) = 11.6, p < 0.001, and Pretreatment with Nicotine, F (1, 116) = 4.7, p < 0.05 (Table 2). No two- or three-way interactions were significant. Subsequent post hoc tests conducted on individual drug sessions found that 20 Bup+Nic mice were more active than Veh+Nic mice on all drug sessions, ps < 0.01. The 20 Bup+Veh mice were more active than Veh+Veh control mice on the last two drug sessions, ps < 0.05, suggesting that the highest bupropion dose produced a locomotor stimulant effect. Furthermore, the 20 Bup+Nic mice were more active than Veh+Veh control mice on all drug sessions, ps < 0.001, suggesting that the combination of the highest bupropion dose and nicotine also produced a locomotor stimulant effect. Moreover, 20 Bup+Nic mice tended to be more active than 20 Bup+Veh mice; however, this difference was not statistically significant, ps > 0.09.
Table 2.
Experiment 2b – Distance traveled (mean ± SEM) in centimeters on Drug Sessions.
| Drug Session | |||||
|---|---|---|---|---|---|
| Pretrmnt With Bupropion | Pretrmnt With Nicotine | 1 | 2 | 3 | 4 |
| Veh | Vehicle | 1980 (± 194) | 1935 (± 156) | 2018 (± 208) | 2082 (± 184) |
| Nicotine | 2563 (± 233) | 2636 (± 331) | 2928 (± 383) | 2846 (± 299) | |
| 5 Bup | Vehicle | 2681 (± 207) | 2599 (± 206) | 2634 (± 266) | 2655 (± 278) |
| Nicotine | 2710 (± 139) | 2738 (± 191) | 2897 (± 224) | 2855 (± 251) | |
| 10 Bup | Vehicle | 3155 (± 290) | 2867 (± 248) | 2833 (± 250) | 3155 (± 246) |
| Nicotine | 2885 (± 359) | 2849 (± 304) | 3227 (± 320) | 3066 (± 236) | |
| 20 Bup | Vehicle | 3534 (± 379) | 3468 (± 482) | 3937 (± 548) * | 4073 (± 519) * |
| Nicotine | 5375 (± 1091) *+ | 5026 (± 790) *+ | 5170 (± 767) *+ | 5265 (± 859) *+ | |
The “*” and “+” symbols denote a significant difference from Veh+Veh and Veh+Nic control mice, respectively, on a specific session, ps < 0.05.
A separate three-way (Pretreatment with Bupropion × Pretreatment with Nicotine × Session) repeated-measures ANOVA conducted on the locomotor activity data of the non-drug sessions revealed no main effects or interactions (data not shown), indicating that group differences in locomotor activity on drug sessions were not complicated by general, non-specific group differences. Moreover, the failure to detect group differences on the non-drug sessions suggests no carry-over effects of bupropion and/or nicotine from the drug sessions.
Experiment 2c (Locomotor Activity – 30 Min Pretreatment Interval)
A three-way (Pretreatment with Bupropion × Pretreatment with Nicotine × Session) repeated-measures ANOVA conducted on the locomotor activity data of the drug sessions revealed significant main effects of Session, F (3, 303) = 11.5, p < 0.001, Pretreatment with Bupropion, F (4, 101) = 12.5, p < 0.001, and Pretreatment with Nicotine, F (1, 101) = 9.0, p < 0.01 (Table 3). The Pretreatment with Bupropion × Session, F (12, 303) = 2.0, p < 0.05, Pretreatment with Nicotine × Session, F (3, 303) = 3.6, p < 0.05, and Pretreatment with Bupropion × Pretreatment with Nicotine, F (4, 101) = 2.9, p < 0.05, interactions also were significant. However, the Pretreatment with Bupropion × Pretreatment with Nicotine × Session interaction was not significant. Subsequent post hoc tests conducted on individual drug sessions found that the 10 Bup+Nic and 20 Bup+Nic mice were more active than Veh+Nic mice on all drug sessions, ps < 0.05. The 5 Bup+Nic mice were more active than Veh+Nic mice only on Drug Sessions 2 and 4, ps < 0.05. The lowest bupropion dose (1 mg/kg) did not differ from Veh+Nic mice. Collectively, these results suggest that bupropion dose-dependently attenuated nicotine-induced hypoactivity. However, this conclusion should be tempered. While Veh+Nic mice tended to be less active than Veh+Veh mice on the initial drug sessions, these group differences were not significant. Thus, nicotine did not reliably produce hypoactivity. None of the bupropion doses administered alone increased locomotor activity relative to Veh+Veh control mice. In contrast, the 20 Bup+Nic mice were more active than Veh+Veh control mice on all drug sessions and the 10 Bup+Nic mice more active than Veh+Veh control mice only on the last drug session, ps < 0.05. These results suggest that these bupropion doses in combination with nicotine produced a locomotor stimulant effect. No other bupropion doses co-administered with nicotine increased locomotor activity relative to Veh+Veh control mice. Furthermore, none of the bupropion+nicotine mice were reliably more active than their bupropion alone counterparts. The 10 Bup+Nic mice tended to be more active than their bupropion alone counterparts by the last drug session; however, these group differences were not significant, p = 0.07.
Table 3.
Experiment 2c – Distance traveled (mean ± SEM) in centimeters on Drug Sessions.
| Drug Session | |||||
|---|---|---|---|---|---|
| Pretrmt with Bupropion | Pretrmt with Nicotine | 1 | 2 | 3 | 4 |
| Vehicle | Vehicle | 2195 (± 141) | 1813 (± 214) | 2129 (± 220) | 1887 (± 262) |
| Nicotine | 1530 (± 234) | 1305 (± 246) | 2057 (± 269) | 1825 (± 210) | |
| 1 Bup | Vehicle | 2194 (± 212) | 1990 (± 307) | 1915 (± 195) | 2106 (± 306) |
| Nicotine | 2231 (± 253) | 2051 (± 157) | 2095 (± 203) | 2530 (± 252) | |
| 5 Bup | Vehicle | 2126 (± 231) | 2403 (± 282) | 2443 (± 254) | 2491 (± 233) |
| Nicotine | 2610 (± 238) | 2487 (± 279) + | 2863 (± 324) | 3181 (± 310) + | |
| 10 Bup | Vehicle | 2256 (± 189) | 2090 (± 202) | 2187 (± 189) | 2157 (± 186) |
| Nicotine | 3325 (± 295) + | 2885 (± 239) + | 3402 (± 230) + | 3447 (± 297) *+ | |
| 20 Bup | Vehicle | 2841 (± 251) | 2568 (± 291) | 3266 (± 358) | 3073 (± 374) |
| Nicotine | 3525 (± 446) *+ | 3197 (± 277) *+ | 4269 (± 488) *+ | 4104 (± 428) *+ | |
The “*” and “+” symbols denote a significant difference from Veh+Veh and Veh+Nic control mice, respectively, on a specific session, p < 0.05.
A separate three-way (Pretreatment with Bupropion × Pretreatment with Nicotine × Session) repeated-measures ANOVA conducted on the locomotor activity data of the non-drug sessions revealed only a main effect of Session, F (3, 303) = 13.5, p < 0.001 (data not shown). No other main effects or interactions were significant.
Experiment 2b (CPP - 15 Min Pretreatment Interval)
A two-way (Pretreatment with Bupropion × Pretreatment with Nicotine) ANOVA conducted on the preference ratios of the pretest session revealed no significant main effects or interactions, Fs < 1.0 (Figure 5, top panel), indicating that groups did not differ prior to the conditioning phase.
Figure 5. (Experiment 2b; CPP Pretest and Test).
Preference ratios (mean + SEM) on the pretest (top panel) or test (bottom panel) sessions in mice that received Pretreatment with Bupropion (Bupropion or Vehicle) followed 15 min later by Pretreatment with Nicotine (Nicotine or Vehicle) during the conditioning phase of the CPP experiment. Except for 10 Bup+Veh mice, the "*" denotes a significant difference from Veh+Veh control mice on the test session, ps < 0.05. n = 15 or 19 mice/group.
A two-way (Pretreatment with Bupropion × Pretreatment with Nicotine) ANOVA conducted on the preference ratios of the test session revealed significant main effects of Pretreatment with Bupropion, F (3, 116) = 4.7, p < 0.01 and Pretreatment with Nicotine, F (1,116) = 5.7, p < 0.05 (Figure 5, bottom panel). The Pretreatment with Bupropion × Pretreatment with Nicotine interaction also was significant, F (3, 116) = 3.3, p < 0.05. Follow-up post hoc tests found that Veh+Nic mice had higher preference ratios compared to Veh+Veh control mice (i.e., a CPP). Furthermore, with the exception of the 10 Bup+Veh mice, the other bupropion doses (5 and 20 mg/kg) administered alone produced CPPs, ps < 0.05. Moreover, all bupropion doses (5, 10 or 20 mg/kg) co-administered with nicotine produced CPPs, ps < 0.05, but did not alter nicotine CPP.
Experiment 2c (CPP - 30 Min Pretreatment Interval)
A two-way (Pretreatment with Bupropion × Pretreatment with Nicotine) ANOVA conducted on the preference ratios of the pretest session revealed no significant main effects or interactions, Fs < 1.0 (Figure 6, top panel), indicating that groups did not differ prior to the conditioning phase.
Figure 6. (Experiment 2c; CPP Pretest and Test).
Preference ratios (mean + SEM) on the pretest (top panel) or test (bottom panel) sessions in mice that received Pretreatment with Bupropion (Bupropion or Vehicle) followed 30 min later by Pretreatment with Nicotine (Nicotine or Vehicle) during the conditioning phase of the CPP experiment. The "*" indicates a significant main effect of Pretreatment with Nicotine (i.e., a CPP) and the "#" denotes a significant difference between Veh+10 Bup and Veh+Veh control mice on the test session, ps < 0.05. n = 9–12 mice/group.
A two-way (Pretreatment with Bupropion × Pretreatment with Nicotine) ANOVA conducted on the preference ratios of the test session revealed a significant main effect Pretreatment with Nicotine, F (1, 101) = 5.5, p = 0.05 (Figure 6, bottom panel). This result suggests that nicotine-conditioned mice had higher preference ratios compared to vehicle-treated mice (i.e., CPPs). The main effect of Pretreatment with Bupropion also was significant, F (4, 101) = 4.6, p < 0.01. Follow-up post hoc contrasts found that only the 20 Bup+Veh mice had higher preference ratios than the Veh+Veh control mice, p < 0.05, suggesting that this bupropion dose administered alone produced a CPP. No other bupropion doses administered alone produced a CPP. The Pretreatment with Bupropion × Pretreatment with Nicotine interaction was not significant, suggesting that no bupropion dose altered nicotine CPP. However, the 10 and 20 Bup+Nic mice tended to have higher preference ratios than Veh+Nic mice but these differences were not significant. Moreover, none of the bupropion+nicotine mice had higher preference ratios than their bupropion alone counterparts.
4. Discussion
The present experiments revealed several findings of interest. First, nicotine dose-dependently produced a CTA, CPP or induced changes in locomotor activity in CD-1 mice. Second, bupropion dose-dependently attenuated or completely blocked nicotine CTA. Third, when bupropion was administered 15 min prior to the conditioning sessions, bupropion dose-dependently produced CPPs and increased locomotor activity when administered alone or in combination with nicotine. Fourth, when bupropion was administered 30 min prior to the conditioning sessions, only the highest bupropion dose (20 mg/kg) produced a CPP when administered alone. Finally, regardless as to the pretreatment interval, no bupropion doses altered nicotine CPP and the combination of bupropion and nicotine did not reliably increase locomotor activity more so than when bupropion was administered alone.
It was found that a one-bottle choice task produced nicotine CTA (Experiment 1a), consistent with other one-bottle choice task nicotine CTA experiments (Pescatore et al., 2005; Risinger and Brown, 1996). Moreover, nicotine doses (0.8 and 2.0 mg/kg) that produced CTAs did not cause suppression of water intake on the non-drug or test sessions. This result suggests that the nicotine CTA was specific to saccharin and was not due to a general suppression in consumption. Finally, the magnitude of the nicotine CTA produced was comparable to that found using the more conventional two-bottle choice task (Kumar et al., 1983; Shoaib et al., 2000; Shoaib et al., 2002; Shoaib et al., 2003). Collectively, these observations suggest that the present procedure was a valid measure of nicotine CTA and demonstrate that CD-1 mice are responsive to the aversive properties of nicotine. Several studies have shown that the rodent strain is an important factor in determining nicotine CTA. Pescatore et al. (2005) found that Fischer 344 rats were slower to acquire and displayed a less robust nicotine CTA compared to Lewis rats. Risinger and Brown (1996) found that C3H/heJ and DBA mice developed dose-dependent nicotine CTAs, whereas BALB demonstrated only minor CTAs and did not differ in a dose-dependent manner. C57BL/6J completely failed to show nicotine CTA. Thus, the present study extends the nicotine CTA literature, showing that CD-1 mice demonstrate nicotine CTA in a dose-dependent manner.
The present study also found that bupropion dose-dependently attenuated or completely blocked nicotine CTA (Experiment 1b). That is, the low bupropion dose (1 mg/kg) failed to attenuate the nicotine CTA, whereas moderate (5 or 10 mg/kg) or high (20 mg/kg) bupropion doses attenuated or completely blocked the nicotine CTA, respectively. This finding is consistent with studies that have found that bupropion attenuates other behavioral effects of nicotine such as antinociception in non-dependent subjects (Slemmer et al., 2000), as well as the affective (Cryan et al., 2003; Hurt et al., 1997; Jorenby et al., 1999; Malin et al., 2006; Shiffman et al., 2000) or somatic (Cryan et al., 2003; Malin et al., 2006) effects of nicotine withdrawal in nicotine-dependent subjects. This finding, however, is at odds with studies that have shown that bupropion enhances (Dwoskin et al., 2006) or does not alter (Shoaib et al., 2003) nicotine CTA in non-dependent rats. The discrepant results may be due to species differences between studies. Indeed, mice and rats metabolize bupropion differently (Suckow et al., 1986; see below for an elaboration of this point).
The neurochemical mechanism mediating the bupropion-induced attenuation in nicotine CTA is unknown. It has been suggested that nicotine exerts its aversive properties via stimulation of centrally-located nicotinic receptors (Kumar et al., 1987; Shoaib and Stolerman, 1995). Furthermore, the competitive nicotinic receptor antagonist, DHβE, which has a high affinity for the α4β2 nicotinic receptor subtype (Harvey and Luetje, 1996; Harvey et al,. 1996; Luetje and Patrick, 1991) has been shown to attenuate nicotine CTA (Shoaib et al., 2000), suggesting that the aversive properties of nicotine appear to be mediated through this nicotinic receptor subtype. The recent observation that β2-knockout mice show a weaker nicotine CTA relative to control wild-type mice provides additional support for the role of the β2 receptor in mediating the aversive properties of nicotine (Shoaib et al., 2002). Because bupropion has been reported to be a non-competitive antagonist at the α4β2 nicotinic receptor subtype (Fryer and Lucas, 1999; Slemmer et al., 2000) and a competitive antagonist at the α3β2 nicotinic receptor subtype (Miller et al., 2002), this is a plausible neurochemical mechanism to account for the effect of bupropion on nicotine CTA, as presently observed.
The contribution of the metabolites of bupropion to the attenuation of nicotine CTA cannot be excluded. Metabolism of bupropion differs between species. For example, hydroxybupropion, a primary metabolite of bupropion, does not accumulate in the brain in significant levels (Suckow et al., 1986), and contributes little to the behavioral effects of bupropion in rats (Cooper et al., 1994). However, bupropion is readily metabolized to hydroxybupropion in mice and humans, which is behaviorally active and has been suggested to contribute largely to the antidepressant (Ascher et al., 1995; Martin et al., 1990) and smoking-cessation effects (Damaj et al., 2004) of bupropion. Indeed, the 2S,3S isomer of hydroxybupropion has been shown recently to more potently inhibit the human α4β2 nicotinic receptor compared to the 2S,3R isomer or racemic mixture of bupropion (Damaj et al., 2004). Thus, the effect of bupropion on nicotine CTA, as presently observed, may be due to the metabolites of bupropion instead of the parent compound.
Previous research has found that nicotine produces a biphasic response on locomotor activity (Clarke and Kumar, 1983). In the present study, nicotine dose-dependently altered locomotor activity with the highest nicotine dose (0.8 mg/kg) initially inducing hypoactivity followed by the development of tolerance to the hypoactive effects (Experiment 2a). No nicotine dose produced hyperactivity with repeated administration (i.e., no sensitization). Several studies have shown that mouse strains are differentially sensitive to the locomotor-altering effects of nicotine (Collins et al., 1988; Marks et al., 1983; Marks et al., 1989; Marks et al., 1991). For example, C57BL and DBA mice are very sensitive to the locomotor-depressant effects of nicotine whereas CH3 mice are sensitive to the locomotor-activating effects of nicotine (Marks et al., 1983). In CD-1 mice, a very low nicotine dose (0.05 mg/kg) induces hyperactivity when administered 15 – 20 min after the injection (Freeman et al., 1987). In addition, Freeman et al. (1987) found that a moderate nicotine dose (0.4 mg/kg) failed to alter locomotor activity whereas a high nicotine dose (0.8 mg/kg) initially induced hypoactivity followed by hyperactivity within a 60-min session in CD-1 mice. However, direct comparisons between the Freeman et al. (1987) study and the present report are complicated by the fact the Freeman et al. (1987) study did not specify if the nicotine dose was based on the freebase or salt weight of the drug. Nevertheless, the present results show that, within the parameters tested, CD-1 mice are responsive to the acute, locomotor-depressant effects of nicotine but are unresponsive to the locomotor-activating effects following repeated nicotine administration (i.e., sensitization).
Using a biased CPP procedure, the present study also found that nicotine CPP was observed (Experiment 2a). The use of a biased CPP procedure is a potential limitation of the present report, as the use of such a procedure complicates the interpretation of the CPP results. It has been suggested that a shift in preference in drug-treated animals may reflect a reduction in the aversiveness of the non-preferred compartment when a biased CPP procedure is used (Tzschentke, 1998). That is, the unconditioned, anxiolytic effects of the drug (e.g., nicotine) may produce a “preference” for the non-preferred compartment by attenuating the animal’s initial perceived dislike of the compartment (i.e., “motivational interaction” hypothesis). Alternatively, a CPP may be observed when a biased CPP procedure is used because the non-preferred compartment is more salient and hence easier to condition than the preferred compartment (for a recent discussion of these alternative accounts, see Le Foll and Goldberg, 2005). Nevertheless, the use of a biased CPP procedure is very common in the nicotine CPP literature. In fact, as reviewed by Le Foll and Goldberg (2005), approximately two-thirds of the studies using a biased nicotine CPP procedure found a CPP. In contrast, only one-third of the studies that observed nicotine CPP used an unbiased procedure. Thus, the present observation of nicotine CPP, when a biased procedure was used, underscores the importance of such a procedure in producing nicotine CPP.
Furthermore, the present observation that adolescent CD-1 mice were responsive to the rewarding effects of a moderate nicotine dose (0.4 mg/kg) are consistent with studies that have suggested other important factors such as age of rodent (i.e., adolescent; Belluzzi et al., 2004; Kota et al., 2007; Vastola et al., 2002) and nicotine dose (i.e., moderate; Grabus et al., 2006), in producing a nicotine CPP. Also, it has been shown that mouse strains are differentially sensitive to the rewarding effects of nicotine. Grabus et al. (2006) found that moderate nicotine doses of 0.3 and 0.5 mg/kg produced a CPP in C57BL/6J and ICR mice, respectively. However, nicotine failed to produce a CPP in DBA/2J mice. The former observation that a moderate nicotine dose (0.5 mg/kg) produced a CPP in ICR mice is particularly relevant for the current study, as CD-1 and ICR mice share a common genetic background (personal communication, Charles River Laboratories).
When administered 15 min prior to the conditioning session (Experiment 2b), only the highest bupropion dose (20 mg/kg) reliably increased locomotor activity in mice when administered alone similar to previous observations in rats (Nielsen et al., 1986; Nomikos et al., 1992). The highest bupropion dose administered 30 min prior to the conditioning sessions, however, did not reliably increase locomotor activity (Experiment 2c). Rather, only the moderate (10 mg/kg) and high (20 mg/kg) bupropion doses co-administered with nicotine reliably increased locomotor activity when a 30-min pretreatment interval was used. However, unlike previous observations, the increased locomotor activity was not enhanced with repeated administrations of bupropion (i.e., no sensitization). Furthermore, with the exception of the moderate bupropion dose (10 mg/kg), the other bupropion doses (5 and 20 mg/kg) produced CPPs in mice when administered alone 15 min prior to the conditioning sessions (Experiment 2b). However, when the pretreatment interval was extended to 30 min, only the highest bupropion dose (20 mg/kg) reliably produced a CPP when administered alone (Experiment 2c). These results are consistent with a previous study that found that bupropion produced a CPP in rats (Ortmann 1985). It is unclear as to the neurochemical mechanism by which bupropion increases locomotor activity or produces a CPP. However, in addition to its interactions with neuronal nicotinic receptors, bupropion inhibits the dopamine and norepinephrine transporters, having relatively low affinity for these sites compared to other selective and high-affinity transporter inhibitors (Ferris et al., 1983). Thus, the locomotor-activating effects of bupropion and the bupropion-produced CPP most likely are related to its facilitation of dopamine, as the dopaminergic system has been shown to be involved in drug-produced hyperactivity and CPP (Di Chiara, 2000; Tzschentke, 1998).
In Experiment 2c, when bupropion was administered 30 min prior to the nicotine during the conditioning phase, bupropion dose-dependently attenuated the hypoactivity initially induced by nicotine, similar to previous observations (Slemmer et al., 2000). This conclusion, however, should be tempered, as nicotine administered alone did not statistically decrease locomotor activity. Furthermore, regardless as to the pretreatment interval, the highest bupropion dose administered in combination with nicotine also increased locomotor activity in mice. However, the combination of the high bupropion dose and nicotine did not increase locomotor activity more so than when bupropion was administered alone. A limitation of Experiment 2b is that the locomotor-activating effects of bupropion when administered alone may have “masked” its impact on locomotor activity when bupropion was co-administered with nicotine. In fact, the highest bupropion dose co-administered with nicotine tended to increase locomotor activity more so than when the highest bupropion dose was administered alone (though this difference was not statistically significant). Dramatic individual differences in the 20 Bup+Nic mice prevented detection of reliable differences compared to 20 Bup+Veh mice in Experiment 2b. Indeed, a recent report found that bupropion (30 mg/kg) administered in combination with nicotine (0.4 mg/kg) increased locomotor activity more so than when bupropion was administered alone in rats (Sidhpura et al., 2007). Despite the fact that the co-administration of the highest bupropion dose and nicotine did not reliably increase locomotor activity beyond bupropion alone, an increased locomotor response was detected earlier in the bupropion + nicotine condition in Experiment 2b. In addition, in Experiment 2c, a statistically significant locomotor stimulant effect was detected only with bupropion doses (10 or 20 mg/kg) co-administered with nicotine but not when these bupropion doses were administered alone. These results suggest that the locomotor-activating effects of these bupropion doses may have summated with those of nicotine (or vice versa). However, the failure to statistically detect a difference between the bupropion+nicotine mice and their respective bupropion alone counterparts, regardless as to the pretreatment interval, prevents a more definitive conclusion on this issue.
In Experiment 2b, when bupropion was administered 15 min prior to the nicotine during the conditioning phase, no bupropion dose altered nicotine CPP. This conclusion is complicated by the fact that all bupropion doses also produced CPPs when administered alone. However, in Experiment 2c, when the pretreatment interval was extended to 30 min, no bupropion dose altered nicotine CPP. Moreover, in this experiment, only the highest bupropion dose (20 mg/kg), administered alone, produced a CPP. These findings are at odds with a recent CPP study that found that bupropion augmented nicotine CPP when co-administered with nicotine (Dwoskin et al., 2006). A number of factors may have contributed to the discrepancy between the CPP studies. Dwoskin et al. (2006) found that bupropion augmented nicotine CPP when a sub-threshold nicotine dose (0.2 mg/kg) was used. Thus, the failure to detect a bupropion-induced augmentation in the present study may have been due to a ceiling effect. Alternatively, the fact that bupropion when administered alone also produced a CPP, may have masked a bupropion-induced augmentation in nicotine CPP. These ideas are unlikely, as the nicotine CPPs observed in Experiments 2b and 2c were not exceptionally robust (i.e., average preference ratios were ~ 5.2). Moreover, in Experiment 2c, bupropion did not augment nicotine CPP at bupropion doses that did not produce CPPs. Most likely, species differences account for the discrepant findings between the present CPP results and those reported in the Dwoskin et al (2006) study. As indicated previously, rats and mice metabolize bupropion differently, with such differences leading to different behavioral effects (Suckow et al., 1986). Regardless as to the reason for the discrepancy, it is clear that bupropion, when administered across a wide dose range (1 – 20 mg/kg), and under different temporal parameters (15 vs. 30 min), fails to alter nicotine CPP in CD-1 mice.
In sum, the present results revealed that bupropion dose-dependently blocked nicotine CTA. Most likely, this effect was mediated by the interaction of bupropion with neuronal nicotinic receptors. Furthermore, bupropion dose-dependently increased locomotor activity and produced CPPs when administered alone or in combination with nicotine under certain temporal parameters. These latter effects of bupropion are most likely related to its interaction with the dopaminergic system. However, regardless as to the pretreatment interval, bupropion failed to reliably alter nicotine CPP. Also, regardless as to the pretreatment interval, the combination of bupropion and nicotine did not reliably increase locomotor activity more so than when bupropion was administered alone. Given the similarity in metabolism of bupropion by mice and humans (Suckow et al., 1986), and the importance of the motivational (Kumar et al., 1983; Picciotto and Corrigall, 2002) and locomotor (Di Chiara, 2000) properties of nicotine in contributing to its addictive potential, the findings that bupropion differentially altered the aversive, locomotor and rewarding properties of nicotine in CD-1 mice provides valuable insight into bupropion’s therapeutic mechanism of action as a smoking-cessation agent.
Acknowledgements
This research was supported by a grant (DA019866) awarded by USPHS to A. S. Rauhut. S.K. Mardekian was financially supported by additional funds provided by Dickinson College. M. Hawrylak and S.K. Mardekian contributed equally to the current project and share second authorship. The authors wish to thank Dr. Meredith Rauhut for a critical reading of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ascher JA, Cole JO, Colin JN, Feighner JP, Ferris RM, Fibiger HC, et al. Bupropion: A review of its mechanism of antidepressant activity. J Clin Psychiatry. 1995;56:395–401. [PubMed] [Google Scholar]
- Balfour DJ, Fagerstrom KO. Pharmacology of nicotine and its therapeutic use in smoking cessation and neurodegenerative disorders. Pharmacol Ther. 1996;72:51–81. doi: 10.1016/s0163-7258(96)00099-x. [DOI] [PubMed] [Google Scholar]
- Belluzzi JD, Lee AG, Oliff HS, Leslie FM. Age-dependent effects of nicotine locomotor activity and conditioned place preference in rats. Psychopharmacology. 2004;174:389–395. doi: 10.1007/s00213-003-1758-6. [DOI] [PubMed] [Google Scholar]
- Brody AL, Mandelkern MA, Lee G, Smith R, Sadeghi M, Saxena S, et al. Attenuation of cue-induced cigarette craving and anterior cingulate cortex activation in bupropion-treated smokers: A preliminary study. Psychiatry Res. 2004;130:269–281. doi: 10.1016/j.pscychresns.2003.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruijnzeel AW, Markou A. Characterization of the effects of bupropion on the reinforcing properties of nicotine and food in rats. Synapse. 2003;50:20–28. doi: 10.1002/syn.10242. [DOI] [PubMed] [Google Scholar]
- Clarke PBS, Kumar R. Characterization of the locomotor stimulant action of nicotine in tolerant rats. Br J Pharmac. 1983;80:587–594. doi: 10.1111/j.1476-5381.1983.tb10733.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins AC, Miner LL, Marks MJ. Genetic influences on acute responses to nicotine and nicotine tolerance in the mouse. Pharmacol Biochem Behav. 1988;30:269–278. doi: 10.1016/0091-3057(88)90455-8. [DOI] [PubMed] [Google Scholar]
- Cooper BR, Wang CM, Cox RF, Norton R, Shea V, Ferris RM. Evidence that the acute behavioral and electrophysiological effects of bupropion (Wellbutrin®) are mediated by a noradrenergic mechanism. Neuropsychopharmacology. 1994;11:133–141. doi: 10.1038/npp.1994.43. [DOI] [PubMed] [Google Scholar]
- Cousins MS, Stamat HM, de Wit H. Acute doses of d-amphetamine and bupropion increase cigarette smoking. Psychopharmacology. 2001;157:243–253. doi: 10.1007/s002130100802. [DOI] [PubMed] [Google Scholar]
- Cryan JF, Bruijnzeel AW, Skhei KL, Markou A. Bupropion enhances brain reward function and reverses the affective and somatic aspects of nicotine withdrawal in the rat. Psychopharmacology. 2003;168:347–358. doi: 10.1007/s00213-003-1445-7. [DOI] [PubMed] [Google Scholar]
- Damaj MI, Carroll FI, Eaton JB, Navarro HA, Blough BE, Mirza S, Lukas RJ, Martin BR. Enantioselective effects of hydroxyl metabolites of bupropion on behavior and on function of monoamine transporters and nicotinic receptors. Molecular Pharmacology. 2004;66:675–682. doi: 10.1124/mol.104.001313. [DOI] [PubMed] [Google Scholar]
- Di Chiara G. Role of dopamine in the behavioural actions of nicotine related to addiction. Eur J Pharmacol. 2000;393:295–314. doi: 10.1016/s0014-2999(00)00122-9. [DOI] [PubMed] [Google Scholar]
- Dwoskin LP, Rauhut AS, King-Pospisil KA, Bardo MT. Review of the pharmacology and clinical profile of bupropion, an antidepressant and tobacco use cessation agent. CNS Drug Rev. 2006;12(3–4):178–207. doi: 10.1111/j.1527-3458.2006.00178.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferris RM, Cooper BR, Maxwell RA. Studies of bupropion's mechanism of antidepressant activity. J Clin Psychiatry. 1983;44:74–78. [PubMed] [Google Scholar]
- Freeman GB, Sherman KA, Gibson GE. Locomotor activity as a predictor of times and dosages for studies of nicotine’s neurochemical actions. Pharmacol Biochem Behav. 1987;26:305–312. doi: 10.1016/0091-3057(87)90123-7. [DOI] [PubMed] [Google Scholar]
- Fryer JD, Lukas RJ. Noncompetitive functional inhibition at diverse, human nicotinic acetylcholine receptor subtypes by bupropion, phencyclidine and ibogaine. J Pharmacol Exp Ther. 1999;288:88–92. [PubMed] [Google Scholar]
- Glick SD, Maisonneuve IM, Kitchen BA. Modulation of nicotine self-administration in rats by combination therapy with agents blocking α3β4 nicotinic receptors. Eur J Pharmacol. 2002;448:185–191. doi: 10.1016/s0014-2999(02)01944-1. [DOI] [PubMed] [Google Scholar]
- Grabus SD, Martin BR, Brown SE, Damaj MI. Nicotine place preference in the mouse: Influences of prior handling, dose and strain and attenuation by nicotinic receptor antagonists. Psychopharmacology. 2006;184:456–463. doi: 10.1007/s00213-006-0305-7. [DOI] [PubMed] [Google Scholar]
- Harvey SC, Luetje CW. Determinants of competitive antagonist sensitivity on neuronal nicotinic receptor beta subunits. J Neurosci. 1996;16:3798–3806. doi: 10.1523/JNEUROSCI.16-12-03798.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey SC, Maddox FN, Luetje CW. Multiple determinants of Dihydro-β-Erythroidine sensitivity on rat neuronal nicotinic receptor α subunits. J Neurochem. 1996;67:1953–1959. doi: 10.1046/j.1471-4159.1996.67051953.x. [DOI] [PubMed] [Google Scholar]
- Hays JT, Hurt RD, Rigotti NA, Niaura R, Gonzales D, Durcan MJ, et al. Sustained-release bupropion for pharmacologic relapse prevention after smoking cessation. Ann Intern Med. 2001;35:423–433. doi: 10.7326/0003-4819-135-6-200109180-00011. [DOI] [PubMed] [Google Scholar]
- Hurt RD, Sachs DPL, Glover PN, Sullivan CR, Croghan IT, Sullivan PM. A comparison of sustained-release bupropion and placebo for smoking cessation. New Eng J Med. 1997;337:1195–1202. doi: 10.1056/NEJM199710233371703. [DOI] [PubMed] [Google Scholar]
- Jorenby DE, Leischow SJ, Nides MA, Rennard SI, Johnston JA, Hughes AR, et al. A controlled trial of sustained-release bupropion, a nicotine patch, or both for smoking cessation. New Eng J Med. 1999;340:685–691. doi: 10.1056/NEJM199903043400903. [DOI] [PubMed] [Google Scholar]
- Kota D, Martin BR, Robinson SE, Damaj MI. Nicotine dependence and reward differ between adolescent and adult male mice. J Pharmacol Exp Ther. 2007;322:399–407. doi: 10.1124/jpet.107.121616. [DOI] [PubMed] [Google Scholar]
- Kumar R, Pratt JA, Stolerman IP. Characteristics of conditioned taste aversion produced by nicotine in rats. Br J Pharmacol. 1983;79:245–253. doi: 10.1111/j.1476-5381.1983.tb10518.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar R, Reavill C, Stolerman IP. Nicotine cue in rats: effects of central administration of ganglionic blocking drugs. Br J Pharmacol. 1987;90:239–246. doi: 10.1111/j.1476-5381.1987.tb16845.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Foll B, Goldberg SR. Nicotine induces conditioned place preferences over a large range of doses in rats. Psychopharmacology. 2005;178:481–492. doi: 10.1007/s00213-004-2021-5. [DOI] [PubMed] [Google Scholar]
- Luetje CW, Patrick J. Both alpha- and beta-subunits contribute to the agonist sensitivity of neuronal nicotinic acetylcholine receptors. J Neurosci. 1991;11:837–845. doi: 10.1523/JNEUROSCI.11-03-00837.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maisonneuve IM, Glick SD. Anti-addictive actions of an iboga alkaloid congener: A novel mechanism for a novel treatment. Pharmacol Biochem Behav. 2003;75:607–618. doi: 10.1016/s0091-3057(03)00119-9. [DOI] [PubMed] [Google Scholar]
- Malin DH, Lake JR, Smith TD, Khambati HN, Meyers-Paal RL, Montellano AL, et al. Bupropion attenuates nicotine abstinence syndrome in the rat. Psychopharmacology. 2006;183:456–463. doi: 10.1007/s00213-005-0135-z. [DOI] [PubMed] [Google Scholar]
- Marks MJ, Burch JB, Collins AC. Genetics of nicotine response in four inbred strains of mice. J Pharmacol Exp Ther. 1983;226:291–302. [PubMed] [Google Scholar]
- Marks MJ, Campbell SM, Romm E, Collins AC. Genotype influences the development of tolerance to nicotine in the mouse. J Pharmacol Exp Ther. 1991;259:392–401. [PubMed] [Google Scholar]
- Marks MJ, Stitzel JA, Collins AC. Genetic influences on nicotine responses. Pharmacol Biochem Behav. 1989;33:667–678. doi: 10.1016/0091-3057(89)90406-1. [DOI] [PubMed] [Google Scholar]
- Martin P, Massol J, Colin JN, Lacomblez L, Puech AJ. Antidepressant profile of bupropion and three metabolites in mice. Pharmacopsychiatry. 1990;23:187–194. doi: 10.1055/s-2007-1014505. [DOI] [PubMed] [Google Scholar]
- Miller DK, Sumithran SP, Dwoskin LP. Bupropion inhibits nicotine-evoked [(3)H]overflow from rat striatal slices preloaded with [(3)H]dopamine and from rat hippocampal slices preloaded with [(3)H]norepinephrine. J Pharmacol Exp Ther. 2002;302:1113–1122. doi: 10.1124/jpet.102.033852. [DOI] [PubMed] [Google Scholar]
- Nielsen JA, Shannon NJ, Berg L, Moore KE. Effects of acute and chronic bupropion on locomotor activity and dopaminergic neurons. Pharmacol Biochem Behav. 1986;24:795–799. doi: 10.1016/0091-3057(86)90413-2. [DOI] [PubMed] [Google Scholar]
- Nomikos GG, Damsma G, Wenkstern D, Fibiger HC. Effects of chronic bupropion on interstitial concentrations of dopamine in rat nucleus accumbens and striatum. Neuropsychopharmacology. 1992;7:7–14. [PubMed] [Google Scholar]
- Ortmann R. The conditioned place preference paradigm in rats: Effect of bupropion. Life Sci. 1985;37:2021–2027. doi: 10.1016/0024-3205(85)90033-5. [DOI] [PubMed] [Google Scholar]
- Pescatore KA, Glowa JR, Riley AL. Strain differences in the acquisition of nicotine-induced conditioned taste aversion. Pharmacol Biochem Behav. 2005;82:751–757. doi: 10.1016/j.pbb.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Picciotto MR, Corrigall WA. Neuronal systems underlying behaviors related to nicotine addiction: Neural circuits and molecular genetics. J Neurosci. 2002;22:3338–3341. doi: 10.1523/JNEUROSCI.22-09-03338.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauhut AS, Dwoskin LP, Bardo MT. Tolerance does not develop to the decrease in nicotine self-administration produced by repeated bupropion administration. Nicotine Tob Res. 2005;7(6):901–907. doi: 10.1080/14622200500381384. [DOI] [PubMed] [Google Scholar]
- Rauhut AS, Neugebauer N, Dwoskin LP, Bardo MT. Effect of bupropion on nicotine self-administration in rats. Psychopharmacology. 2003;169:1–9. doi: 10.1007/s00213-003-1450-x. [DOI] [PubMed] [Google Scholar]
- Richmond E, Zwar N. Review of bupropion for smoking cessation. Drug Alcohol Rev. 2003;22:203–220. doi: 10.1080/09595230100100642. [DOI] [PubMed] [Google Scholar]
- Risinger FO, Brown MM. Genetic differences in nicotine-induced conditioned taste aversion. Life Sci. 1996;58:223–229. doi: 10.1016/0024-3205(96)00051-3. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Johnston JA, Khayrallah M, Elash CA, Gwaltney CJ, Paty JA, et al. The effect of bupropion on nicotine craving and withdrawal. Psychopharmacology. 2000;148:33–40. doi: 10.1007/s002130050022. [DOI] [PubMed] [Google Scholar]
- Shoaib M, Gommans J, Morley A, Stolerman IP, Grailhe R, Changeux JP. The role of nicotinic receptor beta-2 subunits in nicotine discrimination and conditioned taste aversion. Neuropharmacology. 2002;42:530–539. doi: 10.1016/s0028-3908(01)00194-0. [DOI] [PubMed] [Google Scholar]
- Shoaib M, Sidhpura N, Shafait S. Investigating the actions of bupropion on dependence-related effects of nicotine in rats. Psychopharmacology. 2003;165:405–412. doi: 10.1007/s00213-002-1277-x. [DOI] [PubMed] [Google Scholar]
- Shoaib M, Stolerman IP. Conditioned taste aversion in rats after intracerebral administration of nicotine. Behav Pharmacol. 1995;6:375–385. [PubMed] [Google Scholar]
- Shoaib M, Zuburan C, Stolerman IP. Antagonism of stimulus properties of nicotine by dihydro-β-erythroidine (DHβE) in rats. Psychopharmacology. 2000;149:140–146. doi: 10.1007/s002139900348. [DOI] [PubMed] [Google Scholar]
- Sidhpura N, Redfern P, Rowley H, Heal D, Wonnacott S. Comparison of the effects of bupropion and nicotine on locomotor activation and dopamine release in vivo. Biochem Pharmacol. 2007;74:1292–1298. doi: 10.1016/j.bcp.2007.06.025. [DOI] [PubMed] [Google Scholar]
- Slemmer JE, Martin BR, Damaj MI. Bupropion is a nicotinic antagonist. J Pharmacol Exp Ther. 2000;295:321–327. [PubMed] [Google Scholar]
- Suckow RF, Smith TM, Perumal AS, Cooper TB. Pharmacokinetics of bupropion and metabolites in plasma and brain of rats, mice and guinea pigs. Drug Metab Dispo. 1986;14:692–697. [PubMed] [Google Scholar]
- Tzschentke TM. Measuring reward with the conditioned place preference paradigm: A comprehensive review of drug effects, recent progress and new issues. Prog Neurobiol. 1998;56:613–672. doi: 10.1016/s0301-0082(98)00060-4. [DOI] [PubMed] [Google Scholar]
- U.S. Department of Health and Human Services. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2004. The Health Consequences of Smoking: A Report of the Surgeon General; pp. 855–888. [Google Scholar]
- Vastola BJ, Douglas LA, Varlinskaya EI, Spear LP. Nicotine-induced conditioned place preference in adolescent and adult rats. Physio Behav. 2002;77:107–114. doi: 10.1016/s0031-9384(02)00818-1. [DOI] [PubMed] [Google Scholar]