Abstract
We previously demonstrated that the neuropeptide cocaine- and amphetamine-regulated transcript (CART) is protective against focal cerebral ischemia in vivo and against neuronal cell death in culture induced by oxygen-glucose deprivation (OGD). The mechanism of neuroprotection by CART is unknown, in part due to lack of knowledge regarding its putative receptor. Using a yeast two-hybrid system with CART’s carboxy-terminal to screen a mouse brain cDNA library, we uncovered a potential direct interaction between CART and subunit B of the mitochondrial enzyme succinate dehydrogenase (SDHB). We confirmed CART/SDHB binding using in vitro pull-down assay, and tested the effects of CART peptide on SDH activity, Complex II (CII) activity and ATP production in primary cultured cortical neurons under basal conditions and after OGD. At concentrations between 0.2 and 4 nM, CART significantly increased SDH function, CII activity and ATP generation in purified mitochondria and intact neurons under baseline conditions. Furthermore, pretreatment with CART enhanced mitochondrial mechanisms of neuronal survival and prevented the decline in SDH and CII activities and ATP production after OGD. The findings suggest that CART’s neuroprotective mechanism of action may be linked to preservation of mitochondrial function and prevention of energy failure after ischemia–reperfusion injury.
Keywords: CART, mitochondria, neurodegeneration, neuroprotection, succinate dehydrogenase
Introduction
Cocaine- and amphetamine-regulated transcript (CART) is expressed in neural and endocrine tissues. In the brain, CART is widely expressed, with the highest levels found in the hypothalamus; moderate levels in the midbrain and thalamus; and lower, but detectable, levels in the hindbrain, hippocampus, ventral striatum and the cerebral cortex (Douglass et al., 1995). The name CART describes its original discovery by differential display as a transcript inducible in rat brain by psychostimulants, which subsequently lead to extensive research into the role of CART in drug addiction (Kuhar et al., 2005). In normal brain, CART is highly expressed in hypothalamic nuclei responsible for appetite regulation, implicating CART in body weight control and energy metabolism (Kristensen et al., 1998). CART is also expressed in all levels of the hypothalamic–pituitary–adrenal axis (Larsen et al., 2003), which plays an important role in energy homeostasis and the neuroendocrine response to stress (Koylu et al., 2006). CART has also been implicated in a variety of other functions, including pain transmission (Damaj et al., 2003), cardiovascular regulation (Matsumura et al., 2001; Scruggs et al., 2003), sensory processing and locomotor activity (Kimmel et al., 2000). We recently reported that CART expression was induced in the cerebral cortex after focal cerebral ischemia in the rat in vivo and after oxygen-glucose deprivation (OGD) in cultured cortical neurons (Xu et al., 2006). We also demonstrated that CART upregulation in ischemic brain was further enhanced by the female sex hormone 17β-estradiol, and blockade of CART’s action with a CART-neutralizing antibody attenuated estradiol-mediated neuroprotection. Finally, CART peptide administration in vivo reduced infarct size after middle cerebral artery occlusion in mice, and incubation of cortical neurons with CART peptide attenuated OGD-induced ischemic cell death. Despite the many known functions of CART, its mechanisms of action, especially its neuroprotective mechanisms of action, remain unknown. A major issue is that no receptors or interacting partners have been identified for CART, although pharmacological and competitive binding studies have suggested the existence of specific binding sites for CART (Vicentic et al., 2006). To fill this gap in knowledge and begin to explore CART’s molecular mechanisms of actions, in the current study we used a yeast two-hybrid system with a CART functional region as bait to screen a mouse brain cDNA library for potential binding proteins for CART. We here report CART binding to mouse succinate dehydrogenase subunit B (SDHB), a critical enzyme of the Krebs cycle and an integral part of Complex II (CII) of the mitochondrial electron transport chain for oxidative metabolism and ATP synthesis. We further demonstrated that CART’s binding to SDH stimulates its function, increasing CII activity and ATP synthesis in purified mitochondria and intact cells under baseline conditions and, more importantly, preserving mitochondrial functional integrity and ATP synthesis and improving mitochondrial mechanisms of neuronal survival after OGD. The findings suggest that stimulation of mitochondrial energy metabolism is an important part of CART’s mechanism of neuroprotection.
Materials and methods
Yeast two-hybrid screen
The yeast two-hybrid system (Fields & Song, 1989) was performed using a mouse brain cDNA library (pGAD vector, BD Clontech), which contains the GAL4 activation domain. As bait, we used the C-terminus of rat CART (GenBank # NM_017110, amino acids 62–102), which is identical to the mouse amino sequence. The CART C-terminus was amplified by polymerase chain reaction (PCR) using the following primers, which contain EcoR1 sites (underlined): 5′-GGAATTCTACGGCCAAGTCCCCATG-3′ as the forward primer and 5′-GGAATTCTCACAAGCACTTCAAGAGG-3′ as the reverse primer. PCR products were digested with EcoR1 and ligated in-frame into pBDGal4Cam vector (pBD, Stratagene) digested with EcoR1, which contains the GAL4 DNA-binding domain. The new plasmid will be referred to as pBDCART. The yeast two-hybrid screen was performed in yeast strain YRG-2 (Stratagene), following the manufacturer’s instructions with a sequential transformation procedure. Briefly, 100 μg of the cDNA library was used to transform to YRG-2 cells already containing pBDCART. Yeast transformants were incubated at 30 °C for 6 days on agar plates containing synthetic dropout medium with 25 mM of 3-aminotriazol and lacking leucine (Leu), tryptophan (Trp) and histidine (His). Positive clones in the plates were further assayed by β-galactosidase (β-Gal) filter assay. Positive pGAD plasmids (prey) were then isolated using a yeast plasmid DNA isolation kit (BD Clontech) from the yeast after removing the bait plasmid by ‘dropout’ following culture in medium without leucine. Finally, the plasmids of positive clones were co-transformed with either the bait pBDCART or the pBD vector into other yeast strains HF7c and Y190 to eliminate false positive clones. Isolated prey plasmids with mouse cDNA inserts were then amplified by transforming the yeast plasmid into Escherichia coli XL-1 blue MRF (Stratagene) and were identified by DNA sequencing.
In vitro pull-down assay
CART (55–102), where numbers refer to amino acids within the pro-CART, and enhanced green fluorescent protein (EGFP) cDNAs were amplified by PCR and sequenced to confirm sequence accuracy, and inserted into the pHisTAT-2.1 vector (TAT vector kindly provided by Dr Steven Dowdy at UCSD). The following constructs were generated and transformed into E. coli BL21 (DE3) cells (Invitrogen): pTAT-EGFP and pTAT-EGFP-CART. The expressions of HisTAT and HisTAT fusion proteins were induced by 0.1 mM iso-propyl-1-thio-β-D-galactopyranoside, and were purified by Ni-NTA column (Qiagen) according to the manufacturer’s recommended protocol. Band identities were confirmed by Coomassie blue staining and specific antibodies. Cultured HEK293 and PC3 cells were washed with ice-cold phosphate-buffered saline (PBS), and homogenized in CelLytic Reagent (Sigma) buffer containing protease inhibitors (1% protease inhibitor cocktail, Sigma). Samples were incubated on ice for 15–30 min with shaking and then centrifuged at 14 000 g for 10 min. The supernatant was removed to a new tube as the total cell lysate and stored frozen at −80 °C. Thawed lysates (300–500 μg) were first incubated directly with 10 μL of protein G-agarose for 1 h at 4 °C and spun down. The supernatant was then divided equally into two tubes containing 20 μg of TAT-EGFP-CART protein or TAT-EGFP protein, and incubated with 2 μg of anti-CII 30 kDa (SDHB) antibody (Molecular Probes, Eugene, OR, USA) overnight at 4 °C. The reaction was then incubated with 10 μL of protein G-agarose beads (BD Clontech) at 4 °C overnight. Following centrifugation, the beads were washed three times using wash buffer (in mM: Tris–HCl, 10, pH 7.5; NaCl, 150; 1% Triton X-100; dithiothreitol, 1). Bound proteins were eluted with 2 × sodium dodecyl sulfate (SDS) sample buffer and subjected to a 10% SDS–polyacrylamide gel electrophoresis and transferred to a 0.45 micron nitrocellulose membrane. Proteins were analysed by Western blotting using 1 : 400 diluted anti-GFP monoclonal antibody (BD Clontech) and 1 : 10 000 diluted chicken anti-goat IgG horseradish peroxidase conjugated (IgNex). Signals were visualized by chemiluminescence. To confirm that the fusion protein was CART fusion, the blotted membrane was stripped by Restore Western blot stripping buffer (Pierce), according to the manufacturer’s protocol, and reprobed again with anti-CART antibody.
Primary neuronal cell culture
Primary cortical neurons were prepared by dissecting cortices from fetal rat brains on embryonic day 18 (E18), as described in ‘Protocols for neural cell culture’ (Fedoroff & Richardson, 2001). Embryos were retrieved through caesaren section under deep halothane anesthesia and killed by decapitation. Dissociated cells were plated onto a poly-L-ornithine-coated plate or dish (24-well plates, 2.5 × 105 cells/well, 60 × 15 mm culture dish, 26 × 105 cells) and cultured under standard conditions (grown at 37 °C in 100% humidity, 95% room air/5% CO2) in neurobasal medium (NBM) with 2% (v/v) B27 supplement and 2 mM glutamine. Cells were grown for 10–12 days in vitro before they were used in mitochondrial functional studies or subjected to OGD, described below. Studies were approved by the Animal Care and Use Committee at Oregon Health and Science University.
Isolation of mitochondria from cortical neurons
Mitochondria were isolated from primary cortical neurons under baseline conditions and after OGD, as previously described (Verweij et al., 1997; Birch-Machin & Turnbull, 2001; Powell & Jackson, 2003). Briefly, neuronal cells were washed with cold PBS and transferred into ice-cold SEE buffer (in mM: sucrose, 250; HEPES, 10, pH 7.4; EDTA, 0.5; EGTA, 0.5, in double-distilled water). Approximately 1 g of tissue was suspended in 10 mL SEE buffer containing Nagarse protease (3 mg/g tissue), sonicated with a handheld sonicator, and diluted with additional SEE buffer (10 mL) to inactivate the protease. The homogenates were centrifuged for 3 min at 2000 g to pellet the nuclei, and the supernatant was further centrifuged at 12 000 g for 8 min to generate the mitochondrial pellets. After discarding the supernatant, the mitochondrial pellets were resuspended in SEE buffer (10 mL) and centrifuged two additional times in fresh changes of SEE buffer (10 mL) at 12 000 g for 10 min to wash the pellets. The purified mitochondrial pellets were resuspended in 0.25 M sucrose, aliquoted into microcentrifuge tubes, snap frozen in liquid nitrogen and stored at 80°C until analysed (normally less than 2 weeks).
SDH activity assay
SDH activity was determined in a mitochondria-rich preparation of cultured cells using a spectrophotometric method. The measurement was performed in 50 mM Tris–HCl buffer (pH 8.0) containing 3 mM NaCN, 50 μm 2,6-dichloroindiphenol (DCIP), 10 mM succinate and 0.4 mg mitochondrial protein/mL. The reduction of DCIP by SDH was monitored at 650 nm every 3 min for 12 min, as previously described (Kis et al., 2003).
CII mitochondrial respiration assay
Mitochondrial protein from baseline and post-OGD neurons were prepared as previously described (Birch-Machin & Turnbull, 2001). Briefly, CII-specific activity was measured by following the reduction of DCIP. The mitochondria were freeze-thawed three times, and 50 μg of mitochondrial protein was added to a tube containing 100 μL of hypotonic buffer (in mM: KH2PO4, 25, pH 7.2; MgCl2, 5) with 20 mM succinate. After 10 min incubation at 30 °C, antimycin A (2 mg/mL), rotenone (2 mg/mL), KCN (2 mM) and DCIP (50 μM) were added to each tube. A baseline absorbance rate was established at 590 nm on a spectrophotometer for 3 min following the addition of CII inhibitors and the terminal electron acceptor (DCIP). The reaction was started by the addition of ubiquinone (65 μM). Absorbance was measured every 30 s for 5 min following the addition of ubiquinone as the coenzyme for the reaction. 3-Nitropropionic acid (3-NPA, 50 mM) was added prior to the addition of ubiquinone as the positive control to assess the validity of the assay.
ATP assay
To measure the level of ATP, cultured neurons were homogenized in a medium containing 0.3% (w/v) trichloracetic acid and 1 mm EDTA. The homogenate was centrifuged at 10 000 g for 3 min at 4 °C. The supernatant was assayed for ATP levels using a luminescence detection kit (Molecular Probes), as previously described (De Cristobal et al., 2001). ATP levels are expressed in pmol/μg (μmol/g).
OGD and quantification of neuronal cell death
Cells were grown in 24-well plates and each experiment was performed in triplicate. Neuronal cultures were subjected to OGD for 120 min at 37 °C. Cultures were placed in a Coy Hypoxia Chamber filled with a hypoxic gas mixture (5% CO2, 5% H2, 90% N2), and oxygen concentration was controlled at < 0.1%. The culture medium was replaced with deoxygenated glucose-free buffer (PBS with 1 mM CaCl2, 0.8 mM MgCl2). OGD was terminated by replacing OGD media with feeding media containing neurobasal medium (NBM) with 2% (v/v) B27 supplement and 2 mM glutamine, and cultures were maintained in a regular CO2 incubator under normoxic conditions at 37 °C for 24 h. Cell death was determined by the fluorescent markers for live and dead cells, calcein and propidium iodide (PI). Cell death in each well was expressed as the ratio of PI-positive to the sum of PI- and calcein-positive cells in at least three images per well, and three wells for each condition were analysed under a fluorescent microscope (40 ×objective, 10 ×eye piece). Neuronal viability was also determined spectrophotometrically using the 3-(4,5-dimethylthiazol-2-yl) 2,5-di-phenyl-tetrazolium bromide (MTT) assay. The assay is based on the reduction of MTT to purple-colored formazan by mitochondrial dehydrogenases. This assay provides a sensitive assessment of mitochondrial functional integrity, and is most sensitive to mitochondrial mechanisms of cell death. CART (55–102; America Peptide Company) or vehicle (PBS) was added 30 min prior to OGD and maintained during OGD and throughout reoxygenation. Cells were incubated for 24 h after OGD at 37 °C. After the addition of 25 μL of MTT stock solution (5 mg/mL) in 500 μL media for 1 h, 150 μL dimethylsulfoxide was added per well, agitated for 5 min, 15 μL of 2 M Tris (pH 10.5) was then added, and MTT reduction was determined in a Vector III plate reader at 540 nm.
Statistical analysis
Statistical analysis was performed with SigmaStat (SPSS, Chicago, IL, USA). Data are presented as means ± SEM. Differences among groups in SDH and CII activities over time were assessed with two-way ANOVA followed by Fisher LSD test. Differences in MTT and PI death rates were assessed by one-way ANOVA followed by Fisher post hoc analysis. Values are reported as mean + SEM; a P-value of < 0.05 was considered to be statistically significant.
Results
Identification of SDH as a CART-interacting partner
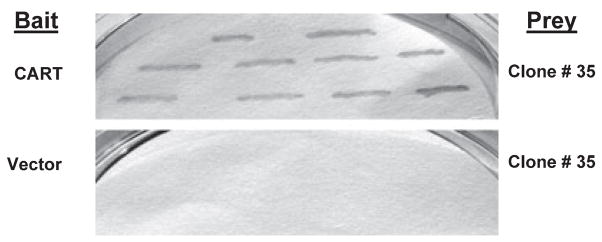
Using the yeast two-hybrid system with a CART functional domain and a mouse brain cDNA library, we screened over 1 million yeast clones. Several clones appeared on the His-negative plates, but only one clone, #35, was positive for both His3 and LacZ phenotypes. The cDNA was recovered from the yeast positive clone, and the DNA sequence analysis showed that the protein encoded by this clone was SDHB (GenBank # NM023374). To eliminate any false positive possibility, the cDNA of the positive clone was reintroduced with the bait pBDCART into the yeast host strain and other yeast strains, which still gave His3- and LacZ-positive phenotypes (Fig. 1, upper panel), while introduction of the cDNA with the empty vector or plasmids containing sequences unrelated to CART was negative for both phenotypes (Fig. 1, lower panel). These results suggest that CART peptide binds to SDHB in yeast cells, and that CART/SDH binding is highly specific.
Fig. 1.
Identification of cocaine- and amphetamine-regulated transcript (CART)-interacting partner using yeast two-hybrid system and β-Gal filter assay. Yeast cells transformed with clone #35 containing the sequence for SDHB (prey) and CART (bait), but not empty vector plasmid (lower panel), induce reporter gene (β-gal) expression (upper panel).
In vitro CART/SDH binding
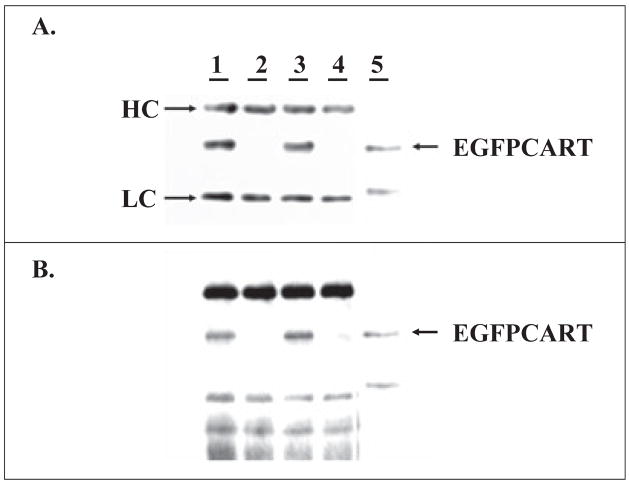
To further confirm the interaction between CART and SDHB, we first generated a TAT-EGFP-CART (EGFPCART) fusion protein that could be interrogated in Western blot analysis by both anti-CART and anti-EGFP antibodies, and which can be compared for binding activity to the fusion protein TAT-EGFP lacking CART. Mitochondrial protein preparations from two mammalian cell lines (HEK293 and PC3) abundantly expressed SDHB, as determined by Western blot with anti-CII antibody (30 kDa, SDHB, not shown). Furthermore, as seen in Fig. 2A and B, when cellular lysates were incubated with TAT-EGFP-CART or TAT-EGFP, the protein G beads with anti-CII antibody were able to pull down the TAT-EGFP-CART fusion protein, but not TATEGFP, confirming the specificity of CART/SDHB binding in vitro.
Fig. 2.
In vitro pull-down assay. Cell lysates were incubated with purified enhanced green fluorescent protein cocaine- and amphetamine-regulated transcript (EGFPCART) proteins (lanes 1 and 3) or EGFP proteins (2 and 4), pulled down by protein G beads linked to anti-CII (SDHB) antibody, and then probed with either anti-EGFP (A) or anti-CART (B) antibodies (same membrane stripped and reprobed). Lysates in lanes 1 and 2 were prepared from PC3 cells, 3 and 4 from HEK293 cells using CelLytic Reagent. Lane 5 is input loaded with approximately 10% of the EGFPCART protein used in the binding reaction. HC, heavy chain of Ig; LC, light chain of Ig.
CART stimulates SDH and CII activities under basal conditions
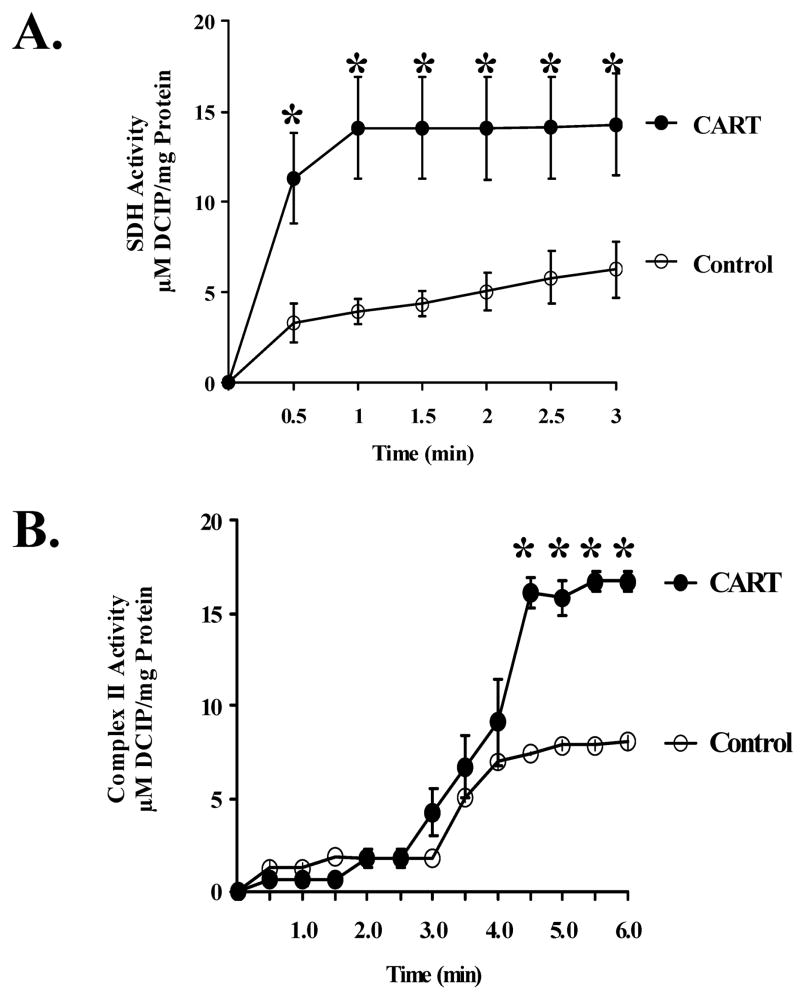
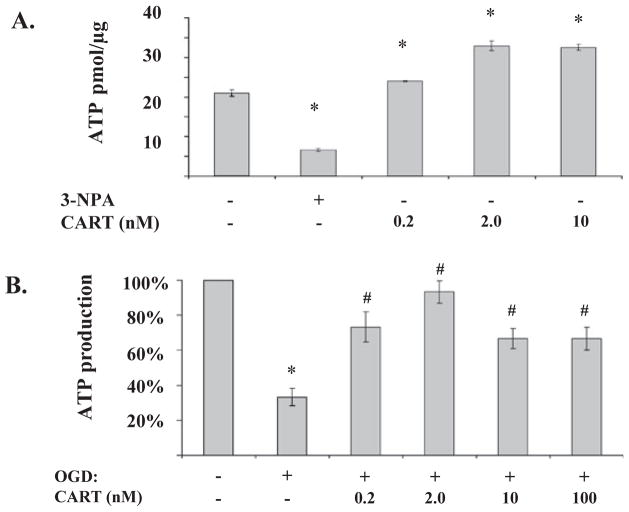
To determine if the physical interaction between CART peptide and SDH alters SDH and CII functions, we tested the effects of CART peptide on SDH and CII activities in mitochondrial extracts from primary cultured cortical neurons under basal conditions. In the first series of experiments, CART or vehicle was added directly to the mitochondrial extract, and SDH activity was determined spectrophotometrically at 650 nm by the reduction of DCIP after addition of the SDH substrate succinate. The results shown in Fig. 3A demonstrate that CART (0.2 nM) significantly increases SDH activity in mitochondrial extracts from primary cultured cortical neurons. We also tested the effect of CART on CII activity in mitochondrial extract measured spectrophotometrically at 595 nm by DCIP reduction after the addition of Coenzyme Q. In agreement with Fig. 3A, the results demonstrate that CART peptide (0.2 nM) increases CII activity (Fig. 3B). Similar increases in SDH and CII activities were observed at CART concentrations up to 4 nM. Interestingly, no further improvement was observed at higher CART concentrations and, at 50 nM, CART actually decreased SDH and CII activities.
Fig. 3.
Cocaine- and amphetamine-regulated transcript (CART) stimulates basal succinate dehydrogenase (SDH) and CII activities in mitochondrial extracts from primary cortical neurons. (A) CART increases SDH activity determined spectrophotometrically at 650 nm by the reduction of 2,6-dichloroindiphenol (DCIP) after addition of the SDH substrate succinate at time 0. (B) CART increases CII activity in mitochondrial extract measured spectrophotometrically at 595 nm by DCIP reduction after the addition of Coenzyme Q at 3 min *P < 0.05 compared with control.
CART prevents mitochondrial dysfunction after OGD in primary cortical neurons
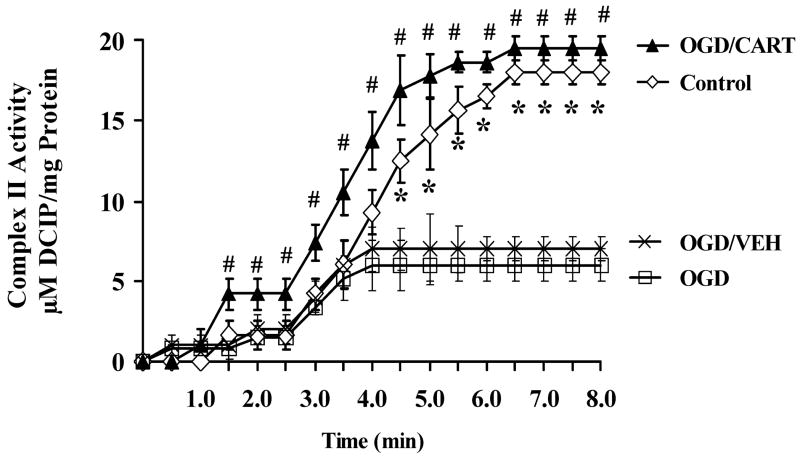
To determine if CART can prevent the decline in mitochondrial function observed after OGD, primary cultured cortical neurons were subjected to 2 h OGD with or without CART (0.2 nM, added 30 min prior to OGD, and maintained during OGD and throughout reoxygenation). At 24 h, cells were washed and homogenized for mitochondrial protein extraction, and SDH and CII activities were measured as in Fig. 3. CII activity was severely depressed at 24 h after OGD compared with control cells (Fig. 4, control vs OGD). However, pretreatment with CART (CART/OGD), but not saline vehicle (OGD/VEH) completely prevented the decline in CII activity after OGD in primary cultured cortical neurons. Similar results were obtained for SDH activity (data not shown).
Fig. 4.
Cocaine- and amphetamine-regulated transcript (CART) prevents the decline in CII activities after oxygen-glucose deprivation (OGD) in mitochondrial extracts from primary cortical neurons. CART was added at 0.2 nM concentration 30 min prior to 2 h OGD, and mitochondria extracted at 24 h after OGD. *P < 0.05 compared with OGD, #P < 0.05 compared with OGD plus vehicle (VEH). DCIP, 2,6-dichloroindiphenol.
CART increases basal ATP production and prevents the decline in ATP after OGD
To determine if increased CII activity translates to increased energy production, we measured ATP levels in primary cortical neuronal cultures. Figure 5A demonstrates that CART peptide stimulated ATP synthesis by mitochondrial extracts from primary cortical neurons. The effect was maximal at 2 nM. Specificity of the assay was determined by the addition of the CII inhibitor 3-NPA, which inhibits ATP synthesis by purified mitochondria. As expected, the rate of ATP synthesis by mitochondrial extracts from post-OGD neurons was severely reduced. However, pretreatment with CART significantly attenuated the decline in ATP formation by mitochondrial extract isolated at 24 h of reoxygenation after 2 h OGD (Fig. 5B). As in Fig. 5A, the effect of CART on neuronal survival was maximal at 2 nM. No further improvement was observed at higher CART concentrations up to 100 nM.
Fig. 5.
Cocaine- and amphetamine-regulated transcript (CART) increases basal ATP production and prevents the decline in ATP after oxygen-glucose deprivation (OGD). (A) CART increases ATP production in mitochondria extracted from normal cultured cortex neuronal cells. ATP levels were measured using a luciferase-based bioluminescence detection assay (n = 4 per group). The CII inhibitor 3-nitropropionic acid (3-NPA, 50 mM) was added to demonstrate specificity. *P < 0.05 compared with control. (B) CART preserves mitochondrial ATP production capacity after OGD and reperfusion (n = 4 per group). CART was added at 0.2 nM 30 min prior to 2 h OGD, and mitochondrial extract isolated at 24 h of reoxygenation after OGD. *P < 0.05 compared with control; #P < 0.05 compared with OGD alone.
CART inhibits OGD-induced neuronal cell death
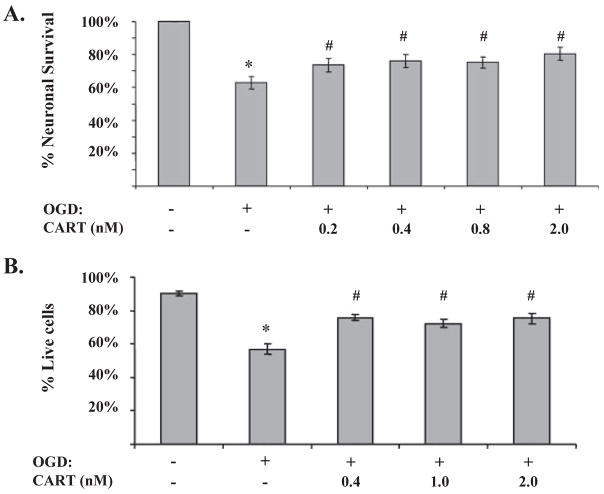
To determine if preservation of mitochondrial integrity translates into enhanced neuronal survival, we examined the effect of CART on OGD-induced cell death using PI staining as well as mitochondria-specific MTT assay. Figure 6A demonstrates that 2 h OGD induced 37.2 ± 3.9% (n = 7) reduction in neuronal viability as determined by the MTT assay. Pretreatment with CART (0.2–2.0 nM) preserved mitochondrial function integrity and improved neuronal survival (74.0 ± 4.1%, n = 7 at 0.2 nM, P < 0.05 compared with 63.0 ± 3.9%, n = 7, in OGD alone). Similarly, Fig. 6B demonstrates that neuronal survival was reduced by 32.4 ± 3.0% (n = 6) at 24 h after 2 h OGD, as determined by PI/calcein staining. The decline in neuronal survival was dose-dependently attenuated by CART at doses between 0.2 and 2.0 nM. In agreement with SDH/CII activity and ATP production assays, the neuroprotective effect of CART was lost at concentrations higher than (10–100 nM).
Fig. 6.
Cocaine- and amphetamine-regulated transcript (CART) improves mitochondrial dysfunction and neuronal viability after oxygen-glucose deprivation (OGD). (A) CART improves neuronal survival as determined by the reduction of MTT by mitochondrial dehydrogenases (n = 7 per group, *P < 0.05 compared with control; #P < 0.05 compared with OGD alone). (B) CART decreases OGD-induced cell death as determined by PI/calcein staining at 24 h after 2 h OGD (n = 6 per group, *P < 0.05 compared with control; #P < 0.05 compared with OGD alone).
Discussion
The main findings of our study are: (1) CART directly interacts with the mitochondrial protein SDH; (2) CART increases basal SDH and CII functional activities in purified neuronal mitochondria; (3) CART increases basal ATP formation by purified neuronal mitochondria; (4) CART prevents the decline in SDH and CII activities and ATP generation in cortical neurons after OGD; (5) CART improves mitochondrial function and neuronal survival after OGD in primary cortical neurons. This is the first identification of a direct interacting partner for CART, and the first report to implicate the mitochondria in the mechanisms of action of CART. The findings suggest that the mechanisms of neuroprotection by CART may in part be linked to preservation of mitochondrial integrity and energy production during hypoxia–ischemia.
CART is a neuropeptide exclusively expressed in neural and neuroendocrine tissues. It was discovered in 1995 as a transcript differentially regulated in the brain by cocaine and amphetamine (Douglass et al., 1995). Since then, CART has been implicated in a variety of other brain functions, including energy metabolism, appetite control and neuroprotection. However, the mechanisms of the actions of CART are poorly understood, in part because no putative receptor or binding partner has yet been identified. The first evidence of CART’s mechanism of action came from a study demonstrating that CART (55–102) inhibited voltage-dependent calcium signaling in hippocampal neurons (Yermolaieva et al., 2001). Other in vivo studies demonstrated that central administration of CART increases the phosphorylation of cAMP response element-binding protein (CREB) in the hypothalamic paraventricular nucleus (Sarkar et al., 2004). More recent studies in the AtT20 cell line demonstrated that CART could activate extracellular signal-regulated kinase (ERK) 1 and 2, which was blocked by pertussis toxin, suggesting the involvement of G-protein signaling in CART’s mechanism of action (Lakatos et al., 2005). Similarly, CART has been shown to potentiate insulin secretion via the cAMP/protein kinase A-dependent pathway in a clonal beta cell line (Wierup et al., 2006). These studies prompted a search for CART’s putative receptor or binding partner (Vicentic et al., 2006). Recent reports suggested the presence of specific binding sites for CART in cell lines and in vivo. In one study, CART binding in AtT20 cells was specific, saturable, time-dependent and altered by pH, temperature and protein concentration (Vicentic et al., 2006). In another study, using a fusion protein containing CART (55–102) and GFP, the authors demonstrated specific binding sites for CART on HepG2 cells and dissociated hypothalamic cells (Keller et al., 2006). The authors in this study also observed hypothalamic displaceable binding sites for CART in brain sections.
We have recently demonstrated that CART peptide exhibits neuroprotective properties against ischemic brain injury in vivo and against OGD-induced cell death in primary cultured cortical neurons. The neuroprotective effect of CART has been linked to the ERK activation (Xu et al., 2006) and to the upregulation of brain-derived neurotrophic factor (BDNF) (Wu et al., 2006). However, CART’s upstream signaling and the identity of its receptor or interacting partner remain elusive. Therefore, in the current study we set out to determine the mechanism of neuroprotection by CART. Using the yeast two-hybrid system, we screened a mouse brain cDNA library for a CART-binding partner, and uncovered a previously unrecognized CART binding to the mitochondrial enzyme SDH.
SDH is a membrane-bound enzyme of both the Krebs TCA cycle and CII of the electron transport chain, a crucial enzyme for intermediary metabolism and energy production. Consequently, CART’s interaction with SDH may potentially have immense physiological and pathophysiological consequences, and may be an important mechanism of its mechanisms of action. Accordingly, we demonstrated here that when added at relevant concentrations demonstrated to be neuroprotective in our previous study (Xu et al., 2006), CART enhanced mitochondrial function and energy production, as well as mitochondrial functional integrity and neuronal survival. CART also rescued the decline in SDH and CII activities after OGD. Suppression of SDH and CII disturbs electron transport, leading to cellular energy deficits and oxidative stress-related neuronal injury (Binienda et al., 2005). By preventing the decline in SDH activity, CART maintains mitochondrial respiration and energy production, reducing oxidative stress, stabilizing mitochondrial membrane potential, and preventing further mitochondrial damage and subsequent neuronal cell death.
In agreement with our previous study (Xu et al., 2006), CART was most effective in stimulating the mitochondrial function and protecting neurons at low nanomolar concentrations. At higher concentrations, CART either lost its ability to stimulate mitochondrial function or actually suppressed mitochondrial activity. This is also in agreement with our previous observation that, at higher concentrations, CART increased neuronal cell death in culture and was associated with higher mortality after experimental stroke in vivo (Xu et al., 2006). CART’s concentration-dependent effects are likely due to the entrainment of non-specific mechanisms that obscure CART’s protective mechanisms in mitochondria. For example, while low concentrations of CART exhibit a protective effect by preventing the decline in energy production after ischemia, the effect of higher CART concentrations on mitochondrial oxygen free radical formation is unknown.
The role of mitochondria in neuronal survival is well documented. Particularly in neurons, where energy demands are extremely high, adequate cellular energy level is critical for neuronal cell survival and function, and energy failure is an important mechanism of neuronal cell death in ischemic stroke (Lipton, 1999). Therefore, enhanced energy formation by CART and preservation of mitochondrial function after OGD are consistent with its neuroprotective action and may be an important mechanism of neuroprotection by CART. Other mitochondrial mechanisms, however, may also play a role in CART’s mechanism of neuroprotection. Specifically, in addition to its central role in coupling the respiratory chain to an oxidative phosphorylation role and ATP production, SDH, unlike other mitochondrial dehydrogenases, also has unique redox properties (Rustin et al., 2002; Yankovskaya et al., 2003). In partnership with ubiquinone (Hoppe et al., 1999; Shults et al., 2002), SDH represents a crucial antioxidant enzyme system in mitochondria. Therefore, CART binding to SDH may potentiate its antioxidant capacity. Other mitochondrial mechanisms may also play a role in mediating the neuroprotective effects of CART, including the maintenance of mitochondrial membrane potential, activation of mitochondrial KATP channel (Van den Top et al., 2006), inhibition of cytochrome C release and subsequent caspase activation. It should be emphasized, however, that CART may exhibit its protective effect through mechanisms other than its actions on the mitochondria. For example, CART’s direct G-protein-coupled receptor (GPCR) may also contribute to its neuroprotective properties, e.g. through inhibition of calcium influx (Yermolaieva et al., 2001), increased CREB phosphorylation (Sarkar et al., 2004), ERK activation (Lakatos et al., 2005) and upregulation of BDNF (Wu et al., 2006).
Considering the many functions of CART, this study not only has the potential to provide new insights into CART’s neuroprotective mechanism of action, but may also indicate a new mechanism for CART’s other functions, especially CART’s well-documented effect on energy metabolism and weight control. For example, it is well known that CART regulates body weight homeostasis by maintaining a balance between food intake and energy expenditure at the cellular and whole-body levels (Larsen & Hunter, 2006), and the mitochondria, which are central to both energy supply and expenditure, are a logical site of action for both processes. Similarly, CART has been shown to induce the expression of mitochondrial uncoupling proteins and increase lipid substrate utilization (Rohner-Jeanrenaud et al., 2002); both actions are likely a result of CART’s action on the mitochondria. Finally, it is important to note that CART’s effect on mitochondrial function may not be aimed teleologically at neuronal preservation during hypoxic and hypoglycemic conditions (OGD). CART is well known to have a physiological role in control of energy metabolism, thermoregulation and body weight, areas that could well entrain mitochondrial mechanisms (Kong et al., 2003), as evidenced, for example, by the observation that mutations in the cart gene are linked to reduced resting energy expenditure in obese children (del Giudice et al., 2001).
One issue that remains unresolved is the mechanism by which exogenous CART reaches the mitochondria. In addition to the actions of CART on a putative membrane-associated receptor, CART has also been shown to cross the blood–brain barrier rapidly, with no apparent self-inhibition or saturation, arguing against a receptor- or carrier-mediated transport (Kastin & Akerstrom, 1999). There are other examples of regulatory peptides similar to CART that interact with mitochondrial proteins. For example, there is evidence that melatonin may interact with mitochondrial electron transport chain proteins (Leon et al., 2004). There is also precedence for peptides with amino acid sequences and structural features that render them uniquely cell-permeable and especially targeted to and concentrated in mitochondria (Zhao et al., 2004). These peptides have been shown to reduce intracellular reactive oxygen species and cell death (Szeto, 2006), and have been demonstrated both in vitro and in vivo to be protective against ischemic brain and heart injury (Cho et al., 2007).
We previously identified CART as an estrogen-regulated gene in ischemic cerebral cortex, which plays an important role in estrogen-mediated neuroprotection. In agreement with our finding here that CART uses mitochondrial mechanisms of neuroprotection, recent studies suggest that the mechanism of neuroprotection by estrogen may also be linked to mitochondrial mechanisms. For example, estrogen increases mitochondrial sequestration of calcium and protects neurons against adverse consequences of excess cytoplasmic calcium. Estrogen has also been shown to promote mitochondrial respiration, ATP generation and antioxidant enzymes that offset the increase in free radical generation induced by increased respiration (Morrison et al., 2006). Furthermore, estrogen protects against amyloid beta neurotoxicity by limiting mitochondrial dysfunction via activation of anti-apoptotic mechanisms (Nilsen et al., 2006), and protects mitochondrial membrane potential collapse from insults by rendering the mitochondria more resistant to pro-oxidants, calcium loading and mitochondrial toxins (Simpkins et al., 2005). Finally, vasoprotection by estrogen is mediated, in part, by modulation of mitochondrial function, resulting in greater energy-producing capacity and decreased reactive oxygen species production (Stirone et al., 2005).
In summary, we here report that CART directly interacts with SDH, increasing SDH and CII activities and enhancing ATP synthesis under baseline conditions. More importantly, we found that CART preserves mitochondrial function and improves neuronal survival in cortical neurons after OGD. These findings suggest that the mitochondria may play a central role in CART’s mechanism of action. The findings also suggest that in addition to its reported protective effect against ischemic stroke, CART may also prove beneficial in neurodegenerative diseases and other conditions with mitochondrial involvement, including ageing.
Acknowledgments
This work was supported by PO1 NS049210 to N.J.A. and P.D.H., RO1 NS33668 to P.D.H., RO1 NS044313 to N.J.A. and by American Heart Association Award 0565527Z to P.M. We thank Dr Steven Dowdy for providing us with pTAT-2.1 and pTATGFP vectors, and Dr Yun Xu for the CART cDNA construct. We would like also to thank Dr Mohammad I. Sabri and Dr Gebretateos Woldegiorgis for their advice and guidance in carrying out these studies.
Abbreviations
- 3-NPA
3-nitropropionic acid
- β-Gal
β-galactosidase
- BDNF
brain-derived neurotrophic factor
- CART
cocaine- and amphetamine-regulated transcript
- CII
Complex II
- CREB
cAMP response element-binding protein
- DCIP
2,6-dichloroindiphenol
- EGFP
enhanced green fluorescent protein
- ERK
extracellular signal-regulated kinase
- MTT
3-(4,5-dimethylthiazol-2-yl)2,5-diphenyl-tetrazolium bromide
- NBM
neurobasal medium
- OGD
oxygen-glucose deprivation
- PBS
phosphate-buffered saline
- PCR
polymerase chain reaction
- PI
propidium iodide
- SDHB
mouse succinate dehydrogenase subunit B
- SDS
sodium dodecyl sulfate
References
- Binienda Z, Przybyla-Zawislak B, Virmani A, Schmued L. L-carnitine and neuroprotection in the animal model of mitochondrial dysfunction. Ann N Y Acad Sci. 2005;1053:174–182. doi: 10.1196/annals.1344.015. [DOI] [PubMed] [Google Scholar]
- Birch-Machin MA, Turnbull DM. Assaying mitochondrial respiratory complex activity in mitochondria isolated from human cells and tissues. In: Pon LA, Schon EA, editors. Mitochondria. Academic Press; San Diego: 2001. pp. 97–117. [DOI] [PubMed] [Google Scholar]
- Cho S, Szeto HH, Kim E, Kim H, Tolhurst AT, Pinto JT. A novel cell-permeable antioxidant peptide, SS31, attenuates ischemic brain injury by down-regulating CD36. J Biol Chem. 2007;282:4634–4642. doi: 10.1074/jbc.M609388200. [DOI] [PubMed] [Google Scholar]
- Damaj MI, Martin BR, Kuhar MJ. Antinociceptive effects of supraspinal rat cart (55)102) peptide in mice. Brain Res. 2003;983:233–236. doi: 10.1016/s0006-8993(03)03094-4. [DOI] [PubMed] [Google Scholar]
- De Cristobal J, Moro MA, Davalos A, Castillo J, Leza JC, Camarero J, Colado MI, Lorenzo P, Lizasoain I. Neuroprotective effect of aspirin by inhibition of glutamate release after permanent focal cerebral ischaemia in rats. J Neurochem. 2001;79:456–459. doi: 10.1046/j.1471-4159.2001.00600.x. [DOI] [PubMed] [Google Scholar]
- Douglass J, McKinzie AA, Couceyro P. PCR differential display identifies a rat brain mRNA that is transcriptionally regulated by cocaine and amphetamine. J Neurosci. 1995;15:2471–2481. doi: 10.1523/JNEUROSCI.15-03-02471.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedoroff S, Richardson A, editors. Protocols for Neural Cell Culture. Human Press; New York: 2001. [Google Scholar]
- Fields S, Song O. A novel genetic system to detect protein–protein interactions. Nature. 1989;340:245–246. doi: 10.1038/340245a0. [DOI] [PubMed] [Google Scholar]
- del Giudice EM, Santoro N, Cirillo G, D’Urso L, Di Toro R, Perrone L. Mutational screening of the CART gene in obese children: identifying a mutation (Leu34Phe) associated with reduced resting energy expenditure and cosegregating with obesity phenotype in a large family. Diabetes. 2001;50:2157–2160. doi: 10.2337/diabetes.50.9.2157. [DOI] [PubMed] [Google Scholar]
- Hoppe U, Bergemann J, Diembeck W, Ennen J, Gohla S, Harris I, Jacob J, Kielholz J, Mei W, Pollet D, Schachtschabel D, Sauermann G, Schreiner V, Stab F, Steckel F. Coenzyme Q10, a cutaneous antioxidant and energizer. Biofactors. 1999;9:71–78. doi: 10.1002/biof.5520090238. [DOI] [PubMed] [Google Scholar]
- Kastin AJ, Akerstrom V. Entry of CART into brain is rapid but not inhibited by excess CART or leptin. Am J Physiol. 1999;277:E901–E904. doi: 10.1152/ajpendo.1999.277.5.E901. [DOI] [PubMed] [Google Scholar]
- Keller PA, Compan V, Bockaert J, Giacobino JP, Charnay Y, Bouras C, Assimacopoulos-Jeannet F. Characterization and localization of cocaine- and amphetamine-regulated transcript (CART) binding sites. Peptides. 2006;27:1328–1334. doi: 10.1016/j.peptides.2005.10.016. [DOI] [PubMed] [Google Scholar]
- Kimmel HL, Gong W, Vechia SD, Hunter RG, Kuhar MJ. Intra-ventral tegmental area injection of rat cocaine and amphetamine-regulated transcript peptide 55)102 induces locomotor activity and promotes conditioned place preference. J Pharmacol Exp Ther. 2000;294:784–792. [PubMed] [Google Scholar]
- Kis B, Rajapakse NC, Snipes JA, Nagy K, Horiguchi T, Busija DW. Diazoxide induces delayed pre-conditioning in cultured rat cortical neurons. J Neurochem. 2003;87:969–980. doi: 10.1046/j.1471-4159.2003.02072.x. [DOI] [PubMed] [Google Scholar]
- Kong WM, Stanley S, Gardiner J, Abbott C, Murphy K, Seth A, Connoley I, Ghatei M, Stephens D, Bloom S. A role for arcuate cocaine and amphetamine-regulated transcript in hyperphagia, thermogenesis, and cold adaptation. FASEB J. 2003;17:1688–1690. doi: 10.1096/fj.02-0805fje. [DOI] [PubMed] [Google Scholar]
- Koylu EO, Balkan B, Kuhar MJ, Pogun S. Cocaine and amphetamine regulated transcript (CART) and the stress response. Peptides. 2006;27:1956–1969. doi: 10.1016/j.peptides.2006.03.032. [DOI] [PubMed] [Google Scholar]
- Kristensen P, Judge ME, Thim L, Ribel U, Christjansen KN, Wulff BS, Clausen JT, Jensen PB, Madsen OD, Vrang N, Larsen PJ, Hastrup S. Hypothalamic CART is a new anorectic peptide regulated by leptin. Nature. 1998;393:72–76. doi: 10.1038/29993. [DOI] [PubMed] [Google Scholar]
- Kuhar MJ, Jaworski JN, Hubert GW, Philpot KB, Dominguez G. Cocaine- and amphetamine regulated transcript peptides play a role in drug abuse and are potential therapeutic targets. AAPS J. 2005;7:E259–E265. doi: 10.1208/aapsj070125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakatos A, Prinster S, Vicentic A, Hall RA, Kuhar MJ. Cocaine- and amphetamine-regulated transcript (CART) peptide activates the extracellular signal-regulated kinase (ERK) pathway in AtT20 cells via putative G-protein coupled receptors. Neurosci Lett. 2005;384:198–202. doi: 10.1016/j.neulet.2005.04.072. [DOI] [PubMed] [Google Scholar]
- Larsen PJ, Hunter RG. The role of CART in body weight homeostasis. Peptides. 2006;8:1981–1986. doi: 10.1016/j.peptides.2005.11.027. [DOI] [PubMed] [Google Scholar]
- Larsen PJ, Seier V, Fink-Jensen A, Holst JJ, Warberg J, Vrang N. Cocaine- and amphetamine-regulated transcript is present in hypothalamic neuroendocrine neurones and is released to the hypothalamic-pituitary portal circuit. J Neuroendocrinol. 2003;15:219–226. doi: 10.1046/j.1365-2826.2003.00960.x. [DOI] [PubMed] [Google Scholar]
- Leon J, Acuna-Castroviejo D, Sainz RM, Mayo JC, Tan DX, Reiter RJ. Melatonin and mitochondrial function. Life Sci. 2004;75:765–790. doi: 10.1016/j.lfs.2004.03.003. [DOI] [PubMed] [Google Scholar]
- Lipton P. Ischemic cell death in brain neurons. Physiol Rev. 1999;79:1431–1568. doi: 10.1152/physrev.1999.79.4.1431. [DOI] [PubMed] [Google Scholar]
- Matsumura K, Tsuchihashi T, Abe I. Central human cocaine- and amphetamine-regulated transcript peptide 55)102 increases arterial pressure in conscious rabbits. Hypertension. 2001;38:1096–1100. doi: 10.1161/hy1101.092968. [DOI] [PubMed] [Google Scholar]
- Morrison JH, Brinton RD, Schmidt PJ, Gore AC. Estrogen, menopause, and the aging brain: how basic neuroscience can inform hormone therapy in women. J Neurosci. 2006;26:10332–10348. doi: 10.1523/JNEUROSCI.3369-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsen J, Chen S, Irwin RW, Iwamoto S, Brinton RD. Estrogen protects neuronal cells from amyloid beta-induced apoptosis via regulation of mitochondrial proteins and function. BMC Neurosci. 2006;7:74. doi: 10.1186/1471-2202-7-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell CS, Jackson RM. Mitochondrial complex I, aconitase, and succinate dehydrogenase during hypoxia-reoxygenation: modulation of enzyme activities by MnSOD. Am J Physiol Lung Cell Mol Physiol. 2003;285:L189–L198. doi: 10.1152/ajplung.00253.2002. [DOI] [PubMed] [Google Scholar]
- Rohner-Jeanrenaud F, Craft LS, Bridwell J, Suter TM, Tinsley FC, Smiley DL, Burkhart DR, Statnick MA, Heiman ML, Ravussin E, Caro JF. Chronic central infusion of cocaine- and amphetamine-regulated transcript (CART 55–102): effects on body weight homeostasis in lean and high-fat-fed obese rats. Int J Obes Relat Metab Disord. 2002;26:143–149. doi: 10.1038/sj.ijo.0801863. [DOI] [PubMed] [Google Scholar]
- Rustin P, Munnich A, Rötig A. Succinate dehydrogenase and human diseases: new insights into a well-known enzyme. Eur J Hum Genet. 2002;10:289–291. doi: 10.1038/sj.ejhg.5200793. [DOI] [PubMed] [Google Scholar]
- Sarkar S, Wittmann G, Fekete C, Lechan RM. Central administration of cocaine- and amphetamine-regulated transcript increases phosphorylation of cAMP response element binding protein in corticotropin-releasing hormone-producing neurons but not in prothyrotropin-releasing hormone-producing neurons in the hypothalamic paraventricular nucleus. Brain Res. 2004;999:181–192. doi: 10.1016/j.brainres.2003.11.062. [DOI] [PubMed] [Google Scholar]
- Scruggs P, Dun SL, Dun NJ. Cocaine- and amphetamine-regulated transcript peptide attenuates phenylephrine-induced bradycardia in anesthetized rats. Am J Physiol Regul Integr Comp Physiol. 2003;285:R1496–R1503. doi: 10.1152/ajpregu.00183.2003. [DOI] [PubMed] [Google Scholar]
- Shults CW, Oakes D, Kieburtz K, Beal MF, Haas R, Plumb S, Juncos JL, Nutt J, Shoulson I, Carter J, Kompoliti K, Perlmutter JS, Reich S, Stern M, Watts RL, Kurlan R, Molho E, Harrison M, Lew M. Effects of coenzyme Q10 in early Parkinson disease: evidence of slowing of the functional decline. Arch Neurol. 2002;59:1541–1550. doi: 10.1001/archneur.59.10.1541. [DOI] [PubMed] [Google Scholar]
- Simpkins JW, Wen Y, Perez E, Yang S, Wang X. Role of nonfeminizing estrogens in brain protection from cerebral ischemia: an animal model of Alzheimer’s disease neuropathology. Ann NY Acad Sci. 2005;1052:233–242. doi: 10.1196/annals.1347.019. [DOI] [PubMed] [Google Scholar]
- Stirone C, Duckles SP, Krause DN, Procaccio V. Estrogen increases mitochondrial efficiency and reduces oxidative stress in cerebral blood vessels. Pharmacology. 2005;68:959–965. doi: 10.1124/mol.105.014662. [DOI] [PubMed] [Google Scholar]
- Szeto HH. Mitochondria-targeted peptide antioxidants: novel neuroprotective agents. AAPS J. 2006;8:E521–E531. doi: 10.1208/aapsj080362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Top M, Lyons DJ, Lee K, Coderre E, Renaud LP, Spanswick D. Pharmacological and molecular characterization of ATP-sensitive K(+) conductances in cart and NPY/AgRP expressing neurons of the hypothalamic arcuate nucleus. Neuroscience. 2006 doi: 10.1016/j.neuroscience.2006.09.059. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Verweij BH, Muizelaar JP, Vinas FC, Peterson PL, Xiong Y, Lee CP. Mitochondrial dysfunction after experimental and human brain injury and its possible reversal with a selective N-type calcium channel antagonist (SNX-111) Neurol Res. 1997;19:334–339. doi: 10.1080/01616412.1997.11740821. [DOI] [PubMed] [Google Scholar]
- Vicentic A, Lakatos A, Jones D. The CART receptors: background and recent advances. Peptides. 2006;27:1934–1937. doi: 10.1016/j.peptides.2006.03.031. [DOI] [PubMed] [Google Scholar]
- Wierup N, Björkqvist M, Kuhar MJ, Mulder H, Sundler F. CART regulates islet hormone secretion and is expressed in the beta-cells of type 2 diabetic rats. Diabetes. 2006;55:305–311. doi: 10.2337/diabetes.55.02.06.db04-1383. [DOI] [PubMed] [Google Scholar]
- Wu B, Hu S, Yang M, Pan H, Zhu S. CART peptide promotes the survival of hippocampal neurons by upregulating brain-derived neurotrophic factor. Biochem Biophys Res Commun. 2006;347:656–661. doi: 10.1016/j.bbrc.2006.06.117. [DOI] [PubMed] [Google Scholar]
- Xu Y, Zhang W, Klaus J, Young J, Koerner I, Sheldahl LC, Hurn PD, Martinez-Murillo F, Alkayed NJ. Role of cocaine- and amphetamine-regulated transcript in estradiol-mediated neuroprotection. Proc Natl Acad Sci USA. 2006;103:14489–14494. doi: 10.1073/pnas.0602932103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yankovskaya V, Horsefield R, Tornroth S, Luna-Chavez C, Miyoshi H, Leger C, Byrne B, Cecchini G, Iwata S. Architecture of succinate dehydrogenase and reactive oxygen species generation. Science. 2003;299:700–704. doi: 10.1126/science.1079605. [DOI] [PubMed] [Google Scholar]
- Yermolaieva O, Chen J, Couceyro PR, Hoshi T. Cocaine- and amphetamine-regulated transcript peptide modulation of voltage-gated Ca2+ signaling in hippocampal neurons. J Neurosci. 2001;21:7474–7480. doi: 10.1523/JNEUROSCI.21-19-07474.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao K, Zhao GM, Wu D, Soong Y, Birk AV, Schiller PW, Szeto HH. Cell-permeable peptide antioxidants targeted to inner mitochondrial membrane inhibit mitochondrial swelling, oxidative cell death, and reperfusion injury. JBC. 2004;279:34682–34690. doi: 10.1074/jbc.M402999200. [DOI] [PubMed] [Google Scholar]