Abstract
Cicatricial contraction of preretinal fibrous membrane is a cause of severe vision loss in proliferative vitreoretinal diseases such as proliferative diabetic retinopathy (PDR) and proliferative vitreoretinopathy (PVR). TGF-β is overexpressed in the vitreous of patients with proliferative vitreoretinal diseases and is also detectable in the contractile membranes. Therefore, TGF-β is presumed to contribute to the cicatricial contraction of the membranes, however, the underlying mechanisms and TGF-β's importance among various other factors remain to be elucidated. Vitreous samples from PDR or PVR patients caused significantly larger contraction of hyalocyte-containing collagen gels, compared with nonproliferative controls. The contractile effect was strongly correlated with the vitreal concentration of activated TGF-β2 (r = 0.82, P < 0.0001). PDR or PVR vitreous promoted expression of α-smooth muscle actin (α-SMA) and phosphorylation of myosin light chain (MLC), a downstream mediator of Rho-kinase (ROCK), both of which were dramatically but incompletely suppressed by TGF-β blockade. In contrast, fasudil, a potent and selective ROCK inhibitor, almost completely blocked the vitreous-induced MLC phosphorylation and collagen gel contraction. Fasudil disrupted α-SMA organization, but it did not affect its vitreal expression. In vivo, fasudil significantly inhibited the progression of experimental PVR in rabbit eyes without affecting the viability of retinal cells by electroretinographic and histological analyses. These results elucidate the critical role of TGF-β in mediating cicatricial contraction in proliferative vitreoretinal diseases. ROCK, a key downstream mediator of TGF-β and other factors might become a unique therapeutic target in the treatment of proliferative vitreoretinal diseases.
Keywords: proliferative diabetic retinopathy, proliferative vitreoretinopathy, fibrosis, wound healing
Proliferative vitreoretinal diseases, such as proliferative diabetic retinopathy (PDR) and proliferative vitreoretinopathy (PVR), remain common causes of severe vision loss despite recent advancements in vitreoretinal surgery (1, 2). A better understanding of the pathogenesis of these diseases is critical for the development of urgently needed new pharmacologic treatments.
Excessive wound healing in proliferative vitreoretinal diseases leads to formation of cicatricial preretinal fibrous membranes. This process is characterized by the migration and proliferation of various cell types, including hyalocytes, retinal pigment epithelial cells (RPE), glial cells, and myofibroblast-like cells of unknown origin. These cells organize into the proliferative membrane (3, 4). Hyalocytes are found in the cortical vitreous in the vicinity of the inner retinal surface (5). Morphological studies suggest that hyalocytes may belong to the monocyte/macrophage lineage (6, 7). Hyalocytes are thought to originate from the bone marrow and differentiate in the vitreous (8). Under physiologic conditions, hyalocytes contribute to the transparency of the vitreous (9), however, they may also be involved in diseases of the vitreoretinal interface (3, 10). Specifically, in diabetic eyes, hyalocytes are found in higher numbers and their morphology is distinct from that in normal eyes (11).
Contraction of the proliferative membranes causes retinal detachment, which leads to photoreceptor apoptosis and ultimately to vision loss. Growth factors, such as TGF-β, PDGF (12, 13), insulin-like growth factor-1 (IGF-1) (13), and endothelins (14) regulate the cicatricial contraction of the preretinal membranes.
TGF-β is a multifunctional cytokine, regulating pivotal biological responses, such as differentiation, apoptosis, migration, immune cell function, and ECM synthesis (15). TGF-β has three isoforms (TGF-β1, -β2, and -β3) and is secreted as a biologically inactive latent complex. Latent TGF-β is activated by various chemical or enzymatic treatments (16–18).
TGF-β has been implicated in tissue contraction in fibrous diseases, such as liver cirrhosis, pulmonary fibrosis, and systemic sclerosis (19). In the eye, TGF-β is overexpressed in the vitreous of patients with PDR and PVR (20, 21) and is also identified in proliferative membranes in these diseases (22). Specifically, the concentration of TGF-β2, the predominant TGF-β isoform in the posterior segment of the eye (23), significantly correlates with the severity of PVR (20). In addition, we previously showed that TGF-β2 contributes to transdifferentiation of hyalocytes into α-smooth muscle actin (α-SMA) positive myofibroblast-like cells that cause collagen gel contraction (12). Taken together, TGF-β is presumed to contribute to the contraction of the cicatricial membranes in PDR and PVR. However, the direct evidence of the effect and the importance of TGF-β among various factors in the vitreous remain to be elucidated.
Phosphorylation of myosin light chain (MLC) is associated with actin-myosin interaction to form stress fibers and contractile rings, facilitating cell contraction and motility (24, 25). Rho-kinase (ROCK), a target protein of Rho, is implicated in cell adhesion, migration, proliferation, and apoptosis. ROCK regulates MLC phosphorylation both directly and via inactivation of the MLC phosphatase (25, 26). Previously we showed that TGF-β2 activates Rho, leading to MLC phosphorylation in hyalocytes (12, 21). A potent and selective ROCK inhibitor, fasudil, suppresses TGF-β2-induced MLC phosphorylation and collagen gel contraction by hyalocytes (12).
In the present study, we investigate the role of TGF-β in mediating cicatricial contraction in proliferative vitreoretinal diseases using vitreous samples. Furthermore, we demonstrate the underlying molecular mechanisms and the central role of ROCK in the cicatrical contraction, downstream of TGF-β and other growth factors.
Results
TGF-β1 and -β2 Concentration in the Vitreous.
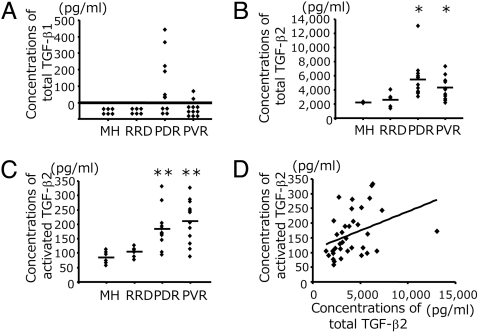
We quantified the concentration of total (activated and latent) TGF-β1 and -β2 protein in vitreous from PDR or PVR patients and nonproliferative controls [macular hole (MH) or rhegmatogenous retinal detachment (RRD)]. Total TGF-β1 was not detectable in MH or RRD samples. In contrast, total TGF-β1 was found in some of PDR and PVR samples (PDR, 115.4 ± 44.6 pg/ml; PVR, 12.7 ± 8.5 pg/ml) (Fig. 1A). Activated TGF-β1 was not detectable in any of the groups (data not shown). Vitreal concentrations of total TGF-β2 in patients with PDR (5563 ± 765 pg/ml) and PVR (4283 ± 455 pg/ml) were significantly higher than those from MH (2213 ± 34.3 pg/ml) and RRD (2643 ± 403 pg/ml) (Fig. 1B). Analogously, the vitreal concentrations of activated TGF-β2 in patients with PDR (180.3 ± 20.4 pg/ml) and PVR (211.9 ± 21.5 pg/ml) were significantly higher than those from MH (86.8 ± 9.1 pg/ml) and RRD (105.2 ± 7.4 pg/ml) (Fig. 1C). In addition, there was a significant correlation between the concentrations of total and activated TGF-β2 protein in the vitreous (r = 0.37, P = 0.026) (Fig. 1D). The average percent ratio of activated TGF-β2 to total TGF-β2 of all vitreal samples was 4.5 ± 0.3%.
Fig. 1.
Concentrations of total and activated TGF-β1 and -β2 protein in the vitreous. (A) Concentrations of total TGF-β1 in the vitreal samples were measured by ELISA (MH, n = 6; RRD, n = 6; PDR, n = 12; and PVR, n = 11). (B and C) Concentrations of total TGF-β2 (B) and activated TGF-β2 (C). (MH, n = 6; RRD, n = 6; PDR, n = 12; and PVR, n = 12). *: PDR vs. MH, P = 0.0007; PDR vs. RRD, P = 0.003; PVR vs. MH, P = 0.002; and PVR vs. RRD, P = 0.049. **: PDR vs. MH, P = 0.005; PDR vs. RRD, P = 0.015; PVR vs. MH, P = 0.002; and PVR vs. RRD, P = 0.005 (Mann-Whitney U-test). (D) Correlation between the concentrations of total and activated TGF-β2 (r = 0.37, P = 0.026).
Role of TGF-β in Vitreous-Induced Collagen Gel Contraction.
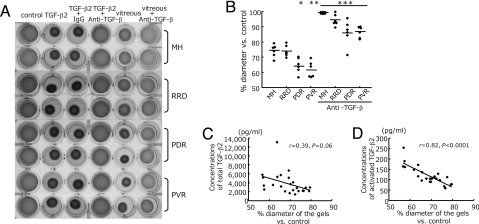
Recombinant TGF-β2 caused significant contraction of hyalocyte-containing collagen gels (58.7 ± 0.4% compared to the diameter of the control gels, n = 24, P < 0.0001; paired t-test), which was effectively blocked (99.8 ± 0.3%, P < 0.0001 vs. TGF-β2) with an anti-TGF-β (-β1, -β2, and -β3) mAb, while a mouse control IgG did not affect the TGF-β2-induced contraction (Fig. 2A).
Fig. 2.
Role of TGF-β in vitreous-induced collagen gel contraction. (A) Hyalocyte-containing collagen gels were exposed to control (DMEM), recombinant TGF-β2 (0.3 nM), or patient vitreous, with anti-TGF-β mAb (10 ng/ml) or control IgG (10 ng/ml) (n = 6 in each group). Two representative wells per condition, 3 days after stimulation, are shown. (B) The diameters of the gels treated with vitreous (lane 5 in A and vitreous with anti-TGF-β mAb lane 6 in A were measured and expressed as a percentage of the diameter of control (lane 1 in A). *, P = 0.01 vs. MH and P = 0.007 vs. RRD; **, P = 0.007 vs. MH and P = 0.004 vs. RRD; ***, P < 0.0001 vs. each disease without anti-TGF-β mAb. (C and D) The correlation of the diameters of the gels with concentration of total TGF-β2 in the vitreous (n = 24, r = 0.39, P = 0.06) (C) and with activated TGF-β2 (n = 24, r = 0.82, P < 0.0001) (D).
Vitreous samples with proliferative (PDR, 64.6 ± 2.1% and PVR, 61.8 ± 2.1%) and nonproliferative (MH, 74.1 ± 1.7% and RRD, 73.9 ± 1.5%) vitreoretinal diseases significantly decreased the diameter of hyalocyte-containing collagen gels, compared with DMEM-treated control (n = 6, P < 0.0001 in each group; Mann-Whitney U-test). Furthermore, PDR or PVR vitreous caused hypercontraction of hyalocyte-containing collagen gels as compared with vitreous with MH (PDR, P = 0.01 and PVR, P = 0.007; Mann-Whitney U-test) or RRD (PDR, P = 0.007 and PVR, P = 0.004). The vitreous-induced collagen gel contraction was significantly suppressed with an anti-TGF-β mAb (MH, 99.1 ± 0.3%; RRD, 94.2 ± 1.6%; PDR, 86.2 ± 3.4% and PVR, 86.6 ± 1.5%, P < 0.0001 in each; paired t-test) (Fig. 2 A and B), but was not affected by a mouse control IgG [supporting information (SI) Fig. S1]. The concentration of activated TGF-β2 in the vitreal samples showed a strong correlation with the reduction in diameter of the gels (r = 0.82, P < 0.0001). However, there was no significant correlation between the concentration of total TGF-β2 and the gel diameter (r = 0.39, P = 0.06) (Fig. 2 C and D).
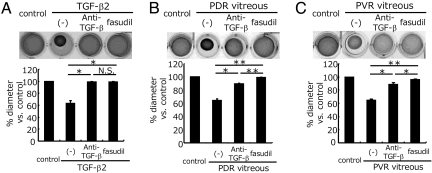
Impact of ROCK Inhibition on PDR/PVR-Induced Collagen Gel Contraction.
Both TGF-β inhibition with a mAb and ROCK inhibition with fasudil almost completely suppressed the TGF-β2-induced collagen gel contraction (average diameter of the gels compared with the medium-treated controls: TGF-β2, 63.4 ± 4.1%; TGF-β2/anti-TGF-β, 99.3 ± 0.4%; TGF-β2/fasudil, 98.9 ± 0.7%) (Fig. 3A). However, TGF-β blockade significantly but incompletely inhibited the collagen gel contraction, induced by PDR/PVR vitreal samples (PDR, 64.4 ± 2.3%; PDR/anti-TGF-β, 89.5 ± 1.1%; PVR, 64.6 ± 0.3%; and PVR/anti-TGF-β, 88.2 ± 2.8%). In comparison, fasudil almost completely suppressed the contraction of collagen gels treated with PDR (99.3 ± 0.5%) and PVR (95.9 ± 1.5%) samples. The impact of fasudil on PDR/PVR-induced collagen gel contraction was significantly higher than that of TGF-β blockade (Fig. 3 B and C).
Fig. 3.
Suppressive effect of fasudil on PDR/PVR-induced collagen gel contraction. Hyalocyte-containing collagen gels were stimulated with recombinant TGF-β2 (A), PDR vitreous (B), or PVR vitreous (C) (n = 3, each group) with or without anti-TGF-β mAb or fasudil (20 μM) for 3 days. Thereafter, the gels were photographed and the diameter of the gels was measured and statistically analyzed. Representative results are shown. *, P < 0.05; **, P < 0.01; NS, not significant (paired t test).
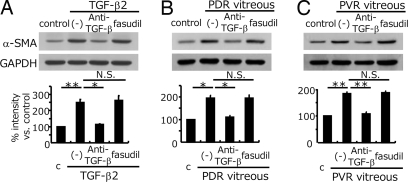
Role of TGF-β in the PDR/PVR-Induced α-SMA Expression.
Recombinant TGF-β2 enhanced the expression of α-SMA protein, an indicator of myofibroblastic transdifferentiation (2.5 ± 0.2 folds compared to medium-treated control), which was significantly reduced by anti-TGF-β mAb treatment (1.1 ± 0.04 folds). However, fasudil did not reduce TGF-β2-induced α-SMA upregulation (2.6 ± 0.3 folds) (Fig. 4A). The PDR and PVR vitreal samples caused a significant 1.9 ± 0.1 and 1.8 ± 0.1 fold increase in α-SMA expression compared with the medium-treated controls. These increases in α-SMA expression were significantly suppressed by TGF-β inhibition (PDR/anti-TGF-β, 1.1 ± 0.1 folds and PVR/anti-TGF-β, 1.1 ± 0.1 folds). However, fasudil did not significantly reduce the increase in α-SMA expression, caused by PDR (1.95 ± 0.1 folds) or PVR (1.9 ± 0.1 folds) vitreous (Fig. 4 B and C). Immunohistochemical analysis of the hyalocyte-containing collagen gels showed increased α-SMA expression with TGF-β2 and PDR vitreous treatment (Fig. S2 A, B, and D). Fasudil disrupted α-SMA organization without affecting its expression (Fig. S2 C and E).
Fig. 4.
Role of TGF-β in the enhanced vitreous-induced α-SMA expression. The gels from Fig. 3 were dissolved and the cells were isolated and lysed. α-SMA expressions were examined by Western blot analysis. Loaded cell lysates in A, B, and C were from the gels in Fig. 3 A, B, and C, respectively. Representative blots are shown. Signal intensities were quantified and expressed as percentages of the α-SMA/GAPDH ratio compared with control (treated with DMEM). *, P < 0.05; **, P < 0.01; NS, not significant (paired t test).
Impact of ROCK Inhibition on Vitreous-Induced MLC Phosphorylation.
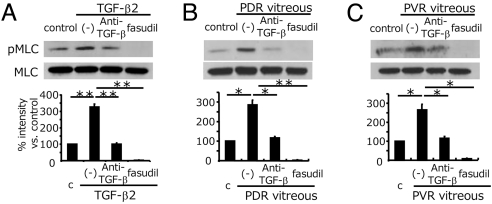
MLC phosphorylation was significantly increased in the presence of recombinant TGF-β2 (3.3 ± 0.2 folds) (Fig. 5A), PDR vitreous (2.8 ± 0.3 folds) (Fig. 5B) and PVR vitreous (2.6 ± 0.3 folds) (Fig. 5C), compared with medium-treated controls. TGF-β blockade significantly suppressed MLC phosphorylation, induced by TGF-β2 (1.01 ± 0.06 folds), PDR (1.2 ± 0.06 folds), and PVR (1.1 ± 0.1 folds) vitreous. In comparison, fasudil nearly abolished MLC phosphorylation, induced by TGF-β (0.03 ± 0.01 fold), PDR (0.03 ± 0.01 fold), and PVR (0.07 ± 0.03 fold) vitreous.
Fig. 5.
Impact of fasudil on vitreous-induced MLC phosphorylation. After pretreatment with or without anti-TGF-β mAb or fasudil, hyalocytes were stimulated with recombinant TGF-β2 (A), vitreous with PDR (B), or vitreous with PVR (C) for 24 h (n = 3, each). Western blot analysis was performed to detect phosphorylated MLC (pMLC). Lane-loading differences were normalized by MLC. Signal intensities were quantified and expressed as percentages of the pMLC/MLC ratio compared with control (treated with DMEM). *, P < 0.05; **, P < 0.01.
Impact of ROCK Inhibition on In Vivo Experimental PVR.
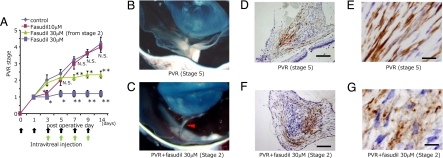
In the vehicle-treated controls, PVR progressed with time up to 14 days after induction (Fig. 6A). Fasudil at a final intravitreal concentration of 10 μM inhibited only the early progression of PVR up to the third day after disease induction. In contrast, fasudil at a concentration of 30 μM effectively suppressed the progression of the PVR from day 3 up to day 14. In addition, treatment of stage-2 PVR with fasudil, from the third or fifth day after induction, when proliferative membranes have already formed and adhered to retinal tissue, effectively blocked PVR progression. The concentrations of both total TGF-β1 and -β2 and activated TGF-β2 were significantly elevated in the vitreous with stage-5 PVR compared with control vitreous collected before disease induction (Fig. S3 A–C). Activated TGF-β1 was not detectable in any sample (data not shown). In the vehicle-treated eyes, contractile proliferative membranes formed (stage-5 PVR), causing total retinal detachment (Fig. 6B). In contrast, in the eyes treated with 30 μM fasudil from stage-2 PVR, stereomicroscopy revealed noncontractile membranes that were adherent to the retinal surface (Fig. 6C). Immunohistochemistry revealed expression and organization of α-SMA in the contractile preretinal proliferative membranes in the vehicle-treated eyes (Fig. 6 D and F). In fasudil-treated eyes, α-SMA was similarly expressed, however, its organization was disrupted and the membranes were noncontractile (Fig. 6 E and G).
Fig. 6.
Experimental PVR in rabbit eyes. (A) Therapeutic potential of fasudil in reducing the progression of experimental PVR. PVR was classified into six stages (0–5). Rhombus, vehicle (n = 5); purple square, fasudil 10 μM (n = 5); trigone, fasudil 30 μM from stage 2 (n = 6); blue square, fasudil 30 μM (n = 5). *, P < 0.05; **, P < 0.01;, not significant vs. vehicle. (B and C) Tractional retinal detachment because of formation and cicatricial contraction of preretinal proliferative membrane was observed by stereomicroscopy in vehicle-treated eyes (stage 5 PVR). (D and F) In contrast, intravitreal membranes adhered to the retina without causing retinal detachment (arrowhead) in 30 μM fasudil-treated eyes with stage 2 PVR. Micrographs depict α-SMA expression (brown) in preretinal proliferative membrane with stage 5 PVR (D) and stage 2 PVR (F) by immunohistochemical analysis. (Scale bar, 200 μm.) (E and G) Magnified images of D and F, respectively. (Scale bar, 10 μm.)
Lack of Apparent Toxicity of Fasudil in Retinal Structure and Function.
Animals were treated with repeated intravitreal injections of fasudil at 10- or 30-μM concentrations and 28 days later electroretinography and histology were performed. Electroretinographic analysis did not show a significant difference between the mean amplitude and latency of 2-Hz b-wave among the vehicle-treated animals (204.8 ± 4.6 mV and 28.1 ± 0.4 msec) and 10 μM (203.6 ± 7.2 mV and 28.0 ± 0.2 msec) and 30 μM among the fasudil-treated animals (200.9 ± 3.9 mV and 28.2 ± 0.4 msec) (Fig. S4A). The morphological examination of the eyes that were injected with 30 μM fasudil did not show signs of retinal damage or inflammatory cell infiltration in light microscopy or transmission EM (Fig. S4 B–E). Furthermore, TUNEL positive cells were found in the outer and inner nuclear layers of detached retinas from eyes with PVR, while no TUNEL positivity was found in 30 μM fasudil-treated eyes (Fig. S4 F and G). Unlike in humans and rodents, in rabbits blood vessels are limited to the medullary ray. Therefore, electroretinographic and histological analysis were also performed in fasudil-treated rats. Intravitreal fasudil did not show any obvious toxicity up to 100 μM concentration (Fig. S5). Furthermore, while 30 μM fasudil slightly but significantly reduced serum-induced proliferation of cultured rabbit conjunctival fibroblasts, the same concentration of fasudil did not affect the number of cells with serum-free DMEM or control vitreous (Fig. S6).
Discussion
This study elucidates the contractile property of human vitreous and provides direct evidence for the critical role of TGF-β in the pathogenesis of proliferative vitreoretinal diseases such as PDR and PVR. Furthermore, we demonstrate the therapeutic potential of fasudil, a potent and selective ROCK inhibitor, in the management of these diseases.
Vitreous from patients with PDR/PVR significantly enhanced contraction of hyalocyte-containing collagen gels, compared with vitreous from patients with nonproliferative diseases. This suggested the existence of vitreal factors that initiate a cascade of events leading to cicatricial contraction of the proliferative membranes in PDR/PVR patients. This insight is of clinical relevance, as it indicates that removal of the vitreous, i.e., through vitrectomy, would diminish the pathogenic factors and might be an effective treatment in a subset of patients with these diseases.
PDR/PVR-induced collagen gel contraction was dramatically suppressed by TGF-β blockade, emphasizing the central role of this cytokine in the events that lead to cicatricial contraction of the proliferative membranes. Our results further indicate that TGF-β2 is the predominantly responsible isoform in the pathogenesis. This may be because of the fact that the concentrations of total and activated TGF-β1 were negligibly low, compared with those of TGF-β2, in line with previous reports (20, 23). Furthermore, there was a strong correlation between the concentrations of activated TGF-β2 and the contractile effects, emphasizing the important role of this isoform in the pathology of PDR or PVR.
Vitreous from patients with nonproliferative diseases such as MH and RRD also caused significant collagen gel contraction, albeit substantially less than vitreous from PDR or PVR patients, which was effectively suppressed by TGF-β blockade. TGF-β is constitutively expressed in the vitreous at much lower concentrations than found in PDR/PVR patients. TGF-β contributes to the ocular immune privilege (27), and its expression under normal conditions suggests that normal vitreous might also have contractile properties. However, even though normal vitreous and vitreous in nonproliferative diseases have contractile potential, without proliferative membranes, retinal traction may not occur in vivo. This suggests that the formation of proliferative membranes, which is regulated by cytokines other than TGF-β, including PDGF (6), hepatocyte growth factor (HGF) (28), and monocyte chemotactic protein-1 (MCP-1) (29), is also critical in the pathogenesis of proliferative vitreoretinal diseases.
Collagen gel contraction strongly correlated with the concentration of activated TGF-β2 in the vitreous, but not with that of total (activated + latent) TGF-β2. Our results suggest that only activated TGF-β2 in the vitreous causes collagen gel contraction, while latent TGF-β2 may serve as a precursor pool for the generation of activated TGF-β2. In addition, there was a mild but significant correlation between activated and total TGF-β2. Therefore, the significantly elevated concentration of total TGF-β2 in the vitreous in proliferative vitreoretinal diseases may also be clinically relevant.
Our results provide novel molecular insight into the mechanism of contraction in PDR/PVR. PDR/PVR vitreous enhanced α-SMA expression and MLC phosphorylation, both of which were dramatically suppressed by TGF-β blockade. This suggests that PDR/PVR vitreous may exert their procontractile property by furthering myofibroblastic transdifferentiation or MLC phosphorylation in targeted cells, such as hyalocytes. Furthermore, our results single out TGF-β as one of the main factors responsible for the procontractile property of PDR/PVR vitreous. However, other factors may also significantly contribute to the cicatricial contraction, as TGF-β blockade does not abolish the procontractile property of PDR/PVR vitreous. PDGF, IGF-1, and endothelins are also thought to be involved in the cicatricial contraction of the proliferative membrane (12–14), albeit to a lesser extent than TGF-β, as suggested in the present study.
Fasudil abolished the PDR/PVR-induced collagen gel contraction. While PDR/PVR-induced α-SMA expression was unaffected by fasudil, α-SMA organization was effectively disrupted. This suggests that fasudil might diminish the contractile property of proliferative membranes by affecting α-SMA organization, in line with previous findings (30). In addition, fasudil almost completely suppressed MLC phosphorylation, a contraction-associated molecule and a mediator of TGF-β signaling. Previously we showed that PDGF causes transient MLC phosphorylation in hyalocytes and that fasudil completely blocks it (12). Furthermore, IGF-1 and endothelin-1-induced contraction are in part dependent on MLC phosphorylation through the activation of the Rho/ROCK pathway and are effectively suppressed by ROCK inhibitors (31–33). Furthermore, ROCK inhibitors reduce cell adhesion, migration, and proliferation, and ECM production, which contribute to the formation of proliferative membranes (21, 25, 26). Our results indicate that ROCK inhibition by fasudil suppresses PVR progression even after the proliferative membranes are formed and have adhered to the retina.
Because ROCK is the common downstream mediator of various growth factors, including TGF-β, which lead to cicatrical contraction, and ROCK inhibition with fasudil reduces PDR/PVR-induced contraction more strongly than TGF-β inhibition, ROCK might be a more attractive therapeutic target than TGF-β in the treatment of cicatricial contraction of preretinal membranes. Furthermore, because TGF-β is a multifunctional cytokine, its inhibition may cause considerable side effects (34). In contrast, fasudil has already proven to be relatively safe and effective in the treatment of cardiovascular diseases, including cerebral and coronary vasospasm, angina, hypertension, pulmonary hypertension, and heart failure (35).
In summary, this work demonstrates the therapeutic potential of fasudil in halting the progression of experimental PVR, without morphological or functional indication of retinal toxicity. Our results suggest that ROCK inhibition might become a therapeutic strategy in the management of proliferative vitreoretinal diseases such as PDR and PVR.
Materials and Methods
Preparation of Vitreal Samples and ELISA.
This study was carried out with approval from the Institutional Review Board and performed in accordance with the ethical standards of the 1989 Declaration of Helsinki. A written informed consent was obtained from each participant. Vitreal samples (1000–1200 μml) were collected from patients who underwent vitrectomy because of MH, RRD, PDR, or PVR. Samples with obvious bleeding were excluded. Vitreal samples were immediately centrifuged and the supernatants were snap frozen. Concentrations of TGF-β1 or -β2 in the vitreous were measured using human TGF-β1 or -β2 immunoassay kits (R&D Systems). The samples were treated with hydrochloric acid before measuring the total (activated + latent) TGF-β. Concentrations of TGF-β isoforms <31.2 pg/ml were considered undetectable.
Cell Culture.
Hyalocytes were isolated and cultured from bovine eyes (21). Cultured hyalocytes obtained between four and seven passages with normal morphology were used in experiments.
Collagen Gel Contraction Assay.
A mixture (0.5 ml) of hyalocytes, type I collagen (Koken Co. Ltd., Tokyo Japan), two different reconstitution buffers, and distilled water (final cell density, 2 × 105 cells/ml) was added to a 24-multiwell plate (36). After 48 h incubation with DMEM containing 10% FBS, the cells were starved with DMEM without any serum overnight. Following the 30-min pretreatment of both the cells and vitreal samples with or without anti-TGF-β (-β1, -β2, and -β3) mAb (10 ng/ml, R&D Systems) or fasudil (20 μM, generous gift of Asahi Kasei Pharma Corp., Tokyo, Japan), the cells were stimulated with recombinant TGF-β2 (0.3 nM, Sigma-Aldrich, Tokyo, Japan) or undiluted vitreal samples (300 μl/well). Mouse IgG (10 ng/ml, Sigma-Aldrich) was added for confirming the absence of nonspecific suppression of the contraction by anti-TGF-β mAb. The collagen gel diameter was measured 3 days after stimulation. The gels were dissolved by collagenase and the cells were isolated and lysed. The protein extracts were subjected to Western blot analysis for the detection of α-SMA (1:2000, Sigma-Aldrich) (36). Lane-loading differences were normalized by GAPDH (1:1000, Santa Cruz Biotechnology, Santa Cruz, CA). Signal intensities were quantified using ImageJ.
MLC Phosphorylation.
Hyalocytes were seeded in collagen-coated 12-well plates. After overnight starvation with serum free DMEM, the cells were treated with PDR or PVR vitreal samples (300 μl/well), with or without anti-TGF-β mAb or fasudil for 24 h. Total cell lysates were extracted and Western blotting was performed to detect phosphorylated MLC (Thr-18/Ser-19, Santa Cruz Biotechnology). Lane-loading differences were normalized by MLC (Santa Cruz Biotechnology).
Rabbit PVR Model.
Approval for experimentation with animals was obtained from the Committee on the Ethics of Animal Experiments, Kyushu University Graduate School of Medical Science, Japan. The procedures adhered to the guidelines from the Association for Research in Vision and Ophthalmology (ARVO) for animal use in research. Experimental PVR was induced by the intravitreal injection of conjunctival fibroblasts (5 × 104 in 0.1 ml DMEM) (37). On days 0, 1, 3, 5, 7, and 9 after fibroblast injection, vehicle or fasudil, which was dissolved with 0.1 ml intraocular irrigating solution (Opeguard-MA, Senju Pharmaceutical, Osaka, Japan) at final intravitreal concentration of 0 (vehicle), 10, or 30 μM, were injected into the vitreous cavity. The eyes were examined by ophthalmoscopy on days 1, 3, 5, 7, 9, and 14 by masked observers. PVR was classified into six stages using the clinical criteria (38) (Table S1 and Fig. S7 A–C). An additional group of animals was treated with fasudil from stage-2 PVR, on day 3 or 5. In that group, fasudil at 30 μM concentration was injected on days 3, 5, 7, and 9. After the last examination, the rabbits were euthanized by i.v. injection of pentobarbital sodium and the eyes were enucleated.
Immunohistochemistry.
Paraffin-embedded sections were incubated with mouse anti-α-SMA mAb (1:100). The rinsed sections were then subjected to peroxidase-labeled secondary Ab (1:250). Peroxidase activity was visualized using diaminobenzidine, and nuclei were counterstained with hematoxylin, observed with light microscopy.
Statistics.
All results were expressed as mean ± SEM. The statistical significance of differences between groups was analyzed by Mann-Whitney U-test or 2-tailed Student's t-test. Differences were considered significant at P < 0.05.
SI.
Further information is available in SI Materials and Methods.
Supplementary Material
Acknowledgments.
We thank Marion W. Knight and Edward F. Knight and Research to Prevent Blindness. We acknowledge Asahi Kasei Pharma Corporation, Tokyo, Japan for its generous provision of fasudil. This study was supported in part by grants from the Ministry of Education, Science, Sports and Culture, Japan (Grant-in-Aid for Scientific Research nos. 19592026, 18791283, and 18591925). This work was supported by National Institutes of Health Grants AI-050775, EY-14104, and HL-086933, a Research Fellowship Award from Bausch & Lomb, and a Fellowship Award from the Japan Eye Bank Association (to S.N.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0804054105/DCSupplemental.
References
- 1.Fong DS, et al. American Diabetes Association: Diabetic retinopathy. Diabetes Care. 2003;26:S99–S102. doi: 10.2337/diacare.26.2007.s99. [DOI] [PubMed] [Google Scholar]
- 2.Pastor JC, de la Rua ER, Martin F. Proliferative vitreoretinopathy: Risk factors and pathology. Prog Retin Eye Res. 2002;21:127–144. doi: 10.1016/s1350-9462(01)00023-4. [DOI] [PubMed] [Google Scholar]
- 3.Kampik A, Kenyon KR, Michels RG, Green WR, de la Cruz ZC. Epiretinal and vitreous membranes: Comparative study of 56 cases. Arch Ophthalmol. 1981;99:1445–1454. doi: 10.1001/archopht.1981.03930020319025. [DOI] [PubMed] [Google Scholar]
- 4.Jerdan JA, et al. Proliferative vitreoretinopathy membranes: An immunohistochemical study. Ophthalmology. 1989;96:801–810. doi: 10.1016/s0161-6420(89)32818-1. [DOI] [PubMed] [Google Scholar]
- 5.Salu P, Claeskens W, De Wilde A, Hijmans W, Wisse E. Light and electron microscopic studies of the rat hyalocyte after perfusion fixation. Ophthalmic Res. 1985;17:125–130. doi: 10.1159/000265363. [DOI] [PubMed] [Google Scholar]
- 6.Noda Y, et al. Functional properties of hyalocytes under PDGF-rich conditions. Invest Ophthalmol Vis Sci. 2004;45:2107–2114. doi: 10.1167/iovs.03-1092. [DOI] [PubMed] [Google Scholar]
- 7.Lazarus HS, Hageman GS. In situ characterization of the human hyalocytes. Arch Ophthalmol. 1994;112:1356–1362. doi: 10.1001/archopht.1994.01090220106031. [DOI] [PubMed] [Google Scholar]
- 8.Qiao H, et al. The characterization of hyalocytes: The origin, phenotype, and turnover. Br J Ophthalmol. 2005;89:513–517. doi: 10.1136/bjo.2004.050658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lang RA, Bishop JM. Macrophages are required for cell death and tissue remodeling in the developing mouse eye. Cell. 1993;74:453–462. doi: 10.1016/0092-8674(93)80047-i. [DOI] [PubMed] [Google Scholar]
- 10.Kampik A, Green WR, Michels RG, Nase PK. Ultrastructual features of progressive idiopathic epiretinal membrane removed by vitreous surgery. Am J Ophthalmol. 1980;90:797–809. doi: 10.1016/s0002-9394(14)75195-5. [DOI] [PubMed] [Google Scholar]
- 11.Faulborn J, Dunker S, Bowald S. Diabetic vitreopathy: Findings using the celloidin embedding technique. Ophthalmologica. 1998;212:369–376. doi: 10.1159/000027370. [DOI] [PubMed] [Google Scholar]
- 12.Hirayama K, et al. The involvement of the Rho-kinase pathway and its regulation in cytokine-induced collagen gel contraction by hyalocytes. Invest Ophthalmol Vis Sci. 2004;45:3896–3903. doi: 10.1167/iovs.03-1330. [DOI] [PubMed] [Google Scholar]
- 13.Hardwick C, et al. Tractional force generation by porcine Müller cells stimulation by growth factors in human vitreous. Invest Ophthalmol Vis Sci. 1997;38:2053–2063. [PubMed] [Google Scholar]
- 14.Guidry C, Hook M. Endothelins produced by endothelial cells promote collagen gel contraction by fibroblasts. J Cell Biol. 1991;115:873–880. doi: 10.1083/jcb.115.3.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Massague J. TGF-beta signal transduction. Annu Rev Biochem. 1998;67:753–791. doi: 10.1146/annurev.biochem.67.1.753. [DOI] [PubMed] [Google Scholar]
- 16.Brown PD, Wakefield LM, Levinson AD, Sporn MB. Physicochemical activation of recombinant latent transforming growth factor-beta's 1, 2 and 3. Growth Factors. 1990;3:35–43. doi: 10.3109/08977199009037500. [DOI] [PubMed] [Google Scholar]
- 17.Lypns RM, Gentry LE, Purchio AF, Moses HL. Mechanism of activation of latent recombinant transforming growth factor-b1 by plasmin. J Cell Biol. 1990;110:1361–1367. doi: 10.1083/jcb.110.4.1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miyazono K, Heldin CH. Role for carbohydrate structures in TGF-b1 latency. Nature. 1989;338:158–160. doi: 10.1038/338158a0. [DOI] [PubMed] [Google Scholar]
- 19.Border WA, Noble NA. Transforming growth factor b in tissue fibrosis. N Engl J Med. 1994;331:1286–1292. doi: 10.1056/NEJM199411103311907. [DOI] [PubMed] [Google Scholar]
- 20.Connor TB, Jr., et al. Correlation of fibrosis and transforming growth factor-b type 2 levels in the eye. J Clin Invest. 1989;83:1661–1666. doi: 10.1172/JCI114065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kita T, et al. Transforming growth factor-b2 and connective tissue growth factor in proliferative vitreoretinal diseases: Possible involvement of hyalocytes and therapeutic potential of Rho kinase inhibitor. Diabetes. 2007;56:231–238. doi: 10.2337/db06-0581. [DOI] [PubMed] [Google Scholar]
- 22.Bochaton-Piallat ML, et al. TGF-beta1, TGF-beta receptor II and ED-A fibronectin expression in myofibroblast of vitreoretinopathy. Invest Ophthalmol Vis Sci. 2000;41:2336–2342. [PubMed] [Google Scholar]
- 23.Pfeffer BA, Flanders KC, Guerin CJ, Danielpour D, Anderson DH. Transforming growth factor beta2 is the predominant isoform in the neural retina, retinal pigment epithelium-choroid and vitreous of the monkey eye. Exp Eye Res. 1994;59:323–333. doi: 10.1006/exer.1994.1114. [DOI] [PubMed] [Google Scholar]
- 24.Kamm KE, Stull JT. The function of myosin and myosin light chain kinase phosphorylation in smooth muscle. Annu Rev Pharmacol Toxicol. 1985;25:593–620. doi: 10.1146/annurev.pa.25.040185.003113. [DOI] [PubMed] [Google Scholar]
- 25.Fukata Y, Amano M, Kaibuchi K. Rho-Rho-kinase pathway in smooth muscle contraction and cytoskeletal reorganization of non-muscle cells. Trends Pharmacol Sci. 2001;22:32–39. doi: 10.1016/s0165-6147(00)01596-0. [DOI] [PubMed] [Google Scholar]
- 26.Seasholtz TM, Majumdar M, Kaplan DD, Brown JH. Rho and rho kinase mediate thrombin-stimulated vascular smooth muscle cell DNA synthesis and migration. Circ Res. 1999;84:1186–1193. doi: 10.1161/01.res.84.10.1186. [DOI] [PubMed] [Google Scholar]
- 27.Streilein JW, Wilbanks GA, Cousins SW. Immunoregulatory mechanisms of the eye. J Neuroimmunol. 1992;39:185–200. doi: 10.1016/0165-5728(92)90253-h. [DOI] [PubMed] [Google Scholar]
- 28.Grierson I, et al. Hepatocyte growth factor/scatter factor in the eye. Prog Retin Eye Res. 2000;19:779–802. doi: 10.1016/s1350-9462(00)00015-x. [DOI] [PubMed] [Google Scholar]
- 29.Han QH, et al. Migration of retinal pigment epithelial cells in vitro modulated by monocyte chemotactic protein-1: Enhancement and inhibition. Graefes Arch Clin Exp Ophthalmol. 2001;239:531–538. doi: 10.1007/s004170000212. [DOI] [PubMed] [Google Scholar]
- 30.Bogatkevich GS, et al. Contractile activity and smooth muscle a-actin organization in thrombin-induced human lung myofibroblasts. Am J Physiol Lung Mol Physiol. 2003;285:L334–L343. doi: 10.1152/ajplung.00417.2002. [DOI] [PubMed] [Google Scholar]
- 31.Zhang X, et al. Multiple signaling pathways are activated during insulin-like growth factor-I (IGF-I) stimulated breast cancer cell migration. Breast Cancer Res Treat. 2005;93:159–168. doi: 10.1007/s10549-005-4626-8. [DOI] [PubMed] [Google Scholar]
- 32.Miao L, Dai Y, Zhang J. Mechanism of RhoA/Rho kinase activation in endothelin-1-induced contraction in rabbit basilar artery. Am J Physiol Heart Circ Physiol. 2002;283:H983–989. doi: 10.1152/ajpheart.00141.2002. [DOI] [PubMed] [Google Scholar]
- 33.Kernochan LE, et al. Endothelin-1 stimulates human colonic myofibroblast contraction and migration. Gut. 2002;50:65–70. doi: 10.1136/gut.50.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Blobe GC, Schiemann WP, Lodish HF. Role of transforming growth factor beta in human disease. N Engl J Med. 2000;342:1350–1358. doi: 10.1056/NEJM200005043421807. [DOI] [PubMed] [Google Scholar]
- 35.Shimokawa H, Rashid M. Development of Rho-kinase inhibitors for cardiovascular medicine. Trends Pharmacol Sci. 2007;28:296–302. doi: 10.1016/j.tips.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 36.Kita T, et al. Functional characteristics of connective tissue growth factor on vitreoretinal cells. Diabetes. 2007;56:1421–1428. doi: 10.2337/db06-1644. [DOI] [PubMed] [Google Scholar]
- 37.Sakamoto T, et al. Inhibition of experimental proliferative vitreoretinopathy by retroviral vector-mediated transfer of suicide gene: Can proliferative vitreoretinopathy be a target of gene therapy? Ophthalmology. 1995;102:1417–1424. doi: 10.1016/s0161-6420(95)30850-0. [DOI] [PubMed] [Google Scholar]
- 38.Fastenberg DM, Diddie KR, Dorey K, Ryan SJ. The role of cellular proliferation in an experimental model of massive periretinal proliferation. Am J Ophthalmol. 1982;93:565–572. doi: 10.1016/s0002-9394(14)77370-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.