Abstract
NK cells are promising effectors for tumor adoptive immunotherapy, particularly when considering the targeting of MHC class I low or negative tumors. Yet, NK cells cannot respond to many tumors, which is particularly the case for nonhematopoietic tumors such as carcinomas or melanoma even when these cells lose MHC class I surface expression. Therefore, we targeted primary human NK cells by gene transfer of an activating chimeric receptor specific for HER-2, which is frequently overexpressed on carcinomas. We found that these targeted NK cells were specifically activated upon recognition of all evaluated HER-2 positive tumor cells, including autologous targets, as indicated by high levels of cytokine secretion as well as degranulation. The magnitude of this specific response correlated with the level of HER-2 expression on the tumor cells. Finally, these receptor transduced NK cells, but not their mock transduced counterpart, efficiently eradicated tumor cells in RAG2 knockout mice as visualized by in vivo imaging. Taken together, these results indicate that the expression of this activating receptor overrides inhibitory signals in primary human NK cells and directs them specifically toward HER-2 expressing tumor cells both in vitro and in vivo.
Keywords: immunotherapy, in vivo imaging, tumor biology, tolerance, retroviral transduction
Tumor cells with defined antigens have been successfully targeted with adoptively transferred T cells (1, 2). This therapy is frequently associated with tumor regression as well as the development of MHC class I (MHC-I) negative or low tumor-cell variants (2–4). Unlike T cells, which express their own antigen specific receptor, the TCR, NK cells are devoid from the expression of such a receptor. In the periphery, self-reactive T cells are inactivated by various tolerance mechanisms. Therefore, expressing a second antigen-specific receptor in T cells (5) might render these cells self-reactive if the introduced targeting receptor activates the recognition through an endogenous self-specific TCR. The potential use of targeted NK cells for adoptive immunotherapy provides an effector cell type with the capacity of recognizing antigen positive as well as MHC-I negative or low tumor cells. NK cells account for ≈10–15% of human blood lymphocytes and constitute an important component of the innate immune system (6). They are characterized by a CD56+ CD3− surface phenotype as well as by the expression of various activating and inhibitory receptors (6, 7). NK cells recognize and kill virus-infected cells and transformed cell lines (6–8). Unlike cytotoxic T lymphocytes, NK cells mediate cytolysis without the need for prior sensitization (6). According to the missing self recognition model suggested by Klas Kärre, NK cells can sense the absence of self MHC-I on other cells and respond by killing these cells (7, 9). Killing is inhibited by the expression of inhibitory receptors that recognize self MHC-I molecules on target cells, which results in transmitting an inhibitory signal that prevents cytotoxic action triggered by activating receptors (7, 10, 11). In the absence of dominant inhibitory signal (7, 8, 12), cytolytic granules are released and various cytokines critical to the immune response, such as IFN-γ, are produced (6–8). In man and mouse, NK cells attain a state of unresponsiveness to self by tolerance mechanisms as recently reviewed (7, 12).
Here we investigated the potential of genetically engineered primary human NK cells to specifically target HER-2+ tumors independently of MHC restriction by an Ab-based chimeric receptor (CR). HER-2 is ubiquitously expressed in many epithelial tumors and overexpressed in a variety of carcinomas, including ovarian and breast cancers (13–15). HER-2 overexpression correlates with increased aggressiveness of malignancy and poor prognosis (13, 15). The first approved immunological treatment of HER-2+ metastatic breast cancer was trastuzumab, a humanized HER-2-specific Ab (13, 14, 16, 17). We here show that primary human NK cells are amenable for specific targeting toward NK-cell-resistant HER-2+ tumor cells by the expression of a specific CR. Hence, these engineered NK cells acquired a new tumor specificity without losing their capacity to recognize MHC-Ilow target cells. Thus, engineered HER-2 specific NK cells can use 2 non-MHC-I-restricted recognition systems to target tumor cells.
Results
Expansion and Transduction of Primary Human NK Cells.
To efficiently transduce NK cells, we optimized the culture conditions that primarily favor the proliferation of NK cells (11, 18). This procedure resulted in NK cell populations of high purity (80–90% CD56+ CD3− NK cells). Using the pMIG vector that expresses GFP as a reporter, we tested 2 retroviral transduction protocols. The first protocol was based on spinoculation, and the second protocol was based on RetroNectin-assisted transduction [supporting information (SI) Fig. S1].
Genetically Engineered NK Cells Express the Transduced Receptor.
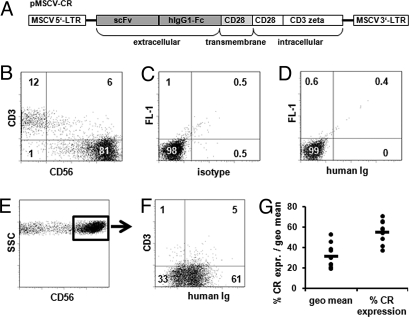
The CR construct specific for HER-2 was cloned into the pMIG vector (Fig. 1A). PBL-derived primary human NK cells of 10 different healthy donors were transduced to express the CR by using the spinoculation protocol. Flow cytometry analysis revealed that PBL-derived NK cells represented 81% of the cell population (Fig. 1 B–D), 61% of which expressed the CR (Fig. 1 E and F) as detected by antibodies specific for human Ig recognizing the extracellular domain of the CR. The average of transduction of the 10 different donors was 55 ± 11%, and the geometric mean of the fluorescence signal was 31 ± 10 (Fig. 1G). The CR expression on NK cells remained stable for more than 1 month from the transduction date (data not shown).
Fig. 1.
Her-2 -specific CR is efficiently expressed on transduced NK cells. (A) Schematic representation of the CR used in this study. The construct was cloned into pMIG replacing IRES and GFP. The resulting construct is designated pMSCV-CR. scFv, single-chain fragment variable; hIgG1-Fc, human IgG1 crystallizable fragment. (B–F) Staining of CR-transduced primary human NK cells from 1 representative donor. Cells were stained with a mouse anti human CD56 Ab conjugated to APC, a mouse anti-human CD3 Ab conjugated to Cy-5, and a goat anti-human Ig Ab conjugated to PE. (B) CD3 and CD56 expression on CR-transduced cells. (C) Isotype control and (D) mock-transduced cells stained for CR. Cells were gated on the CD56+ population (E) and were then analyzed for the expression of CR and CD3 (F). (G) Percent and geometric mean of the expression of the CR on primary NK cells from 10 different donors analyzed by flow cytometry.
NK Cells Genetically Engineered to Express a HER-2-Specific Receptor Recognize HER-2-Expressing Target Cells.
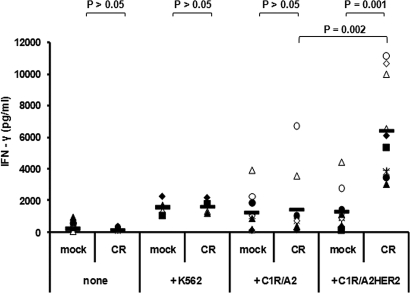
To assess the function and specificity of CR-NK cells, we analyzed the ability of these cells to recognize HER-2-expressing target cells in an IFN-γ release assay. CR-transduced or control-transduced PBLs were CD3 depleted to remove residual T and NKT cells resulting in 95–99% of the cells displaying the CD56+ CD3− NK cell phenotype (data not shown). CR or control engineered NK cells were cocultured with either the HER-2-negative tumor cell line C1R/A2 or the HER-2-expressing transfectant of this cell line, C1R/A2HER2. K562 cells were included as control target cells. Fig. 2 summarizes the results obtained from NK cells of 10 healthy donors included in this assay. CR-NK as well as mock-NK cells were induced by C1R/A2 cells to produce on average 1,300 pg/ml of IFN-γ (Fig. 2). Significantly higher levels of IFN-γ were produced by CR-NK cells, but not by mock-NK cells, in response to stimulation by C1R/A2HER2 cells with an average of 6,400 pg/ml (Fig. 2). As expected, when cultured with K562 cells, all gene-engineered NK cells produced significant levels of IFN-γ (1,600 pg/ml) that were similar to those produced by nonengineered NK cells (Fig. 2 and data not shown). Neither CR- nor mock-NK cells produced IFN-γ spontaneously (Fig. 2).
Fig. 2.
CR-NK cells are specifically stimulated by HER-2-expressing cells. IFN-γ was measured in the supernatant of stimulated NK cells from 10 donors. Values shown represent mean values of triplicates obtained from IFN-γ-specific ELISA. Effector cells (5 × 104) were mock-transduced or CR-transduced NK cells. They were either cultured without target cells (none) or cocultured with equal number of K562 cells (4 donors), C1R/A2 cells, or C1R/A2HER2 cells (10 donors). P values were calculated by using the Wilcoxon–Mann–Whitney test and indicate the difference between the groups.
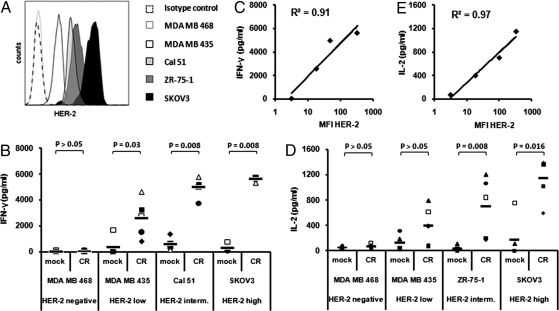
To determine the ability of CR-NK cells to specifically recognize the endogenously expressed HER-2 on cancer cells, we tested a panel of breast- and ovarian-carcinoma cells lines (Fig. 3A), 4 of which are HER-2+ and 1 of which is HER-2−, for their ability to induce cytokine production by CR-NK cells. CR-NK cells from 5 donors were able to recognize all HER-2+ cell lines and produced significantly higher levels of IFN-γ and IL-2 than mock-NK cells (Fig. 3 B and D). Interestingly, IL-2 production by CD3− CR-NK cells was confined to a small subset of these cells representing 10–14% of the CD3− CD56+-transduced cells as determined by IL-2 secretion assay (Fig. S2C). There was a good correlation between the level of IFN-γ or IL-2 production by the CR-NK cells and HER-2 expression on the cancer cells (Fig. 3 C and E). While the recognition of cancer cell lines expressing higher levels of HER-2 was better than that of cells with lower HER-2 expression level, those cells that did not express HER-2 were not recognized, confirming the specificity of the recognition pattern of CR-NK cells. Furthermore, additional HER-2+ breast carcinoma cell lines were tested for the ability of inducing IFN-γ or IL-2 production by CR-NK cells derived from different donors and were found to induce high levels of IFN-γ and IL-2 production by CR- but not mock-NK cells (Fig. S2). MHC-I expression on the different tumor cell lines varied but did not correlate with the level of CR-NK cell response (Fig. S3).
Fig. 3.
CR-NK cells recognize HER-2+ but not HER-2− carcinoma. Effector cells (5 × 104) were pMIG (mock)-transduced or CR-transduced NK cells. They were cocultured with equal numbers of the indicated carcinoma cell line. Supernatants were harvested and measured for IFN-γ or IL-2 using specific ELISA. (A) Five different carcinoma cell lines were selected based on the increasing level of HER-2 expression on their surface. IFN-γ (B) and IL-2 (D) production by CR-NK cells derived from 5 different donors in response to stimulation and correlation between HER-2 expression levels on the different carcinoma lines and IFN-γ (C) or IL-2 (E) production levels by CR-NK cells in response to stimulation by these lines are shown. P values were calculated by using the Wilcoxon–Mann–Whitney test and indicate the difference between the groups.
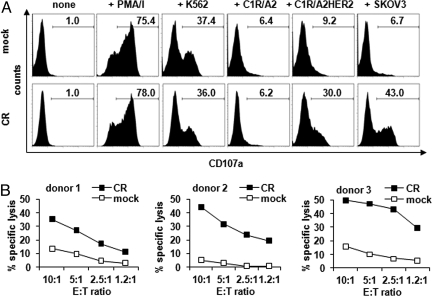
In addition to cytokine release, HER-2-specific NK-mediated cytotoxicity was induced by HER-2+ target cells as indicated by the degranulation assay. In this assay, mock-NK cells significantly degranulated in response to mitogenic (75%) and K562 cell stimulation (37–53%) and to some extent to the carcinoma lines MDA MB 453 and Cal 51 (22% and 38%, respectively) (Fig. 4A and Fig. S4). CR-NK cells, while degranulated in response to mitogenic (78%) and K562 (36–39%) cell stimulation, also responded well to all HER-2 expressing targets to a much higher extent (up to 64%) than mock-NK cells. Notably, CR-NK cells did not degranulate in response to the HER-2 negative targets C1R/A2 or MDA MB 468. The level of degranulation by CR-NK cells correlated with the level of HER-2 expression on the target carcinoma cell lines, which was in accordance with the cytokine release data. We also analyzed the ability of CR-NK cells to lyse HER-2+ tumor cells. Therefore, a 51Cr release assay was performed by using SKOV3 cell line as target. CR-NK cells but not mock-NK cells from 3 different donors specifically lysed HER-2+ SKOV3 cells (Fig. 4B).
Fig. 4.
CR-NK degranulate in response to HER-2-specific stimulation and lyse HER-2+ target cells. (A) Effector cells identified by size and granularity were pMIG (mock)-transduced or CR-transduced NK cells from 1 donor. They were either cultured without target cells (none), or with PMA and ionomycin (+PMA/I), or cocultured with the indicated cell line for 5 h while being stained for CD107a conjugated with PE. One representative experiment of five performed is depicted. (B) Effector cells were pMIG (mock)-transduced or CR-transduced NK cells from 3 donors. They were cocultured with 51Cr-labeled SKOV3 cells in different E:T ratios for 4 h, and supernatants were analyzed for 51Cr release.
CR-NK Cells Recognize Autologous HER-2 Expressing Target Cells as well as Freshly Isolated Ovarian Carcinomas ex Vivo.
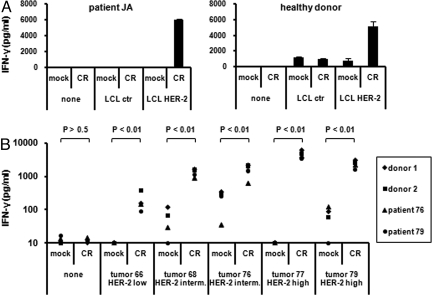
Paired mini LCL and HER-2 mini LCL from 1 healthy donor and 1 ovarian cancer patient were produced. These LCLs were obtained by transforming B cells from the same donor with either mini Epstein–Barr virus (miniEBV) construct or a mini-EBV construct that additionally expresses HER-2. HER-2 expression on these cells was confirmed by FACS (Fig. S5). CR-NK cells from the same donors were able to specifically recognize the HER-2, but not control mini LCL (Fig. 5A). Similarly, autologous and allogenic CR-NK cells obtained from ovarian carcinoma patients or healthy donors, but not mock-NK cells, were able to recognize freshly isolated ovarian tumor cells ex vivo (Fig. 5B and Fig. S6).
Fig. 5.
CR-NK cells specifically recognize autologous HER-2 mini-LCLs and ovarian tumor cells ex vivo. (A) Control or HER-2 mini-LCLs were cocultured with autologous CR-NK cells. (B) Freshly isolated ovarian tumor cells were cocultured with autologous or allogenic CR-NK cells. Supernatants were harvested and measured for IFN-γ by using specific ELISA. HER-2 status was estimated by measuring the percentage of tumor cells in samples and the level of HER-2 expression by FACS. P values were calculated by using the Wilcoxon–Mann–Whitney test and indicate the difference between the groups.
Tumor Challenge Model and in Vivo Imaging.
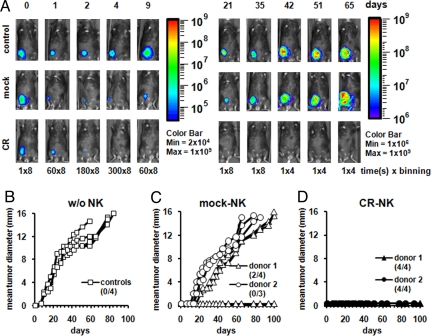
To evaluate the in vivo potential of these antigen-specific primary human NK cells, we chose the SKOV3 cell line because of its ability to grow in RAG2 knockout mice (Fig. 6B and data not shown) and transduced it to express the luciferase gene to follow its survival and outgrowth. After inoculating RAG2 knockout mice s.c. with either SKOV/CBG cells alone (control) or together with mock- or CR-NK cells from 2 different donors, the animals were imaged for bioluminescence. This bioluminescence imaging model allows monitoring of tumor cell fate during the first few days after injection, at the time that tumor formation cannot be detected by palpation. In Fig. 6A, 1 mouse of each group is shown. If SKOV/CBG cells were injected alone, light signal decreased during the first 6 days but recovered on day 9 and increased over time, which was followed by increase in mean tumor diameter (Fig. 6 A Top and B and Fig. S7). A similar signal kinetic was seen in most of the mice that were coinjected with SKOV/CBG cells together with mock-NK cells. In these mice, light signal decreased more dramatically but nonetheless started to recover on day 9 and increased over time, followed by an increase in the mean tumor diameter (Fig. 6 A Middle and C and Fig. S7). All mice that have received coinjection with mock-NK cells from donor 2 succumbed to tumor outgrowth. Two of four mice that received similar coinjection but with mock-NK cells from donor 1 also succumbed, whereas the other 2 mice survived the tumor challenge (Fig. 6C and Fig. S7). When SKOV/CBG cells were injected together with CR-NK cells, the light signal decreased initially to the same extent as the group with mock-NK coinjection but by day 4, no signal above background (Fig. S7) was detectable from the SKOV/CBG cells despite prolonged imaging, and no tumor could be palpated in these mice (Fig. 6 A Lower and D). All mice that have received the coinjection of SKOV/CBG cells together with CR-NK cells survived and remained tumor-free.
Fig. 6.
CR-NK cells efficiently eradicate HER-2+ carcinoma in a RAG2−/− mouse model. RAG2−/− mice were inoculated s.c. with 5 × 106 SKOV/CBG cells alone or together with the same number of CR+-NK cells or mock-NK cells from 2 different donors. (A) In vivo imaging of tumor cell outgrowth or death in 1 representative mouse from each group. Because of increase in signal intensity correlating with tumor outgrowth, acquisition time and binning were changed as indicated, and 2 pseudocolor scales corresponding to signal intensity are depicted with a minimum of 2 × 104 for days 1–9 and 1 × 106 for days 21–65. Exact quantification of signal intensity is provided in Fig. S7. (B–D) Mean tumor diameter. Tumors were measured 1–2 times per week by using a digital caliper. Survival comparison was performed by using the Wilcoxon–Mann–Whitney test. The P values for the difference between the groups are P < 0.001 and P = 0.02 for differences between B and D and between C and D, respectively. The difference between B and C is not statistically significant. Numbers of live mice in each group are described above the abscissa.
Discussion
Adoptively transferred T cells are regarded today as the most effective treatment for patients with metastatic melanoma (2). Earlier studies with this type of therapy have reported a subsequent frequent loss of MHC-I expression (3, 4) on melanoma tissue sections as well as on melanoma cell lines, which were later shown to be recognized and killed by IL-2-activated NK cell lines and clones (19). NK cells initially identified by their ability to recognize transformed cells are limited by their recognition mechanisms that depend on sensing the fine-tuned expression of activation versus inhibitory ligands on their target cells (6, 7, 12, 20–22). Although MHC-I loss or down-regulation is often associated with NK recognition, some tumors that lack MHC-I are resistant to NK killing. Various approaches were evaluated to overcome tumor-cell resistance to NK cells. These included the use of allo NK cells (23), blocking the inhibitory signal (24), or triggering the activation receptors by using monoclonal antibodies or by genetic modification of tumor cells (11, 25, 26).
Here we tested a genetic approach to enhance specific tumor recognition by NK cells based on the recognition of the tumor-associated antigen HER-2 via a CR (27). HER-2-specific CR-NK cells recognized C1R/A2HER2 cells in a HER-2-dependent fashion. However, unlike transformed cells from hematopoietic origin expressing several activation ligands (7, 11, 28), cells derived from solid tumors are rather resistant to NK recognition. Nonetheless, all HER-2-expressing breast carcinomas that we have tested were recognized by CR-NK cells, and the extent of recognition correlated with the level of HER-2 expression on these tumor cells.
Taken together, the data indicate that CR-NK cells have the capacity to respond specifically to HER-2-expressing target by IFN-γ and IL-2 production, the 2 cytokines essential for tumor rejection and NK cell survival, as well as degranulation and specific lysis of HER-2+ target cells. These responses occurred while CR-NK cells maintained their classical NK specificity as indicated by the response to K562 cells. Hence, these cells use 2 independent mechanisms, which are non-MHC-I restricted, to recognize their target cells.
Remarkably, CR-NK cells recognized autologous HER-2 miniLCLs but not their HER-2-negative counterpart, indicating that the activation provided by the CR signaling was sufficient to override the collective inhibitory signals provided by the corresponding inhibitory ligands on the autologous targets. Additionally, HER-2-positive ovarian carcinoma explanted from 5 patients expressed HER-2 at levels that permitted recognition by CR-NK cells ex vivo.
This universal recognition of HER-2+ target cells may be explained by the level of activation provided by the CR used in our study, which may have overridden the sum of inhibitory signals provided by any of the tested cell lines. Apart from the qualitative difference in signaling, the affinity of the binding domain (Kd = 10−8 M) of the CR is much higher than that of the reported interactions between known inhibitory NK-receptors and their ligands (29). Therefore, it will be interesting to study whether NK cells engineered with CRs with lower affinity would also be able to overcome NK inhibition.
HER-2 is an attractive target for immunotherapy, and Ab (trastuzumab)-based therapy targeting HER-2 is clinically approved (16). Trastuzumab is believed to manifest its effect through direct Ab binding, NK-mediated Ab-dependent cell cytotoxicity, and by blocking angiogenesis through inhibition of VEGF expression (13, 14). Distinguishing the CR-NK cell approach from the use of trastuzumab or other strategies targeting HER-2 is that not all HER-2+ tumors or cell lines are responsive to this Ab-based therapy (13, 14, 16, 30, 31) or to this extent to siRNA-mediated HER-2 targeting that we have earlier evaluated (31). Only tumor cells that have gene amplified HER-2, accounting for 1/3 of the HER-2+ tumors, represent good targets for these Ab and siRNA treatments. In contrast, the CR-NK cells were able to recognize all tested HER-2+ carcinomas regardless of their gene amplification status. Interestingly, Neve et al. (32) have recently reported that breast cancer cell lines have molecular features that mirror primary breast tumors, which permitted prediction of response to targeted therapy by trastuzumab. Based on this study, the majority of breast carcinoma lines that we have tested and shown to be sensitive to CR-NK effector function would not be responsive to trastuzumab. Indeed, both MCF-7 and MDA MB 453 cell lines that were shown to be resistant to trastuzumab treatment (32) did induce a robust CR-NK cell response. Furthermore, cell lines expressing lower levels of HER-2, such as MCF-7, cannot be specifically recognized by trastuzumab-directed NK cells via Ab-dependent cell cytotoxicity (33, 34), unless transfected to overexpress HER-2 (35). Notably, MCF-7 was efficiently recognized by CR-NK cells.
Haploidentical hematopoietic transplantation for the treatment of leukemia was reported to exploit alloreactive NK cells to increase the probability of survival of high-risk acute myeloid leukemia, representing a positive precedent for adoptive NK cell therapy (23). Additionally, acute lymphoblastic leukemia cells were shown to be a good target for NK cells transduced with CD19 specific receptor (36). Few reports have investigated the potential of using genetically engineered NK-like lymphoma cells in adoptive immunotherapy targeting solid tumors such as breast cancer. Transduction of an NK-like lymphoma line (NK-92) targeted these cells to HER-2-expressing tumor cells. These attempts demonstrated that tumor outgrowth was transiently delayed in mice coinjected with a HER-2-positive tumor cells and targeted NK-92 cells, but mice protection could not be achieved (37, 38). Moreover, these NK-92 cells are transformed and therefore not ideal for immunotherapy and do not reflect the biology of primary NK cells expressing a CR. Adoptively transferred primary NK cells have the potential of long-term persistence and proliferation in the recipient and do compete well for the utilization of homeostasis growth factors (39), a prerequisite for successful adoptive immunotherapy (1, 2). Our initial in vivo study shows that CR-engineered NK cells can overcome their intrinsic inhibition, which limited their function in most of the animals that received mock-transduced NK cells, and these CR-NK cells did prevent tumor outgrowth in all mice that have received this treatment.
The ability to genetically engineer primary NK cells, apart from providing an opportunity to further the analysis of NK cell biology, can represent an effective alternative or a complement to the currently used approaches in cancer immunotherapy.
Materials and Methods
Retroviral Vectors.
The CR construct specific for HER-2, C6.5-scFv-Fc-CD3ζ-CD28, was earlier described (40, 41). The CR was cloned into the modified retroviral vector pMIG described elsewhere (1, 42) replacing the GFP and IRES fragment. The resulting construct was designated pMSCV-CR. The pMIG vector encoding GFP, or the pMSCVred vector encoding red fluorescent protein, which was generated by replacing the IRES and the GFP encoding region of pMIG by the RFP encoding region from the DSred-Express plasmid (Clontech, BD Biosciences), were used as controls. The click beetle green (CBG) luciferase encoding retroviral vector was constructed by cloning the luciferase cDNA obtained from the pCBG99 plasmid (Promega) and shuttled through the phRL-null plasmid into the pMIG vector. The resulting plasmid was designated pMIG-CBG.
Cloning of the various constructs was confirmed by restriction mapping, partial sequencing, and transient transfection into 293T cells. The 10A1-pseudotyped retrovirus was generated by cotransfection of 293T cells with the above mentioned plasmids and the gag, pol, and env encoding pCL-10A1 vector by using Lipofectamine 2000 (Invitrogen). Briefly, 1 × 106 293T cells were seeded into a T 25 flask 1 day before transfection. The next day, medium was replaced with 4 mL of fresh medium. Ten microliters of Lipofectamine 2000 and 3 μg of DNA of each plasmid were used (6 μg of total DNA) diluted in 1 mL of Opti-MEM for transfection of 1 T 25 flask of 293T cells according to the manufacturer's manual. The next day, medium was changed to 5 mL of RPMI medium 1640, 10% (vol/vol) FBS, and the cells were moved to a 32 °C, 5% CO2 humidified incubator. Virus supernatant was collected on 3 successive days and filtered through a 0.45-μm sterile filter before use.
Expansion of NK Cells and Retroviral Transduction.
NK cells were expanded as previously described (11, 18) with the following modification: a 30-min plastic adherence step was used and cells were cultured in RPMI medium 1640, 10% (vol/vol) FBS in 6-well tissue culture plates. Transduction was performed on day 6 and was repeated on 2 subsequent days using either of the 2 following protocols: a RetroNectin-assessed protocol and a spinoculation-assessed protocol. For RetroNectin-based transduction, 6-well tissue culture plates were coated with 50 μg per well RetroNectin (TaKaRa) as recommended by the manufacturer. One day later, 4 mL of virus supernatant was added to each well and plates were incubated for 30 min at 32 °C and then for an additional 24 h at 4 °C (43). Virus supernatant was removed and replaced by cells (1 × 106 cells/mL) in RPMI medium 1640, 10% (vol/vol) FBS, containing 200 IU/mL IL-2 (kindly provided by O. Krieter, Chiron GmbH, Marburg, Germany). A half mililiter of fresh virus supernatant was added to each well, and cells were incubated at 32 °C for 24 h. Transduction was repeated on 2 successive days. After the third transduction, cells were maintained in RPMI medium 1640, 10% (vol/vol) FBS, and 200 IU/ml IL-2 at 37 °C.
Spinoculation-based transduction was performed in 24-well tissue culture plates (1) using 2 × 105 cells per well in a total volume of 2 mL of virus supernatant diluted 1:1 in culture medium in the presence of 8 μg/ml polybrene (Sigma–Aldrich) and 200 IU/mL IL-2. Cells were centrifuged at 805 × g at 32 °C for 90 min. Plates were placed afterward in a 32 °C, 5% CO2 humidified incubator for 24 h. Transduction was repeated on 2 successive days. After the third transduction, cells were maintained in RPMI medium 1640, 10% (vol/vol) FBS, and 200 IU/mL IL-2 at 37 °C.
Tumor Cell Challenge and in Vivo Imaging.
All mouse studies have been approved by the institutional review board and local authorities. RAG2 knockout mice were obtained from Jackson (Charles River Laboratories) and bred in our animal facility. SKOV3 cells were virally transduced with pMIG-CBG to express the CBG luciferase (SKOV/CBG). The cells were sorted based on GFP expression to be 100% GFP positive on a FACSAria cell sorter (BD Biosiences). NK cells from 2 donors were transduced by using virus-containing supernatants of either the pMSCVred or pMSCV-CR transfected 293T cells. SKOV3-CBG cells (5 × 106) were mixed in 200 μL of PBS with either mock- or CR-NK cells in an effector to target ratio of 1:1 of SKOV/CBG cells to CR+ NK cells, mock-NK cells were adjusted to the same cell number. The cell mixture was inoculated s.c. on the flank of 3–4 mice per group. In total, 4 mice received control tumor cells and 7–8 mice received either mock-NK or CR-NK cells, respectively, together with tumor cells. For in vivo imaging, animals were injected with 100 μL of an aqueous solution of the substrate d-luciferin (150 μg/g, Biosynth) i.p. and imaged using IVIS 200 in vivo imaging system (Xenogen, Caliper Life Sciences GmbH). Twenty five percent of the mice in each group were imaged for the CBG-Luc signal from days 1–6. From day 7 to the end of the experiment all mice were imaged. A pseudocolor image representing light intensity was generated and the relative light intensity from each mouse was quantified by using LivingImage 2.6.1 software (Xenogen). Tumor measurements were performed as described earlier (44), and mice were killed once tumors reached 15 mm in any of the 3 perpendiculars.
See SI Materials and Methods for additional details.
Supplementary Material
Acknowledgments.
We thank Stephanie Kupsch and Markus Hensel for providing excellent technical assistance and Dr. Hans-Peter Rahn for cell sorting. This work was supported by the European Community, FP6 program ‘ATTACK’, and by the Deutsche Forschungsgemeinschaft (Transregio-Sonderforschungsbereich TR 36).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0804788105/DCSupplemental.
References
- 1.Charo J, et al. Bcl-2 overexpression enhances tumor-specific T-cell survival. Cancer Res. 2005;65:2001–2008. doi: 10.1158/0008-5472.CAN-04-2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rosenberg SA, Restifo NP, Yang JC, Morgan RA, Dudley ME. Adoptive cell transfer: A clinical path to effective cancer immunotherapy. Nat Rev Cancer. 2008;8:299–308. doi: 10.1038/nrc2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Khong HT, Restifo NP. Natural selection of tumor variants in the generation of “tumor escape” phenotypes. Nat Immun. 2002;3:999–1005. doi: 10.1038/ni1102-999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Restifo NP, et al. Loss of functional β 2-microglobulin in metastatic melanomas from five patients receiving immunotherapy. J Natl Cancer Inst. 1996;88:100–108. doi: 10.1093/jnci/88.2.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gladow M, Uckert W, Blankenstein T. Dual T cell receptor T cells with two defined specificities mediate tumor suppression via both receptors. Eur J Immunol. 2004;34:1882–1891. doi: 10.1002/eji.200425041. [DOI] [PubMed] [Google Scholar]
- 6.Trinchieri G. Biology of natural killer cells. Adv Immunol. 1989;47:187–376. doi: 10.1016/S0065-2776(08)60664-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lanier LL. NK cell recognition. Annu Rev Immunol. 2005;23:225–274. doi: 10.1146/annurev.immunol.23.021704.115526. [DOI] [PubMed] [Google Scholar]
- 8.Yokoyama WM, Kim S. Licensing of natural killer cells by self-major histocompatibility complex class I. Immunol Rev. 2006;214:143–154. doi: 10.1111/j.1600-065X.2006.00458.x. [DOI] [PubMed] [Google Scholar]
- 9.Karre K, Ljunggren HG, Piontek G, Kiessling R. Selective rejection of H-2-deficient lymphoma variants suggests alternative immune defence strategy. Nature. 1986;319:675–678. doi: 10.1038/319675a0. [DOI] [PubMed] [Google Scholar]
- 10.Moretta A, Moretta L. HLA class I specific inhibitory receptors. Curr Opin Immunol. 1997;9:694–701. doi: 10.1016/s0952-7915(97)80051-9. [DOI] [PubMed] [Google Scholar]
- 11.Wilson JL, et al. NK cell triggering by the human costimulatory molecules CD80 and CD86. J Immunol. 1999;163:4207–4212. [PubMed] [Google Scholar]
- 12.Lanier LL. Natural killer cells: Roundup. Immunol Rev. 2006;214:5–8. doi: 10.1111/j.1600-065X.2006.00464.x. [DOI] [PubMed] [Google Scholar]
- 13.Menard S, Pupa SM, Campiglio M, Tagliabue E. Biologic and therapeutic role of HER2 in cancer. Oncogene. 2003;22:6570–6578. doi: 10.1038/sj.onc.1206779. [DOI] [PubMed] [Google Scholar]
- 14.Meric-Bernstam F, Hung MC. Advances in targeting human epidermal growth factor receptor-2 signaling for cancer therapy. Clin Cancer Res. 2006;12:6326–6330. doi: 10.1158/1078-0432.CCR-06-1732. [DOI] [PubMed] [Google Scholar]
- 15.Slamon DJ, et al. Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science. 1989;244:707–712. doi: 10.1126/science.2470152. [DOI] [PubMed] [Google Scholar]
- 16.Finn RS, Slamon DJ. Monoclonal antibody therapy for breast cancer: Herceptin. Cancer Chemother Biol Response Modif. 2003;21:223–233. doi: 10.1016/s0921-4410(03)21010-3. [DOI] [PubMed] [Google Scholar]
- 17.Slamon DJ, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783–792. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 18.Perussia B, et al. Preferential proliferation of natural killer cells among peripheral blood mononuclear cells cocultured with B lymphoblastoid cell lines. Nat Immun Cell Growth Regul. 1987;6:171–188. [PubMed] [Google Scholar]
- 19.Porgador A, Mandelboim O, Restifo NP, Strominger JL. Natural killer cell lines kill autologous β2-microglobulin-deficient melanoma cells: Implications for cancer immunotherapy. Proc Natl Acad Sci USA. 1997;94:13140–13145. doi: 10.1073/pnas.94.24.13140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Raulet DH. Missing self recognition and self tolerance of natural killer (NK) cells. Semin Immunol. 2006;18:145–150. doi: 10.1016/j.smim.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 21.Parham P. Taking license with natural killer cell maturation and repertoire development. Immunol Rev. 2006;214:155–160. doi: 10.1111/j.1600-065X.2006.00462.x. [DOI] [PubMed] [Google Scholar]
- 22.Johansson MH, Hoglund P. The dynamics of natural killer cell tolerance. Semin Cancer Biol. 2006;16:393–403. doi: 10.1016/j.semcancer.2006.07.013. [DOI] [PubMed] [Google Scholar]
- 23.Ruggeri L, Aversa F, Martelli MF, Velardi A. Allogeneic hematopoietic transplantation and natural killer cell recognition of missing self. Immunol Rev. 2006;214:202–218. doi: 10.1111/j.1600-065X.2006.00455.x. [DOI] [PubMed] [Google Scholar]
- 24.Koh CY, et al. Augmentation of antitumor effects by NK cell inhibitory receptor blockade in vitro and in vivo. Blood. 2001;97:3132–3137. doi: 10.1182/blood.v97.10.3132. [DOI] [PubMed] [Google Scholar]
- 25.Cerwenka A, Baron JL, Lanier LL. Ectopic expression of retinoic acid early inducible-1 gene (RAE-1) permits natural killer cell-mediated rejection of a MHC class I-bearing tumor in vivo. Proc Natl Acad Sci USA. 2001;98:11521–11526. doi: 10.1073/pnas.201238598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Diefenbach A, Jensen ER, Jamieson AM, Raulet DH. Rae1 and H60 ligands of the NKG2D receptor stimulate tumour immunity. Nature. 2001;413:165–171. doi: 10.1038/35093109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Abken H, Hombach A, Heuser C, Kronfeld K, Seliger B. Tuning tumor-specific T-cell activation: A matter of costimulation? Trends Immunol. 2002;23:240–245. doi: 10.1016/s1471-4906(02)02180-4. [DOI] [PubMed] [Google Scholar]
- 28.Bryceson YT, March ME, Ljunggren HG, Long EO. Activation, coactivation, and costimulation of resting human natural killer cells. Immunol Rev. 2006;214:73–91. doi: 10.1111/j.1600-065X.2006.00457.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vales-Gomez M, Reyburn H, Strominger J. Molecular analyses of the interactions between human NK receptors and their HLA ligands. Hum Immunol. 2000;61:28–38. doi: 10.1016/s0198-8859(99)00159-7. [DOI] [PubMed] [Google Scholar]
- 30.Robert N, et al. Randomized phase III study of trastuzumab, paclitaxel, and carboplatin compared with trastuzumab and paclitaxel in women with HER-2-overexpressing metastatic breast cancer. J Clin Oncol. 2006;24:2786–2792. doi: 10.1200/JCO.2005.04.1764. [DOI] [PubMed] [Google Scholar]
- 31.Choudhury A, et al. Small interfering RNA (siRNA) inhibits the expression of the Her2/neu gene, upregulates HLA class I and induces apoptosis of Her2/neu positive tumor cell lines. Int J Cancer. 2004;108:71–77. doi: 10.1002/ijc.11497. [DOI] [PubMed] [Google Scholar]
- 32.Neve RM, et al. A collection of breast cancer cell lines for the study of functionally distinct cancer subtypes. Cancer Cell. 2006;10:515–527. doi: 10.1016/j.ccr.2006.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stein MN, Shin J, Gudzowaty O, Bernstein AM, Liu JM. Antibody-dependent cell cytotoxicity to breast cancer targets despite inhibitory KIR signaling. Anticancer Res. 2006;26:1759–1763. [PubMed] [Google Scholar]
- 34.Yamaguchi Y, et al. HER2-specific cytotoxic activity of lymphokine-activated killer cells in the presence of trastuzumab. Anticancer Res. 2005;25:827–832. [PubMed] [Google Scholar]
- 35.Carson WE, et al. Interleukin-2 enhances the natural killer cell response to Herceptin-coated Her2/neu-positive breast cancer cells. Eur J Immunol. 2001;31:3016–3025. doi: 10.1002/1521-4141(2001010)31:10<3016::aid-immu3016>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 36.Imai C, Iwamoto S, Campana D. Genetic modification of primary natural killer cells overcomes inhibitory signals and induces specific killing of leukemic cells. Blood. 2005;106:376–383. doi: 10.1182/blood-2004-12-4797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Suck G. Novel approaches using natural killer cells in cancer therapy. Semin Cancer Biol. 2006;16:412–418. doi: 10.1016/j.semcancer.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 38.Uherek C, et al. Retargeting of natural killer-cell cytolytic activity to ErbB2-expressing cancer cells results in efficient and selective tumor cell destruction. Blood. 2002;100:1265–1273. [PubMed] [Google Scholar]
- 39.Miller JS, et al. Successful adoptive transfer and in vivo expansion of human haploidentical NK cells in patients with cancer. Blood. 2005;105:3051–3057. doi: 10.1182/blood-2004-07-2974. [DOI] [PubMed] [Google Scholar]
- 40.Chmielewski M, Hombach A, Heuser C, Adams GP, Abken H. T cell activation by antibody-like immunoreceptors: increase in affinity of the single-chain fragment domain above threshold does not increase T cell activation against antigen-positive target cells but decreases selectivity. J Immunol. 2004;173:7647–7653. doi: 10.4049/jimmunol.173.12.7647. [DOI] [PubMed] [Google Scholar]
- 41.Hombach A, Heuser C, Abken H. Generation, expression, and monitoring of recombinant immune receptors for use in cellular immunotherapy. Methods Mol Biol. 2003;207:365–381. doi: 10.1385/1-59259-334-8:365. [DOI] [PubMed] [Google Scholar]
- 42.Van Parijs L, Refaeli Y, Abbas AK, Baltimore D. Autoimmunity as a consequence of retrovirus-mediated expression of C-FLIP in lymphocytes. Immunity. 1999;11:763–770. doi: 10.1016/s1074-7613(00)80150-8. [DOI] [PubMed] [Google Scholar]
- 43.Morgan RA, et al. High efficiency TCR gene transfer into primary human lymphocytes affords avid recognition of melanoma tumor antigen glycoprotein 100 and does not alter the recognition of autologous melanoma antigens. J Immunol. 2003;171:3287–3295. doi: 10.4049/jimmunol.171.6.3287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Charo J, et al. A long-term memory obtained by genetic immunization results in full protection from a mammary adenocarcinoma expressing an EBV gene. J Immunol. 1999;163:5913–5919. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.