Abstract
The purpose of the present study was to evaluate the effectiveness of a 3-carboranyl thymidine analogue (3CTA), 3-[5-{2-(2,3-dihydroxyprop-1-yl)-o-carboran-1-yl}pentan-1-yl] thymidine, designated N5–2OH, for boron neutron capture therapy (BNCT) of brain tumors using the RG2 rat glioma model. Target validation was established using the thymidine kinase (TK) 1(+) wild-type, murine L929 cell line and its TK1(−) mutant counterpart, which were implanted s.c. (s.c.) into nude mice. Two intratumoral (i.t.) injections of 10B-enriched N5–2OH were administered to tumor-bearing mice at 2-hour intervals, after which BNCT was carried out at the Massachusetts Institute of Technology (MIT) Research Reactor. Thirty days after BNCT, mice bearing TK1(+) L929 tumors had a 15× reduction in tumor volume compared with TK1(−) controls. Based on these favorable results, BNCT studies were then initiated in rats bearing intracerebral (i.c.) RG2 gliomas, after i.c. administration of N5–2OH by Alzet osmotic pumps, either alone or in combination with i.v. (i.v.) boronophenylalanine (BPA), a drug that has been used clinically. The mean survival times (MSTs) of RG2 glioma bearing rats were 45.6 ± 7.2 days, 35.0 ± 3.3days, and 52.9 ± 8.9 days, respectively, for animals that received N5–2OH, BPA, or both. The differences between the survival plots of rats that received N5–2OH and BPA alone were highly significant (P = 0.0003). These data provide proof-of-principle that a 3CTA can function as a boron delivery agent for NCT. Further studies are planned to design and synthesize 3CTAs with enhanced chemical and biological properties, and increased therapeutic efficacy.
Keywords: 3-carboranyl thymidine analogues
Boron neutron capture therapy (BNCT) is one of many experimental approaches that has been used to treat patients with glioblastoma multiforme (GBM), the most malignant of all human brain tumors (1, 2). It is a radiotherapeutic modality that is based on the selective delivery of nonradioactive boron-10 (10B), followed by irradiation with either low energy thermal or intermediate energy epithermal neutrons to the site of the tumor. Besides GBMs, BNCT has been used to treat patients with recurrent malignant meningiomas (3), either cutaneous primaries or cerebral metastases of melanoma (1), and most recently, patients with recurrent carcinomas of the head and neck (4), and hepatic metastases of colon cancer (5). Interested readers are referred to several recent reviews (1) and monographs relating to various aspects of BNCT (6, 7).
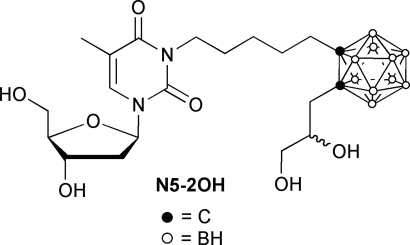
A prerequisite for successful BNCT is the selective accumulation of 10B within tumor cells. To accomplish this, a wide variety of low and high molecular weight boron delivery agents have been designed and synthesized to specifically target malignant cells (8). However, only two agents have been used clinically, a dihydroxyboryl derivative of phenylalanine, referred to as boronphenylalanine (BPA), and an anionic polyhedral boron cluster, sodium borocaptate (BSH). Our most recent studies have focused on two specific molecular targets. The first is the epidermal growth factor receptor (EGFR), the gene for which is frequently overexpressed in gliomas (9). In these studies, either boronated EGF or the anti-EGFR monoclonal antibodies (mAbs), cetuximab and L8A4, were used as the targeting vehicles (10–12). The second molecular target is thymidine (Thd) kinase 1 (TK1), a cytosolic deoxynucleoside kinase of the nucleic acid synthesis salvage pathway that 5′-monophosphorylates Thd and dUrd (13). TK1 activity only is found in proliferating cells, and it is distributed and expressed in a wide variety of malignant tumors (13). As recently reported by us (14–17), a panel of 3-carboranyl Thd analogues (3CTAs) have been designed and synthesized. These are substrates of TK1, and after 5′-monophosphorylation, they are selectively trapped in tumor cells. Based on earlier in vitro and in vivo studies (14, 15, 17), the 3CTA, designated N5–2OH (Fig. 1), was evaluated as a boron delivery agent for NCT of brain tumors, and as described below, our data provide proof-of-principle for its effectiveness in the RG2 rat glioma model.
Fig. 1.
Structure of 3-[5-{2-(2,3-dihydroxyprop-1-yl)-o-carboran-1-yl}pentan-1-yl] thymidine (N5–2OH). Nonenriched and 10B enriched N5–2OH were synthesized as an epimeric mixture, as previously described by us (17).
Results
Target Validation Studies with the L929 Tumor Model.
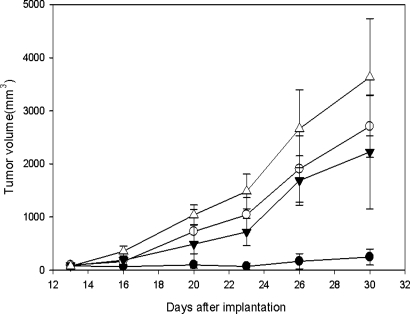
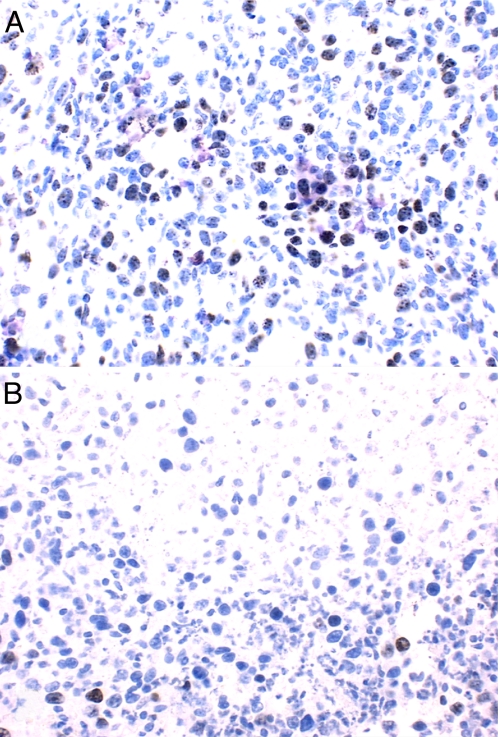
Labeling of TK1(+) L929 cells with BUdR, which also is a TK1 substrate as N5–2OH (18), resulted in 13 ± 3%, 22 ± 5%, 35 ± 6%, and 43 ± 6% incorporation at 4, 6, 8, and 10 h., respectively. From these data the calculated length of the cell cycle (Tc), the duration of S phase (Ts), the labeling index (LI0), and the growth fraction (GF) were 17.2 h, 5.2 h, 13%, and 45%, respectively. Because BUdR can be considered a surrogate of N5–2OH, these data suggest that sustained delivery for at least 12 h and possibly longer, would be required to obtain maximum in vivo uptake of N5–2OH, especially for human brain tumors that have much lower LI and GF values. Based on these data, in vivo biodistribution studies with N5–2OH first were initiated in L929 tumor-bearing nude mice. Four hours after the first i.t. injection of N5–2OH, the boron concentrations in TK1(+) wild-type and TK1(−) mutant L929 tumors were 22.8 ± 5.0 and 8.4 ± 3.6 μg/g, respectively. The significance of this is that after BNCT the latter would receive a lower physical radiation dose attributable to the 10B [n,α]7Li capture reaction. Boron levels in the adjacent normal skin and blood were undetectable (<0.5 μg/g), and the tumor to blood (T:Bl) ratio for animals bearing TK1(+) tumors was at least 47:1, which was highly favorable for BNCT. The same neutron fluence was administered to each mouse during BNCT, which resulted in a calculated total absorbed dose (beam only) to the tumor of 4.50 ± 0.16 Gy. The 10B containing TK1(+) and TK1(−) tumors received an additional 0.55 ± 0.03 Gy per μg 10B during irradiations (17.0 versus 9.1 Gy, respectively). Animals bearing TK1(+) tumors, which had received 10B-enriched N5–2OH by i.t. injection followed by BNCT, had an average 15-fold reduction in mean tumor volume on day 30 after implantation. This was 247 ± 151 mm3 compared with 3603 ± 1103 mm3 for matched control animals that did not receive BNCT and 2225 ± 1074 mm3 for irradiated controls that did not receive N5–2OH (Fig. 2). Animals bearing L929 TK1(−) tumors showed modest reductions in tumor volumes, which were not significantly different from those of irradiated animals bearing TK1(+) tumors that did not receive N5–2OH. Because hypothetically N5–2OH preferentially targets proliferating tumor cells, these reductions in tumor volumes correlated with a marked decrease in the number of proliferating cells, as evidenced by immunostaining for Ki67, which recognizes a nuclear protein involved in the proliferative phases of the cell cycle (Fig. 3). L929 TK1(+) tumors from animals that received N5–2OH, followed by BNCT, had 1.2 ± 1 Ki67 (+) cells per high power field (hpf) with extensive areas of necrosis. In contrast, tumors from animals that did not receive N5–2OH, but had been irradiated with thermal neutrons, had 154.4 ± 2.8 cells per hpf without any necrosis, and those from irradiated control mice had 188.8 ± 8.5 cells per hpf, and in both of these groups, there was no evidence of necrosis.
Fig. 2.
Growth of L929 TK1(+) and TK1(−) tumors after i.t. injection of N5–2OH and BNCT TK1(+) (●); TK1(−) (○); TK1(+) radiation control (→); TK1(+) untreated control (▵). The vertical lines indicate the standard deviations (SD) of the mean tumor volumes.
Fig. 3.
Ki67 expression in L929 TK1(+) tumors. (A) A section of L929 tumor from one irradiated animal that did not receive N5–2OH. (B) Section from an animal that received N5–2OH. (200× magnification).
Biodistribution Studies and Dosimetry in Glioma Bearing Rats.
To determine whether i.c. delivery of N5–2OH was nontoxic, a group of five non-tumor-bearing rats received 500 μg of N5–2OH, administered i.c. by Alzet osmotic pumps over 24 h. These animals were followed clinically for 2 weeks and weighed 3× per week. Initially there was <10% weight loss, but this was regained within a few days. There was no clinical evidence of neurologic deficits, and 2 weeks later, the rats were euthanized and their brains were removed and processed for neuropathologic examination. This did not reveal any microscopic findings associated with the i.c. administration of N5–2OH.
Boron concentrations in tumor, brain, and blood of RG2 glioma bearing rats after administration of N5–2OH are summarized in Table 1. The tumor and normal brain boron concentrations were 27.6 ± 9.5 μg/g and 2.6 ± 2.4 μg/g, respectively, and the blood value was undetectable (<0.5 μg/g). Because it is unlikely that any single boron delivery agent could target all tumor cells, a group of RG2 glioma-bearing rats received i.v. BPA in combination with i.c. administration of N5–2OH to target cells that had not taken up sufficient quantities N5–2OH. The corresponding tumor and normal brain boron values for the combination at 1 h after termination of delivery of N5–2OH and 2.5 h after i.v. injection of BPA were 45.6 ± 14.1 μg/g and 7.3 ± 1.0 μg/g, respectively. In contrast, the tumor and normal brain boron values for BPA alone were 14.2 ± 7.7 μg/g and 5.0 ± 1.0 μg/g, respectively. Tumor to normal brain (T:Br) boron ratios for rats that received either N5–2OH or BPA alone were 10.6:1 and 1.9:1, respectively. Based on these boron concentrations, the unweighted, absorbed physical radiation doses were calculated (Table 1). Irradiated control animals received the same physical radiation dose as those that received either N5–2OH or BPA alone, but in addition they received a dose attributable to the 10B [n, α]7Li capture reaction. This was 8.1 Gy for rats that received N5–2OH, 5.0 Gy for those that received BPA, and 12.2 Gy for animals that received the combination.
Table 1.
Boron biodistribution and physical radiation doses delivered to tumor, brain, and blood in RG2 glioma bearing rats
| Group‡ | Boron uptake, μg/g wt |
Physical dose, Gy† |
|||||
|---|---|---|---|---|---|---|---|
| Tumor | Brain§ | Blood | Tumor/brain conc. ratio | Tumor | Brain | Blood | |
| RG2/CED N5–2OH | 27.6 ± 9.5 | 2.6 ± 2.4 | <0.5 | 10.6 | 8.1 | 2.3 | 1.9 |
| RG2/CED N5–2OH + i.v. BPA | 45.6 ± 14.1 | 7.3 ± 1.0 | 7.6 ± 1.9 | 6.2 | 12.2 | 3.4 | 3.5 |
| RG2/i.v. BPA | 14.2 ± 7.7 | 5.0 ± 1.0 | 7.5 ± 2.1 | 1.9 | 5.0 | 2.9 | 3.5 |
| RG2/Irradiation control (CED of DMSO) | None | None | None | — | 1.8 | 1.8 | 1.8 |
| RG2/Untreated control (CED N5–2OH) | 17.3 ± 4.3 | <0.5 | <0.5 | 0 | 0 | 0 | |
Boron-10 enriched N5–2OH was administered i.c. by means of convection enhanced delivery over 24 h using Alzet pumps at a flow rate of 8.33 μl/h. BPA was administered intravenously 2.5 h prior to BNCT.
*Boron content was quantified by means of direct current plasma-atomic emission spectroscopy (DCP-AES) and has been reported as micrograms per gram of wt.
†Physical dose estimates include contributions from γ photons, 14N (n,p)14C, and 10B (n,α) 7Li reactions.
‡RG2 glioma cells were implanted into rats intracerebrally. The rats were irradiated 14 days after implantation.
§Boron concentrations for the tumor bearing cerebral hemisphere after excision of the tumor.
Responses After BNCT of RG2 Glioma-Bearing Rats.
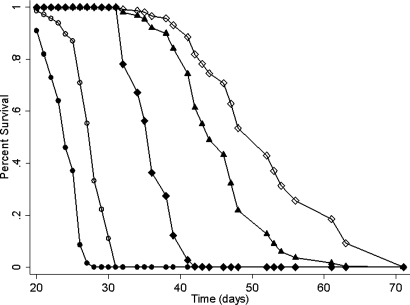
Survival data are summarized in Table 2, and the corresponding Cox survival plots are shown in Fig. 4. The longest MST ± standard error (SE) was 52.9 ± 8.9 days for animals that received the combination of N5–2OH and BPA compared with 45.6 ± 7.2 days for the rats that received N5–2OH alone and 35.9 ± 3.3 d for those that received BPA alone. The differences between the Cox survival plots (Fig. 4) of RG2 glioma-bearing rats that received i.c. N5–2OH vs. i.v. BPA were highly significant (P = 0.0003) while in contrast, there was only borderline significance in the survival plots of animals that received i.v. BPA + N5–2OH vs. N5–2OH alone (P = 0.054). The MSTs of irradiated and untreated controls were 28.1 ± 1.8 d and 23.8 ± 2.3 d, respectively. The greatest percent increase in life span (%ILS) (122%) was seen in RG2 glioma-bearing rats that received the combination of N5–2OH and BPA, and this correlated with the fact that the tumor in these animals received the highest physical radiation doses. The tumor size indices for all experimental and control groups were all almost identical and ranged from 3.3 to 3.6. Neuropathologic examination of the brains of RG2 glioma-bearing rats showed invasive growth of malignant cells and rare foci of necrosis and hemorrhage. In some instances, there were central clear zones in which viable tumor cells were dispersed and focal areas of leptomeningeal extension of the tumor.
Table 2.
Survival times of RG2 glioma bearing rats following CED of N5–2OH with or without i.v. BPA
| Group* | Number of animals | Survival time, d† |
% Increased life span‡ |
|||
|---|---|---|---|---|---|---|
| Mean ± SE | Median | Mean | Median | |||
| CED of N5–2OH + i.v. BPA | 10 | 52.9 ± 8.9 | 52.5 | 42–71 | 122 | 119 |
| CED of N5–2OH | 10 | 45.6 ± 7.2 | 45 | 36–61 | 92 | 88 |
| i.v. BPA | 8 | 35.9 ± 3.3 | 35.5 | 32–41 | 51 | 48 |
| Irradiated controls | 8 | 28.1 ± 1.8 | 28 | 26–31 | 18 | 17 |
| Untreated controls | 10 | 23.8 ± 2.3 | 24 | 20–27 | — | — |
*N5–2OH and BPA were administered as described in a footnote in Table 1.
†Mean and median survival times were determined for each group of 8–10 rats.
‡Percent increased life span (% ILS) was defined relative to mean and median survival times of untreated controls.
Fig. 4.
Cox survival plots for RG2 glioma bearing rats. Survival times have been plotted for untreated animals (●), irradiated controls (○), and animals that received i.v. BPA (◆) or N5–2OH either alone (▴) or in combination with BPA (◇).
Discussion
The experimental data presented in this study provide proof-of-principle that a 3CTA can function as an effective in vivo boron delivery agent for NCT. Ki67(+) immunostaining of L929 tumors indicated that TK1(+) proliferating cells were the primary targets for N5–2OH based BNCT. The %ILS of BNCT-treated, RG2 glioma-bearing rats that received N5–2OH was 2.4× greater than in those that received BPA. Kinase mediated trapping (19) appears to have been an effective mechanism for the selective entrapment of N5–2OH in the TK1(+) L929 tumor and the RG2 glioma. However, it was less so in the F98 glioma (20), which possibly might have been due to differences in TK1 activity and/or the expression levels of multidrug resistance proteins. Because serum concentrations of Thd, which compete with N5–2OH at the active site of TK1, are at least 10 times lower in humans than in mice and rats (21), N5–2OH might achieve higher tumor boron concentrations in humans than in rodents.
Schinazi et al. (22) have evaluated the efficacy of a pyrimidine nucleoside analogue, β-5-o-carboranyl-2′-deoxyuridine (D-CDU) as a boron delivery agent in 9L gliosarcoma-bearing rats. Non-10B-enriched D-CDU at concentrations of either 30 or 150 mg/kg was administered by either i.p. or i.v. injection, and the measured tumor boron concentrations at 2 h were 2.3 and 7.4 μg/g of tumor, respectively, with concomitant normal brain concentrations of 1.13 and 0.17 μg/g. In contrast, by administering N5–2OH at a much lower dose (500 μg vs. 30 mg for D-CDU) i.c. over 24 h by Alzet pumps, we have markedly increased tumor boron uptake (27.6 μg/gm tumor) with undetectable amounts in normal brain and blood. Furthermore, our survival data demonstrated that N5–2OH can function as an effective boron delivery agent, as evidenced by a highly significant improvement in MST compared with that of irradiated control rats.
There are, however, limitations and problems associated with N5–2OH and other currently available 3CTAs. First, the most promising 3CTAs are not water-soluble and require DMSO for solubilization. This could be obviated by designing 3CTA-specific drug delivery systems based on lipid nanotechnology or the design and synthesis of water-soluble third generation 3CTA prodrugs. Second, they appear to be significantly less effective than Thd in competing for the substrate binding site of TK1-like enzymes (19). This is a major limitation of the currently available 3CTAs, and could explain their lack of in vivo tumor selectivity after i.v. administration (W.Y. and R.F.B., unpublished data) and the necessity to administer them i.c. by means of Alzet osmotic pumps to produce high tumor drug concentrations. Third, virtually nothing is known about the metabolism of 3CTAs, including their possible incorporation into DNA. Fourth, there is very little information relating to the mechanisms of cellular influx and efflux of 3CTAs, and their metabolites.
On the other hand, the subcellular localization of 3 CTAs after in vivo administration may prove to be a significant advantage in their future development as boron delivery agents. Using the technique of secondary ion mass spectrometry (SIMS), it has been demonstrated that another 3CTA, designated N4 (14, 15), was distributed throughout interphase and mitotic T9 glioma cells, including their chromosomes (23). The latter observation suggests that it possibly was incorporated into DNA. In contrast, no nuclear localization was seen in T9 cells that had been exposed to BPA, which is not incorporated into DNA but whose uptake is increased in metabolically active, proliferating tumor cells. These observations support our hypothesis that the 3CTAs have a propensity to deliver boron to the cell nucleus, where the 10B [n,α]7Li capture reaction products have a greater likelihood of lethally damaging cells. In contrast to N5–2OH, i.c. delivery of BPA resulted in transiently high tumor and normal brain boron concentrations with rapid clearance, which would have precluded BNCT (15). The combination of boron delivery agents that target different subpopulations of tumor cells has been used by us (24) and others (25). In future studies, it would be of interest to evaluate 3CTAs in combination with BSH, which is taken up passively by nonproliferating (quiescent) cells (25), as well as with BPA. In conclusion, our data have demonstrated that a carboranyl nucleoside, N5–2OH, can function as boron delivery agent for NCT of a rat glioma. Based on this, we plan to move forward with the design and synthesis of 3CTAs with improved physico-chemical and biological properties and therapeutic effectiveness.
Materials and Methods
Target Validation Studies Using the L929 Tumor Model.
Both unenriched and 10B-enriched N5–2OH were synthesized as epimeric mixtures, as previously described (17). Because N5–2OH was designed to specifically target cells producing the TK1 enzyme, its potential to selectively accumulate in tumors was established using the L929TK1(+) wild-type cell line [American Type Culture Collection (ATCC) #CCL1, NCTC clone 929] and its L929 TK1(−) mutant counterpart [ATCC#CCL1.3 L-M (TK-)].
To obtain some estimate of the potential maximum in vivo uptake of N5–2OH, studies were carried out with TK1(+) and TK1(−) L929 cells, using a standardized labeling procedure with the Thd analogue, 5-bromo-2-deoxyuridine (BUdR), which is a TK1 substrate and is incorporated into DNA during S phase of the cell cycle. All animal studies were carried out in accordance with National Research Council guidelines (26) and approved by the Institutional Laboratory Animal Use Committee of The Ohio State University. L929 cells were implanted s.c. into the dorsum of NIH NCr-nu/nu nude mice, and ∼2 weeks later, when tumors had attained a sufficient size, the animals were injected i.p. with a mixture of BUdR and 5′ fluorodeoxyuridine (FUdR) at 2- h intervals for a total of up to 6 injections. FUdR was administered to suppress the de novo synthesis of thymidylate and, thus, DNA by inhibiting thymidylate synthetase. Two animals were killed by exposure to halothane vapor 30 min after each injection. The tumors then were excised, fixed in ethanol, and embedded in paraffin, and then 4-μm sections were cut. These were immunostained for BUdR (+) cells using a commercially available kit (ZYMED Laboratory, Inc.) and counterstained with hematoxylin and eosin (H&E), and the percentage of BUdR(+) cells was determined microscopically by counting 3–5 medium power fields (mpf).
To validate the hypothesis that tumor cells, which constitutively produced the TK1 enzyme, would be selectively targeted by N5–2OH, an in vivo biodistribution study was performed in which 106 TK1(+) or (−) L929 cells were implanted s.c. into the flanks of NIH nu/nu mice. Once the tumors had attained a size of ∼0.3–0.5 cm in diameter, in vivo uptake studies were initiated. Non 10B-enriched N5–2OH (250 μg) at a concentration of 31.6 mM (50 μg of boron) was solubilized in 70% dimethyl sulfoxide (DMSO). Because it previously was shown by us that systemic injection of N5–2OH resulted in low tumor and high nontumor boron concentrations (W.Y. and R.F.B., unpublished data), this was administered by direct i.t. injection in a volume of 15 μl over 2 min, and again 2 h later. Animals were killed at 2 h after the second injection; tumors and samples of skin and blood were taken, and subsequently processed for boron determination by means of direct current plasma-atomic emission spectroscopy (DCP-AES), as previously described (27).
Intracerebral Delivery and Toxicity of N5–2OH in Glioma Bearing Rats.
To determine whether i.c. delivery of N5–2OH was nontoxic, a group of five non-tumor-bearing Fischer rats received 500 μg of N5–2OH (100 μg of B) at a concentration of 4.7 mM, solubilized in 200 μl of 30% DMSO. This was administered over 24 h by means of Alzet pumps (model #2001D, Durect Corp., Cupertino, CA) at a flow rate of 8.33 μl/h. After administration of N5–2OH, the animals were monitored clinically for 2 weeks and weighed three times per week, after which they were killed. Their brains were removed, fixed in formalin, and then cut at 2-mm intervals. Selected sections were processed for microscopic neuropathologic examination and stained with H&E.
BNCT of L929 Tumor-Bearing Nude Mice.
One million L929 TK1(+) or TK1(−) cells were implanted s.c. into NIH NCr-nu/nu mice. Thirteen days later, the tumors had attained a volume of ∼80 mm3, as determined by measuring the greatest length (a) and width (b) [V = a·b2/2]. The mice then were transported to the MIT Research Reactor (MITR-II), where BNCT was performed by using the M-011 thermal neutron beam. Anesthetized animals were positioned in a 6Li enriched polyethylene box with a removable lid in which a 13 × 2 cm2 aperture had been machined. This served as a beam delimiter and provided whole body shielding from the thermal neutrons during an irradiation. Four mice were secured side-by-side, head-to-tail, to the underside of the box lid and positioned to align their tumors in the middle of the aperture. 10B-enriched N5–2OH was administered as previously described. Four hours after the first dose, the animals were irradiated with a collimated beam of thermal neutrons at a reactor power of 4.8 megawatts (MW). To determine the response to BNCT, tumors were removed from L929 TK1(+) tumor bearing animals, two of which had received N5–2OH followed by BNCT and two of which did not, but had been similarly irradiated. The tumors were fixed in formalin, processed for histology, and then immunostained with Ki67 mAb, which recognizes a nuclear antigen expressed during cell proliferation. Reductions in Ki67 expression have been correlated with the radiochemotherapeutic response of some neoplasms (28).
RG2 Brain Tumor Model, Biodistribution, and BNCT Studies.
The RG2 (CRL-2433, ATCC, Manassus, VA) rat glioma, which in vitro expressed TK1, was used to assess the response to BNCT. This was carried out 14 d after stereotactic implantation of 103 glioma cells (29). One week before irradiation, the rats were shipped by air to the MIT Nuclear Reactor Laboratory for irradiation at the MITR-II facility. They were randomized into experimental groups of 8–10 animals each as follows: (1) i.c. delivery of N5–2OH plus i.v. BPA and BNCT; (2) i.c. delivery of N5–2OH and BNCT; (3) i.v. BPA and BNCT; (4) i.c. delivery of DMSO and BNCT; and (5) untreated controls. Animals in Groups 1 and 2 received 500 μg of 10B-enriched N5–2OH at the same concentration as that used in the biodistribution studies. It contained 100 μg of boron, solubilized in 35% DMSO in a volume of 200 μl, and was administered by means of Alzet osmotic pumps (model #2001D) over 24 h at a flow rate of 8.33 μl/h, after which BNCT was carried out. Animals in Groups 1 and 3 received 500 mg of 10B-enriched BPA (Katchem), formulated as a fructose complex (29), and administered i.v. via the penile vein 2.5 h. before neutron irradiation. All irradiated rats were anesthetized with a mixture of ketamine and xylazine, after which they were irradiated at the MITR-II reactor, as previously described by us (11, 30).
Dosimetry and Clinical Monitoring.
Dosimetric measurements were performed, as previously described (11, 30). The measured absorbed dose rates in mice (muscle −3.5% nitrogen by weight) at the position of the tumor and normalized to the reactor operating at 5 MW were 0.20 ± .01 Gy·min−1 for γ photons, 0.13 ± 0.01 Gy·min−1 for thermal neutrons (principally capture in nitrogen 14N [n, p] 14C), and 0.04 Gy·min−1 for neutron capture per μg of 10B in tissue. The measured dose rates in rats (brain −2.2% nitrogen by weight), normalized to the reactor operating at a power of 5 MW, were 0.185 Gy·min−1 for photons, 0.08 Gy·min−1 for thermal neutrons, and 0.034 Gy·min−1 per μg 10B in tissues, which was based on 10B concentrations determined in the biodistribution studies. After completion of BNCT, the animals were held at MIT for ∼3 d to allow induced radioactivity to decay before they were returned to Columbus, OH. All animals were weighed three times per week, and their clinical status was evaluated at the same time. Once the animals had progressively growing tumors, they were euthanized to minimize discomfort. The brains of all animals in the therapy studies were removed after death, fixed in 10% buffered formalin, and then cut coronally at the level of the optic chiasm and 2 mm anterior and posterior to it. Representative brains from each group were processed for neuropathologic examination. The tumor size index was determined by measuring with calipers the tumor's greatest cross-sectional diameter in 2-mm coronal sections of brain under a dissecting microscope. As previously described (30), a semiquantitative grading scale ranging from 0 to 4 was used. The mean survival time (MST), standard error (SE), and median survival times (MeST) were calculated for each group by using the Kaplan–Meier method, and simultaneous Cox survival plots also were plotted (31). The latter performs a simultaneous fit of the survival plots by using all of the data points using a partial likelihood approach. Therefore, the number of data points in each plot includes all of the death times, rather than only those animals in a specific group. Because proportional hazards were satisfied, pairwise Wald log rank tests were performed comparing the Cox survival plots of the groups by using a Bonferroni method of adjustment for multiple comparisons (32).
Acknowledgments.
This work was supported in part by National Institutes of Health Grant 1R01 CA09845 (to R.F.B.); the Dardinger Neuro-Oncology Center Endowment of The Ohio State University (R.F.B.); Unites States Department of Energy through the program of Innovations in Nuclear Infrastructure and Education, Office of Nuclear Energy, Science and Technology [contract no. DE-FG07-02ID14420DE-FG07–02 (K14420)] (to P.J.B. and K.J.R.); Office of Environmental and Biological Research (contract nos. DE-FG02-02ER63358 (to R.F.B.) and DE-FG02-90ER60972 (to W.T.); and the Swedish Research Council (S.E.).
Footnotes
This work was presented in part at the Annual Meeting of the American Association for Cancer Research, Los Angeles, April 14–18, 2007.
The authors declare no conflict of interest.
References
- 1.Barth RF, Coderre JA, Vicente MG, Blue TE. Boron neutron capture therapy of cancer: Current status and future prospects. Clin Cancer Res. 2005;11:3987–4002. doi: 10.1158/1078-0432.CCR-05-0035. [DOI] [PubMed] [Google Scholar]
- 2.Barth RF, Joensuu H. Boron neutron capture therapy for the treatment of glioblastomas and extracranial tumours: As effective, more effective or less effective than photon irradiation? Radiother Oncol. 2007;82:119–122. doi: 10.1016/j.radonc.2007.01.010. [DOI] [PubMed] [Google Scholar]
- 3.Miyatake S-I, et al. Boron neutron capture therapy for malignant tumors related to meningiomas. Neurosurg. 2007;60:1–11. doi: 10.1227/01.neu.0000279727.90650.24. [DOI] [PubMed] [Google Scholar]
- 4.Kankaanranta L, et al. Boron neutron capture therapy in the treatment of locally recurred head and neck cancer. Int J Rad Oncol Biol Phys. 2007;69:475–482. doi: 10.1016/j.ijrobp.2007.03.039. [DOI] [PubMed] [Google Scholar]
- 5.Zonta A, et al. Clinical lessons from the first applications of BNCT on unresectable liver metastases. J Physics: Conference series. 2006;41:484–495. [Google Scholar]
- 6.Zamenhof RG, Coderre JA, Rivard MJ, Patel H. Topics in Neutron Capture Therapy. Appl Radiat Isotop; Proceedings of the Eleventh World Congress on Neutron Capture Therapy; 2004. pp. 731–1130. [Google Scholar]
- 7.Vicente MGH. Boron in medicinal chemistry. Anti-Cancer Agents Med Chem. 2006;6:73–181. [Google Scholar]
- 8.Soloway AH, et al. The chemistry of neutron capture therapy. Chem Rev. 1998;98:1515–1562. doi: 10.1021/cr941195u. [DOI] [PubMed] [Google Scholar]
- 9.Mendelsohn J, Baselga J. Epidermal growth factor receptor targeting in cancer. Semin Oncol. 2006;33:369–385. doi: 10.1053/j.seminoncol.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 10.Wu G, et al. Boron containing macromolecules and nanovehicles as delivery agents for neutron capture therapy. Anti-Cancer Agents Med Chem. 2006;6:167–184. doi: 10.2174/187152006776119153. [DOI] [PubMed] [Google Scholar]
- 11.Wu G, et al. Molecular targeting and treatment of an epidermal growth factor receptor-positive glioma using boronated cetuximab. Clin Cancer Res. 2007;13:1260–1268. doi: 10.1158/1078-0432.CCR-06-2399. [DOI] [PubMed] [Google Scholar]
- 12.Yang W, Barth RF, Wu G, et al. Molecular targeting and treatment of EGFRvIII-positive gliomas using boronated monoclonal antibody L8A4. Clin Cancer Res. 2006;12:3792–3802. doi: 10.1158/1078-0432.CCR-06-0141. [DOI] [PubMed] [Google Scholar]
- 13.Arner ES, Eriksson S. Mammalian deoxyribonucleoside kinases. Pharmacol Therapeut. 1995;67:155–186. doi: 10.1016/0163-7258(95)00015-9. [DOI] [PubMed] [Google Scholar]
- 14.Al-Madhoun AS, Johnsamuel J, Barth RF, Tjarks W, Eriksson S. Evaluation of human thymidine kinase 1 substrates as new candidates for boron neutron capture therapy. Cancer Res. 2004;64:6280–6286. doi: 10.1158/0008-5472.CAN-04-0197. [DOI] [PubMed] [Google Scholar]
- 15.Barth RF, et al. Boron-containing nucleosides as potential delivery agents for neutron capture therapy of brain tumors. Cancer Res. 2004;64:6287–6295. doi: 10.1158/0008-5472.CAN-04-0437. [DOI] [PubMed] [Google Scholar]
- 16.Byun Y, et al. 3-carboranyl thymidine derivatives and other boronated nucleosides for neutron capture. Curr Med Chem Anti-Cancer Agents. 2006;6:127–144. doi: 10.2174/187152006776119171. [DOI] [PubMed] [Google Scholar]
- 17.Byun Y, et al. Preparation and biological evaluation of 10B-enriched 3-[5-{2-(2,3-dihydroxyprop-1-yl)-o-carboran-1-yl}pentan-1-yl]thymidine (N5–2OH), a new boron delivery agent for boron neutron capture therapy of brain tumors. J Med Chem. 2006;49:5513–5523. doi: 10.1021/jm060413w. [DOI] [PubMed] [Google Scholar]
- 18.Johansson NG, Eriksson S. Structure-activity relationships for phosphorylation of nucleoside analogs to monophosphates by nucleoside kinases. Acta Biochim Pol. 1996;43:143–160. [PubMed] [Google Scholar]
- 19.Tjarks W, Tiwari R, Byun J, Naranyanasamy S, Barth RF. Carboranyl nucleoside analogues for neutron capture therapy. Chem Comm (London) 2007;47:4978–4991. doi: 10.1039/b707257k. [DOI] [PubMed] [Google Scholar]
- 20.Barth RF, et al. In vivo evaluation of the 3-carboranyl thymidine analogue (3-CTA), N5–2OH, for neutron capture therapy. In: Nakagawa Y, Kobayashi T, Fukada H, editors. Proc. 12th International Congress on Neutron Capture Therapy; 2006. pp. 109–112. [Google Scholar]
- 21.Nottebrock H, Then R. Thymidine concentrations in serum and urine of different animal species and man. Biochem Pharmacol. 1977;26:2175–2179. doi: 10.1016/0006-2952(77)90271-4. [DOI] [PubMed] [Google Scholar]
- 22.Schinazi RF, et al. Treatment of isografted 9L rat brain tumors with β-5-o-carboranyl-2′-deoxyuridine neutron capture therapy. Clin Cancer Res. 2000;6:725–730. [PubMed] [Google Scholar]
- 23.Chandra S, Tjarks W, Lorey DR, Barth RF. Quantitative subcellular imaging of boron compounds in individual mitotic and interphase human glioblastoma cells with imaging secondary ion mass spectrometry (SIMS) J Microscopy. 2008;229:92–103. doi: 10.1111/j.1365-2818.2007.01869.x. [DOI] [PubMed] [Google Scholar]
- 24.Barth RF, et al. Boron neutron capture therapy of brain tumors: Enhanced survival and cure following blood-brain barrier disruption and intracarotid injection of sodium borocaptate and boronophenylalanine. Int J Radiat Oncol Biol Phys. 2000;47:209–218. doi: 10.1016/s0360-3016(00)00421-1. [DOI] [PubMed] [Google Scholar]
- 25.Ono K, et al. The combined effect of boronphenylalanine and borocaptate in boron neutron capture therapy for SccVII tumors in mice. Int J Rad Oncol Biol Phys. 1999;43:431–436. doi: 10.1016/s0360-3016(98)00421-0. [DOI] [PubMed] [Google Scholar]
- 26.Guide for the Care and Use of Lab Anim. Washington, DC: National Academy Press; 1996. Anonymous. [Google Scholar]
- 27.Barth RF, et al. Determination of boron in tissues and cells using direct-current plasma atomic emission spectroscopy. Anal Chem. 1991;63:890–893. doi: 10.1021/ac00009a010. [DOI] [PubMed] [Google Scholar]
- 28.Rodel C, et al. Apoptosis, p53, bcl-2, and Ki-67 in invasive bladder carcinoma: Possible predictors for response to radiochemotherapy and successful bladder preservation. Intl J Rad Oncol Bio Phys. 2000;46:1213–1221. doi: 10.1016/s0360-3016(99)00544-1. [DOI] [PubMed] [Google Scholar]
- 29.Yang W, Barth RF, Carpenter DE, Moeschberger ML, Goodman JH. Enhanced delivery of boronophenylalanine for neutron capture therapy by means of intracarotid injection and blood-brain barrier disruption. Neurosurg. 1996;38:985–992. doi: 10.1097/00006123-199605000-00027. [DOI] [PubMed] [Google Scholar]
- 30.Yang W, et al. Molecular targeting and treatment of composite EGFRvIII and EGFR-positive gliomas using boronated monoclonal antibodies. Clin Cancer Res. 2008;14:883–891. doi: 10.1158/1078-0432.CCR-07-1968. [DOI] [PubMed] [Google Scholar]
- 31.Klein JP, Moeschberger ML. Survival analysis: Techniques for censored and truncated data, 2nd ed. Springer, New York), pp 92–104, 205–. 2003;221:276–283. [Google Scholar]
- 32.Madsen RW, Moeschberger ML. Englewood Cliffs, New Jersey: Prentice-Hall; 1986. Statistical concepts; pp. 538–541.pp. 583 [Google Scholar]