Abstract
Cloning animals by nuclear transfer provides an opportunity to preserve endangered mammalian species. However, it has been suggested that the “resurrection” of frozen extinct species (such as the woolly mammoth) is impracticable, as no live cells are available, and the genomic material that remains is inevitably degraded. Here we report production of cloned mice from bodies kept frozen at −20 °C for up to 16 years without any cryoprotection. As all of the cells were ruptured after thawing, we used a modified cloning method and examined nuclei from several organs for use in nuclear transfer attempts. Using brain nuclei as nuclear donors, we established embryonic stem cell lines from the cloned embryos. Healthy cloned mice were then produced from these nuclear transferred embryonic stem cells by serial nuclear transfer. Thus, nuclear transfer techniques could be used to “resurrect” animals or maintain valuable genomic stocks from tissues frozen for prolonged periods without any cryopreservation.
Keywords: brain, cloning, cryopreservation, nuclear transfer, reprogramming
Current animal cloning techniques using nuclear transfer (1) can reconstruct animals from donor cells; clones so produced differ only in their mitochondrial DNA (2). This technology has produced a variety of cloned animals for scientific and commercial purposes. However, it is not known whether this method could be applied to the recovery of animals from frozen extinct species. Although most, if not all, mammalian cells lose their functional integrity if frozen without cryoprotective reagents, it is conceivable that some aspects of genomic information remain intact even in such dead bodies. By contrast, the genomes of spermatozoa are resistant to freezing (3), and even freeze-drying (4) without cryoprotectants. However, it has been difficult to demonstrate this in somatic cells, due to technical difficulties in assessing genomic integrity.
One recent paper has reported on the nuclear integrity of dead cells after freeze-thawing by improved techniques (5). This study successfully produced cloned mouse embryos and generated germ line chimerae via nuclear transferred embryonic stem cell (ntES) cells. However, although all donor cells were dead after thawing, the donor cells were frozen in a medium including polyvinylpyrrolidone, which is known to exhibit cryoprotectant effect (6). Freeze-dried cells were also used for these nuclear transfer attempt, and all cells were dead after rehydration (7, 8). Although cloned embryos were obtained from those dead cells following nuclear transfer, no cloned offspring were obtained. Moreover, so far all studies of nuclear transfer using “dead” material have used isolated single cells frozen in the laboratory using special media. In dead specimens frozen in natural conditions such as permafrost tundra, the cells of tissue will presumably bind strongly to each other and freeze gradually after death due to the large body size. It remains to be shown whether nuclei can be collected from whole bodies frozen without cryoprotectants and whether they will be viable for use in generating offspring following nuclear transfer. This is an important question with potential application in the cloning of extinct animals frozen in permafrost, or specimens collected opportunistically from endangered species in the field without access to sophisticated laboratory facilities.
In this study, we first attempted to determine the conditions needed to restore nuclei from frozen bodies and to assess their viability for use in cloning by nuclear transfer. In one typical cloning procedure, live donor cells are fused with enucleated oocytes, which requires the donors to have viable cell membranes. Thus, obtaining suitable cells for nuclear transfer from naturally frozen tissues is extremely difficult, if not impossible. We have developed an alternative nuclear transfer method in which we inject denuded donor nuclei into oocytes directly (9). We hypothesized that this method might allow us to produce cloned embryos using nuclear transfer from frozen bodies or tissues
Results
Adaptation of the Nuclear Transfer Technique for Frozen Dead Donor Mice.
To apply our nuclear transfer methods for production of cloned mice from frozen tissues, we tested the efficiency of producing cloned embryo development in vitro from different organs of mice frozen at −20 °C for one week. We collected denuded nuclei (Fig. 1 D and E) by homogenizing the organs in nuclear isolation medium (NIM) (10) instead of making single cell suspensions by enzyme digestion. Blood was also collected from the tail vein and diluted simply with NIM. To minimize nuclear damage and improve the survival rate of oocytes after injection, nuclear transfer into enucleated oocytes was carried out using a large diameter injection pipette in modified NIM [supporting information (SI) Fig. S1 a and b]. The rate of development to morula/blastocyst stage embryos using the nuclei from cells taken from most organs of the frozen mice was very low (Table 1 and Fig. S1c) and not as efficient as when using live cumulus or fibroblast cell nuclei (11–13). However, cloned embryos derived from brain tissue nuclei developed into morulae/blastocysts (39%) at a rate similar to or even better (Fig. S1d) than a previous report (9). Moreover, nuclei from tail blood cells also supported morula/blastocyst development (18%), albeit at a lower efficiency (Table 1). To determine if this success was attributable to the presence of surviving whole cells, or only subcellular components that still included the genome, we assessed the extent to which functional cells were present in these frozen body tissues. No live cells could be detected from blood samples using flow cytometry and vital staining, (Fig. S2 and Fig. S3). Likewise, for brain tissues, no live cells were detectable after gentle homogenization (Fig. S4), suggesting that our success was due to the preservation of subcellular components rather than living cells. Accordingly, despite the absence of viable cells, we concentrated on these two organs (brain and blood) as potential sources of nuclei for the production of cloned mice from frozen tissues.
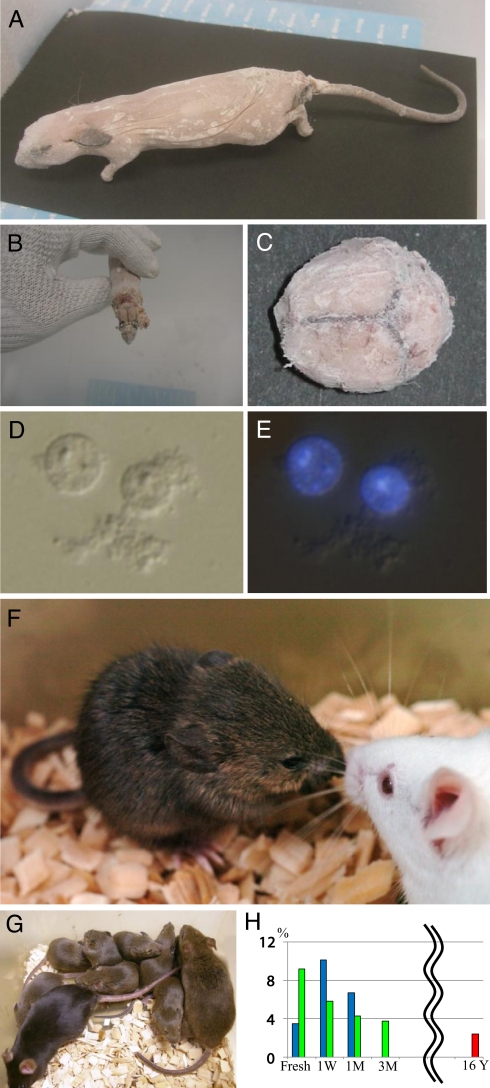
Fig. 1.
Mouse frozen for 16 years and its clone. (A) C3H/He strain male mouse frozen at −20 °C for 16 years. (B and C), Frozen brain tissues were collected on dry ice. (D and E) Donor nuclei were collected from a brain fragment by gentle homogenization. (E) Nuclei were stained with Hoechst 33342. (F) A cloned mouse derived from C3H/He strain male mouse frozen at −20 °C for 16 years via secondary nuclear transfer from an ntES cell. One clone died from respiratory failure just after birth and one clone showed open eyelids at birth but was cannibalized the next day by the foster mother. However, the remaining two clones grew to adulthood. (G) The first C3H/He strain cloned mouse (agouti) has demonstrated normal fertility (all offspring were agouti) when mated with a female (black). The female clone was derived from BDF1 frozen brain via ntES cell nuclear transfer. (H) Although the rate of establishment of ntES cell lines decreased with the increased storage period, the sample frozen for 16 years retained the potential to establish ntES cell lines after nuclear transfer. Blue bar, derived from brain nuclei; green bar, derived from tail blood; red bar, derived from C3H strain brain nuclei.
Table 1.
Attempts at generating cloned embryos and establishment of ntES cell lines by nuclear transfer from several organs of frozen mice stored at −20°C for one week without cryoprotectants
| Tissue | No. enucleated oocytes | No. surviving oocytes after nuclear injection | No. activated oocytes | No. embryos developing to morula/ blastocyst, %* | No. established ntES cell lines, %** |
|---|---|---|---|---|---|
| Brain | 133 | 110 | 109 | 43 (39.4)a | 11 (10.1)g |
| Tail blood | 447 | 409 | 326 | 58 (17.8)b | 19 (5.8) |
| Pancreas | 111 | 90 | 85 | 13 (15.3)c | 1 (1.2)h |
| Kidney | 117 | 84 | 76 | 11 (14.5)c | 3 (3.9) |
| Bone M | 116 | 83 | 71 | 9 (12.7)d | 2 (2.8) |
| Spleen | 115 | 85 | 75 | 9 (12.0)d | 3 (4.0) |
| Thymus | 110 | 84 | 77 | 5 (6.5)e | 1 (1.3)h |
| Lung | 136 | 83 | 78 | 5 (6.4)e | 1 (1.3) |
| Heart | 110 | 89 | 82 | 5 (6.1)e | 0h |
| Liver | 111 | 75 | 66 | 0f | 0h |
| Intestine | 100 | 65 | 55 | 0f | 0h |
| Total | 1606 | 1257 | 1100 | 158 | 34 |
All experiments were repeated more than four times; percentages are based on the numbers of activated oocytes.
*a vs b, c, d, e, f and b, c vs f: P < 0.01; b vs e and d vs f: P < 0.05.
**g vs h: P < 0.05.
Production of Cloned Mice Directly from Frozen Mice.
To produce cloned mice by nuclear transfer, brain tissues Fig. 1 B and C from two different frozen mouse strains were collected (BDF1 females and males frozen for one week or one month and two C3H/He male mice frozen for 16 years at −20 °C; Fig. 1A and nuclei were transferred to enucleated oocytes Fig. S1 a and b. The resulting 2-cell embryos were transferred into recipient pseudopregnant females. From the BDF1 nuclei, we obtained three and five live cloned mice from male samples frozen for one week and one month, respectively (Table 2). These cloned mice did not show any abnormalities and grew to adulthood. However, no cloned mouse was produced using donor nuclei from the brain from C3H strain mice frozen for 16 years. It should be noted that these mice were from an inbred strain and that cloning has never been achieved from inbred mice, even when using fresh donor cells (14, 15). Thus, the failure of cloning in this instance was probably an effect of the mouse strain, rather than because of freezing damage. Because the genetic background inevitably affects experimental outcomes in cloning, results should ideally be compared within the same strain to exclude strain effects. However, as mentioned above, inbred mice cannot be used to set up a control study using nuclear transfer, and we could obtain only C3H strain mice as donor samples frozen for prolonged periods. Thus, in the following experiments we were limited to making indirect comparisons of the BDF1 and C3H results.
Table 2.
Production of cloned mice from brain cell nuclei recovered from mice frozen without cryoprotectants
| Freezing duration | Donor strain | Sex | No. enucleated oocytes | No. activated oocytes | No. embryos developing to the 2-cell stage, % | No. offspring, %* |
|---|---|---|---|---|---|---|
| Fresh | BDF1 | M | 273 | 172 | 163 (94.8) | 0 |
| 1 week | F | 108 | 77 | 67 (87) | 0 | |
| M | 162 | 116 | 103 (88.8) | 3 (2.6) | ||
| 1 month | F | 142 | 101 | 75 (74) | 0 | |
| M | 208 | 182 | 160 (87.9) | 5 (2.7) | ||
| 16 years | C3H | M | 489 | 266 | 126 (47.4) | 0 |
*Percentages based on the numbers of 2-cell embryos.
Establishment of Nuclear Transfer Embryonic Stem (ntES) Cells from Frozen Mice.
In parallel experiments, we tried to establish ntES cell lines (16) using frozen tissues as a nuclear donor source. As shown in Tables 1 and 3 and Table S1, we established 46 ntES cell lines from all brain samples including both two C3H/He mice frozen for 16 years, 42 ntES cell lines from tail blood samples, and 11 ntES cell lines from several organs, such as pancreas or kidney. The rate of establishment decreased with the duration of freezing (Fig. 1H), but all established ntES cell lines were positive for the ES cell-specific pluripotency markers, Oct3/4 (Fig. S1 e, and f), Nanog (data not shown), and alkaline phosphatase (Fig. S1g). Ten randomly selected ntES cell lines had normal karyotypes (Fig. S1h). To test the normality of these ntES cell lines, we injected them into diploid albino strain mouse blastocysts Table S2. These chimeric mice had a high rate of ntES cell contribution (Fig. S5a), including to the germline (Fig. S5b), demonstrating the pluripotency of the ntES cells.
Table 3.
Establishment of ntES cell lines from brain cell nuclei of mice frozen without cryoprotectants
| Freezing duration | Donor strain | Sex | No. enucleated oocytes | No. activated oocytes | No. embryos developing to morula/blastocyst, % | No. established ntES cell lines, %* |
|---|---|---|---|---|---|---|
| Fresh | BDF1 | M | 88 | 57 | 7 (13) | 2 (4)a |
| 1 week** | F | 66 | 57 | 24 (14) | 8 (14)b,c | |
| M | 67 | 52 | 19 (21) | 3 (6) | ||
| 1 month | F | 203 | 172 | 40 (15.7) | 13 (7.6)c | |
| M | 192 | 170 | 49 (28.8) | 10 (5.9)c | ||
| 16 years | C3H | M | 560 | 412 | 81 (19.7) | 10 (2.4)d |
*a vs b and c vs d: P < 0.05.
**These data and those on the brain in Table 1 are the same but given in more detail.
Production of Cloned and Chimeric Clonal Mice from ntES Cell Nuclei.
Using these ntES cells, we attempted to produce cloned or chimeric clonal mice by a second round of nuclear transfer and tetraploid complementation. In this procedure, ntES cells are aggregated with tetraploid embryos, and the resulting chimeric offspring are nearly completely of ntES cell origin (17, 18). As shown in Table 4, we obtained cloned mice from all experimental groups by secondary transfer of ntES cell nuclei into enucleated oocytes, irrespective of donor sex or storage duration. Importantly, we produced a total of 4 cloned mice (Fig. 1F and 9 chimeric clonal mice (Fig. S5c) via ntES cells from both C3H/He mice frozen for 16 years. Two of these clones showed normal fertility after being mated with a BDF1 female clone (Fig. 1G). These C3H/He cloned and chimeric clonal mice show the agouti coat color of the donor strain (Fig. 1F), whereas the oocyte donors and surrogate mothers do not carry the agouti gene. The coat color, sex and microsatellite analysis of the genotypes showed that all of the ntES cell lines as well as the cloned and chimeric clonal mice were indeed derived from the donor mice (Fig. S6a). In addition, no gene rearrangement was found in any ntES cell line (Fig. S6b), which demonstrated that donor nuclei were not T- or B-lymphocytes and that the complete genomes of the donor mice had been recovered successfully, as in the ntES cells. Thus, we could obtain cloned mice by nuclear transfer from frozen brain tissue either directly or via ntES cell nuclei. Although, as with all cloning technology, the success rate was low, we also obtained cloned or chimeric clonal mice from frozen tail blood cells via ntES cell nuclei (Tables S3 and S4).
Table 4.
Production of cloned or clonal mice from ntES cells derived from mice frozen without cryoprotectants
| Freezing duration | Donor | Sex | Type | No. enucleated oocytes | No. activated oocytes | No. embryos developing to 2-cell, %* | No. chimeric embryos | No. offspring, %** |
|---|---|---|---|---|---|---|---|---|
| Fresh | BDF1 | M | Clone | 162 | 117 | 59 (50.4) | — | 1 (1.7) |
| 1 week | F | 208 | 130 | 41 (31.5) | — | 4 (9.8) | ||
| M | 151 | 92 | 34 (37) | — | 1 (2.9) | |||
| 1 month | F | 144 | 103 | 64 (62.1) | — | 1 (1.6) | ||
| M | 167 | 116 | 69 (59.5) | — | 2 (2.9) | |||
| 16 years | C3H | M | Clone | 453 | 274 | 95 (34.7) | — | 4 (4.2) |
| Clonal | — | — | — | 171 | 9 (5.3) |
*Percentages based on the numbers of activated oocytes.
**Percentages based on the numbers of 2-cell embryos.
Discussion
We have demonstrated here that healthy cloned mice and chimeric clonal mice could be obtained by nuclear transfer using donor nuclei from cells obtained from bodies frozen without cryoprotectants for up to 16 years. None of the cells in animals frozen in this manner remain intact. However, as the cloning efficiency from the frozen tissues was similar to that using live normal tissues, it is evident that some genomic integrity was preserved even after such extended storage. Unexpectedly, the best organ for source of donor nuclei was the brain, although the specific cell type (or types) of the donor cells has not been determined. Cloned mice have been obtained using the nuclei of embryonic or newborn neural cells (19–21). However, to our knowledge, no cloned mice have been generated from adult brain cells, and we have also failed to generate cloned mice from fresh adult brain tissues.
Why did frozen-thawed brain nuclei show such good developmental potential after nuclear transfer? One possibility is that this outcome may reflect differential cold tolerance dependent on the organ or cell type (22). It is known that polysaccharides, such as sucrose or trehalose, can be used as cryoprotectants and brain function is heavily dependent on glucose. One testable possibility is that endogenous glucose improved the ability of the brain to withstand freezing without cryoprotection. Paradoxically, freeze-thawing might even have allowed the brain cells to show better reprogrammable potential than live cell nuclei. It has been suggested that the use of denatured somatic cells for cloning might permit enhanced access of oocyte reprogramming factors into the nuclei and thereby increase the cloning success rate (23, 24). These frozen-thawed brain nuclei also had denatured genomes, which may have permitted the accessibility of reprogramming factors from the oocyte cytoplasm.
Most of our success in generating cloned or chimeric clonal mice from frozen samples was dependent on a secondary round of nuclear transfer from established ntES cells. This indirect route is simpler than the direct production of cloned mice (25, 26). Such ntES cells are functionally indistinguishable from ES cells derived by fertilization (27, 28). For any attempts at the resurrection of extinct animals or preservation of endangered species, the establishment of ntES cells should be attempted first rather than the direct production of clones (26). Once ntES cell lines are established, sufficient numbers of cells with intact genomic DNA can be obtained easily. Although we failed to produce cloned mice directly from C3H/He strain brain nuclei frozen for 16 years, this could be an effect of the strain rather than the storage period, because we were able to produce C3H/He cloned mice from ntES cells by a second round of nuclear transfer. Using such ntES cells as a genomic resource would allow us to carry out a variety of experiments, such as whole genome sequencing or establishment of an animal genome repository similar to those developed for plant seeds. Although many of the techniques in this study have succeeded only in the mouse and, so far, ES cell lines have been established only from mice, primates, and humans, ES-like cell lines have been derived from many other species (29–32). Such cell lines could be used to prepare sufficient numbers of donor cells for serial nuclear transfer studies, as demonstrated in experiments with cattle (29). Thus, the combination of cloning and ntES cell techniques offers a distinct chance to resurrect extinct animals or preserve endangered species with appropriate frozen tissues.
Recently, our group and others have demonstrated that live mice could be obtained using intracytoplasmic injections of dead sperm (4, 33) or even using sperm from bodies kept frozen for 15 years (3). However, it is not possible to clone complete animals from spermatozoa alone. Nuclear transfer techniques, in contrast, allow us in effect to “resurrect” entire genomes, irrespective of the donor sex. Although brain tissues proved the most effective for producing cloned mice in this study, it is possible that other organs or tissues, such as frozen leukocytes, could be used as sources of donor nuclei. This would increase the chances of finding tissues in good condition. At present, the lack of suitable species for recipient oocytes and for surrogate mothers is one of the major problems that needs to be solved for the method to be applied in extinct or endangered animals. However, the use of interspecies nuclear transfer techniques (34, 35) could solve this problem and provide a possibility of reconstituting genomes or animals from samples frozen without access to sophisticated laboratory facilities.
Methods
Preparation of Donor Cells.
To identify cells resistant against freezing, 11 mouse organs were stored in cryotubes at −20 °C for one week without any cryoprotection. For prolonged preservation experiments, whole mouse bodies were kept at −20 °C for up to 3 months without defrosting (BDF1 strain, healthy males and females, 2–3 months old, euthanized by vertebral dislocation and placed into a plastic bag), or at −20 °C for 16 years (C3H/He strain, healthy males, 3 months old, euthanized by an overdose of pentobarbital sodium and placed into a paper box before freezing). Frozen brain tissues were extracted from the heads on dry ice just before nuclear transfer. Tissues were thawed by adding 400 μL of NIM (10) and then homogenized gently to collect denuded nuclei. The exception was blood cells, which were collected by squeezing 1–2 μL of blood from the tail; these were simply washed and diluted in NIM. Those nuclei were kept at 4 °C until use, and fresh donor nuclei were added to the micromanipulation chamber every hour from this stock.
Nuclear Transfer and Production of Cloned Mice.
Nuclear transfer and oocyte activation was performed as described (36, 37). However, in this experiment, to avoid damage to denuded nuclei we performed nuclear transfer into enucleated oocytes using a large injection pipette and used modified NIM for nuclear injection instead of polyvinylpyrrolidone-containing medium. When any cloned embryos developed to the 2-cell stage, they were transferred into pseudopregnant ICR females at 0.5 days post copulation (dpc), and live offspring were collected by Caesarean section at 19.5 dpc.
ntES Cell Derivation.
When embryos had developed to the morula or blastocyst stage, they were used to establish ntES cell lines as described (16). Briefly, embryos were treated with acid Tyrode's solution to remove the zona pellucida and placed in 96-well dishes with mouse embryonic fibroblasts (ICR strain origin) for more than 10 days. Proliferating outgrowths were dissociated using trypsin digestion and replated on fibroblasts until stable cell lines grew out. All established ntES cells were evaluated for signs of pluripotency by alkaline phosphatase staining, immunostaining for Oct3/4 and Nanog, and by karyotyping 10 randomly selected ntES cell lines using SKY-FISH staining according to the manufacturer's protocol (Spectral Imaging Ltd.).
Production of Diploid and Tetraploid Chimeras.
Diploid and tetraploid embryos were obtained from ICR strain females mated with ICR males. Tetraploid embryos were produced by the electrofusion of 2-cell embryos (18). To make diploid chimeras, ntES cells were injected into the blastocoels of blastocysts. To construct tetraploid chimeras—clonal embryos—3 tetraploid embryos at the 8-cell stage and a small block of ntES cells were aggregated (38). Next day, the chimeric blastocysts were transferred into pseudopregnant females.
Genotyping of Cloned Mice and Examination of Gene Rearrangements.
The microsatellite markers D1Mit26, D3Mit18, and D3Mit21 were amplified using primer pair sequences obtained from the National Center for Biotechnology Information (NCBI, http://www.ncbi.nlm.nih.gov/). DNA was extracted from ntES cells and tail tips of cloned and clonal mice. Thirty cycles of PCR amplification were performed and the products were separated on 3% agarose gel before visualization.
DNA samples from T and B cell-derived ntES cell lines 1–10 and from cell lines established from frozen C3H strain mice were analyzed for any rearrangements of the gene sequences for the T cell receptor and Ig κ light chain. The primer pairs used for PCR amplify a gene segment upstream of the Db chain, and 40% of the ES cell lines derived from T cells showed a deletion of the segment. For Ig rearrangements, the primer pairs used amplify a region upstream of the Jk complex (39) and 45% of the B cell-derived ES cells had a deletion of this segment.
Statistical Procedures.
Outcomes were evaluated using χ2 test, and P < 0.05 was assumed to be statistically significant.
Supplementary Material
Acknowledgments.
We thank S-I. Nishikawa, S. Nishikawa, D. Sipp, J. Cummins, P. Mombaerts, M. Takase, N. Ogonuki, A. Ogura, S. Kishigami and E. Kanagawa for critical and useful comments on the manuscript. We also thank T. Oyanagi, Y. Sakaide, K. Yamagata, C. Li, and T. Ono for preparing this manuscript. We are grateful to the Laboratory for Animal Resources and Genetic Engineering for housing the mice. This work was supported by Scientific Research in Priority Areas Grant 15080211 and a Project for the Realization of Regenerative Medicine grant (to T.W.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0806166105/DCSupplemental.
References
- 1.Wilmut I, et al. Viable offspring derived from fetal and adult mammalian cells. Nature. 1997;385:810–813. doi: 10.1038/385810a0. [DOI] [PubMed] [Google Scholar]
- 2.Evans MJ, et al. Mitochondrial DNA genotypes in nuclear transfer-derived cloned sheep. Nat Genet. 1999;23:90–93. doi: 10.1038/12696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ogonuki N, et al. Spermatozoa and spermatids retrieved from frozen reproductive organs or frozen whole bodies of male mice can produce normal offspring. Proc Natl Acad Sci USA. 2006;103:13098–13103. doi: 10.1073/pnas.0605755103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wakayama T, Yanagimachi R. Development of normal mice from oocytes injected with freeze-dried spermatozoa. Nat Biotechnol. 1998;16:639–641. doi: 10.1038/nbt0798-639. [DOI] [PubMed] [Google Scholar]
- 5.Li J, Mombaerts P. Nuclear transfer-mediated rescue of the nuclear genome of nonviable mouse cells frozen without cryoprotectant. Biol Reprod. 2008;79:588–593. doi: 10.1095/biolreprod.108.069583. [DOI] [PubMed] [Google Scholar]
- 6.Kim CG, Yong H, Lee G, Cho J. Effect of the Polyvinylpyrrolidone Concentration of Cryoprotectant on Mouse Embryo Development and Production of Pups: 7.5% of PVP is Beneficial for In Vitro and In Vivo Development of Frozen-Thawed Mouse Embryos. J Reprod Dev. 2008;54:250–253. doi: 10.1262/jrd.19185. [DOI] [PubMed] [Google Scholar]
- 7.Loi P, et al. Freeze-dried somatic cells direct embryonic development after nuclear transfer. PLoS ONE. 2008;3:e2978. doi: 10.1371/journal.pone.0002978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ono T, et al. Nuclear transfer preserves the nuclear genome of freeze-dried mouse cells. J Reprod. Dev. 2008 doi: 10.1262/jrd.20112. in press. [DOI] [PubMed] [Google Scholar]
- 9.Wakayama T, et al. Full-term development of mice from enucleated oocytes injected with cumulus cell nuclei. Nature. 1998;394:369–374. doi: 10.1038/28615. [DOI] [PubMed] [Google Scholar]
- 10.Kuretake S, Kimura Y, Hoshi K, Yanagimachi R. Fertilization and development of mouse oocytes injected with isolated sperm heads. Biol Reprod. 1996;55:789–795. doi: 10.1095/biolreprod55.4.789. [DOI] [PubMed] [Google Scholar]
- 11.Kishigami S, et al. Successful mouse cloning of an outbred strain by trichostatin A treatment after somatic nuclear transfer. J Reprod Dev. 2007;53:165–170. doi: 10.1262/jrd.18098. [DOI] [PubMed] [Google Scholar]
- 12.Wakayama T. Production of cloned mice and ES cells from adult somatic cells by nuclear transfer: how to improve cloning efficiency? J Reprod Dev. 2007;53:13–26. doi: 10.1262/jrd.18120. [DOI] [PubMed] [Google Scholar]
- 13.Wakayama T, Yanagimachi R. Cloning of male mice from adult tail-tip cells. Nat Genet. 1999;22:127–128. doi: 10.1038/9632. [DOI] [PubMed] [Google Scholar]
- 14.Wakayama T, Yanagimachi R. Mouse cloning with nucleus donor cells of different age and type. Mol Reprod Dev. 2001;58:376–383. doi: 10.1002/1098-2795(20010401)58:4<376::AID-MRD4>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 15.Inoue K, et al. Effects of donor cell type and genotype on the efficiency of mouse somatic cell cloning. Biol Reprod. 2003;69:1394–1400. doi: 10.1095/biolreprod.103.017731. [DOI] [PubMed] [Google Scholar]
- 16.Wakayama T, et al. Differentiation of embryonic stem cell lines generated from adult somatic cells by nuclear transfer. Science. 2001;292:740–743. doi: 10.1126/science.1059399. [DOI] [PubMed] [Google Scholar]
- 17.Li J, Ishii T, Wen D, Mombaerts P. Non-equivalence of cloned and clonal mice. Curr Biol. 2005;15:R756–R757. doi: 10.1016/j.cub.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 18.Nagy A, et al. Embryonic stem cells alone are able to support fetal development in the mouse. Development. 1990;110:815–821. doi: 10.1242/dev.110.3.815. [DOI] [PubMed] [Google Scholar]
- 19.Mizutani E, et al. Developmental ability of cloned embryos from neural stem cells. Reproduction. 2006;132:849–857. doi: 10.1530/rep.1.01010. [DOI] [PubMed] [Google Scholar]
- 20.Osada T, et al. Developmental pluripotency of the nuclei of neurons in the cerebral cortex of juvenile mice. J Neurosci. 2005;25:8368–8374. doi: 10.1523/JNEUROSCI.1591-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yamazaki Y, et al. Assessment of the developmental totipotency of neural cells in the cerebral cortex of mouse embryo by nuclear transfer. Proc Natl Acad Sci USA. 2001;98:14022–14026. doi: 10.1073/pnas.231489398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fuller B, Paynter S. Fundamentals of cryobiology in reproductive medicine. Reprod Biomed Online. 2004;9:680–691. doi: 10.1016/s1472-6483(10)61780-4. [DOI] [PubMed] [Google Scholar]
- 23.Lacham-Kaplan O, et al. Developmental competence of nuclear transfer cow oocytes after direct injection of fetal fibroblast nuclei. Cloning. 2000;2:55–62. doi: 10.1089/152045500436078. [DOI] [PubMed] [Google Scholar]
- 24.Loi P, et al. Nuclei of nonviable ovine somatic cells develop into lambs after nuclear transplantation. Biol Reprod. 2002;67:126–132. doi: 10.1095/biolreprod67.1.126. [DOI] [PubMed] [Google Scholar]
- 25.Wakayama S, et al. Establishment of male and female nuclear transfer embryonic stem cell lines from different mouse strains and tissues. Biol Reprod. 2005;72:932–936. doi: 10.1095/biolreprod.104.035105. [DOI] [PubMed] [Google Scholar]
- 26.Wakayama S, et al. Mice cloned by nuclear transfer from somatic and ntES cells derived from the same individuals. J Reprod Dev. 2005;51:765–772. doi: 10.1262/jrd.17061. [DOI] [PubMed] [Google Scholar]
- 27.Wakayama S, et al. Equivalency of nuclear transfer-derived embryonic stem cells to those derived from fertilized mouse blastocysts. Stem Cells. 2006;24:2023–2033. doi: 10.1634/stemcells.2005-0537. [DOI] [PubMed] [Google Scholar]
- 28.Brambrink T, Hochedlinger K, Bell G, Jaenisch R. ES cells derived from cloned and fertilized blastocysts are transcriptionally and functionally indistinguishable. Proc Natl Acad Sci USA. 2006;103:933–938. doi: 10.1073/pnas.0510485103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cibelli JB, et al. Transgenic bovine chimeric offspring produced from somatic cell-derived stem-like cells. Nat Biotechnol. 1998;16:642–646. doi: 10.1038/nbt0798-642. [DOI] [PubMed] [Google Scholar]
- 30.Piedrahita JA, Anderson GB, Bondurant RH. On the isolation of embryonic stem cells: Comparative behavior of murine, porcine and ovine embryos. Theriogenology. 1990;34:879–901. doi: 10.1016/0093-691x(90)90559-c. [DOI] [PubMed] [Google Scholar]
- 31.Sukoyan MA, et al. Isolation and cultivation of blastocyst-derived stem cell lines from American mink (Mustela vison) Mol Reprod Dev. 1992;33:418–431. doi: 10.1002/mrd.1080330408. [DOI] [PubMed] [Google Scholar]
- 32.Wang L, et al. Generation and characterization of pluripotent stem cells from cloned bovine embryos. Biol Reprod. 2005;73:149–155. doi: 10.1095/biolreprod.104.037150. [DOI] [PubMed] [Google Scholar]
- 33.Ohta H, Sakaide Y, Wakayama T. Long-term preservation of mouse spermatozoa as frozen testicular sections. J Reprod Dev. 2008;54:295–298. doi: 10.1262/jrd.20027. [DOI] [PubMed] [Google Scholar]
- 34.Lanza RP, et al. Cloning of an endangered species (Bos gaurus) using interspecies nuclear transfer. Cloning. 2000;2:79–90. doi: 10.1089/152045500436104. [DOI] [PubMed] [Google Scholar]
- 35.Loi P, et al. Genetic rescue of an endangered mammal by cross-species nuclear transfer using post-mortem somatic cells. Nat Biotechnol. 2001;19:962–964. doi: 10.1038/nbt1001-962. [DOI] [PubMed] [Google Scholar]
- 36.Kishigami S, et al. Production of cloned mice by somatic cell nuclear transfer. Nat Prot. 2006;1:125–138. doi: 10.1038/nprot.2006.21. [DOI] [PubMed] [Google Scholar]
- 37.Kishigami S, Wakayama T. Efficient strontium-induced activation of mouse oocytes in standard culture media by chelating calcium. J Reprod Dev. 2007;53:1207–1215. doi: 10.1262/jrd.19067. [DOI] [PubMed] [Google Scholar]
- 38.Ohta H, Sakaide Y, Yamagata K, Wakayama T. Increasing the cell number of host tetraploid embryos can improve the production of ES mice. Biol Reprod. 2008;79:486–492. doi: 10.1095/biolreprod.107.067116. [DOI] [PubMed] [Google Scholar]
- 39.Novobrantseva TI, et al. Rearrangement and expression of immunoglobulin light chain genes can precede heavy chain expression during normal B cell development in mice. J Exp Med. 1999;189:75–88. doi: 10.1084/jem.189.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.