Abstract
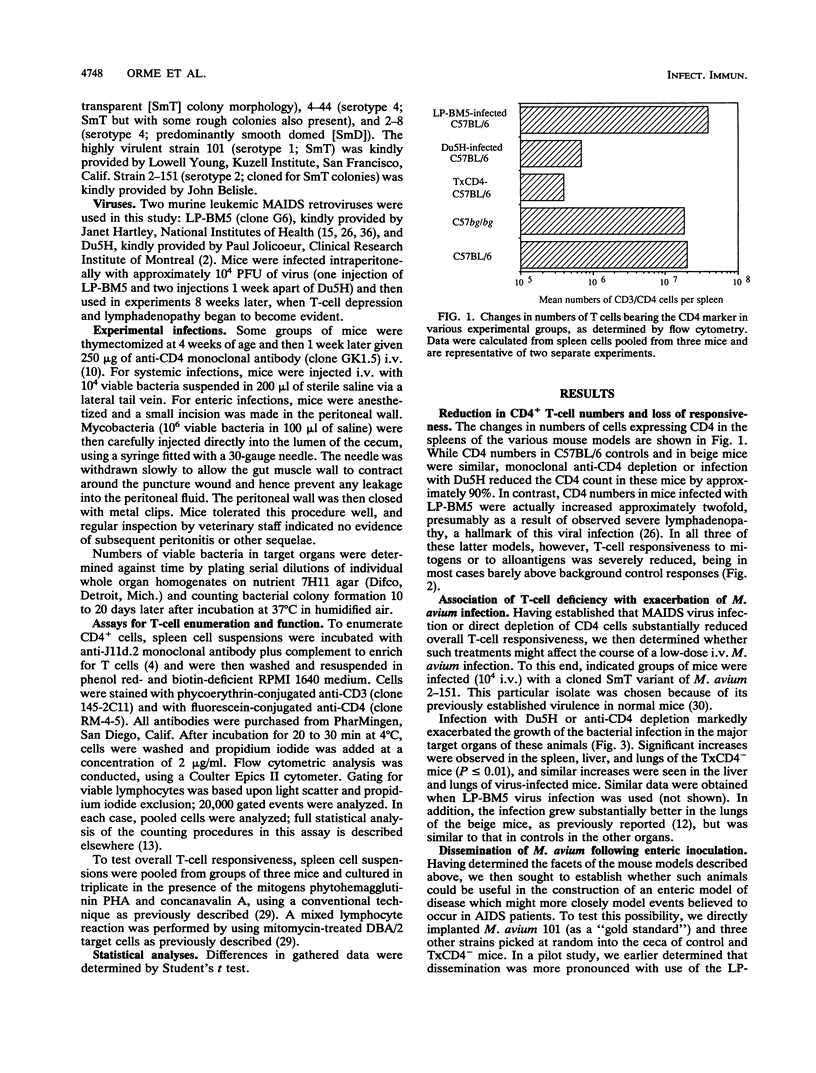
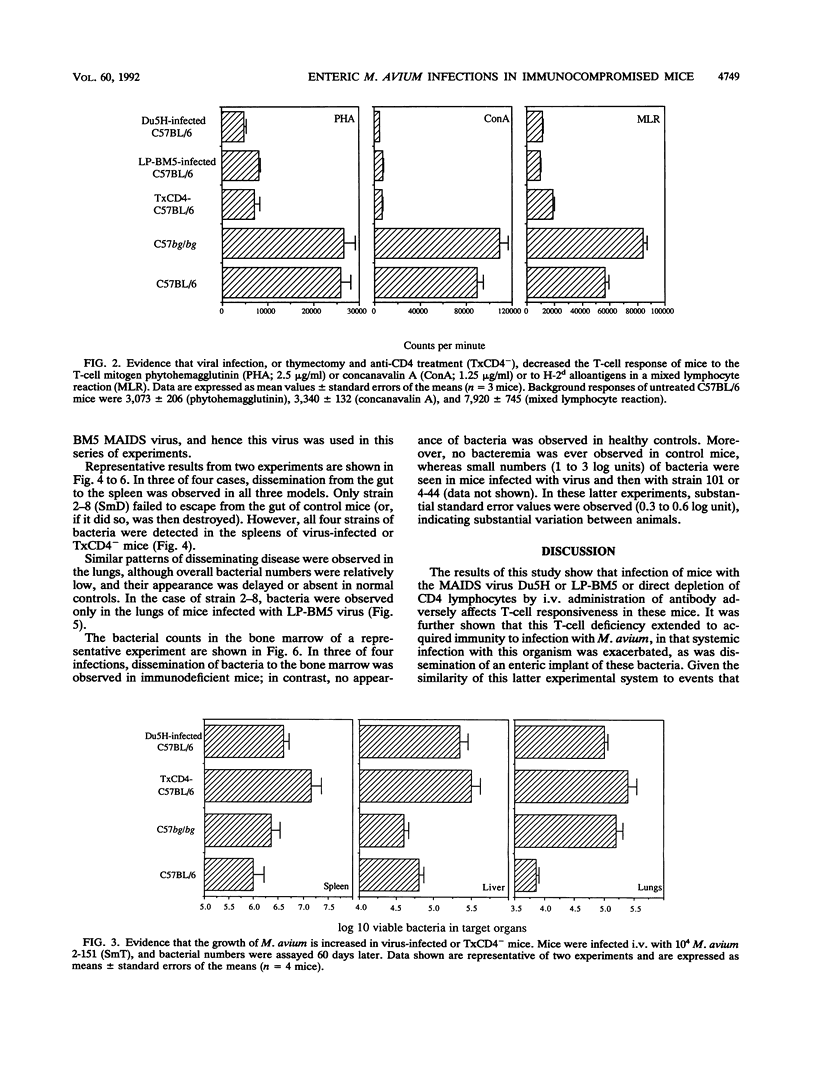
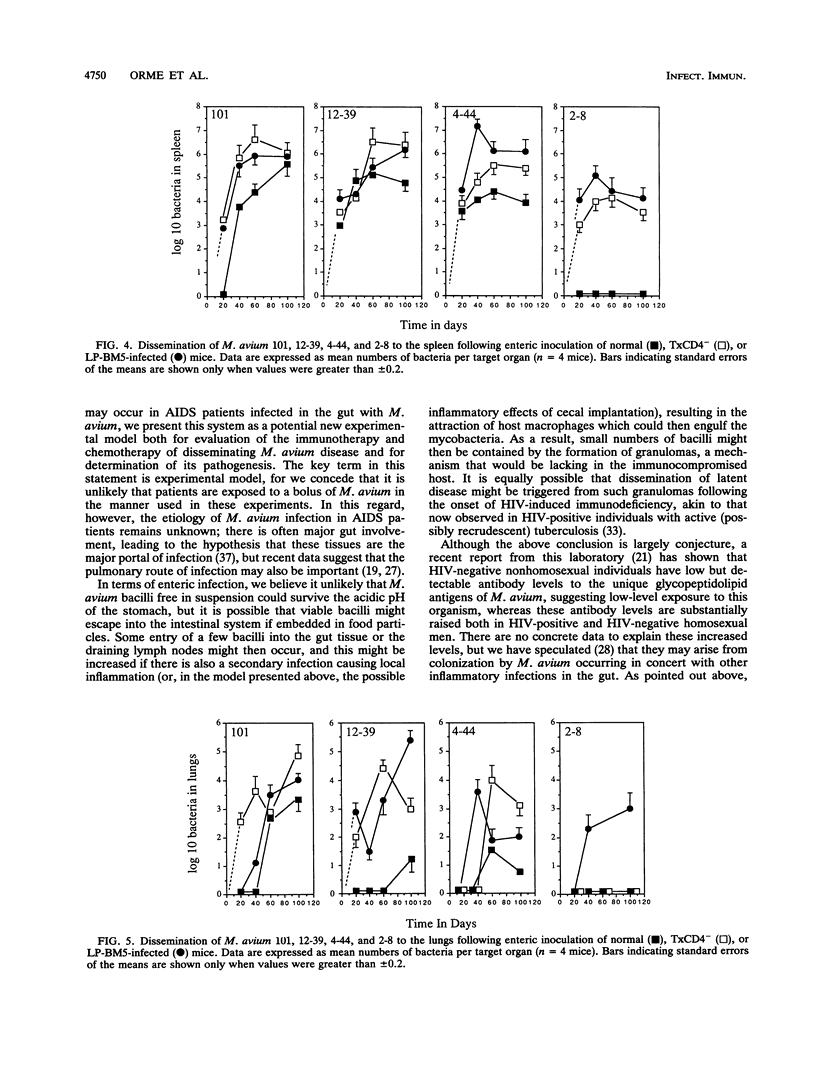
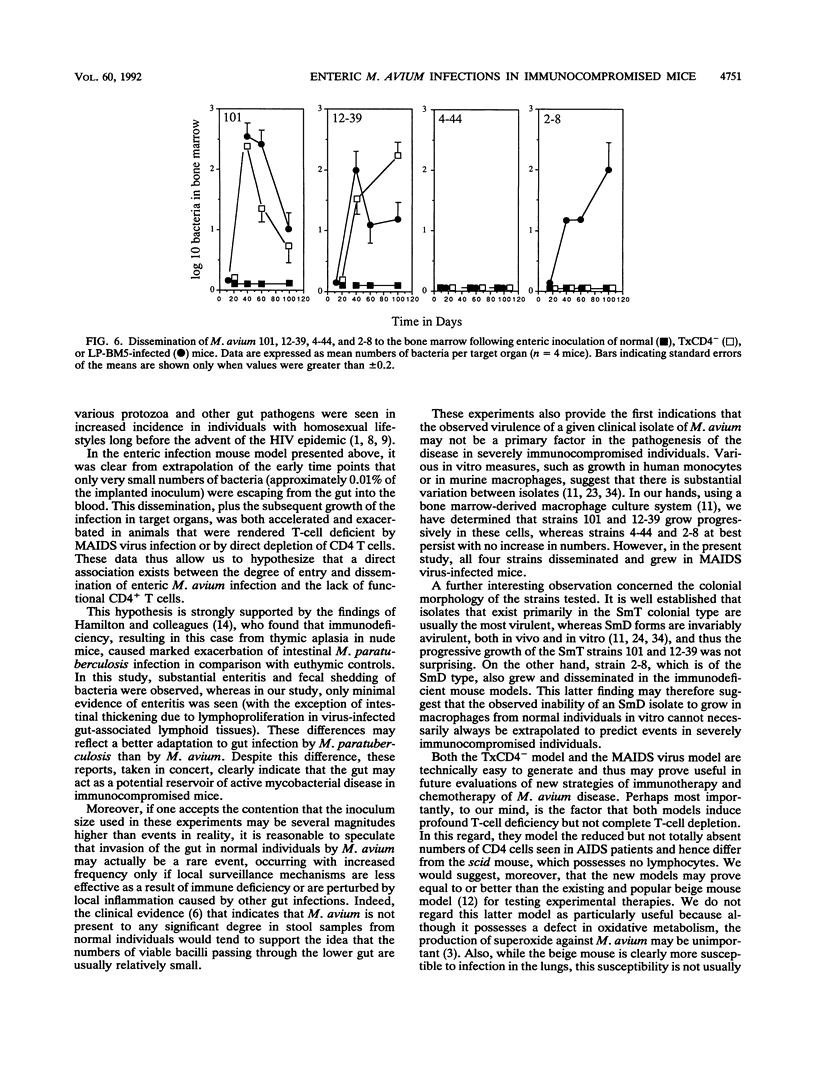
This study shows that infection of mice with the murine AIDS virus LP-BM5 or Du5H profoundly depressed the capacity of splenic T cells from these animals to respond to the T-cell mitogen phytohemagglutinin or concanavalin A or to alloantigens. Similar effects were also observed if mice were thymectomized and then infused with monoclonal anti-CD4 antibody (TxCD4- mice). When such mice were infected intravenously with Mycobacterium avium, growth of the infection was markedly exacerbated in the TxCD4- mice or in mice given murine AIDS virus 2 months earlier. In view of these data, we then investigated whether such treatments might cause dissemination of M. avium following enteric implantation of bacteria into the mouse cecum; this route was chosen in an attempt to model events in AIDS patients, in which the gut appears to be one of the major portals of M. avium infection. In this model, the entry and hematogenous dissemination of four clinical isolates of M. avium were monitored against time and found to be accelerated and enhanced in T-cell-deficient mice. In view of this finding, these novel approaches for enteric infection that use immunodeficient mice are presented as potential new models for the evaluation of immunotherapy and chemotherapy in a setting that bears some similarity to events believed to occur in AIDS patients.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aziz D. C., Hanna Z., Jolicoeur P. Severe immunodeficiency disease induced by a defective murine leukaemia virus. Nature. 1989 Apr 6;338(6215):505–508. doi: 10.1038/338505a0. [DOI] [PubMed] [Google Scholar]
- Bermudez L. E., Young L. S. Tumor necrosis factor, alone or in combination with IL-2, but not IFN-gamma, is associated with macrophage killing of Mycobacterium avium complex. J Immunol. 1988 May 1;140(9):3006–3013. [PubMed] [Google Scholar]
- Bruce J., Symington F. W., McKearn T. J., Sprent J. A monoclonal antibody discriminating between subsets of T and B cells. J Immunol. 1981 Dec;127(6):2496–2501. [PubMed] [Google Scholar]
- Chaisson R. E., Hopewell P. C. Mycobacteria and AIDS mortality. Am Rev Respir Dis. 1989 Jan;139(1):1–3. doi: 10.1164/ajrccm/139.1.1. [DOI] [PubMed] [Google Scholar]
- Coker R. J., Hellyer T. J., Brown I. N., Weber J. N. Clinical aspects of mycobacterial infections in HIV infection. Res Microbiol. 1992 May;143(4):377–381. doi: 10.1016/0923-2508(92)90049-t. [DOI] [PubMed] [Google Scholar]
- Damsker B., Bottone E. J. Mycobacterium avium-Mycobacterium intracellulare from the intestinal tracts of patients with the acquired immunodeficiency syndrome: concepts regarding acquisition and pathogenesis. J Infect Dis. 1985 Jan;151(1):179–181. doi: 10.1093/infdis/151.1.179. [DOI] [PubMed] [Google Scholar]
- Dritz S. K., Goldsmith R. S. Sexually transmissible protozoal, bacterial and viral enteric infections. Compr Ther. 1980 Jan;6(1):34–40. [PubMed] [Google Scholar]
- Furney S. K., Roberts A. D., Orme I. M. Effect of rifabutin on disseminated Mycobacterium avium infections in thymectomized, CD4 T-cell-deficient mice. Antimicrob Agents Chemother. 1990 Sep;34(9):1629–1632. doi: 10.1128/aac.34.9.1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furney S. K., Skinner P. S., Roberts A. D., Appelberg R., Orme I. M. Capacity of Mycobacterium avium isolates to grow well or poorly in murine macrophages resides in their ability to induce secretion of tumor necrosis factor. Infect Immun. 1992 Oct;60(10):4410–4413. doi: 10.1128/iai.60.10.4410-4413.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gangadharam P. R., Edwards C. K., 3rd, Murthy P. S., Pratt P. F. An acute infection model for Mycobacterium intracellulare disease using beige mice: preliminary results. Am Rev Respir Dis. 1983 May;127(5):648–649. doi: 10.1164/arrd.1983.127.5.648. [DOI] [PubMed] [Google Scholar]
- Griffin J. P., Harshan K. V., Born W. K., Orme I. M. Kinetics of accumulation of gamma delta receptor-bearing T lymphocytes in mice infected with live mycobacteria. Infect Immun. 1991 Nov;59(11):4263–4265. doi: 10.1128/iai.59.11.4263-4265.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton H. L., Follett D. M., Siegfried L. M., Czuprynski C. J. Intestinal multiplication of Mycobacterium paratuberculosis in athymic nude gnotobiotic mice. Infect Immun. 1989 Jan;57(1):225–230. doi: 10.1128/iai.57.1.225-230.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley J. W., Fredrickson T. N., Yetter R. A., Makino M., Morse H. C., 3rd Retrovirus-induced murine acquired immunodeficiency syndrome: natural history of infection and differing susceptibility of inbred mouse strains. J Virol. 1989 Mar;63(3):1223–1231. doi: 10.1128/jvi.63.3.1223-1231.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horsburgh C. R., Jr Epidemiology of mycobacterial diseases in AIDS. Res Microbiol. 1992 May;143(4):372–377. doi: 10.1016/0923-2508(92)90048-s. [DOI] [PubMed] [Google Scholar]
- Horsburgh C. R., Jr Mycobacterium avium complex infection in the acquired immunodeficiency syndrome. N Engl J Med. 1991 May 9;324(19):1332–1338. doi: 10.1056/NEJM199105093241906. [DOI] [PubMed] [Google Scholar]
- Horsburgh C. R., Jr, Selik R. M. The epidemiology of disseminated nontuberculous mycobacterial infection in the acquired immunodeficiency syndrome (AIDS). Am Rev Respir Dis. 1989 Jan;139(1):4–7. doi: 10.1164/ajrccm/139.1.4. [DOI] [PubMed] [Google Scholar]
- Jacobson M. A., Hopewell P. C., Yajko D. M., Hadley W. K., Lazarus E., Mohanty P. K., Modin G. W., Feigal D. W., Cusick P. S., Sande M. A. Natural history of disseminated Mycobacterium avium complex infection in AIDS. J Infect Dis. 1991 Nov;164(5):994–998. doi: 10.1093/infdis/164.5.994. [DOI] [PubMed] [Google Scholar]
- Kazal H. L., Sohn N., Carrasco J. I., Robilotti J. G., Delaney W. E. The gay bowel syndrome: clinico-pathologic correlation in 260 cases. Ann Clin Lab Sci. 1976 Mar-Apr;6(2):184–192. [PubMed] [Google Scholar]
- Kotler D. P., Gaetz H. P., Lange M., Klein E. B., Holt P. R. Enteropathy associated with the acquired immunodeficiency syndrome. Ann Intern Med. 1984 Oct;101(4):421–428. doi: 10.7326/0003-4819-101-4-421. [DOI] [PubMed] [Google Scholar]
- Lee B. Y., Chatterjee D., Bozic C. M., Brennan P. J., Cohn D. L., Bales J. D., Harrison S. M., Andron L. A., Orme I. M. Prevalence of serum antibody to the type-specific glycopeptidolipid antigens of Mycobacterium avium in human immunodeficiency virus-positive and -negative individuals. J Clin Microbiol. 1991 May;29(5):1026–1029. doi: 10.1128/jcm.29.5.1026-1029.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macher A. M., Kovacs J. A., Gill V., Roberts G. D., Ames J., Park C. H., Straus S., Lane H. C., Parrillo J. E., Fauci A. S. Bacteremia due to Mycobacterium avium-intracellulare in the acquired immunodeficiency syndrome. Ann Intern Med. 1983 Dec;99(6):782–785. doi: 10.7326/0003-4819-99-6-782. [DOI] [PubMed] [Google Scholar]
- Meylan P. R., Richman D. D., Kornbluth R. S. Characterization and growth in human macrophages of Mycobacterium avium complex strains isolated from the blood of patients with acquired immunodeficiency syndrome. Infect Immun. 1990 Aug;58(8):2564–2568. doi: 10.1128/iai.58.8.2564-2568.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michelini-Norris M. B., Blanchard D. K., Pearson C. A., Djeu J. Y. Differential release of interleukin (IL)-1 alpha, IL-1 beta, and IL-6 from normal human monocytes stimulated with a virulent and an avirulent isogenic variant of Mycobacterium avium-intracellulare complex. J Infect Dis. 1992 Apr;165(4):702–709. doi: 10.1093/infdis/165.4.702. [DOI] [PubMed] [Google Scholar]
- Mildvan D., Gelb A. M., William D. Venereal transmission of enteric pathogens in male homosexuals. Two case reports. JAMA. 1977 Sep 26;238(13):1387–1389. [PubMed] [Google Scholar]
- Mosier D. E., Yetter R. A., Morse H. C., 3rd Retroviral induction of acute lymphoproliferative disease and profound immunosuppression in adult C57BL/6 mice. J Exp Med. 1985 Apr 1;161(4):766–784. doi: 10.1084/jem.161.4.766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nassos P. S., Yajko D. M., Sanders C. A., Hadley W. K. Prevalence of Mycobacterium avium complex in respiratory specimens from AIDS and non-AIDS patients in a San Francisco hospital. Am Rev Respir Dis. 1991 Jan;143(1):66–68. doi: 10.1164/ajrccm/143.1.66. [DOI] [PubMed] [Google Scholar]
- Orme I. M., Chatterjee D. Antibody levels to the type-specific glycopeptidolipid antigens of Mycobacterium avium in homosexual men: possible implications. Res Microbiol. 1992 May;143(4):381–385. doi: 10.1016/0923-2508(92)90050-x. [DOI] [PubMed] [Google Scholar]
- Orme I. M., Collins F. M. Immune response to atypical mycobacteria: immunocompetence of heavily infected mice measured in vivo fails to substantiate immunosuppression data obtained in vitro. Infect Immun. 1984 Jan;43(1):32–37. doi: 10.1128/iai.43.1.32-37.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards J. M., Bedford J. M., Witkin S. S. Rectal insemination modifies immune responses in rabbits. Science. 1984 Apr 27;224(4647):390–392. doi: 10.1126/science.6608789. [DOI] [PubMed] [Google Scholar]
- Roder J. C. The beige mutation in the mouse. I. A stem cell predetermined impairment in natural killer cell function. J Immunol. 1979 Nov;123(5):2168–2173. [PubMed] [Google Scholar]
- Selwyn P. A., Hartel D., Lewis V. A., Schoenbaum E. E., Vermund S. H., Klein R. S., Walker A. T., Friedland G. H. A prospective study of the risk of tuberculosis among intravenous drug users with human immunodeficiency virus infection. N Engl J Med. 1989 Mar 2;320(9):545–550. doi: 10.1056/NEJM198903023200901. [DOI] [PubMed] [Google Scholar]
- Shiratsuchi H., Johnson J. L., Ellner J. J. Bidirectional effects of cytokines on the growth of Mycobacterium avium within human monocytes. J Immunol. 1991 May 1;146(9):3165–3170. [PubMed] [Google Scholar]
- Wicher V., Wicher K. Immune response of rabbits to intrarectal injections of particulate and soluble antigens with and without enemas. Clin Immunol Immunopathol. 1986 Dec;41(3):443–452. doi: 10.1016/0090-1229(86)90015-2. [DOI] [PubMed] [Google Scholar]
- Yetter R. A., Buller R. M., Lee J. S., Elkins K. L., Mosier D. E., Fredrickson T. N., Morse H. C., 3rd CD4+ T cells are required for development of a murine retrovirus-induced immunodeficiency syndrome (MAIDS). J Exp Med. 1988 Aug 1;168(2):623–635. doi: 10.1084/jem.168.2.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young L. S. Mycobacterium avium complex infection. J Infect Dis. 1988 May;157(5):863–867. doi: 10.1093/infdis/157.5.863. [DOI] [PubMed] [Google Scholar]



