Abstract
Understanding how risk genes cumulatively impair brain function in schizophrenia could provide critical insights into its pathophysiology. Working memory impairment in schizophrenia has been associated with abnormal dopamine signaling in the prefrontal cortex, which is likely under complex genetic control. The catechol-O-methyltransferase (COMT) 158Val → Met polymorphism (rs4680), which affects the availability of prefrontal dopamine signaling, consistently stratifies prefrontal activation during working memory performance. However, the low-dopamine COMT 158Val allele does not confer increased risk for schizophrenia, and its effects on prefrontal function are not specific to the disorder. In the setting of other genetic variants influencing prefrontal dopamine signaling, COMT 158Val → Met genotype may exert disease-specific effects. A second polymorphism, methylenetetrahydrofolate reductase (MTHFR) 677C → T (rs1801133), has been associated with overall schizophrenia risk and executive function impairment in patients, and may influence dopamine signaling through mechanisms upstream of COMT effects. We found that the hypofunctional 677T variant was associated with decreased working memory load-dependent activation in the prefrontal and insular cortices in 79 schizophrenia patients, but not in 75 demographically matched healthy controls. Further, significant MTHFR × COMT genotype interactions were observed, which differed by diagnostic group: Reduced prefrontal activation was associated with the 677T and 158Val alleles in patients, but with 677C/C and 158Met/Met genotype in controls. These findings are consistent with epistatic effects of the COMT and MTHFR polymorphisms on prefrontal dopamine signaling, and suggest that in schizophrenia patients, the MTHFR 677T allele exacerbates prefrontal dopamine deficiency. The findings also suggest the importance of weighing COMT effects on prefrontal function within the context of MTHFR genotype.
Keywords: dopamine, folate, functional MRI, genetics, methylation
Cognitive impairment accounts for greater long-term disability than any other aspect of schizophrenia (1), but it remains incompletely understood and largely unresponsive to treatment. Working memory dysfunction, a core cognitive deficit in schizophrenia, is strongly heritable and likely under complex genetic control (2). Understanding the contributions of specific genes and specific gene combinations to neural function during working memory could guide the development of cognition enhancing treatments for schizophrenia.
Neuroimaging studies of the catechol-O-methyltransferase (COMT) 158Val → Met polymorphism (rs4680) demonstrate the importance of dopamine signaling to brain function during working memory. Within the dorsolateral prefrontal cortex (DLPFC), a region critical to working memory, COMT contributes substantially to synaptic dopamine inactivation. Individuals with the high-activity (low-dopamine) COMT 158Val allele consistently exhibit less-efficient DLPFC activation during working memory performance than those with the low-activity (high-dopamine) 158Met variant (3–5). Further, healthy individuals homozygous for the Val allele exhibit increased prefrontal D1 receptor binding compared with Met-allele carriers, consistent with lower dopaminergic tone (6). However, following administration of amphetamine, which augments catecholamine signaling, healthy Met/Met individuals performing a demanding working memory task revert to an inefficient pattern of DLPFC activation (5). These and other findings support an “inverted U”-shaped relationship between dopamine transmission and DLPFC activation, wherein dopamine signaling either below or above an optimal range impairs DLPFC function during working memory performance. This model can be applied to understand working memory dysfunction in schizophrenia, which may reflect deficient dopamine signaling in the DLPFC (supporting information (SI) Fig. S1).
However, despite the reliable effects of the COMT Val → Met polymorphism on prefrontal physiology and its plausible role in modulating prefrontal dopamine, it does not consistently influence risk for either schizophrenia (7) or working memory dysfunction in patients (8). Further, 158Val → Met effects on DLPFC activation during working memory performance are not specific to schizophrenia, as analogous findings have been reported among patients (4), unaffected siblings (3), and healthy individuals (5). Effects of 158Val → Met on DLPFC activation and working memory function may be stronger, and more diagnostically specific, in the context of other genetic variants that influence COMT activity.
Several aspects of COMT function, including its transcription (9) and its inactivation of dopamine via transmethylation, depend on the availability of one-carbon moieties, which in turn is strongly influenced by the activity of methylenetetrahydrofolate reductase (MTHFR). Severe MTHFR deficiency, although rare, has been associated with psychosis, developmental delay, and other neuropsychiatric sequelae (10). A more common single-nucleotide polymorphism in a coding region of the MTHFR gene, 677C → T [rs1801133, T-allele frequency = 0.3 in Caucasians (11)], causes a less complete (35%) reduction in MTHFR function (12), but has also been consistently associated with overall schizophrenia risk (11, 12) and specifically with executive dysfunction severity (13). Moreover, we recently observed an epistatic interaction between COMT 158Val → Met and MTHFR 677C → T genotype in which patients who carried both the Val and (low-methyl) T alleles exhibited executive function deficits that were more than additive (14). This pattern suggests that the T allele may exacerbate the low-dopamine state of patients who also carry the Val allele, however, the effect of MTHFR genotype on prefrontal physiology has not yet been directly assessed.
Here, we used fMRI to provide the first evidence for an MTHFR genotype effect on brain activation during working memory performance in schizophrenia patients. We also addressed specifically whether MTHFR genotype influences dopamine-related DLPFC activation, as probed through its interaction with COMT genotype. Based on previous working memory performance findings (14), we anticipated that among patients, the T allele would have the greatest detrimental effect on DLPFC recruitment (i.e., activation as a function of increasing task demand) in those with the low-dopamine Val allele and the least effect on high-dopamine Met/Met patients. Although joint effects of COMT and MTHFR on cognition in healthy subjects had not previously been described, interactive gene effects on DLPFC activation were expected to be weaker than in patients, presuming a more optimal background range of prefrontal dopamine signaling (Fig. S1a).
Results
Demographic information is summarized in Table 1. Groups did not differ in age, gender, or handedness, and among patient genotype groups, there were no differences in duration of illness, severity of clinical symptoms, or antipsychotic medication use. Consistent with prior reports [reviewed in (8) and (12)] Caucasian participants were over-represented in T and Met allele-carrier groups, but DLPFC activation did not differ between Caucasian and nonCaucasian participants (Table S1). Regardless, to further control for population stratification, race and ethnicity were entered as covariates in the main analysis.
Table 1.
Demographic and clinical data for each compound genotype group, within each diagnosis
| Groups | Healthy controls |
Schizophrenia patients |
All con. n = 75 | All pat. n = 79 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| COMT genotype | Val/Val |
Val/Met |
Met/Met |
Val/Val |
Val/Met |
Met/Met |
||||||||
| MTHFR genotype | T carrier n = 11 | C/C n = 11 | T carrier n = 19 | C/C n = 17 | T carrier n = 10 | C/C n = 7 | T carrier n = 8 | C/C n = 9 | T carrier n = 22 | C/C n = 22 | T carrier n = 8 | C/C n = 10 | ||
| Demographics | ||||||||||||||
| Age | 36.8 | 38.5 | 32.1 | 38.6 | 38.6 | 39.9 | 35.4 | 39.3 | 37.0 | 38.9 | 37.9 | 38.8 | 36.8 | 37.9 |
| Gender, % female | 36.3 | 36.3 | 47.4 | 47.1 | 0.0 | 71.4 | 12.5 | 33.3 | 31.8 | 18.2 | 25.0 | 40.0 | 40.0 | 26.6 |
| Site, total n | ||||||||||||||
| Harvard | 3 | 5 | 5 | 5 | 1 | 1 | 0 | 4 | 5 | 8 | 3 | 3 | 20 | 23 |
| Iowa | 4 | 4 | 8 | 8 | 1 | 3 | 3 | 3 | 4 | 7 | 1 | 2 | 28 | 20 |
| Minnesota | 1 | 1 | 2 | 1 | 4 | 2 | 1 | 2 | 5 | 6 | 2 | 4 | 11 | 20 |
| New Mexico | 3 | 1 | 4 | 3 | 4 | 1 | 4 | 0 | 8 | 1 | 2 | 1 | 16 | 16 |
| Race*, % Caucasian | 72.7 | 90.0 | 88.9 | 81.3 | 90.0 | 100 | 87.5 | 33.3 | 90.5 | 81.8 | 100 | 80.0 | 85.9 | 80.7 |
| Handedness, % RH | 90.9 | 100 | 83.3 | 88.2 | 100 | 85.7 | 75.0 | 87.5 | 81.8 | 95.2 | 87.5 | 88.9 | 90.5 | 86.8 |
| Clinical | ||||||||||||||
| Years of illness | 11.7 | 15.9 | 14.0 | 16.8 | 15.3 | 17.9 | 15.3 | |||||||
| Antipsychotic use | ||||||||||||||
| Typical, % | 28.6 | 25.0 | 19.0 | 20.0 | 12.5 | 22.2 | 21.9 | |||||||
| Atypical, % | 85.7 | 87.5 | 95.2 | 85.0 | 87.5 | 88.9 | 89.0 | |||||||
| CPZ equivalents | 50.8 | 25.9 | 69.9 | 55.3 | 28.0 | 76.8 | 51.1 | |||||||
| Positive symptoms | 4.6 | 4.6 | 5.3 | 3.8 | 4.1 | 5.9 | 4.7 | |||||||
| Negative symptoms | 7.1 | 7.1 | 7.3 | 6.8 | 7.8 | 8.2 | 7.4 | |||||||
| Disorganization | 1.0 | 1.6 | 1.8 | 2.0 | 2.3 | 1.4 | 1.7 | |||||||
If present, any significant between-group differences are indicated below the table. RH = right handed, CPZ = chlorpromazine.
*Race: χ2 determined whether distribution of race differed among genotype groups. For MTHFR, χ2=10.29, P = 0.036 (T carrier > C/C for % Caucasian); for COMT, χ2=16.11, P = 0.041 (Met/Met > Val/Met > Val/Val for % Caucasian).
Participants underwent fMRI scanning while performing the Sternberg Item Recognition Paradigm (SIRP), which is reliably associated with DLPFC activation that increases as a function of working memory load. Our primary outcome measure was the increase in DLPFC activation from a low to high level of working memory load (from 1 to 5 digits). Although all participants performed well on the SIRP (Table S2), patients made significantly more errors at both the one-digit (1D) recall and five-digit (5D) conditions, regardless of genotype. Accordingly, accuracy at 1D and 5D were also entered as covariates in the main analysis.
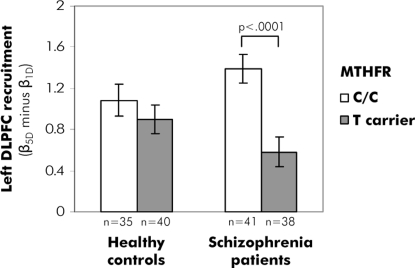
A significant main effect of MTHFR genotype was observed in the left DLPFC region-of-interest (ROI), with C/C participants exhibiting stronger recruitment than T carriers (F = 10.14, P < 0.005) as memory load increased from 1–5 digits. However, this effect was entirely driven by C/C versus T carrier differences in patients (MTHFR × diagnosis interaction F = 8.13, P = 0.005; post-hoc comparison of C/C versus T: In patients, P < 0.0001; in controls, P = 0.57; see Fig. 1 and Table S3). For the 5D condition, accuracy correlated positively with left DLPFC activation in T-carrier patients (r = 0.36, P < 0.05), but not in C/C patients (r = −0.27, P = 0.09, see Fig. S3) nor in control subjects (C/C controls: r = −0.12, P = 0.50; T carrier controls: r = −0.14, P = 0.39).
Fig. 1.
Effects of MTHFR genotype (T carrier versus C/C) on working memory load-dependent activation in the left DLPFC in healthy controls and schizophrenia patients.
COMT genotype by itself was not associated with DLPFC activation differences during SIRP performance. However, when stratifying MTHFR effects by COMT genotype, further striking differences between patients and controls emerged in the left DLPFC (MTHFR × COMT × diagnosis interaction, F = 3.14, P < 0.05, see Fig. 2 and Table S3). In patients, the effect of the T allele in reducing DLPFC recruitment was strongest in Val homozygotes, intermediate in Val/Met patients, and weakest in Met homozygotes (Fig. 2 A–C). To the extent that prefrontal dopamine signaling is impaired in patients as a group, whether because of schizophrenia pathophysiology or chronic antipsychotic exposure, these findings suggest that MTHFR genotype strongly modulates prefrontal function in patients with the greatest risk of low dopamine (i.e., Val homozygotes). In patients who were presumably closer to the flatter part of the inverted U curve because of Met/Met genotype, MTHFR effects were minimal (Fig. 2D).
Fig. 2.
Effects of MTHFR genotype (T carrier versus C/C) on working memory load-dependent left DLPFC ROI activation. Effects are shown in schizophrenia patients (A–C) and in healthy controls (E–G). Groups are further stratified by COMT genotype (Val/Val, Val/Met, or Met/Met). Error bars indicate standard error of the mean. Activation maps below each graph present voxelwise statistical maps of C/C minus T effects within the left DLPFC (outlined in yellow) for the corresponding COMT genotype group. A qualitative model of compound genotype effects on prefrontal dopamine signaling (inverted U curve) is proposed for patients (D) and healthy participants (H). The arrows at the left edge illustrate the difference between MTHFR C/C- and T-carrier genotype at each level of COMT genotype.
Conversely, in controls, whereas C/C genotype was associated with slight increases in recruitment among Val allele carriers, the strongest effect of MTHFR genotype was seen among Met homozygotes—and within this group, C/C genotype was associated with reduced recruitment (Fig. 2 E–G). The observed effects of C/C genotype in relation to COMT in healthy subjects appear analogous to the amphetamine-COMT relationship described by Mattay and colleagues (5), although that study involved a different working memory task (n-back). Mattay et al. (5) reported a beneficial effect of amphetamine on DLPFC activation among healthy Val allele carriers, but a decline in prefrontal efficiency and performance among Met/Met subjects given amphetamine during a high working memory load (3-back), presumably because exogenous augmentation of dopamine signaling pushed these Met/Met individuals down the rightward slope of the inverted U curve (as with the proposed effects of C/C genotype in Fig. 2H).
A total of 20% of the variance in left DLPFC activation was accounted for by MTHFR and COMT genotype and diagnosis (adjusted r2). Trend-level interactions of MTHFR × COMT (F = 2.95, P = 0.056) and MTHFR × COMT × diagnosis (F = 2.91, P = 0.058) were also seen in the right DLPFC, with overall patterns similar to those observed in the left DLPFC (see Table S3).
Exploratory voxelwise analyses were conducted to determine whether MTHFR exerted significant effects outside of the DLPFC. By using conservative criteria for multiple comparisons (family-wise error corrected P < 0.05), a bilateral effect was seen in the insular cortex of patients, where C/C participants exhibited greater activation than T carriers (Fig. 3 and Table 2). The insula is one of several regions consistently activated by the SIRP (15), and the present findings suggest that MTHFR genotype contributes to this effect in schizophrenia patients. Of note, in patients, a finding of C/C greater than T-carrier activation in the right DLPFC (Brodmann area 46) also survived family-wise error correction. No additional main effects of MTHFR genotype were seen in patients, and none were observed at all in controls. Consistent with previous observations that COMT effects on working memory occur primarily within the DLPFC (3–5), no MTHFR × COMT interactions were observed outside of this region in either diagnostic group.
Fig. 3.
Effects of MTHFR genotype (T carrier versus C/C) on working memory load-dependent activation in the bilateral insula, following whole-brain correction for multiple comparisons with family-wise error (P < 0.05).
Table 2.
Exploratory whole-brain (voxelwise) analysis of working memory load-dependent recruitment in C/C versus T carrier schizophrenia patients (C/C > T carriers)
| Cluster | Volume, mm3 | Max coordinates, x, y, z | Max Z score | P value |
|---|---|---|---|---|
| Right DLPFC (BA 46) | 54 | 48, 54, 6 | 4.76 | 0.031 |
| Right insula | 54 | 45, −3, −3 | 4.72 | 0.036 |
| Left insula | 54 | −42, −9, 0 | 4.69 | 0.040 |
Clusters indicated met family-wise error corrections for multiple comparisons across the entire brain volume (corrected P < 0.05).
Discussion
The COMT 158Val → Met and MTHFR 677C → T polymorphisms have been extensively studied in schizophrenia, and although their effects on dopamine and folate metabolism are well established, the mechanisms by which they influence cognitive function in schizophrenia have remained uncertain. To our knowledge, no other study has investigated either the effects of MTHFR genotype on brain activation or the epistatic interaction of any two polymorphisms on neural activity in schizophrenia patients.
In agreement with our previous studies of executive function in schizophrenia (13, 14), we found that the MTHFR 677T allele was associated with decreased load-dependent recruitment of the DLPFC during working memory performance; however, this effect was not present in healthy controls. COMT 158Val → Met genotype further stratified MTHFR findings, but in a different manner in patients and healthy controls. Among patients, 677T-allele effects on reducing DLPFC recruitment were exacerbated as a function of 158Val allele dose. However, in healthy participants, MTHFR genotype effects were strongest in Met/Met individuals, and for these subjects, C/C genotype reduced recruitment. These findings are consistent with the idea that C/C genotype augments (and the T allele detracts from) prefrontal dopamine signaling, a pattern with different implications on prefrontal activation in patients and controls by virtue of their respective hypothesized positions on the inverted U curve (Fig. 2 D and H).
These findings also have important implications for other studies of the COMT 158Val → Met polymorphism, as they suggest that previously unrecognized variance in MTHFR genotype contributes to COMT-related prefrontal activation patterns. Whereas all working memory tasks likely rely on prefrontal dopamine signaling, many factors [including other allelic variation in COMT (16)] contribute to synaptic dopamine availability. Further, the relative contribution of the 158Val → Met polymorphism may vary as a function of the specific working memory process required by the task. For instance, compared to its strong role in temporal updating as during the n-back task (3–5), the 158Val → Met polymorphism contributes relatively weakly to DLPFC activation during maintenance-related aspects of working memory (17). In the present study, no significant main effects of the COMT polymorphism on DLPFC activation were observed during SIRP performance, which relies heavily on maintenance of a digit set in working memory. However, as suggested by the interaction of MTHFR and COMT genotype, effects of MTHFR variation may have amplified the effects of COMT.
One potential mechanism for the interactive effects of MTHFR, COMT, and diagnosis on DLPFC activation is suggested by a recent investigation (18) that demonstrated reduced COMT promoter methylation in DLPFC tissue taken from schizophrenia patients, with a concordant increase in COMT expression. COMT promoter methylation is strongly heritable (19), and the MTHFR T allele has been associated with reduced methylation of genomic DNA (20). Consequently, it is possible that the T allele contributes to hypomethylation of the COMT promoter, increased COMT expression, and subsequent abatement of dopamine signaling in the DLPFC. These effects would be magnified in the presence of the high-activity COMT Val allele (Fig. S4). By the same token, increased methylation of the COMT promoter in healthy C/C + Met/Met participants may contribute to excessive dopamine signaling in these individuals, akin to the pattern observed by Mattay and colleagues when healthy Met/Met participants were given amphetamine (5). However, effects of MTHFR genotype at the level of COMT promoter methylation have not yet been reported and, whether even present in the hypothesized direction, they may not be directly responsible for the observed brain activation findings. Rather, the findings could reflect in part the effects of MTHFR genotype on the expression of other genes salient to schizophrenia and cognition, or perhaps they could reflect interactive effects of MTHFR and COMT on homocysteine metabolism [as recently described by Tunbridge and colleagues (21)]. Additional work may thus clarify the molecular mechanisms by which COMT and MTHFR genotype interactively affect neural activity and cognition in schizophrenia.
It is worth noting that when we examined group differences in DLPFC function in ROIs defined by using individual participant anatomy and functional activation we found a significant genotype effect in left DLPFC and a trend in right DLPFC. However, in the averaged group data, only right DLPFC showed a significant effect. Discrepancies in findings from group comparisons of DLPFC activation based on ROI-based vs. voxel-wise group data have been noted previously in schizophrenia (22). Because patients with schizophrenia have greater morphological variability than controls (23) and are more heterogeneous in the location of peak fMRI activation (22), group averaging, which assumes overlap in regional brain activation across participants, may be misleading. This is particularly true in the DLPFC, which shows a high degree of inter-individual regional variability even in healthy brains (24), and is among the most highly evolved and plastic of cortical regions. The ROI-based approach of the present study avoids signal loss attributable to variability in region location between participants and increases statistical power as a result of signal averaging within participants.
Previous studies of DLPFC activation using the n-back have reported increased activation of the DLPFC during working memory performance in schizophrenia patients (compared to healthy subjects) (25), as well as in COMT Val allele carriers (compared to Met homozygotes) (3–5, 26). These patterns of increased activity have been described as “inefficient” because in the patient and Val allele groups, increased activation is required to achieve the same (or worse) level of performance as controls and Met homozygotes (25, 26). However, whether increased DLPFC activation during working memory performance represents inefficient versus optimal recruitment likely depends on a variety of factors, including the type and level of task demand, and the participants' working memory capacity [for a detailed discussion, please see (27)]—and as previously demonstrated, patients can demonstrate relative hyper- or hypoactivation depending on working memory load (27, 28). In the present study, increased load-dependent activation of DLPFC during SIRP performance was considered beneficial for working memory function in patients a priori, based on the replicated, positive correlation between better performance and increased activation in schizophrenia patients (15, 22). This pattern contrasts with the n-back, a more difficult task for which accuracy has been negatively correlated with DLPFC activation in schizophrenia patients (25).
Although controls performed significantly better than patients in the present study, there were no differences in accuracy among genotype groups. This pattern suggests that within each diagnostic group, genotype-related differences in brain activation were not confounded by differences in performance. Thus, we interpret DLPFC hypoactivation in T allele-carrier patients (compared with C/C patients) as reflecting suboptimal recruitment. Strengthening this argument are the findings that first, T allele-related hypoactivation in patients is mitigated in an allele dose-dependent manner by COMT Met, which itself has consistently been associated with more efficient patterns of DLPFC activation during the n-back (3–5, 26); and second, only within T allele-carrier patients was the previously reported positive relationship between DLPFC activation and accuracy observed, suggesting that the ability to recruit DLPFC was rate-limiting for accuracy only in T allele-carrier patients. This interpretation is also consistent with our previous findings associating the T allele with poorer executive function (13, 14) and greater negative symptoms (29) in schizophrenia, and suggests that T allele-related DLPFC hypoactivation contributes to these clinical phenotypes.
Several limitations to this study are important to consider. First, as with other fMRI investigations of schizophrenia patients and healthy comparison subjects, medication use among patients presents a potential confound. Unmedicated schizophrenia patients are difficult to study with fMRI, as they are less clinically stable and less able to tolerate the scanning environment. The confounds posed by medications must be weighed against the potential benefit of studying genetic effects on brain function in patients, as opposed to extrapolating these effects based on their known actions in healthy individuals or unaffected siblings. However, in the present study, patterns of antipsychotic medication use were similar among patient genotype groups (Table 1), suggesting that medications are unlikely to account for fMRI differences among these groups. Second, although the number of participants in each diagnostic and MTHFR genotype group was large for a functional neuroimaging study, the group sizes of some compound genotype groups were relatively small. Significant interactive effects of COMT and other genotypes on brain activation have been reported in several other studies with even smaller group sizes (30–32), suggesting a large cumulative effect of these compound genotypes on brain activation profiles. Indeed, in the present study, COMT and MTHFR genotype together with diagnosis accounted for 20% of the variance in left DLPFC activation. Third, because our task was not designed to parse working memory into its component cognitive subprocesses, we cannot ascribe genotype effects to a unique subprocess. Similarly, given the block design, we were not able to separate correct from error trials. This is unlikely to be a confounding factor given the near ceiling levels of accuracy and the lack of significant accuracy differences among genotype groups. Finally, with the use of participants of varying race and ethnicity, it is possible that differences in brain activation between genotype groups could reflect an artifact of population stratification. However, the lack of brain activity differences between subjects of varying racial and ethnic groups diminishes this concern.
In summary, the present findings demonstrate that MTHFR genotype influences DLPFC function during working memory performance in schizophrenia. Moreover, they support the hypothesis that MTHFR C/C genotype augments prefrontal dopamine—but whether this has beneficial or detrimental effects on DLPFC function may depend on several other factors influencing prefrontal dopamine signaling, including COMT genotype. Additional work examining the effects of MTHFR genotype on COMT promoter methylation profiles and on more direct measures of dopamine signaling could further illuminate the interactive contributions of MTHFR and COMT genotype to prefrontally mediated cognitive dysfunction in schizophrenia and suggest targets for treatment.
Materials and Methods
Participants.
A total of 79 patients with chronic schizophrenia and 75 demographically matched healthy controls enrolled in the multisite MIND clinical imaging consortium participated in this study. All participants provided informed consent, and the study protocol was approved by the human research committees at each site (Universities of Iowa, Minnesota, and New Mexico and Massachusetts General Hospital.) Schizophrenia diagnosis was confirmed by using DSM-IV-TR (American Psychiatric Association) based structured clinical interviews and review of case files by experienced raters. All patients were stabilized with antipsychotic medications before the fMRI scan. Healthy controls were free of any Axis I disorder, as assessed with the Structured Clinical Interview for DSM-IV-TR (33). Severity of schizophrenia symptoms was assessed by trained raters by using the Positive and Negative Syndrome Scale (34). Demographic and performance differences among genotype × diagnosis groups were assessed by using analysis of variance (ANOVA) or χ2 as appropriate, and subsequent analyses were covaried by factors that differed significantly among participant groups.
Genotyping.
Genotyping for the MTHFR 677C → T and COMT 158Val → Met polymorphisms was conducted with the Taqman (Asuragen) platform by using primers previously described (14). Participants were grouped by diagnosis and genotype for all analyses. Because of the low frequency of T/T genotype, C/T and T/T participants were combined into T allele-carrier groups.
Behavioral Task.
The SIRP is a continuous performance task that requires the maintenance and scanning of items held in working memory (35). During SIRP performance, activation in the DLPFC increases as a function of working memory load (15, 22, 28). The same SIRP paradigm (described in SI Methods and Fig. S2) was used across all 4 sites. Participants practiced the paradigm before scanning until they understood the task well enough to perform at a greater-than-chance level of accuracy. Effects of diagnosis and genotype on accuracy and reaction time (RT) at 1D and 5D were examined by using repeated measures ANOVA. Because of an acquisition problem at one of the sites, reliable RT data were only available for a subset of subjects (n = 42 controls and 56 patients).
Functional Neuroimaging Acquisition.
For all sites, functional images were acquired by using single-shot echo-planar imaging with identical parameters [orientation: AC–PC line; number of slices = 27; slice thickness = 4 mm, 1-mm gap; TR = 2,000 ms; TE = 30 ms (3T) or 40 ms (1.5T), FOV = 22 cm; matrix 64 × 64; flip angle = 90°; voxel dimensions = 3.4375 mm × 3.4375 mm × 4 mm]. Massachusetts General Hospital, University of Iowa, and University of Minnesota all used Siemens Trio 3.0 T magnets, and University of New Mexico used a Siemens Sonata 1.5 T scanner. Datasets were preprocessed by using SPM5 software. Preprocessing methods are described in SI Methods.
Region-of-Interest Analysis.
We analyzed activations in the probe epochs, which involve mentally scanning the memorized set, deciding whether the probe was a target or foil, and choosing, planning, and executing a motor response. Given our a priori interest in the DLPFC, we chose a block rather than event-related design because it results in a much stronger DLPFC signal during SIRP performance (36). ROI in the right and left DLPFC were defined for each subject by using anatomical and functional constraints. The anatomical search territory was defined by using WFU Pickatlas software and comprised Brodmann's areas 9 and 46, excluding the medial frontal cortex. Within this territory, the ROI was functionally constrained to voxels whose beta weights fell within the upper quartile in the contrast of all load conditions (averaged together) versus fixation. For each subject, an average beta weight for the 1D and 5D conditions was computed by averaging across the voxels in the ROI, and a DLPFC recruitment value (reflecting that individual's load-dependent activation) was determined by subtracting the beta value at 1D from that at 5D.
For each ROI (right and left DLPFC), an analysis of covariance was conducted by using all participants, with DLPFC recruitment from 1D to 5D as the dependent variable, and diagnosis, MTHFR 677C → T genotype, and COMT 158Val → Met genotype as between-group factors. Race, ethnicity, age, site of scanning, and SIRP accuracy at 1D and 5D were entered as covariates, as was signal-to-fluctuation noise value [described in SI Methods and (37)].
Alpha (two-tailed) was set at P < 0.05 for main effects of diagnosis and genotype, as well as all genotype and diagnosis × genotype interactions. Correlations between DLPFC activation and accuracy at 5D were performed with Pearson's r as previously described (15, 22).
Voxel-Wise (Whole Brain) Analysis.
An exploratory analysis was conducted to determine whether genotype affected brain activation in regions outside of the DLPFC. Parallel analyses were conducted in patients and controls. The first analysis contrasted activation (5D minus 1D) among C/C minus T carrier participants. A second analysis examined interactions between MTHFR and COMT genotype by separately considering the C/C minus T carrier contrast among Val/Val, Val/Met, and Met/Met participants. A conservative correction for multiple comparisons (family-wise error) (38) was applied for each contrast with a significance threshold set at a corrected P value of 0.05.
Supplementary Material
Acknowledgments.
The authors thank all of the participants as well as the investigators and support staff of the MIND Institute who made this study possible. We also thank Dr. Doug Greve for his assistance with the SFNR analysis. This research was supported by a grant from the Department of Energy (DE-F02-99ER62764-A012) to the MIND Research Network and National Institutes of Health Grant EB001632-04 (to J.L.R.).
Footnotes
This work was presented in part at the Winter Conference on Brain Research, Snowbird, UT, January 26–February 1, 2008.
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0803727105/DCSupplemental.
References
- 1.Green MF. What are the functional consequences of neurocognitive deficits in schizophrenia? Am J Psychiatry. 1996;153:321–330. doi: 10.1176/ajp.153.3.321. [DOI] [PubMed] [Google Scholar]
- 2.Gur RE, et al. The Consortium on the Genetics of Schizophrenia: Neurocognitive endophenotypes. Schizophr Bull. 2007;33:49–68. doi: 10.1093/schbul/sbl055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Egan MF, et al. Effect of COMT Val108/158 Met genotype on frontal lobe function and risk for schizophrenia. Proc Natl Acad Sci USA. 2001;98:6917–6922. doi: 10.1073/pnas.111134598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bertolino A, et al. Interaction of COMT (Val(108/158)Met) genotype and olanzapine treatment on prefrontal cortical function in patients with schizophrenia. Am J Psychiatry. 2004;161:1798–1805. doi: 10.1176/ajp.161.10.1798. [DOI] [PubMed] [Google Scholar]
- 5.Mattay VS, et al. Catechol-O-methyltransferase val158-met genotype and individual variation in the brain response to amphetamine. Proc Natl Acad Sci USA. 2003;100:6186–6191. doi: 10.1073/pnas.0931309100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Slifstein M, et al. COMT genotype predicts cortical-limbic D1 receptor availability measured with [(11)C]NNC112 and PET. Mol Psychiatry. 2008;13:821–827. doi: 10.1038/mp.2008.19. [DOI] [PubMed] [Google Scholar]
- 7.Munafo MR, Bowes L, Clark TG, Flint J. Lack of association of the COMT (Val158/108 Met) gene and schizophrenia: A meta-analysis of case-control studies. Mol Psychiatry. 2005;10:765–770. doi: 10.1038/sj.mp.4001664. [DOI] [PubMed] [Google Scholar]
- 8.Barnett JH, Jones PB, Robbins TW, Muller U. Effects of the catechol-O-methyltransferase Val(158)Met polymorphism on executive function: A meta-analysis of the Wisconsin Card Sort Test in schizophrenia and healthy controls. Mol Psychiatry. 2007;12:502–509. doi: 10.1038/sj.mp.4001973. [DOI] [PubMed] [Google Scholar]
- 9.Sasaki M, Kaneuchi M, Sakuragi N, Dahiya R. Multiple promoters of catechol-O-methyltransferase gene are selectively inactivated by CpG hypermethylation in endometrial cancer. Cancer Res. 2003;63:3101–3106. [PubMed] [Google Scholar]
- 10.Freeman JM, Finkelstein JD, Mudd SH. Folate-responsive homocystinuria and “schizophrenia”. A defect in methylation due to deficient 5,10-methylenetetrahydrofolate reductase activity. N Engl J Med. 1975;292:491–496. doi: 10.1056/NEJM197503062921001. [DOI] [PubMed] [Google Scholar]
- 11.Allen NC, et al. Systematic meta-analyses and field synopsis of genetic association studies in schizophrenia: The SzGene database. Nat Genet. 2008;40:827–834. doi: 10.1038/ng.171. [DOI] [PubMed] [Google Scholar]
- 12.Gilbody S, Lewis S, Lightfoot T. Methylenetetrahydrofolate reductase (MTHFR) genetic polymorphisms and psychiatric disorders: A HuGE review. Am J Epidemiol. 2007;165:1–13. doi: 10.1093/aje/kwj347. [DOI] [PubMed] [Google Scholar]
- 13.Roffman JL, et al. Effects of the methylenetetrahydrofolate reductase (MTHFR) C677T polymorphism on executive function in schizophrenia. Schizophr Res. 2007;92:181–188. doi: 10.1016/j.schres.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 14.Roffman JL, et al. Interactive effects of COMT Val108/158Met and MTHFR C677T genotype on executive function in schizophrenia. Am J Med Genet B. 2008;147B:990–995. doi: 10.1002/ajmg.b.30684. [DOI] [PubMed] [Google Scholar]
- 15.Manoach DS, et al. Schizophrenic subjects activate dorsolateral prefrontal cortex during a working memory task, as measured by fMRI. Biol Psychiatry. 1999;45:1128–1137. doi: 10.1016/s0006-3223(98)00318-7. [DOI] [PubMed] [Google Scholar]
- 16.Tunbridge EM, Harrison PJ, Weinberger DR. Catechol-O -methyltransferase, cognition, and psychosis: Val158Met and beyond. Biol Psychiatry. 2006;60:141–151. doi: 10.1016/j.biopsych.2005.10.024. [DOI] [PubMed] [Google Scholar]
- 17.Tan HY, et al. Catechol-O-methyltransferase Val158Met modulation of prefrontal-parietal-striatal brain systems during arithmetic and temporal transformations in working memory. J Neurosci. 2007;27:13393–13401. doi: 10.1523/JNEUROSCI.4041-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abdolmaleky HM, et al. Hypomethylation of MB-COMT promoter is a major risk factor for schizophrenia and bipolar disorder. Hum Mol Genet. 2006;15:3132–3145. doi: 10.1093/hmg/ddl253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mill J, et al. Evidence for monozygotic twin (MZ) discordance in methylation level at two CpG sites in the promoter region of the catechol-O-methyltransferase (COMT) gene. Am J Med Genet B. 2006;141:421–425. doi: 10.1002/ajmg.b.30316. [DOI] [PubMed] [Google Scholar]
- 20.Friso S, et al. A common mutation in the 5,10-methylenetetrahydrofolate reductase gene affects genomic DNA methylation through an interaction with folate status. Proc Natl Acad Sci USA. 2002;99:5606–5611. doi: 10.1073/pnas.062066299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tunbridge EM, et al. Polymorphisms in the catechol-O-methyltransferase (COMT) gene influence plasma total homocysteine levels. Am J Med Genet B. 2008;147B:996–999. doi: 10.1002/ajmg.b.30700. [DOI] [PubMed] [Google Scholar]
- 22.Manoach DS, et al. Schizophrenic subjects show aberrant fMRI activation of dorsolateral prefrontal cortex and basal ganglia during working memory performance. Biol Psychiatry. 2000;48:99–109. doi: 10.1016/s0006-3223(00)00227-4. [DOI] [PubMed] [Google Scholar]
- 23.Park HJ, et al. An MRI study of spatial probability brain map differences between first-episode schizophrenia and normal controls. NeuroImage. 2004;22:1231–1246. doi: 10.1016/j.neuroimage.2004.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rajkowska G, Goldman-Rakic PS. Cytoarchitectonic definition of prefrontal areas in the normal human cortex: I. Remapping of areas 9 and 46 using quantitative criteria. Cereb Cortex. 1995;5:307–322. doi: 10.1093/cercor/5.4.307. [DOI] [PubMed] [Google Scholar]
- 25.Callicott JH, et al. Physiological dysfunction of the dorsolateral prefrontal cortex in schizophrenia revisited. Cereb Cortex. 2000;10:1078–1092. doi: 10.1093/cercor/10.11.1078. [DOI] [PubMed] [Google Scholar]
- 26.Bertolino A, et al. Prefrontal dysfunction in schizophrenia controlling for COMT Val158Met genotype and working memory performance. Psychiatry Res. 2006;147:221–226. doi: 10.1016/j.pscychresns.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 27.Manoach DS. Prefrontal cortex dysfunction during working memory performance in schizophrenia: reconciling discrepant findings. Schizophr Res. 2003;60:285–298. doi: 10.1016/s0920-9964(02)00294-3. [DOI] [PubMed] [Google Scholar]
- 28.Johnson MR, et al. A functional magnetic resonance imaging study of working memory abnormalities in schizophrenia. Biol Psychiatry. 2006;60:11–21. doi: 10.1016/j.biopsych.2005.11.012. [DOI] [PubMed] [Google Scholar]
- 29.Roffman JL, et al. Contribution of methylenetetrahyrdofolate reductase (MTHFR) polymorphisms to negative symptoms in schizophrenia. Biol Psychiatry. 2008;63:42–48. doi: 10.1016/j.biopsych.2006.12.017. [DOI] [PubMed] [Google Scholar]
- 30.Bertolino A, et al. Additive effects of genetic variation in dopamine regulating genes on working memory cortical activity in human brain. J Neurosci. 2006;26:3918–3922. doi: 10.1523/JNEUROSCI.4975-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Caldu X, et al. Impact of the COMT Val(108/158) Met and DAT genotypes on prefrontal function in healthy subjects. NeuroImage. 2007;37:1437–1444. doi: 10.1016/j.neuroimage.2007.06.021. [DOI] [PubMed] [Google Scholar]
- 32.Tan HY, et al. Epistasis between catechol-O-methyltransferase and type II metabotropic glutamate receptor 3 genes on working memory brain function. Proc Natl Acad Sci USA. 2007;104:12536–12541. doi: 10.1073/pnas.0610125104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DMS-IV-TR Axis I Disorders, Research Version, Nonpatient edition. New York State Psychiatric Institute, New York: Biometrics Research; 2002. [Google Scholar]
- 34.Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- 35.Sternberg S. High-speed scanning in human memory. Science. 1966;153:652–654. doi: 10.1126/science.153.3736.652. [DOI] [PubMed] [Google Scholar]
- 36.Manoach DS, Greve DN, Lindgren KA, Dale AM. Identifying regional activity associated with temporally separated components of working memory using event-related functional MRI. NeuroImage. 2003;20:1670–1684. doi: 10.1016/j.neuroimage.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 37.Friedman L, Glover GH. Reducing interscanner variability of activation in a multicenter fMRI study: Controlling for signal-to-fluctuation-noise-ratio (SFNR) differences. NeuroImage. 2006;33:471–481. doi: 10.1016/j.neuroimage.2006.07.012. [DOI] [PubMed] [Google Scholar]
- 38.Nandy R, Cordes D. A semi-parametric approach to estimate the family-wise error rate in fMRI using resting-state data. NeuroImage. 2007;34:1562–1576. doi: 10.1016/j.neuroimage.2006.10.025. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.