Abstract
The transcription factor NFκB is activated by phosphorylation and acetylation and plays important roles in inflammatory and immune responses in the cell. Additionally, posttranslational modification of the NFκB p65 subunit by O-linked N-acetylglucosamine (O-GlcNAc) has been reported, but the modification site of O-GlcNAc on NFκB p65 and its exact function have not been elucidated. In this work, we show that O-GlcNAcylation of NFκB p65 decreases binding to IκBα and increases transcriptional activity under hyperglycemic conditions. Also, we demonstrate that both Thr-322 and Thr-352 of NFκB p65 can be modified with O-GlcNAc, but modification on Thr-352, not Thr-322, is important for transcriptional activation. Our findings suggest that site-specific O-GlcNAcylation may be a reason why NFκB activity increases continuously under hyperglycemic conditions.
Keywords: diabetes, O-GlcNAc transferase, O-GlcNAcase
Transcription factor NFκB plays important roles in inflammatory, immune, and antiapoptotic responses (1–3). In mammals, NFκB is present as a dimer composed of various combinations of Rel proteins such as p65 (RelA), RelB, c-Rel, p50/p105, and p52/p100. In most cell types, NFκB is composed of p65 and p50 and is localized in the cytosol where it binds inhibitor (IκB). Treatment with NFκB-activating agents such as tumor necrosis factor α (TNFα) activates IκB kinase (IKK) complexes, inducing phosphorylation in the N terminus of IκB. The phosphorylation event induces IκB degradation via a ubiquitin-dependent proteolysis. Free NFκB translocates to the nucleus and activates the expression of target genes (1, 2).
Posttranslational modifications such as phosphorylation (4–9) and acetylation (10, 11) regulate the transcriptional activity of NFκB. The activity of NFκB is influenced also by the hexosamine biosynthetic pathway, which produces a substrate of O-GlcNAcylation, UDP-GlcNAc (12). Many nucleocytoplasmic proteins are known to be dynamically modified with O-GlcNAc. This modification is modulated by O-GlcNAc transferase (OGT) and O-GlcNAcase (OGA) (13–18). O-GlcNAcylation levels play an important role in transcription, translation, nuclear transport, protein stability, and protein–protein interactions and can be increased under hyperglycemic conditions caused by diabetes (16–18). Although it is known that O-GlcNAcylation of NFκB is involved in hyperglycemia-induced NFκB activation (12) and is required for lymphocyte activation (19), the specific sites and the function of O-GlcNAcylation on NFκB are not well understood.
In this work, we show that OGA overexpression down-regulates O-GlcNAcylation and inhibits hyperglycemia-induced NFκB activation in rat vascular smooth muscle cells (VSMCs). In contrast, up-regulation of O-GlcNAcylation after OGT overexpression and treatment of cells with the O-GlcNAcase inhibitors streptozotocin (STZ) (20) and O-(2-acetamido-2-deoxy-d-glucopyranosylidene)amino-N-phenylcarbamate (PUGNAc) (21) increase NFκB transcriptional activity. Additionally, we identify Thr-322 and Thr-352 as O-GlcNAcylation sites in a mutation study and with mass spectrometry analysis. Our data show that an increased amount of O-GlcNAcylation on NFκB p65 increases the transcriptional activity of NFκB. Furthermore, O-GlcNAcylation on Thr-352 inhibits the interaction between NFκB and IκB. This O-GlcNAcylated NFκB is translocated to the nucleus and has a longer half-life in the nucleus than unmodified protein. This finding may partly answer why NFκB is continuously activated in diabetic conditions.
Results
OGA Overexpression-Mediated Down-Regulation of O-GlcNAcylation Inhibits Hyperglycemia-Induced NFκB Activation.
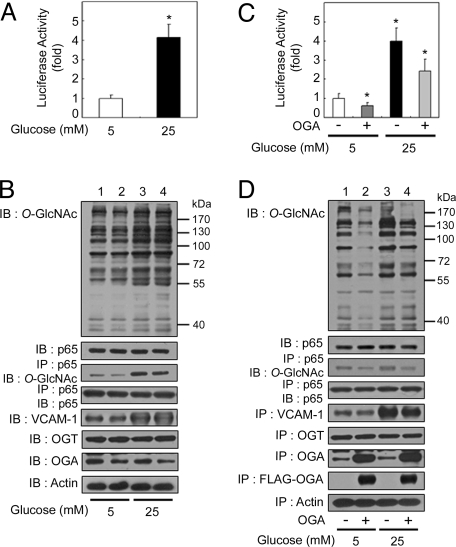
We first confirmed that hyperglycemic conditions induced NFκB activation in rat VSMCs (22). To examine NFκB-mediated gene regulation, we analyzed the expression of a κB-luciferase reporter gene construct under normal glucose (5 mM) and high-glucose (25 mM) conditions. When cells were exposed to high glucose for 24 h, an increased amount of κB-luciferase reporter gene expression was observed (Fig. 1A). At this time point, exposure to high-glucose levels increased levels of O-GlcNAcylation on total protein and NFκB p65 (Fig. 1B, 1st and 3rd panels from top); however, O-GlcNAcylation of NFκB p50 was not detected (data not shown). Furthermore, expression of vascular cell adhesion molecule 1 (VCAM-1), which is known to be controlled by NFκB, increased under high-glucose conditions (Fig. 1B, 5th panel).
Fig. 1.
OGA overexpression-mediated decrease in O-GlcNAcylation suppresses hyperglycemia-induced NFκB activation. (A) Rat VSMCs were transfected with the κB-luciferase reporter gene plasmid and then incubated for 24 h under normal (5 mM) or high-glucose (25 mM) conditions. The luciferase activity was measured and normalized to β-galactosidase activity from a cotransfected control plasmid. The data shown represent the mean ± SD (n = 3); *, P < 0.01 by Student's t test. (B) VSMCs were exposed to normal-glucose (lanes 1 and 2) or high-glucose (lanes 3 and 4) levels for 24 h. The levels of O-GlcNAc, NFκB p65, VCAM-1, OGT, and OGA were determined in total cell lysates by immunoblotting (IB; 1st, 2nd, and 5th–7th panels, respectively). NFκB p65 immunoprecipitates (IP) obtained from cellular extracts were analyzed by immunoblotting for O-GlcNAc and NFκB p65 (3rd and 4th panels, respectively). Actin was included as a loading control (8th panel). (C) VSMCs were cotransfected with the κB-luciferase reporter gene plasmid and the plasmid expressing FLAG-tagged human OGA, and incubated for 24 h under normal- or high-glucose conditions. The luciferase activity was measured and normalized to β-galactosidase activity, and the data shown represent the mean ± SD (n = 3); *, P < 0.01 by Student's t test. (D) VSMCs transfected with empty vector (lanes 1 and 3) or that encoding FLAG-tagged OGA (lanes 2 and 4) were exposed to normal-glucose (lanes 1 and 2) or high-glucose (lanes 3 and 4) levels for 24 h. The levels of O-GlcNAc, NFκB p65, VCAM-1, OGT, OGA, and FLAG-tagged OGA were determined in total cell lysates by immunoblotting (1st, 2nd, and 5th–8th panels, respectively). NFκB p65 immunoprecipitates obtained from the cellular extracts were analyzed by immunoblotting for O-GlcNAc and NFκB p65 (3rd and 4th panels, respectively). Actin was included as a loading control (9th panel).
To determine the role of O-GlcNAcylation in hyperglycemia-induced NFκB activation, we transfected a vector expressing FLAG-tagged human OGA into VSMCs and found that OGA overexpression reduced hyperglycemia-induced NFκB activation as measured by luciferase assays (Fig. 1C). Also, OGA overexpression decreased the observed hyperglycemia-induced increase in VCAM-1 expression (Fig. 1D, 5th panel). As expected, OGA overexpression decreased levels of O-GlcNAcylation on total proteins and NFκB p65 (Fig. 1D, 1st and 3rd panels). Taken together, these results indicate that an increased amount of O-GlcNAcylation has an important role in hyperglycemia-induced NFκB activation.
OGA Overexpression Blocks Hyperglycemia-Induced Reduction in NFκB p65–IκBα Interactions and Hyperglycemia-Induced Nuclear Translocation of NFκB p65.
Generally, the phosphorylation, ubiquitination, and proteolytic degradation of IκB releases NFκB, which can then enter the nucleus, bind to DNA, and activate the transcription of various genes (1–3). Therefore, we investigated the expression level of IκB, the interaction between NFκB and IκB, and the nuclear translocation of NFκB under high-glucose conditions.
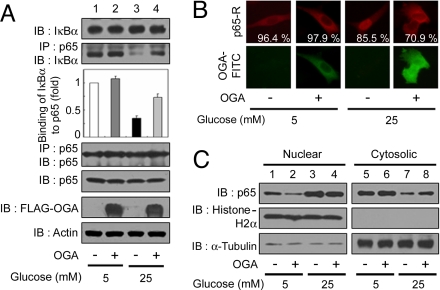
When VSMCs were exposed to high glucose for 24 h, the IκBα expression level was similar to that found under normal-glucose conditions (Fig. 2A, 1st panel). To investigate how high-glucose conditions influence the interaction between IκBα and NFκB, we analyzed the amount of IκBα that coimmunoprecipitated with NFκB p65 and found that the interaction between NFκB p65 and IκBα was decreased dramatically under high-glucose conditions (Fig. 2A, 2nd and 3rd panels).
Fig. 2.
OGA overexpression inhibits the reduction in NFκB p65–IκBα interactions and nuclear translocation of NFκB p65 under high-glucose conditions. VSMCs were transfected with either vector or a plasmid expressing FLAG-tagged OGA and exposed to high glucose for 24 h. (A) Immunoblotting (IB) for NFκB p65, IκBα, and FLAG-tagged OGA was performed with an anti-NFκB p65 antibody, an anti-IκBα antibody, and an anti-FLAG antibody (1st, 5th, and 6th panels, respectively). NFκB p65 immunoprecipitates (IP) were analyzed for IκBα and NFκB p65 expression by using immunoblotting with the corresponding antibodies (2nd and 4th panels, respectively). Actin was used as a loading control (7th panel). (B) (Upper) VSMCs were immunostained by using antibodies against NFκB p65 followed by anti-rabbit Ig covalently conjugated to rhodamine (R) to determine the subcellular localization. (Lower) OGA overexpression was detected with an anti-FLAG antibody conjugated to FITC. The percentages of cells showing NFκB p65 localization are derived from at least 150 transfected cells with FLAG-OGA in microscopic fields. (C) Immunoblotting of nuclear or cytosolic extracts of VSMCs by using antibodies against NFκB p65 was performed to determine NFκB p65 nuclear translocation (1st row). The purity of the nuclear and cytosolic extracts was determined by immunoblotting of histone H2A (nuclear fractions, 2nd row) and α-tubulin (cytosolic fractions, 3rd row).
We then investigated how OGA overexpression affects the interaction between NFκB p65 and IκBα. Interestingly, OGA overexpression inhibited the hyperglycemia-induced decrease in NFκB p65–IκBα interactions but did not significantly affect total IκBα levels (Fig. 2A, 1st 3 panels).
Next, we investigated the nuclear translocation of NFκB p65 in cells under high-glucose conditions by using immunostaining and immunoblotting. NFκB p65 was present primarily in the cytosol of the cells under normal-glucose conditions but was translocated to the nucleus under high-glucose conditions (Fig. 2B). Interestingly, the translocated NFκB p65 was found in the cytosol when FLAG-tagged OGA was transfected into the cells under high-glucose conditions (Fig. 2B). This observation was confirmed by a Western blotting experiment using nuclear extracts (Fig. 2C). Therefore, O-GlcNAcylation is involved in hyperglycemia-induced NFκB activation by inducing a change in NFκB p65–IκBα interactions, resulting in an increase in the nuclear translocation of NFκB p65.
OGT Overexpression-Mediated Up-Regulation of O-GlcNAcylation Increases the Transcriptional Activity of NFκB.
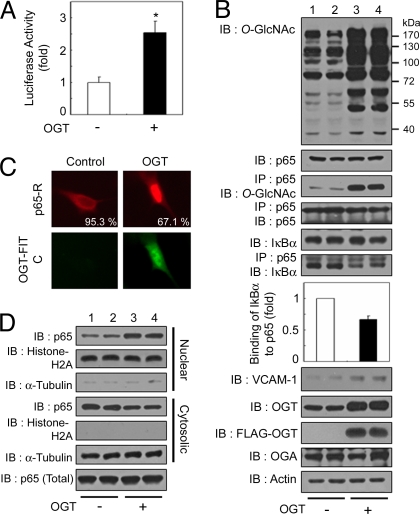
To investigate the influence of increased intracellular O-GlcNAcylation on NFκB activity, we transfected a vector expressing FLAG-tagged human OGT into VSMCs. As expected, OGT overexpression increased levels of total protein and NFκB p65 O-GlcNAcylation levels (Fig. 3B, 1st and 3rd panels). Interestingly, OGT overexpression also increased a κB-luciferase reporter gene expression (Fig. 3A) and the expression of VCAM-1 (Fig. 3B, 8th panel). Furthermore, OGT overexpression inhibited NFκB p65–IκBα interactions (Fig. 3B, 6th and 7th panels) and increased the nuclear translocation of NFκB p65 (Fig. 3 C and D) in a manner similar to that observed under hyperglycemia-induced NFκB activation. Interestingly, O-GlcNAcylation of NFκB p65 and its transcriptional activity are increased in STZ-induced diabetes in mice [supporting information (SI) Fig. S1] (23). Therefore, these results suggest that an increased amount of O-GlcNAcylation up-regulates NFκB transcriptional activity, and O-GlcNAcylation-induced NFκB activation is closely involved in diabetes.
Fig. 3.
OGT overexpression-mediated increase in O-GlcNAcylation induces the up-regulation of NFκB activity. (A) VSMCs were transfected with the κB-luciferase reporter gene plasmid and the plasmid encoding FLAG-tagged OGT and incubated for 24 h under normal conditions. The luciferase activity was measured and normalized to β-galactosidase activity, and the data shown represent the mean ± SD (n = 3); *, P < 0.01 by Student's t test. (B) VSMCs were cotransfected with the plasmid encoding FLAG-tagged OGT (+) or vector control (−) and incubated for 24 h under normal glucose conditions. Immunoblotting (IB) for O-GlcNAc, NFκB p65, IκBα, VCAM-1, OGT, FLAG-OGT, and OGA was performed with the corresponding antibodies (1st, 2nd, 5th, and 8th–11th panels, respectively). NFκB p65 immunoprecipitates (IP) were analyzed for O-GlcNAc, NFκB p65, and IκBα (3rd, 4th, and 6th panels, respectively). Actin was used as a loading control (12th panel). (C) (Upper) VSMCs were immunostained by using antibodies against NFκB p65 to determine the subcellular localization. (Lower) OGT overexpression was detected with an anti-FLAG antibody conjugated to FITC. The percentages of cells showing NFκB p65 localization are derived from at least 150 transfected cells with FLAG-OGT in microscopic fields. (D) Immunoblotting of nuclear or cytosolic extracts of VSMCs by using antibodies against NFκB p65 was performed to determine NFκB p65 nuclear translocation (1st and 4th panels) or total levels of NFκB p65 (7th panel). The purity of the nuclear and cytosolic extracts was determined by immunoblotting of histone H2A (nuclear fractions, 2nd and 5th panels) and α-tubulin (cytosolic fractions, 3rd and 6th panels).
O-GlcNAcylation of NFκB p65 Occurs at Multiple Sites.
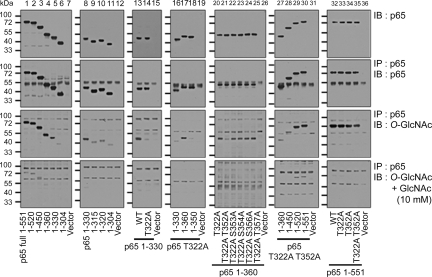
To identify O-GlcNAcylated sites on NFκB p65, we studied His-tagged NFκB p65 C-terminal deletion mutants and site-directed substitution mutants of NFκB p65, in which Ser or Thr was changed into Ala, expressed in NFκB p65 knockout murine embryo fibroblasts (NFκB p65 KO MEFs) (24) (Fig. 4, 1st row). His-NFκB p65 mutant proteins were immunoprecipitated from cells overexpressing OGT with an anti-NFκB p65 antibody (Fig. 4, 2nd row), and O-GlcNAcylation levels were identified with an anti-O-GlcNAc antibody (Fig. 4, 3rd row). The specificity of O-GlcNAcylation on NFκB p65 mutant proteins was confirmed by adding 10 mM GlcNAc during immunoblotting (Fig. 4, 4th row). As expected, full-length His-NFκB p65 (1–551) was modified with O-GlcNAc, but the modification of truncated His-NFκB p65 (1–304) was greatly reduced (Fig. 4, 3rd row). Therefore, O-GlcNAcylation occurs between amino acids 305 and 551. To map the site further, we tested other truncated constructs and found that O-GlcNAcylation occurred on His-NFκB p65 (1–330), but the amount of modification was greatly decreased on His-NFκB p65 (1–320) (Fig. 4, 3rd row). These results show that O-GlcNAcylation occurs on Thr-322 of NFκB p65 because this is the only possible modification site in NFκB p65 between amino acids 321 and 330.
Fig. 4.
O-GlcNAcylation of NFκB p65 occurs at Thr-322 and Thr-352. O-GlcNAcylation of NFκB p65 C-terminal deletion mutant proteins and Ser or Thr to Ala substitution mutant proteins. NFκB p65 KO MEFs were cotransfected with the vector alone or plasmids encoding the His-NFκB p65 deletion (lanes 1–12) or substitution mutants (lanes 13–36), along with the plasmid encoding FLAG-tagged OGT for 12 h. His-NFκB p65 immunoprecipitates (2nd row) were obtained from cellular extracts by using the anti-NFκB p65 polyclonal antibody and were analyzed by immunoblotting for O-GlcNAc (3rd row). Total levels of His-NFκB p65 mutants in these extracts were examined by using the anti-NFκB p65 monoclonal antibody (1st row), and the specificity of O-GlcNAcylation on NFκB p65 mutants was confirmed by decreasing the modification via the addition of 10 mM GlcNAc (4th row).
To test this further, we replaced Thr-322 with Ala and found that the amount of O-GlcNAcylation of His-NFκB p65 (1–330) was greatly decreased (Fig. 4, 3rd row). However, full-length NFκB p65 T322A was modified still with O-GlcNAc (Fig. 4, 3rd row). Therefore, more deletion constructs were made by using the full-length His-NFκB p65 T322A to find additional O-GlcNAcylation sites. From this investigation, we found that His-NFκB p65 (1–360) T322A was largely modified by O-GlcNAc compared with His-NFκB p65 (1–330) T322A and His-NFκB p65 (1–350) T322A (Fig. 4, 3rd row). This result shows that O-GlcNAcylation also occurs between amino acids 351 and 360 of NFκB p65. There are 5 Ser or Thr residues in this region: Thr-352, Ser-353, Ser-354, Ser-356, and Thr-357. To define further the modification site in 351–360 of NFκB p65, additional Ser or Thr to Ala substitution mutations T352A, S353A, S354A, S356A, and T357A were made in the His-NFκB p65 (1–360) T322A protein. O-GlcNAcylation of His-NFκB p65 (1–360) T322A/T352A was greatly decreased compared with the other substitution mutants (Fig. 4, 3rd row). From these results, we conclude that Thr-352 of NFκB p65 is modified with O-GlcNAc. Additional O-GlcNAcylation sites other than Thr-322 and Thr-352 may exist because O-GlcNAcylation of the full-length and His-NFκB p65 (1–520) proteins occurs despite mutations in the identified sites (T322A/T352A) (Fig. 4, 3rd row). Also, by using mass spectrometry (25, 26), we identified that O-GlcNAcylation occurs in peptide 337–360 of NFκB p65 (Fig. S2).
O-GlcNAcylation of NFκB p65 on Thr-352, but Not on Thr-322, Is Important for O-GlcNAc-Induced NFκB Transcriptional Activation.
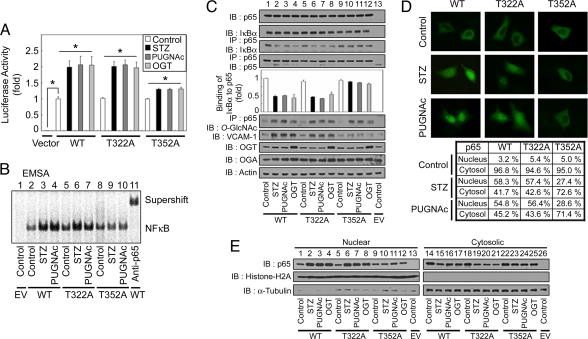
We then investigated the functional importance of O-GlcNAcylation on both Thr-322 and Thr-352. We used Thr to Ala substitution mutants of NFκB p65 expressed in NFκB p65 KO MEFs to rule out endogenous NFκB p65 activity (24). Also, we used OGT overexpression and treatment of cells with O-GlcNAcase inhibitors (STZ and PUGNAc) to increase NFκB p65 O-GlcNAcylation. First, we investigated whether the mutations in NFκB p65 changed the transcriptional activity of NFκB by using the luciferase assay. Plasmids encoding the His-tagged wild-type NFκB p65 (His-NFκB p65 WT) or NFκB p65 containing site-specific Thr to Ala substitution mutations (His-NFκB p65 T322A or T352A) were cotransfected with FLAG-tagged IκBα into NFκB p65 KO MEFs. In the case of either His-NFκB p65 WT or T322A, O-GlcNAcylation increased κB-luciferase reporter gene expression, but in the case of T352A, it did not (Fig. 5A). Additionally, in an electrophoretic mobility-shift assay (EMSA) experiment using nuclear extracts, O-GlcNAcylation increased the DNA-binding affinity of both His-NFκB p65 WT and T322A, but O-GlcNAc did not increase the DNA-binding affinity of T352A (Fig. 5B).
Fig. 5.
Mutation of the NFκB p65 O-GlcNAcylation site at Thr-352 abrogates O-GlcNAc-induced NFκB transcriptional activation. (A) NFκB p65 KO MEFs were transfected with empty vector, the κB-luciferase reporter gene plasmid, the plasmid encoding wild-type FLAG-tagged IκBα, and the WT or mutant NFκB p65 (T322A and T352A) His-tagged expression vectors. The transfected cells were then incubated for 12 h in the absence (control) or presence of STZ (2 mM), PUGNAc (100 μM), or transfected with the plasmid expressing FLAG-tagged OGT as indicated, and the luciferase activity was measured and normalized to β-galactosidase activity. The data shown represent the mean ± SD (n = 3); *, P < 0.01 by Student's t test. (B) NFκB p65 KO MEFs were cotransfected with empty vector (EV) or plasmids encoding FLAG-tagged IκBα, and plasmids encoding the WT or mutant His-tagged NFκB p65 proteins were incubated for 12 h in the absence (control) or presence of STZ or PUGNAc. Nuclear NFκB DNA-binding affinity was analyzed by EMSA using 32P-radiolabeled κB-enhancer probes. The supershift assay was examined with antibodies against NFκB p65 (rabbit polyclonal). (C) Plasmids encoding His-NFκB p65 (WT or mutants) and FLAG-IκBα were cotransfected into NFκB p65 KO MEFs. After a 12-h incubation in the absence (control) or presence of STZ, PUGNAc, or after transfection with the plasmid expressing FLAG-tagged OGT, immunoblotting for NFκB p65, IκBα, VCAM-1, OGA, and OGA was performed by using the corresponding antibodies (1st, 2nd, and 7th–9th panels, respectively). NFκB p65 WT and mutant proteins were also immunoprecipitated from cell lysates, and the immunoprecipitates were analyzed for IκBα, NFκB p65, and O-GlcNAc by using immunoblotting (3rd, 4th, and 6th panels, respectively). Actin was used as a loading control (10th panel). (D) Plasmids encoding His-NFκB p65 (WT or mutants) and FLAG-IκBα were cotransfected into NFκB p65 KO MEFs and incubated for 12 h in the absence (control) or presence of STZ or PUGNAc. Immunostaining for NFκB p65 was performed to determine the subcellular localization of His-NFκB p65 WT and mutants. The table indicates percentage localization in each compartment, and the percentages are derived from at least 200 transfected cells in microscopic fields. (E) NFκB p65 KO MEFs were cotransfected with empty vector (EV) or plasmids containing His-NFκB p65 (WT or mutants) and FLAG-tagged IκBα, and incubated for 12 h in the presence or absence of STZ, PUGNAc, or transfected with a plasmid expressing FLAG-tagged OGT. Immunoblotting of nuclear or cytosolic extracts by using antibodies against NFκB p65 was performed to determine NFκB p65 nuclear translocation (1st row). The purity of the nuclear and cytosolic extracts was determined by immunoblotting of histone H2A (nuclear fractions, 2nd row) and α-tubulin (cytosolic fractions, 3rd row).
Next, we examined the interactions between the His-NFκB p65 mutants and FLAG-tagged IκBα. When NFκB p65 KO MEFs were cultured in the presence of STZ or PUGNAc or were transfected with a plasmid expressing OGT, interactions between either NFκB p65 WT or T322A and FLAG-IκBα were reduced dramatically, but those between His-NFκB p65 T352A and FLAG-IκBα did not change to the same degree (Fig. 5C). Under these conditions, the total levels of His-NFκB p65 and FLAG-IκBα did not change, but O-GlcNAcylation on His-NFκB p65 was increased dramatically after STZ or PUGNAc treatment or upon OGT transfection (Fig. 5C).
We then studied the nuclear translocation of His-NFκB p65 and its mutated forms by using immunostaining. In the case of either His-NFκB p65 WT or T322A, the proteins were translocated into the nucleus under elevated O-GlcNAc conditions, but in the case of His-NFκB p65 T352A, most of the protein was not translocated (Fig. 5D). These results were confirmed with immunoblotting experiments by using an anti-NFκB p65 antibody on nuclear extracts (Fig. 5E). Therefore, O-GlcNAcylation on Thr-352, but not on Thr-322, can inhibit the interaction between NFκB p65 and IκBα. Taken together, these results show that inhibition of the interaction between NFκB p65 and IκBα caused by O-GlcNAcylation on Thr-352 of NFκB p65 induces the nuclear translocation of free NFκB p65, resulting in increased transcriptional activity. Also, these results show that site-specific O-GlcNAcylation can regulate NFκB activity in hyperglycemic conditions caused by diabetes.
Discussion
Since the discovery of O-GlcNAcylation (27), it has been reported that O-GlcNAcylation on Ser or Thr residues plays an important role in the function of nucleocytoplasmic proteins (16–18). For example, the activity of transcription factors such as Sp-1 and FoxO1 can be modulated by O-GlcNAcylation (28–31), and the stability and activity of p53 are also controlled by O-GlcNAcylation (26). Although it is known that NFκB is O-GlcNAcylated (12) and that this modification is important for T and B lymphocyte activation (19), the specific sites of modification and the functions are not well understood. In this work, we confirmed that the p65 subunit of NFκB is O-GlcNAcylated, and we found that this modification plays an important role in modulating hyperglycemia-induced NFκB activation. Furthermore, we also found that O-GlcNAcylation on Thr-352 of NFκB p65 interrupts the interaction between NFκB and IκBα, which increases the nuclear translocation of O-GlcNAcylated NFκB. These observations suggest that O-GlcNAcylation plays an important role in protein–protein interactions. Finally, we showed that an increased amount of O-GlcNAcylation on NFκB p65 under hyperglycemic conditions might explain, in part, the transcriptional activation of NFκB in STZ-induced type 1 diabetic mice.
In our studies, increased levels of O-GlcNAcylated-NFκB p65 were observed in cells under high-glucose conditions and in cells treated with STZ, PUGNAc, or overexpressing OGT, and the interaction between NFκB p65 and IκBα dramatically decreased under these conditions. These results could be caused by O-GlcNAcylation on NFκB p50 and IκBα, but we could not find any O-GlcNAcylation on these 2 proteins (data not shown). Also, by studying Thr to Ala substitution mutants of NFκB p65, we found that O-GlcNAcylation of Thr-352 played a crucial role in the interaction between NFκB and IκBα.
As reported, some proteins such as p53 and murine estrogen receptor β are reciprocally modified with O-GlcNAc and O-phosphate (26, 32). Because NFκB p65 is also modified with O-phosphate and this modification plays an important role in modulating its activity (2), it is possible that the changes in O-GlcNAcylation level affect not only its activity but also its phosphorylation states. In our experiments, the phosphorylation level on NFκB p65 did not change significantly even though its O-GlcNAcylation level was largely increased by treatment with STZ, PUGNAc, or OGT (Fig. S3). Also, there are no existing reports of modification of the O-GlcNAcylation sites, Thr-322 and Thr-352, by O-phosphate, and we could not identify any phosphorylation on these sites with MS analysis. Therefore, these results indicate that O-GlcNAcylation on NFκB p65 is a key regulator of NFκB activation, at least in hyper-O-GlcNAcylated states such as hyperglycemic conditions.
By using C-terminal deletion and substitution mutants of NFκB p65, we identified O-GlcNAcylation of amino acids Thr-322 and Thr-352 of NFκB p65, and the modification of Thr-352 was confirmed with MS analysis. By studying the site-directed mutants, we found that O-GlcNAcylation of NFκB p65 on Thr-352, but not on Thr-322, was important for the transcriptional activation of NFκB through the inhibition of the interaction between NFκB p65 and IκBα. However, it has been reported that the binding domain, or Rel homology domain (RHD), to IκBα exists in the 300 aa residues at the N terminus of NFκB p65 (33, 34). Interestingly, although Thr-322 is closer to the RHD than Thr-352, O-GlcNAcylation on Thr-352 of NFκB p65 decreases the interaction with IκBα, but modification on Thr-322 does not likely influence the interaction. It is possible that the O-GlcNAcylation on Thr-352 is closer to the IκBα-binding site than Thr-322 in a solution state. Therefore, future studies of the 3-dimensional structure of NFκB p65 and the modification-induced changes of the interaction with other proteins would be beneficial.
According to previous reports, hyperglycemic conditions induce NFκB activation via the degradation of IκBα in VSMCs, and the degradation occurs in relatively quickly (≈180 min) (22). However, in our experiments, the nuclear localization of NFκB was increased under high-glucose conditions at a relatively later time point (≈24 h), although the level of IκBα was similar to that observed under normal glucose conditions in our experiments. Moreover, hyperglycemia-induced NFκB activation was inhibited by OGA overexpression-induced down-regulation of O-GlcNAcylation. Therefore, this observation supports the possibility that O-GlcNAcylation-induced inhibition of NFκB–IκBα interactions is involved in the sustained activation of NFκB that is associated with diabetes (35). Additionally, in the STZ-induced diabetic mouse model, we found that NFκB p65 O-GlcNAcylation largely increased, and less IκBα was bound to NFκB p65 in each organ in diabetic mice compared with normal mice. Furthermore, according to a previous report, NFκB activation is involved in complications developed by type 1 or 2 diabetic patients (36). In fact, NFκB was activated dramatically in peripheral blood cells isolated from diabetic nephropathy patients (35). Therefore, O-GlcNAcylation on p65 of NFκB appears to be related to its sustained activation in diabetic conditions and the development of complications in diabetic patients. It is likely that other factors also contribute to the diabetes-associated NFκB activation.
In our proposed model, O-GlcNAcylation of the NFκB p65 subunit, particularly at Thr-352, induces NFκB nuclear translocation through the inhibition of the interaction between NFκB and IκBα, resulting in NFκB transcriptional activation (Fig. S4). We observed that O-GlcNAcylation still occurred on NFκB p65 when Thr to Ala substitutions were present at Thr-322 and Thr-352. Just as each phosphorylation of p65 of NFκB modulates its activity (2, 37), it is possible that O-GlcNAcylation on other sites also can modulate NFκB activity. Therefore, the effects of O-GlcNAcylation at other sites on NFκB activity should be investigated in the future.
Methods
Please see SI Methods for the following detailed methods: (i) cell culture, DNA transfection, and plasmids; (ii) reagents and antibodies; (iii) immunoblotting, immunoprecipitation, and immunostaining; (iv) luciferase assays; (v) EMSA; (vi) mapping O-GlcNAc sites using ESI-MS/MS; and (vii) diabetic mouse models.
Supplementary Material
Acknowledgments.
We thank Dr. Alexander Hoffmann (University of California at San Diego, La Jolla, CA) for the NFκB p65 knockout mouse embryonic fibroblasts cell lines, and Dr. Tae Ho Lee (Yonsei University, Seoul, Korea) for various vectors. This work was supported by grants from the Korea Science and Engineering Foundation (KOSEF) funded by the Ministry of Education, Science and Technology Grant R0A-2007-000-20011-0, SRC programs of MEST/KOSEF R112000078020010 and R11200301901001, Korea Research Foundation Grant KRF-2004-005-C00112, and, in part, by Grant A030003 from the Health 21 research and development project, via the Ministry of Health and Welfare, Republic of Korea (to J.W.C.). Additionally, W.H.Y., S.Y.P., and D.H.K. are fellowship awardees of the Brain Korea 21 program. This work was made possible through the use of research facilities in the Yonsei Center for Biotechnology.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The sequences reported in this paper have been deposited in the GenBank database [accession nos. M62399 (NFκB p65), BC014434 (OGT), and NP036347 (OGA)].
This article contains supporting information online at www.pnas.org/cgi/content/full/0806198105/DCSupplemental.
References
- 1.Ghosh S, Karin M. Missing pieces in the NF-κB puzzle. Cell. 2002;109:S81–S96. doi: 10.1016/s0092-8674(02)00703-1. [DOI] [PubMed] [Google Scholar]
- 2.Li Q, Verma IM. NF-κB regulation in the immune system. Nat Rev Immunol. 2002;2:725–734. doi: 10.1038/nri910. [DOI] [PubMed] [Google Scholar]
- 3.Hayden MS, Ghosh S. Signaling to NF-κB. Genes Dev. 2004;18:2195–2224. doi: 10.1101/gad.1228704. [DOI] [PubMed] [Google Scholar]
- 4.Zhong H, SuYang H, Erdjument-Bromage H, Tempst P, Ghosh S. The transcriptional activity of NF-κB is regulated by the IκB-associated PKAc subunit through a cyclic AMP-independent mechanism. Cell. 1997;89:413–424. doi: 10.1016/s0092-8674(00)80222-6. [DOI] [PubMed] [Google Scholar]
- 5.Bird TA, Schooley K, Dower SK, Hagen H, Virca GD. Activation of nuclear transcription factor NF-κB by interleukin-1 is accompanied by casein kinase II-mediated phosphorylation of the p65 subunit. J Biol Chem. 1997;272:32606–32612. doi: 10.1074/jbc.272.51.32606. [DOI] [PubMed] [Google Scholar]
- 6.Sakurai H, Chiba H, Miyoshi H, Sugita T, Toriumi W. IκB kinases phosphorylate NF-κB p65 subunit on serine 536 in the transactivation domain. J Biol Chem. 1999;274:30353–30356. doi: 10.1074/jbc.274.43.30353. [DOI] [PubMed] [Google Scholar]
- 7.Wang D, Westerheide SD, Hanson JL, Baldwin AS., Jr Tumor necrosis factor α-induced phosphorylation of RelA/p65 on Ser-529 is controlled by casein kinase II. J Biol Chem. 2000;275:32592–32597. doi: 10.1074/jbc.M001358200. [DOI] [PubMed] [Google Scholar]
- 8.Vermeulen L, De Wilde G, Van Damme P, Vanden Berghe W, Haegeman G. Transcriptional activation of the NF-κB p65 subunit by mitogen- and stress-activated protein kinase-1. EMBO J. 2003;22:1313–1324. doi: 10.1093/emboj/cdg139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Duran A, Diaz-Meco MT, Moscat J. Essential role of RelA Ser-311 phosphorylation by ζPKC in NF-κB transcriptional activation. EMBO J. 2003;22:3910–3918. doi: 10.1093/emboj/cdg370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen LF, Fischle W, Verdin E, Greene WC. Duration of nuclear NF-κB action regulated by reversible acetylation. Science. 2001;293:1653–1657. doi: 10.1126/science.1062374. [DOI] [PubMed] [Google Scholar]
- 11.Chen LF, Mu Y, Greene WC. Acetylation of RelA at discrete sites regulates distinct nuclear functions of NF-κB. EMBO J. 2002;21:6539–6548. doi: 10.1093/emboj/cdf660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.James LR, et al. Flux through the hexosamine pathway is a determinant of nuclear factor κB-dependent promoter activation. Diabetes. 2002;51:1146–1156. doi: 10.2337/diabetes.51.4.1146. [DOI] [PubMed] [Google Scholar]
- 13.Kreppel LK, Blomberg MA, Hart GW. Dynamic glycosylation of nuclear and cytosolic proteins. Cloning and characterization of a unique O-GlcNAc transferase with multiple tetratricopeptide repeats. J Biol Chem. 1997;272:9308–9315. doi: 10.1074/jbc.272.14.9308. [DOI] [PubMed] [Google Scholar]
- 14.Lubas WA, Hanover JA. Functional expression of O-linked GlcNAc transferase: Domain structure and substrate specificity. J Biol Chem. 2000;275:10983–10988. doi: 10.1074/jbc.275.15.10983. [DOI] [PubMed] [Google Scholar]
- 15.Gao Y, Wells L, Comer FI, Parker GJ, Hart GW. Dynamic O-glycosylation of nuclear and cytosolic proteins: Cloning and characterization of a neutral, cytosolic β-N-acetylglucosaminidase from human brain. J Biol Chem. 2001;276:9838–9845. doi: 10.1074/jbc.M010420200. [DOI] [PubMed] [Google Scholar]
- 16.Wells L, Vosseller K, Hart GW. Glycosylation of nucleocytoplasmic proteins: Signal transduction and O-GlcNAc. Science. 2001;291:2376–2378. doi: 10.1126/science.1058714. [DOI] [PubMed] [Google Scholar]
- 17.Love DC, Hanover JA. The hexosamine signaling pathway: Deciphering the “O-GlcNAc code”. Sci STKE. 2005;2005:re13. doi: 10.1126/stke.3122005re13. [DOI] [PubMed] [Google Scholar]
- 18.Hart GW, Housley MP, Slawson C. Cycling of O-linked β-N-acetylglucosamine on nucleocytoplasmic proteins. Nature. 2007;446:1017–1022. doi: 10.1038/nature05815. [DOI] [PubMed] [Google Scholar]
- 19.Golks A, Tran TT, Goetschy JF, Guerini D. Requirement for O-linked N-acetylglucosaminyltransferase in lymphocytes activation. EMBO J. 2007;26:4368–4379. doi: 10.1038/sj.emboj.7601845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Toleman C, Paterson AJ, Shin R, Kudlow JE. Streptozotocin inhibits O-GlcNAcase via the production of a transition state analog. Biochem Biophys Res Commun. 2006;340:526–534. doi: 10.1016/j.bbrc.2005.12.041. [DOI] [PubMed] [Google Scholar]
- 21.Haltiwanger RS, Grove K, Philipsberg GA. Modulation of O-linked N-acetylglucosamine levels on nuclear and cytoplasmic proteins in vivo using the peptide O-GlcNAc-β-N-acetylglucosaminidase inhibitor O-(2-acetamido-2-deoxy-d-glucopyranosylidene)amino-N-phenylcarbamate. J Biol Chem. 1998;273:3611–3617. doi: 10.1074/jbc.273.6.3611. [DOI] [PubMed] [Google Scholar]
- 22.Ramana KV, Friedrich B, Srivastava S, Bhatnagar A, Srivastava SK. Activation of nuclear factor-κB by hyperglycemia in vascular smooth muscle cells is regulated by aldose reductase. Diabetes. 2004;53:2910–2920. doi: 10.2337/diabetes.53.11.2910. [DOI] [PubMed] [Google Scholar]
- 23.Parker G, Taylor R, Jones D, McClain D. Hyperglycemia and inhibition of glycogen synthase in streptozotocin-treated mice: Role of O-linked N-acetylglucosamine. J Biol Chem. 2004;279:20636–20642. doi: 10.1074/jbc.M312139200. [DOI] [PubMed] [Google Scholar]
- 24.Gapuzan ME, Schmah O, Pollock AD, Hoffmann A, Gilmore TD. Immortalized fibroblasts from NF-κB RelA knockout mice show phenotypic heterogeneity and maintain increased sensitivity to tumor necrosis factor α after transformation by v-Ras. Oncogene. 2005;24:6574–6583. doi: 10.1038/sj.onc.1208809. [DOI] [PubMed] [Google Scholar]
- 25.Chalkley RJ, Burlingame AL. Identification of novel sites of O-N-acetylglucosamine modification of serum response factor using quadrupole time-of-flight mass spectrometry. Mol Cell Proteomics. 2003;2:182–190. doi: 10.1074/mcp.M300027-MCP200. [DOI] [PubMed] [Google Scholar]
- 26.Yang WH, et al. Modification of p53 with O-linked N-acetylglucosamine regulates p53 activity and stability. Nat Cell Biol. 2006;8:1074–1083. doi: 10.1038/ncb1470. [DOI] [PubMed] [Google Scholar]
- 27.Torres CR, Hart GW. Topography and polypeptide distribution of terminal N-acetylglucosamine residues on the surfaces of intact lymphocytes: Evidence for O-linked GlcNAc. J Biol Chem. 1984;259:3308–3317. [PubMed] [Google Scholar]
- 28.Yang X, et al. O-linkage of N-acetylglucosamine to Sp1 activation domain inhibits its transcriptional capability. Proc Natl Acad Sci USA. 2001;98:6611–6616. doi: 10.1073/pnas.111099998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kang HT, Ju JW, Cho JW, Hwang ES. Down-regulation of Sp1 activity through modulation of O-glycosylation by treatment with a low-glucose mimetic, 2-deoxyglucose. J Biol Chem. 2003;278:51223–51231. doi: 10.1074/jbc.M307332200. [DOI] [PubMed] [Google Scholar]
- 30.Majumdar G, et al. Insulin stimulates and diabetes inhibits O-linked N-acetylglucosamine transferase and O-glycosylation of Sp1. Diabetes. 2004;53:3184–3192. doi: 10.2337/diabetes.53.12.3184. [DOI] [PubMed] [Google Scholar]
- 31.Housley MP, et al. O-GlcNAc regulates FoxO activation in response to glucose. J Biol Chem. 2008;283:16283–16292. doi: 10.1074/jbc.M802240200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cheng X, Hart GW. Alternative O-glycosylation/O-phosphorylation of serine-16 in murine estrogen receptor β: Posttranslational regulation of turnover and transactivation activity. J Biol Chem. 2001;276:10570–10575. doi: 10.1074/jbc.M010411200. [DOI] [PubMed] [Google Scholar]
- 33.Jacobs MD, Harrison SC. Structure of an IκBα/NF-κB complex. Cell. 1998;95:749–758. doi: 10.1016/s0092-8674(00)81698-0. [DOI] [PubMed] [Google Scholar]
- 34.Huxford T, Huang DB, Malek S, Ghosh G. The crystal structure of the IκBα/NF-κB complex reveals mechanisms of NF-κB inactivation. Cell. 1998;95:759–770. doi: 10.1016/s0092-8674(00)81699-2. [DOI] [PubMed] [Google Scholar]
- 35.Bierhaus A, et al. Diabetes-associated sustained activation of the transcription factor nuclear factor-κB. Diabetes. 2001;50:2792–2808. doi: 10.2337/diabetes.50.12.2792. [DOI] [PubMed] [Google Scholar]
- 36.Hegazy DM, et al. NFκB polymorphisms and susceptibility to type 1 diabetes. Genes Immun. 2001;2:304–308. doi: 10.1038/sj.gene.6363776. [DOI] [PubMed] [Google Scholar]
- 37.Viatour P, Merville MP, Bours V, Chariot A. Phosphorylation of NF-κB and IκB proteins: Implications in cancer and inflammation. Trends Biochem Sci. 2005;30:43–52. doi: 10.1016/j.tibs.2004.11.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.