Abstract
Tolerance, described as the loss of drug effectiveness over time, is an important component of addiction. The degree of acute behavioral tolerance to alcohol exhibited by a naïve subject can predict the likelihood of alcohol abuse. Thus, the determinants of acute tolerance are important to understand. Calcium- and voltage-gated (BK) potassium channels, consisting of pore forming α and modulatory β subunits, are targets of ethanol (EtOH) action. Here, we examine the role, at the molecular, cellular, and behavioral levels, of the BK β4 subunit in acute tolerance. Single channel recordings in HEK-293 cells show that, in the absence of β4, EtOH potentiation of activity exhibits acute tolerance, which is blocked by coexpressing the β4 subunit. BK channels in acutely isolated medium spiny neurons from WT mice (in which the β4 subunit is well-represented) exhibit little tolerance. In contrast, neuronal BK channels from β4 knockout (KO) mice do display acute tolerance. Brain slice recordings showed tolerance to EtOH's effects on spike patterning in KO but not in WT mice. In addition, β4 KO mice develop rapid tolerance to EtOH's locomotor effects, whereas WT mice do not. Finally, in a restricted access ethanol self-administration assay, β4 KO mice drink more than their WT counterparts. Taken together, these data indicate that the β4 subunit controls ethanol tolerance at the molecular, cellular, and behavioral levels, and could determine individual differences in alcohol abuse and alcoholism, as well as represent a therapeutic target for alcoholism.
Keywords: electrophysiology, knockout mice, striatum, addiction, plasticity
Alcohol abuse is the third largest cause of preventable mortality in the world. Tolerance, described as the gradual loss of drug effectiveness over time, is a hallmark of abused drugs. This phenomenon is particularly important in the response to acute alcohol because the degree of tolerance exhibited by a naïve subject can predict the likelihood to develop alcohol abuse (1–4). Thus, identifying the mechanistic and molecular underpinnings of tolerance is essential for understanding the pathophysiology of alcoholism, as well as determining potential therapeutic targets for alcohol abuse. The neurobiology of tolerance is thought to involve several types of adaptation, ranging from alteration in membrane lipid composition (5) to neuroadaptative changes in target proteins (6, 7).
In recent years, large conductance calcium- and voltage-gated potassium (BK) channels have emerged as one of the key targets of ethanol action, yet their role in the physiological and behavioral response to alcohol are unknown. Invertebrate studies suggest that BK channels may be important for the development of tolerance to ethanol (8, 9). In mammals, BK channels exist as a complex formed by the association of the pore-forming α subunit with the auxiliary β subunit. The α subunit is encoded by only one gene (slo) with several splice variants (STREX, P27, insertless, etc.), whereas the β subunit is the product of four distinct genes (β1-β4). BK α subunits, unlike β, form functional BK channels (10–12). BK α subunit expression is robust and widespread throughout the brain, with particularly high levels in the neo-, olfactory, and hippocampal cortices, striatum, habenula, and cerebellum (11, 13–15). Other prominent sites for BK α are thalamus, amygdala, and, to a lesser degree, the brainstem, and spinal cord (14). In contrast, the β4 subunit, although highly expressed, appears to be restricted to specific brain regions like the lateral hypothalamus, the purkinje layer and the striatum (13, 14). Whereas β1 expression is found at low levels in brain, β2 and β3 do not appear to be expressed in the central nervous system (16, 17). In previous work, we showed that low EtOH concentrations (10–50 mM) potentiated BK channel open probability in a number of brain regions (hypothalamo-hypophyseal axis and nucleus accumbens) (18–20). Recently, we also reported that EtOH effects depend on BK channel subunit composition in ventral striatum. We found that αβ4 BK channels were potentiated by EtOH, whereas αβ1 channels were not (19). In the present study, we tested the hypothesis that BK subunit composition can control the degree and duration of ethanol sensitivity and, because of robust expression in striatum—a brain region implicated in addiction—we predicted that differences in BK subunit expression can translate into altered ethanol-induced behaviors. We focused on the β4 subunit because of our previous work that indicates it is widely expressed in ventral striatum and co-assembles with BK α to form functional, ethanol sensitive channels in the soma of medium spiny neurons (MSNs).
Results
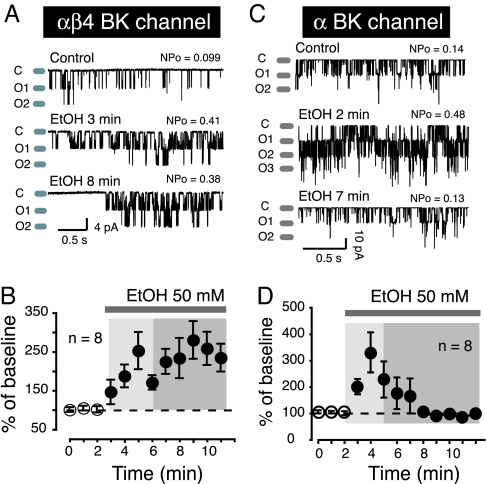
We transfected HEK-293 cells with α alone or in combination with β4, and recorded BK single channel activity in cell-attached patch clamp mode for 20 sec every minute for up to 20 min. 50 mM EtOH, a concentration known to strongly influence channel activity (19), increased αβ4 BK channel open probability (Fig. 1A; middle trace EtOH 3 min) compared to control (Fig. 1A; Top Trace Control). This effect persisted up to 8 min after the start of EtOH exposure (Fig. 1A Bottom Trace). The lack of tolerance was not voltage-dependent because we observed a similar phenomenon when large (+150 mV) depolarizing voltage steps evoked outward BK currents on three additional patches (see supporting information (SI) Fig. S1A). In five patches (two from HEK cells and three from freshly isolated neurons), we found that BK channel activity remained potentiated (about 3.4 fold) for up to 14 min (the longest tested) after the beginning of EtOH perfusion compared to control values (data not shown). A ceiling effect that would distort the magnitude of EtOH's effects is very unlikely as we systematically set BK channel NPo to low values before exposing cells to the drug. Furthermore, even in presence of EtOH, BK channels typically spent only a small fraction of their total open time in the second (Fig. 1A; O2 Middle Trace) or third open states (Fig. 1C; O3 Middle Trace), indicating that EtOH had not maxed out BK channel activity. In control experiments, we measured BK channel activity for up to 15 min in the absence of ethanol and found no change in baseline (data not shown). On average (n = 8), 50 mM EtOH increased BK channel activity by about 2.5 fold (Fig. 1B).
Fig. 1.
BK β4 subunit influences tolerance: (A) αβ4 BK Single channel activity recorded at hyperpolarized potentials from HEK293 cells. (B) EtOH's effects on αβ4 BK channel activity averaged over several cells (n values indicated in graphs). (C) shows α BK single channel activity before (control) and during EtOH exposure. (D) Magnitude of EtOH's effects on α BK activity averaged over several cells (n values are indicated in graphs). Representative traces of BK activity before (control) and during EtOH exposure (EtOH). “C” and “O” refer to BK closed and open states, respectively. NPo indicates BK channel open probability under each experimental condition. In graphs (B and D) lightly shaded areas indicate where BK channel potentiation typically occurs, while the darker shaded areas show where tolerance is observed. EtOH effects are expressed as % of baseline.
We examined the effects of 50 mM EtOH on BK channels consisting of only α subunits. We found that EtOH effects on both inward (Fig. 1C) and outward (Fig. S1B) currents were similar. Thus, under these conditions, 50 mM EtOH also boosted channel activity compared to control. In contrast to its effect on αβ4 BK channels, EtOH potentiation disappeared minutes after the beginning of alcohol exposure, demonstrating acute tolerance of the response (Fig. 1C Lower Trace EtOH 7 min). When averaged over 8 patches, EtOH initially increased α BK channel activity by about 3 fold (Fig. 1D, light shaded box), before returning to control levels (Fig. 1D; darker shaded box). To evaluate whether EtOH potentiation was voltage dependent, we tested EtOH's effect on both α and αβ4 BK activity by recording macroscopic currents in whole cell mode at multiple potentials. Plotting current amplitude (normalized to Imax, typically observed around +150 mV) vs. voltage revealed that EtOH increased both α and αβ4 BK current amplitude over a range of potentials (Fig. S2), indicating it was not voltage-dependent.
To determine the time course of α and αβ4 BK EtOH response, we recorded macroscopic currents in response to a single voltage step (between 110 and 140 mV from a holding potential of −70 mV) every 10 sec for several minutes. We found that the response of α BK channels to EtOH (Fig. S3 A and C) developed much faster than that of αβ4 channels (Fig. S3 B and C). The effect of EtOH on α BK channels peaked approximately 2 min after drug exposure (Fig. S3C). In contrast, it took about twice as long for αβ4 BK channel responses to peak under similar experimental conditions. In addition, as in cell-attached recordings, tolerance was observed with α, but not αβ4 BK channels, demonstrating that this does not depend on the recording mode.
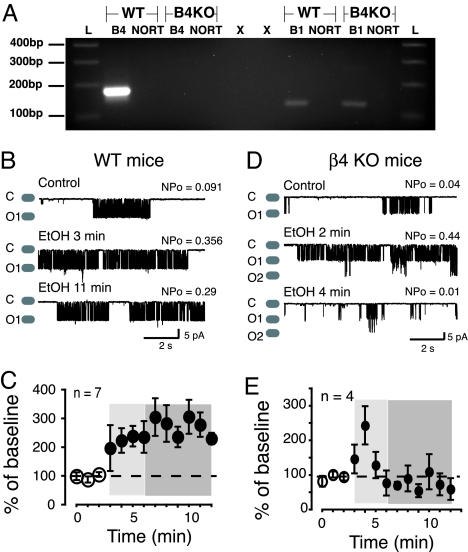
From previous work, we know that the BK β4 subunit is coexpressed with the α subunit in rat ventral striatum MSNs (19) and that these channels are dose-dependently potentiated by EtOH. RT-PCR amplification of β4 mRNA confirms that this subunit is robustly expressed in mouse striatum along with much lower levels of the β1 subunit (Fig. 2A). Therefore, we hypothesized that MSNs from WT mice should functionally express αβ4 BK channels and that they should be potentiated by EtOH, mirroring αβ4 BK activity in heterologous expression studies. Indeed, the WT BK channel response to 50 mM EtOH was very similar to that observed with αβ4 BK channels in HEK293 cells: EtOH potentiated BK channel activity (Fig. 2B, Middle Trace and Fig. 2C, light shaded box) and this was sustained throughout the recording session (Fig. 2B, Bottom Trace and Fig. 2C, darker shaded box). To determine if this persistent EtOH mediated channel potentiation was dependent on β4 expression, we recorded from BK channels in MSNs isolated from mice that do not express KCNMβ4, the gene encoding the β4 subunit (β4 KO) (15). Interestingly, MSNs from KO mice exhibited BK channel activity that was potentiated by EtOH but rapidly returned to control levels (Fig. 2 D and E, 4/5 neurons), indicating that, in the absence of β4, acute tolerance develops. This effect mirrored what we found when BK α subunit alone was expressed in HEK293 cells (Fig. 1 D and E).
Fig. 2.
β4 subunit controls tolerance of BK single channel activity in freshly isolated striatal MSNs. (A) DNA agarose gel shows that only β4 expression is lacking in striatum isolated from β4 KO mice; whereas β1 mRNA is present in both WT and KO animals. “L” denotes the 100 bp marker on molecular weight ladder. Base pair number is indicated in the left hand margin. “B1” and “B4” refer to BK β1 and β4 subunits, respectively. “NORT” are negative controls with omitted reverse transcriptase and “X” indicates columns where no material was loaded. Single channel activity recorded from striatal MSNs acutely isolated from WT (B and C) or β4 KO (D and E) mice, respectively. (B and D) Representative traces of BK activity from WT and KO mice, before (control) and during EtOH exposure (EtOH). “C” and “O” refer to closed and open states. NPo indicates BK channel open probability. (C and E) graphs show magnitude of EtOH's effects averaged over several cells (n values are indicated in graphs). Lightly shaded areas indicate where BK channel potentiation typically occurs, while the darker shaded areas show where tolerance is observed. EtOH effects are expressed as a percentage of baseline.
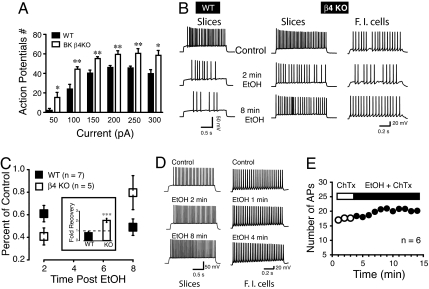
To better understand the physiological role of BK β4 subunit expression on neuronal excitability, we evoked action potentials (APs) in WT and β4 KO mice using whole cell patch clamp recordings in striatal slice and freshly isolated MSNs. Similar to previous reports from hippocampal neurons (21), the number of APs evoked by current injection was increased in β4 KO MSNs compared to WT (Fig. 3A). Since BKs contribute to determining MSNs AP patterning in WT mice, we tested the idea that EtOH-mediated modulation of BK channels should alter the excitability of these neurons and that this effect should show little tolerance. In contrast, in KO mice where most BK channels are presumably composed of α subunits only, we expected to see a transient effect of EtOH on AP patterning. In WT mice, 50 mM EtOH markedly decreased the number of APs 2 min after EtOH perfusion (Fig. 3B; Middle Trace, Left) and this effect persisted 8 min after the start of EtOH application, indicating a lack of tolerance. In β4 KO MSNs, the number of APs was also reduced 2 min after EtOH perfusion. However, unlike WT responses, significant tolerance developed to EtOH induced suppression of excitability within the 8 min EtOH exposure (Fig. 3B, Middle, Bottom Trace). In freshly isolated MSNs from KO mice (n = 3, Fig. 3B; Right), EtOH similarly transiently reduced the number of APs, mirroring results obtained in slices. This latter experiment demonstrates that EtOH effects on MSN spike patterning are intrinsic to these neurons. On average, 2 min after EtOH exposure, the number of evoked APs in β4 KO MSNs decreased by 60% of control compared to 40% for neurons from WT mice (Fig. 3C). While the number of APs in KO mice was almost back to control level after 8 min exposure, it was smaller in WT mice compared to the 2 min time point (Fig. 3C). The development of tolerance (or its absence) is also shown in the inset of Fig. 3C as the ratio of APs at 8 min over the number of APs 2 min after EtOH exposure. In WT mice, the ratio was below 1 (broken line, Fig. 3C; Inset) while it was significantly higher in MSNs from KO mice (approximately 2, P < 0.001).
Fig. 3.
EtOH-mediated decrease of MSN excitability exhibits tolerance in KO, but not WT mice. (A) Number of APs recorded from WT (filled columns) and KO (open columns) MSNs following a series of incremental (50 pA) current steps (50–300 pA). (B) Representative action potential trains evoked in a slice preparation (Slices) by a single 100 pA current step in WT (Left) and KO (Middle) mice before (control) and after 50 mM EtOH exposure (2 or 8 min). Two minutes after EtOH, the number of APs is smaller in both WT and KO mice. While KO mice MSN excitability partially recovers 8 min after EtOH exposure (Bottom Trace; Right), WT neuronal excitability remains depressed (Left, Bottom Trace). Results obtained in slices were reproduced on freshly isolated neurons from KO mice (Right; β4 KO/F.I. cells). (C) Averaged change in action potential number recorded in MSNs in slices and freshly isolated after 2 or 8 min EtOH exposure, presented as percent of control before EtOH exposure in MSNs from WT and β4 KO striatal slices; 5/7 neurons were ethanol sensitive and developed tolerance in β4 KO MSNs, whereas 7/9 MSNs from WT were ethanol sensitive and did not develop tolerance (*P < 0.05). The Inset shows the ratio of AP number at 2 and 8 min reported as fold recovery from ethanol; value below the broken bar indicates a further decrease of APs number at 8 min compared to 2 min EtOH (solid column; WT), while value above the line indicate a recovery (KO); F1,10 = 27.6, P < 0.001. (D) 100 nM ChTx blocks EtOH effects on striatal MSNs AP patterns in slices (Left) and freshly isolated neurons (F.I cells, Right). (E) Number of APs measured every minute before (open circles) and during 50 mM EtOH exposure (solid circles) in presence of 100 nM ChTx. Data from slices and freshly isolated MSNs were combined.
To further establish a functional link between β4 expression and the development of tolerance of spike patterning, we exposed β4 KO neurons in slice and dissociated culture to 100 nM charybdotoxin (ChTx), known to block α and αβ1 BK, the two BK channel subtypes found in these KO mice. In both slices (n = 2) and freshly isolated MSNs (n = 4), not only did 50 mM EtOH fail to decrease spiking, but it slightly increased it (n = 6; Fig. 3D). Fig. 3E shows the average number of APs before (open circles) and during EtOH exposure in the presence of 100 nM ChTx. We also tested the effect of 1.5 μM tetrandrine, a blocker of αβ4 BK channels. Tetrandrine completely prevented EtOH from altering excitability of WT MSNs. The number of APs remained unchanged up to 3 min after EtOH exposure (Fig. S4), confirming that EtOH effects are mediated by αβ4 BK.
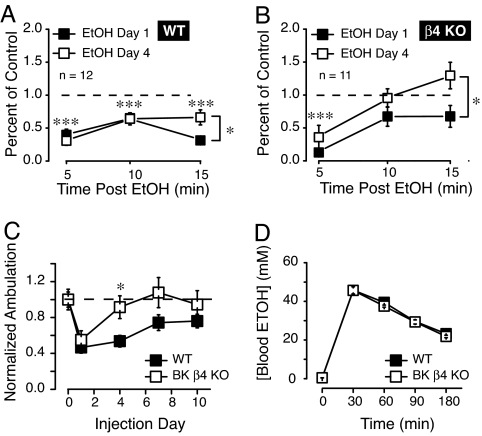
Because striatum is a brain region known to be involved in both the motivational and locomotor properties of drugs of abuse (for reviews see refs. 22, 23), we wondered if the stark difference in physiology at the single channel and whole cell levels could also be observed in the behavioral response to EtOH. Thus, we challenged β4 KO and WT mice with 2g/kg EtOH once a day and monitored their ambulatory activity 5, 10, and 15 min after injection. Following EtOH injection on day 1, WT mice showed a marked (70%) reduction of their activity levels 5 min after injection (Fig. 4A; black square symbols, F1,22 = 25.0, P < 0.001) compared to a saline injection. Activity remained depressed when monitored over the following 10 and 15 min time blocks. On day 4, the response of WT mice to EtOH 5 and 10 min after injection were comparable to that of day 1 and only showed tolerance at the 15 min time point (Fig. 4A; open squares, F1,22 = 7.36, P < 0.05 day 1 compared to day 4). However, in sharp contrast to WT mice, ambulatory activity in KO mice displayed rapid tolerance to the locomotor suppressing effects of EtOH. Thus, on day 1, although EtOH significantly depressed locomotor activity (Fig. 4B; day 1) 5 min after injection compared to control (F1,20 = 11.7, P < 0.01), when tested at 10 and at 15 min after EtOH injection on day 1, ambulatory activity had returned to control levels (Fig. 4B; filled squares). In the same KO mice, by the fourth day of ethanol challenge, nearly complete tolerance to ethanol-induced hypolocomotion was observed. In addition, we compared summed locomotor activity 15 min after EtOH injection on days 1, 4, 7, and 10 between β4 and WT mice. On day 1 (first injection), the activity of both WT and KO mice decreased (Fig. 4C). However, by the fourth injection day (day 4), complete tolerance to ethanol-induced hypolocomotion developed in β4 KO mice (Fig. 4C; day 4 significance between genotypes, F1,21 = 7.1, P < 0.05), whereas suppression remained evident in WT mice through the 10th day of injection (Fig. 4C). The difference in acute locomotor tolerance could not be explained by a difference in the pharmacokinetics of ethanol between β4 KO and WT mice because the peak blood ethanol concentration (BEC), as well as the clearance rate of ethanol after an i.p. injection of 2 g/kg was identical in the two genotypes (Fig. 4D).
Fig. 4.
Effects of EtOH on locomotor activity in male WT and β4 KO mice. (A) Ambulatory activity of WT mice 5, 10, and 15 min after a 2 g/kg i.p. EtOH injection on days 0, 1, and 4. Each data point represents 5 min summed activity. Asterisks above symbols indicate significant locomotor activity on both days 1 and 4 of EtOH injection at each time point (5, 10, and 15 min) compared to saline injection baseline (represented by the dashed line). Asterisk next to bracket indicates difference between days 1 and 4 at 15 min time point only. (B) Effects of the same EtOH dose at the same time points in KO mice. (C) Fifteen minute summed activity immediately after a daily ethanol injection. Note the rapid development of tolerance for KO mice upon repeated injections of 2 g/kg EtOH, compared to WT mice. (D) Blood alcohol concentration in WT and KO mice (n = 4) before (time 0) and at 30-min intervals after a single i.p. injection of 2 g/kg ethanol. One-way ANOVA, Tukey posthoc, * P < 0.05, *** P < 0.001.
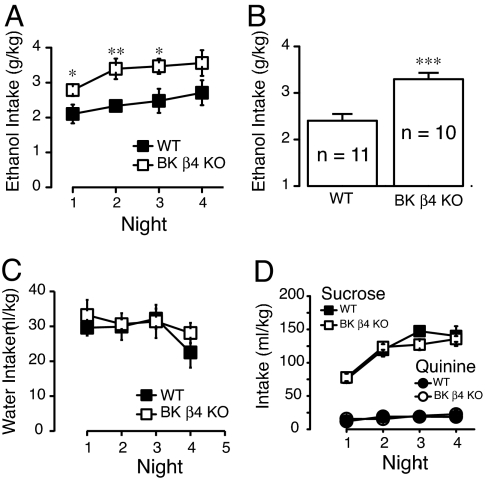
Because rapid tolerance is predictive of increased alcohol consumption, we compared voluntary ethanol intake in WT and β4 KO mice, using a restricted access EtOH self-administration paradigm termed “drinking in the dark” (24, 25). This assay produces robust EtOH intake in C57BL/6J mice, the background strain of the β4 KOs. Remarkably, β4 KO mice consumed significantly greater levels of EtOH compared to consumption in WT mice during each of the first three nights of the assay (Fig. 5A). In addition, the ethanol intake averaged over four nights was significantly higher in β4 KO mice (Fig. 5B, F1,82 = 19.7, P < 0.001). We measured blood alcohol levels (BAL) immediately following ethanol exposure on night 2 of the DID assay, where we observed the largest difference in intake between genotypes. As expected, BALs of KO mice (26.62 ± 7.53; n = 4) were much higher than that of WT mice (5.85 ± 0.53 mM, n = 4). Water intake between WT and KO mice was not different (Fig. 5C), suggesting that changes in ethanol consumption were not due to differences in drinking volume. Importantly, there was also no significant difference between genotypes in sucrose intake, indicating that changes in ethanol drinking were specific for the drug (Fig. 5D). WT and KO mice had similar aversion for quinine, suggesting that the difference of EtOH intake was not due to aversive taste (Fig. 5D).
Fig. 5.
EtOH consumption is higher in β4 KO. (A) EtOH consumption of WT (black squares) and β4 KO mice (white squares) using a restricted access single bottle self-administration assay. Measurements were taken every day for four days for 2 h after lights were turned off. (B) Average daily EtOH intake in male and female WT and KO mice. (C and D) Water, sucrose, and quinine intake of WT (black squares and circles) and KO (white squares and circles) mice, respectively (n = 5–8 per genotype). No significant difference was observed in either condition. One Way ANOVA, Tukey posthoc, * P < 0.05, ** P < 0.01, *** P < 0.001.
Discussion
Our results suggest remarkable parallels in the effects of the BK β4 subunit on acute alcohol tolerance at the level of single channel recording, spike patterning, and behavioral studies. The development of tolerance was apparent within a few minutes at each level of analysis in β4 KO but not WT mice.
The bridge between molecular events and behavioral outcome is always difficult to establish. We believe that our finding of β4-dependent tolerance at the single channel and action potential levels is a compelling candidate for mediating effects we see on behavior (locomotor tolerance and alcohol consumption). Because the β4 subunit is expressed in a number of brain regions (15, 16), we cannot rule out that regions outside the striatum may participate in the changes in ethanol-related behavior. However, our focus on striatum is based in part on the known role this circuitry plays in these behavioral outcomes. Ethanol, via both direct and indirect activation of DAergic neurons in the ventral tegmental area (26–29), increases dopamine release in the striatum, which is associated with both the motivational and locomotor properties of most abused drugs. In addition, our data are consistent with recent reports in c-elegans indicating a role for BK channels in depression of locomotor effects of alcohol (11). MSNs make up approximately 95% of neurons in striatum and receive inputs from DAergic neurons in the VTA and substantia nigra pars compacta. MSNs express BK channels consisting of α and β4 subunits that are potentiated by ethanol, an effect that develops little tolerance in response to acute alcohol. In the absence of the β4 subunit, the rate of tolerance to ethanol potentiation is dramatically enhanced at both the single channel and whole cell level. This is associated with an increase in the rate of tolerance to locomotor suppression elicited by both acute and chronic ethanol exposure in β4 KO mice compared to WT mice. The fact that β4 KO mice also self-administer more alcohol than WT animals corroborates the important role β4 subunit expression has on alcohol tolerance. This dramatic difference in tolerance and alcohol consumption is specific for ethanol because β4 KO mice consume equal amounts of water, quinine, and sucrose solution compared to WT mice. In addition, the pharmacokinetics of ethanol does not differ between genotypes.
At the macroscopic level, the influence of the BK channel in shaping APs in MSNs of the dorsal striatum is not surprising. Studies carried out in CA1 pyramidal neurons from the hippocampus (30, 31), dorsal vagal neurons (32), lateral amygdala (33) and purkinje cells (34, 35), report that toxin-mediated BK channel blockade widens APs, suggesting that BK channels facilitate repolarization. In striatal interneurons and MSNs, BK contributions to shaping APs has also been reported (36). Interestingly, our data showing that spike frequency is significantly higher in β4 KO mice compared to WT mice, is consistent with a recent study in CA1 neurons by Brenner et al. (21) with the same knockout animals. The similarity with the Brenner study confirms the role of β4 subunit mRNA, since it is highly expressed in both striatum and hippocampus (37). Although MSNs express other channels involved in shaping APs and neuronal excitability, the effects of EtOH on MSN excitability are likely mediated by BK channels. First, delayed-rectifier and rapidly inactivating IA K+ channels, the two other main potassium channels activated by depolarization in striatal MSNs, have been shown in other preparations to be insensitive to 50 mM EtOH (38), the concentration tested here. Additionally, tolerance to EtOH-mediated effects of AP patterns in MSNs observed in KO mice occurs over a similar time course to tolerance to EtOH-mediated potentiation of BK single channel activity in the same mice. Finally, the effects of ethanol on AP spike patterns are inhibited by charybdotoxin in β4 KO mice suggesting a BKα dependent mode of drug action.
Our data indicate that the BK β4 subunit controls tolerance to alcohol at both the molecular and behavioral levels. Since a dramatic association between tolerance to alcohol and the propensity to develop alcoholism exists, our data suggest that the gene encoding the BK channel β4 subunit, KCNMB4 should be evaluated as a candidate gene for susceptibility to alcohol abuse and alcoholism.
Materials and Methods
Cell Culture.
Our methods are essentially the same as previously published (39). Briefly, α BK channels were derived from stable cell lines (40), a gift from Peter Ahring (NeuroSearch A/S, Ballerup, Denmark) (41). αβ4 channels were derived from cell lines stably expressing α and transiently expressing β4.
Slice Preparation and Freshly Isolated Striatal Neurons.
This is described in detail in Martin et al. (42). Briefly, mouse brains were sliced (350 μm) using a Vibratome 3000 (Vibratome) and incubated for up to 6 h at room temp (20–22 °C) in a gassed (95% O2 and 5% CO2) saline solution. Following enzymatic digestion with protease XIV (1 mg/ml), the tissue was mechanically triturated using fire-polished Pasteur pipettes, and cells were plated into a 35 mm Petri dish.
Electrophysiological Recording.
Single-cell cell-attached patch clamp recording used standard methods (43). We pulled patch electrodes from 1.5 mm OD borosilicate capillary glass (Warner Instrument) to a resistance of 4–6 MΩ. The recording pipette solution was (in mM) 130 K2MeSO4, 2 MgCl2, 2 CaCl2, 15 Hepes. We set sampling rate and low-pass filter at 10 and 2 kHz, respectively, using an EPC10 double amplifier (HEKA Elektroniks). Voltage and current were digitized and stored using PatchMaster 2.1 (HEKA Elektroniks). We recorded BK activity for 20 sec, every minute, three times to ensure a stable baseline. We averaged the open probability of these three controls, and all subsequent NPo values were expressed relative to this average. Drugs were applied and BK channel activity was recorded in successive blocks of 20 sec, every minute, for up to 10 min. Data were expressed as mean ±SEM (with the number of cells or patches in parentheses).
For whole cell recording in slices, MSNs were visually identified and characterized electrophysiologically. Series resistance (Rs) was monitored throughout experiments. Recordings showing Rs changes of more than 15–20% were discarded. We used MultiClamp 700 B and EPC10 double amplifiers, at a rate of 20 kHz, to record APs. Voltage and current traces were acquired and analyzed with pClamp 10 (Molecular Devices) and FitMaster 2.15 (HEKA Elektronik) software packages.
Charybdotoxin Treatment.
To ensure we recorded exclusively αβ4 channels in HEK-293 cells and in WT striatal neurons, we added low concentrations of charybdotoxin (ChTx), a toxin that rapidly and selectively inhibits activity of α and αβ1 BK channels at 100 nM (37).
Calculation of the Steady-State Channel Activity, NPo.
We used all-points amplitude histograms to calculate BK activity, determined from the product of the total number of functional channels present in the membrane patch (N) and the probability that a particular channel was open under steady-state conditions (Po). Calculations of NPowere performed with TAC analysis software (Bruxton Inc). NPo ratios generated from the first ethanol exposure were used for normalization of the data. BK activity was measured as NPo ratio percent ((NPoethanol/NPocontrol) × 100).
Behavioral Experiments.
Male and female C57BL/6J mice (Jackson Laboratory) between 8–14 weeks of age were housed 3–4 animals per cage until the start of each experiment. For drinking in the dark, mice purchased from Jackson Labs were habituated to BNRI colony rooms for at least two weeks and the DID procedure room for at least one week before the start of experiments. Mice used for locomotor studies were bred and raised at the BNRI. β4 KO mice were back-crossed at least ten generations to the C57BL/6J strain. We kept mice on a standard 12 h light/dark cycle with lights on at 7:00 a.m. and off at 7:00 p.m., and given food and water ad libitum. We conducted all experiments in accordance with the guidelines for care and use of animals provided by the National Institute of Health, as well as with an approved animal protocol from the Institutional Animal Care and Use Committee of the UMass Medical School.
Drugs and Drinking Solutions.
Ethanol solution was prepared from 190 proof absolute anhydrous ethanol (Pharmco-Aaper brand) diluted to 10% ethanol (vol/vol) using tap water. Sucrose (EMD) and quinine hydrochloride (Sigma-Aldrich) was dissolved in tap water to make a 10% (wt/vol) and 1 mM concentration solution, respectively.
Drinking in the Dark (DID).
Two hours after lights out, water bottles were removed and replaced with 10% ethanol bottles and left in place for 2 h. Control animals had water replaced with another water bottle or 10% sucrose or 1 mM quinine solution. An empty cage was set up and a water bottle was replaced with ethanol to control for evaporation.
Activity Monitoring.
Locomotor activity was measured by a photobeam system (San Diego Instruments). Mice were placed in activity cages and allowed to habituate for 90 min before first i.p. injection of either saline or ethanol (2 g/kg, 20% vol/vol with saline, 10 μl/g body weight).
Ethanol Metabolism.
Before an ethanol injection, blood was obtained from the tail vein (approximately 30 μl each time point) to provide a zero point. After a 2 g/kg i.p. injection of EtOH, blood samples were taken at 30, 60, 90, and 120 min. For BEC measurements after DID, mice were culled immediately after a 2 h EtOH exposure on night 2 and trunk blood was collected in heparinized capillary tubes. Blood was centrifuged (1500×g for 5 min) and analyzed using an alcohol oxidase-based assay. We measured BALs on a GM7 MicroStat Analyzer (Analox Inst Ltd.).
Supplementary Material
Acknowledgments.
This work was supported by National Institute on Alcohol Abuse and Alcoholism (AA008003 to SNT)
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0801068105/DCSupplemental.
References
- 1.Erwin VG, McClearn GE, Kuse AR. Interrelationships of alcohol consumption, actions of alcohol, and biochemical traits. Pharmacol Biochem Behav 13 Suppl. 1980;1:297–302. doi: 10.1016/s0091-3057(80)80045-1. [DOI] [PubMed] [Google Scholar]
- 2.Chrostek L, Szmitkowski M. Genetic evaluation of tolerance to alcohol. Postepy Hig Med Dosw. 1998;52:35–47. [PubMed] [Google Scholar]
- 3.Heath AC, et al. Genetic differences in alcohol sensitivity and the inheritance of alcoholism risk. Psychol Med. 1999;29:1069–1081. doi: 10.1017/s0033291799008909. [DOI] [PubMed] [Google Scholar]
- 4.Fillmore MT, Marczinski CA, Bowman AM. Acute tolerance to alcohol effects on inhibitory and activational mechanisms of behavioral control. J Stud Alcohol. 2005;66:663–672. doi: 10.15288/jsa.2005.66.663. [DOI] [PubMed] [Google Scholar]
- 5.Yuan C, et al. Acute alcohol tolerance is intrinsic to the BKCa protein, but is modulated by the lipid environment. J Biol Chem. 2007;283:5090–5098. doi: 10.1074/jbc.M708214200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Crews FT, Morrow AL, Criswell H, Breese G. Effects of ethanol on ion channels. Int Rev Neurobiol. 1996;39:283–367. doi: 10.1016/s0074-7742(08)60670-4. [DOI] [PubMed] [Google Scholar]
- 7.Woodward JJ, Ron D, Winder D, Roberto M. From blue states to up states: A regional view of NMDA-ethanol interactions. Alcohol Clin Exp Res. 2006;30:359–367. doi: 10.1111/j.1530-0277.2006.00040.x. [DOI] [PubMed] [Google Scholar]
- 8.Cowmeadow RB, Krishnan HR, Atkinson NS. The slowpoke gene is necessary for rapid ethanol tolerance in Drosophila. Alcohol Clin Exp Res. 2005;29:1777–1786. doi: 10.1097/01.alc.0000183232.56788.62. [DOI] [PubMed] [Google Scholar]
- 9.Davies AG, et al. A central role of the BK potassium channel in behavioral responses to ethanol in C. elegans. Cell. 2003;115:655–666. doi: 10.1016/s0092-8674(03)00979-6. [DOI] [PubMed] [Google Scholar]
- 10.Coetzee WA, et al. Molecular diversity of K+ channels. Ann N Y Acad Sci. 1999;868:233–285. doi: 10.1111/j.1749-6632.1999.tb11293.x. [DOI] [PubMed] [Google Scholar]
- 11.Kaczorowski GJ, et al. High-conductance calcium-activated potassium channels; structure, pharmacology, and function. J Bionenerg Biomembr. 1996;28:255–267. doi: 10.1007/BF02110699. [DOI] [PubMed] [Google Scholar]
- 12.Vergara C, Latorre R, Marrion NV, Adelman JP. Calcium-activated potassium channels. Curr Opin Neurobiol. 1998;8:321–329. doi: 10.1016/s0959-4388(98)80056-1. [DOI] [PubMed] [Google Scholar]
- 13.Brenner R, et al. Cloning and functional characterization of novel large conductance calcium-activated potassium channel beta subunits, hKCNMB3 and hKCNMB4. J Biol Chem. 2000;275:6453–6461. doi: 10.1074/jbc.275.9.6453. [DOI] [PubMed] [Google Scholar]
- 14.Chang CP, Dworetzky SI, Wang J, Goldstein ME. Differential expression of the alpha and beta subunits of the large- conductance calcium-activated potassium channel: implication for channel diversity. Brain ResMolBrain Res. 1997;45:33–40. doi: 10.1016/s0169-328x(96)00230-6. [DOI] [PubMed] [Google Scholar]
- 15.Gribkoff VK, Starrett JEJ, Dworetzky SI. Maxi-K potassium channels: Form, function, and modulation of a class of endogenous regulators of intracellular calcium. Neuroscientist. 2001;7:166–177. doi: 10.1177/107385840100700211. [DOI] [PubMed] [Google Scholar]
- 16.Uebele VN, et al. Cloning and functional expression of two families of beta-subunits of the large conductance calcium-activated K+ channel. J Biol Chem. 2000;275:23211–23218. doi: 10.1074/jbc.M910187199. [DOI] [PubMed] [Google Scholar]
- 17.Wallner M, Meera P, Toro L. Molecular basis of fast inactivation in voltage and Ca2+-activated K+ channels: A transmembrane beta-subunit homolog. Proc Natl Acad Sci USA. 1999;96:4137–4142. doi: 10.1073/pnas.96.7.4137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dopico AM, Lemos JR, Treistman SN. Ethanol increases the activity of large conductance, Ca(2+)-activated K+ channels in isolated neurohypophysial terminals Mol Pharmacol. 1996;49:40–48. [PubMed] [Google Scholar]
- 19.Martin G, et al. Somatic localization of a specific large-conductance calcium-activated potassium channel subtype controls compartmentalized ethanol sensitivity in the nucleus accumbens. J Neurosci. 2004;24:6563–6572. doi: 10.1523/JNEUROSCI.0684-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pietrzykowski AZ, et al. Alcohol tolerance in large-conductance, calcium-activated potassium channels of CNS terminals is intrinsic and includes two components: Decreased ethanol potentiation and decreased channel density. J Neurosci. 2004;24:8322–8332. doi: 10.1523/JNEUROSCI.1536-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brenner R. BK channel beta4 subunit reduces dentate gyrus excitability and protects against temporal lobe seizures. Nat Neurosci. 2005;8:1752–1759. doi: 10.1038/nn1573. [DOI] [PubMed] [Google Scholar]
- 22.Hyman SE, Malenka RC, Nestler EJ. Neural mechanisms of addiction: The role of reward-related learning and memory. Annu Rev Neurosci. 2006;29:565–598. doi: 10.1146/annurev.neuro.29.051605.113009. [DOI] [PubMed] [Google Scholar]
- 23.Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: From actions to habits to compulsion. Nat Neurosci. 2005;8:1481–1489. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- 24.Rhodes JS, et al. Evaluation of a simple model of ethanol drinking to intoxication in C57BL/6J mice. Physiol Behav. 2005;84:53–63. doi: 10.1016/j.physbeh.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 25.Rhodes JS, et al. Mouse inbred strain differences in ethanol drinking to intoxication. Genes Brain Behav. 2007;6:1–18. doi: 10.1111/j.1601-183X.2006.00210.x. [DOI] [PubMed] [Google Scholar]
- 26.Blomqvist O, Engel JA, Nissbrandt H, Soderpalm B. The mesolimbic dopamine-activating properties of ethanol are antagonized by mecamylamine. Eur J Pharmacol. 1993;249:207–213. doi: 10.1016/0014-2999(93)90434-j. [DOI] [PubMed] [Google Scholar]
- 27.Ericson M, Blomqvist O, Engel JA, Soderpalm B. Voluntary ethanol intake in the rat and the associated accumbal dopamine overflow are blocked by ventral tegmental mecamylamine. Eur J Pharmacol. 1998;358:189–196. doi: 10.1016/s0014-2999(98)00602-5. [DOI] [PubMed] [Google Scholar]
- 28.Larsson A, Svensson L, Soderpalm B, Engel JA. Role of different nicotinic acetylcholine receptors in mediating behavioral and neurochemical effects of ethanol in mice. Alcohol. 2002;28:157–167. doi: 10.1016/s0741-8329(02)00244-6. [DOI] [PubMed] [Google Scholar]
- 29.Okamoto T, Harnett MT, Morikawa H. Hyperpolarization-activated cation current (Ih) is an ethanol target in midbrain dopamine neurons of mice. J Neurophysiol. 2006;95:619–626. doi: 10.1152/jn.00682.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shao LR, Halvorsrud R, Borg-Graham L, Storm JF. The role of BK-type Ca2+-dependent K+ channels in spike broadening during repetitive firing in rat hippocampal pyramidal cells. J Physiol 521 Pt. 1999;1:135–146. doi: 10.1111/j.1469-7793.1999.00135.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gu N, Vervaeke K, Storm JF. BK potassium channels facilitate high-frequency firing and cause early spike frequency adaptation in rat CA1 hippocampal pyramidal cells. J Physiol. 2007;580:859–882. doi: 10.1113/jphysiol.2006.126367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pedarzani P, et al. Molecular determinants of Ca2+-dependent K+ channel function in rat dorsal vagal neurones. J Physiol. 2000;527(Pt 2):283–290. doi: 10.1111/j.1469-7793.2000.t01-1-00283.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Faber ES, Sah P. Physiological role of calcium-activated potassium currents in the rat lateral amygdala. J Neurosci. 2002;22:1618–1628. doi: 10.1523/JNEUROSCI.22-05-01618.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Edgerton JR, Reinhart PH. Distinct contributions of small and large conductance Ca2+-activated K+ channels to rat Purkinje neuron function. J Physiol. 2003;548:53–69. doi: 10.1113/jphysiol.2002.027854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sausbier M, et al. Cerebellar ataxia and Purkinje cell dysfunction caused by Ca2+-activated K+ channel deficiency. Proc Natl Acad Sci USA. 2004;101:9474–9478. doi: 10.1073/pnas.0401702101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bennett BD, Callaway JC, Wilson CJ. Intrinsic membrane properties underlying spontaneous tonic firing in neostriatal cholinergic interneurons. J Neurosci. 2000;20:8493–8503. doi: 10.1523/JNEUROSCI.20-22-08493.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Behrens R, et al. hKCNMB3 and hKCNMB4, cloning and characterization of two members of the large-conductance calcium-activated potassium channel beta subunit family. FEBS Lett. 2000;474:99–106. doi: 10.1016/s0014-5793(00)01584-2. [DOI] [PubMed] [Google Scholar]
- 38.Camacho-Nasi P, Treistman SN. Ethanol effects on voltage-dependent membrane conductances: comparative sensitivity of channel populations in Aplysia neurons. Cell Mol Neurobiol. 1986;6:263–279. doi: 10.1007/BF00711113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Feinberg-Zadek PL, Treistman SN. Beta-subunits are important modulators of the acute response to alcohol in human BK channels. Alcohol Clin Exp Res. 2007;31:737–744. doi: 10.1111/j.1530-0277.2007.00371.x. [DOI] [PubMed] [Google Scholar]
- 40.Tseng-Crank J, et al. Cloning, expression, and distribution of a Ca(2+)-activated K+ channel beta-subunit from human brain. Proc Natl Acad Sci USA. 1996;93:9200–9205. doi: 10.1073/pnas.93.17.9200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ahring PK, et al. Stable expression of the human large-conductance Ca2+-activated K+ channel alpha- and beta-subunits in HEK293 cells. FEBS Lett. 1997;415:67–70. doi: 10.1016/s0014-5793(97)01096-x. [DOI] [PubMed] [Google Scholar]
- 42.Martin G, Fabre V, Siggins GR, de Lecea L. Interaction of the hypocretins with neurotransmitters in the nucleus accumbens. Regul Pept. 2002;104:111–117. doi: 10.1016/s0167-0115(01)00354-8. [DOI] [PubMed] [Google Scholar]
- 43.Hamill OP, et al. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.