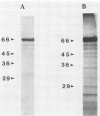
Abstract
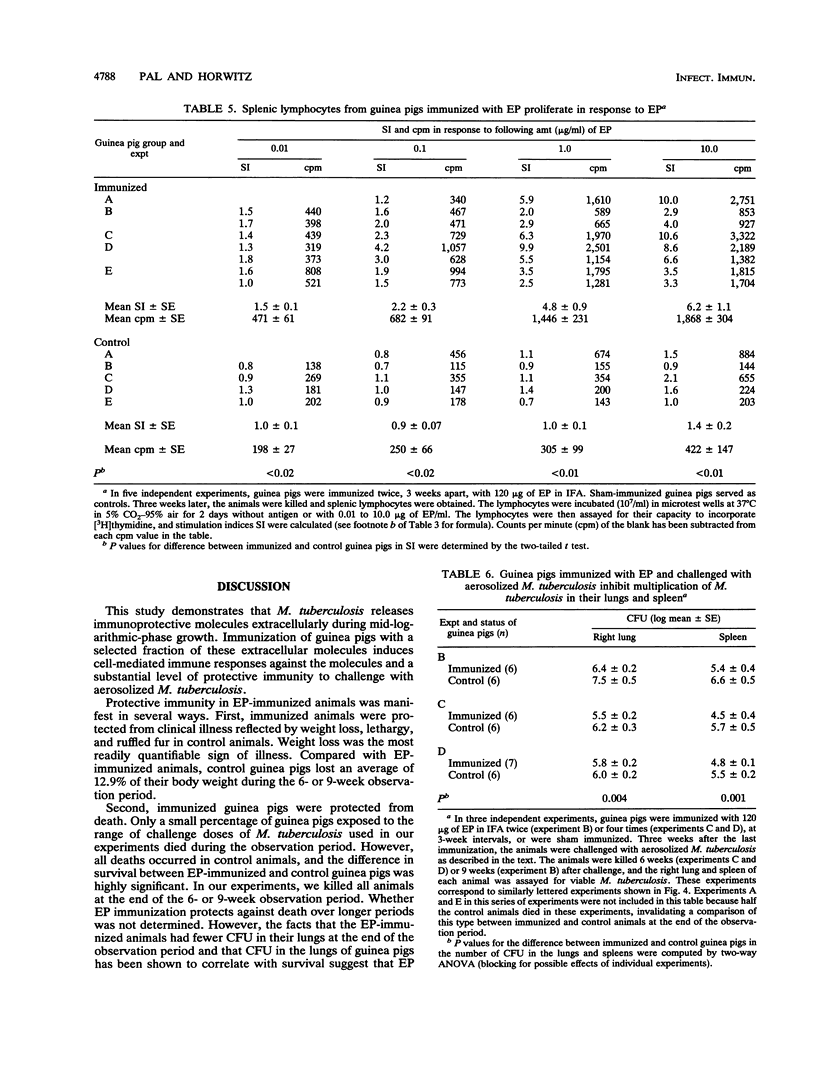
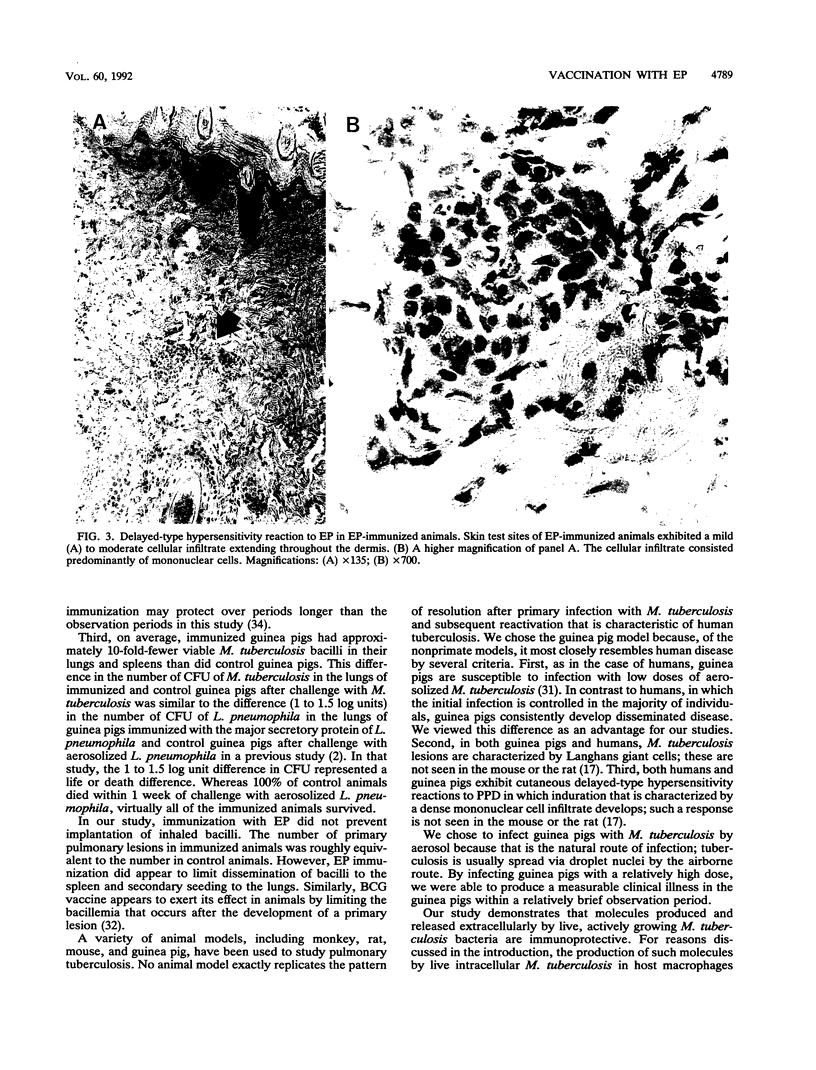
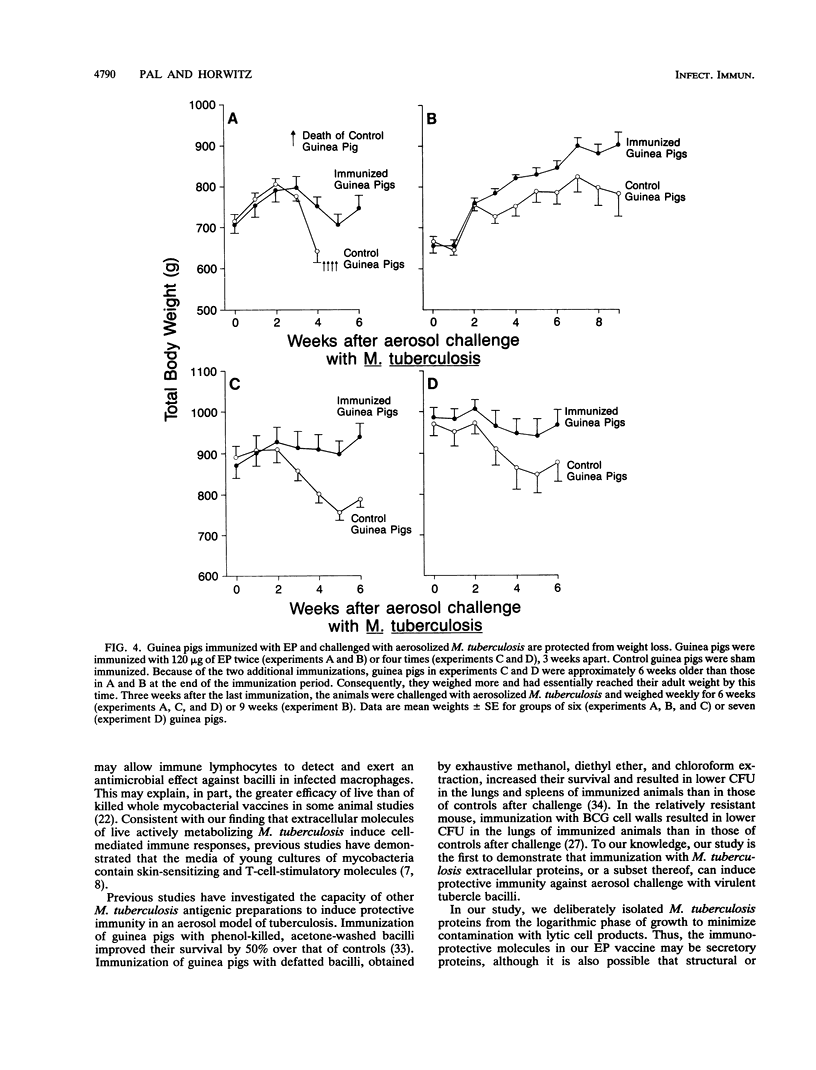
We have studied the capacity of a selected fraction of Mycobacterium tuberculosis extracellular proteins (EP) released into broth culture by mid-logarithmic-growth-phase organisms to induce cell-mediated immune responses and protective immunity in a guinea pig model of pulmonary tuberculosis. Guinea pigs infected with M. tuberculosis by aerosol but not uninfected control guinea pigs exhibit strong cell-mediated immune responses to EP, manifest by dose-dependent cutaneous delayed-type hypersensitivity and splenic lymphocyte proliferation. Guinea pigs immunized subcutaneously with EP but not sham-immunized control guinea pigs also develop strong cell-mediated immune responses to EP, manifest by dose-dependent cutaneous delayed-type hypersensitivity and splenic lymphocyte proliferation. EP is nonlethal and nontoxic to guinea pigs upon subcutaneous immunization. Guinea pigs immunized with EP and then challenged with aerosolized M. tuberculosis exhibit protective immunity. In five independent experiments, EP-immunized guinea pigs were consistently protected against clinical illness, including weight loss. Compared with EP-immunized guinea pigs, sham-immunized control guinea pigs lost 12.9 +/- 2.0% (mean +/- SE) of their total weight. EP-immunized guinea pigs also had a 10-fold reduction in viable M. tuberculosis bacilli in their lungs and spleens (P = 0.004 and 0.001, respectively) compared with sham-immunized control animals. In the two experiments in which some guinea pigs died after aerosol challenge, EP-immunized animals were protected from death. Whereas all 12 (100%) EP-immunized guinea pigs survived challenge with aerosolized M. tuberculosis, only 6 of 12 (50%) sham-immunized control guinea pigs survived challenge (P = 0.007, Fisher exact test). This study demonstrates that actively growing M. tuberculosis cells release immunoprotective molecules extracellularly, that a subunit vaccine against tuberculosis is feasible, and that extracellular molecules of M. tuberculosis are potential candidates for a subunit vaccine.
Full text
PDF



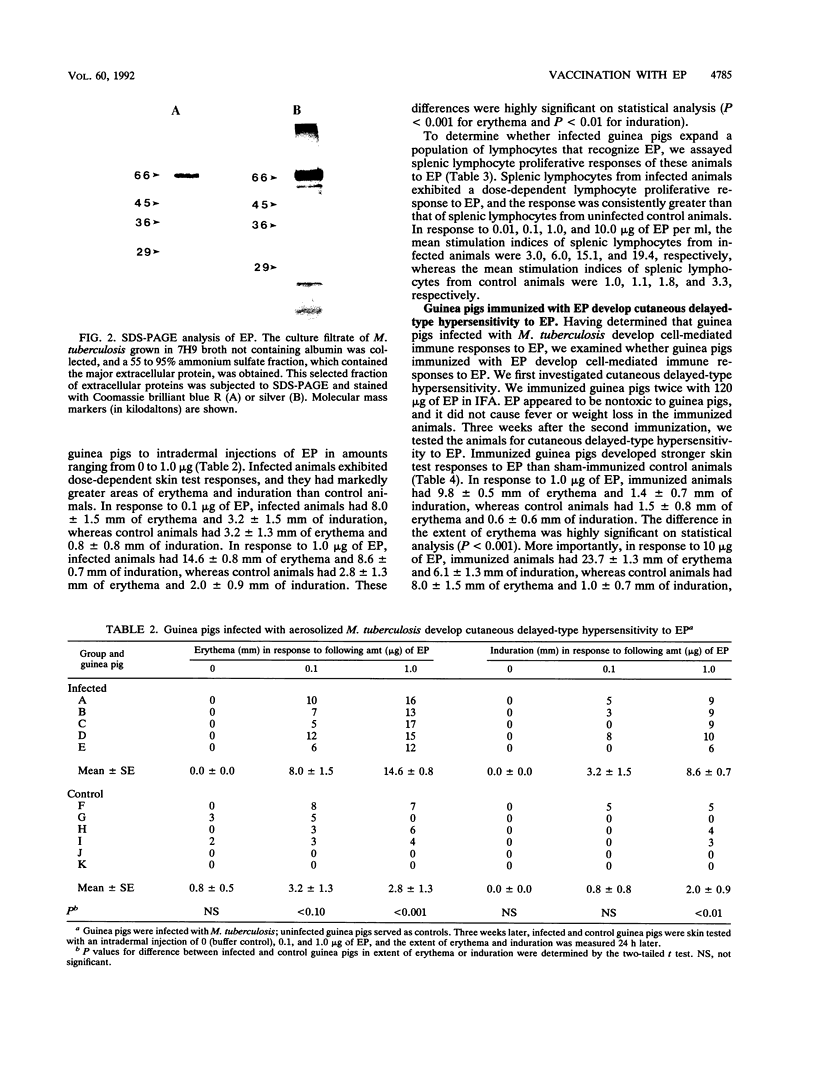
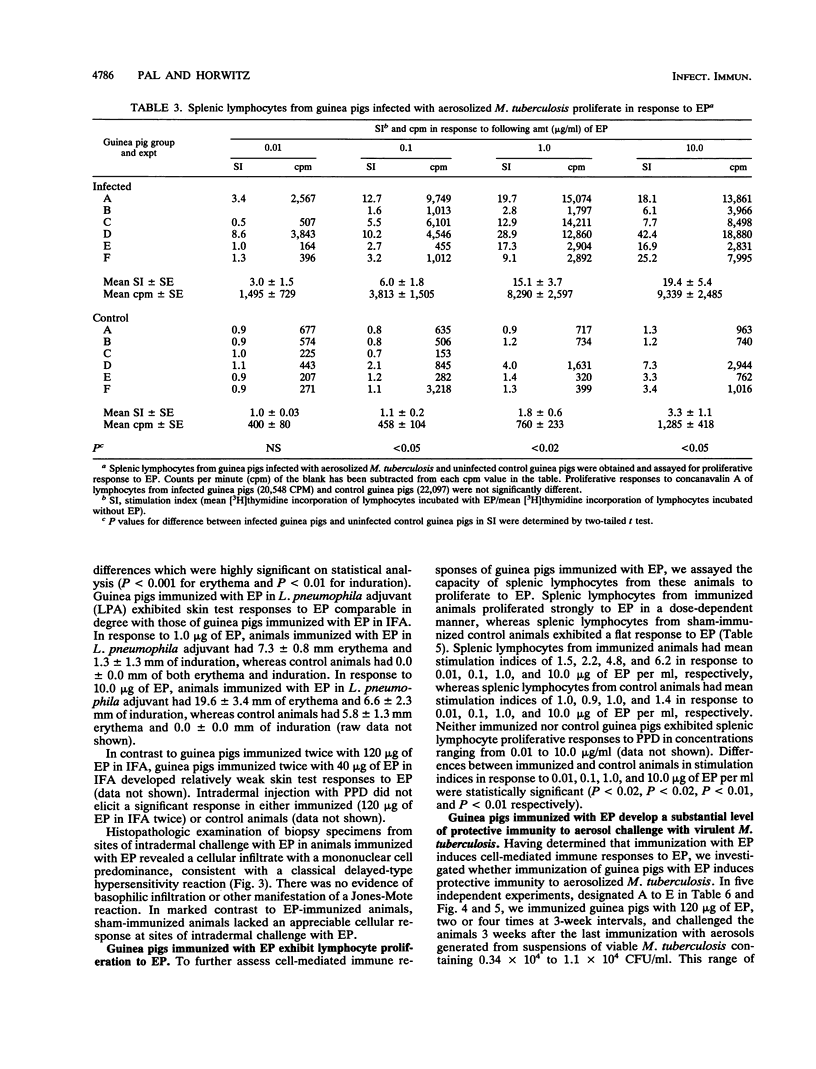
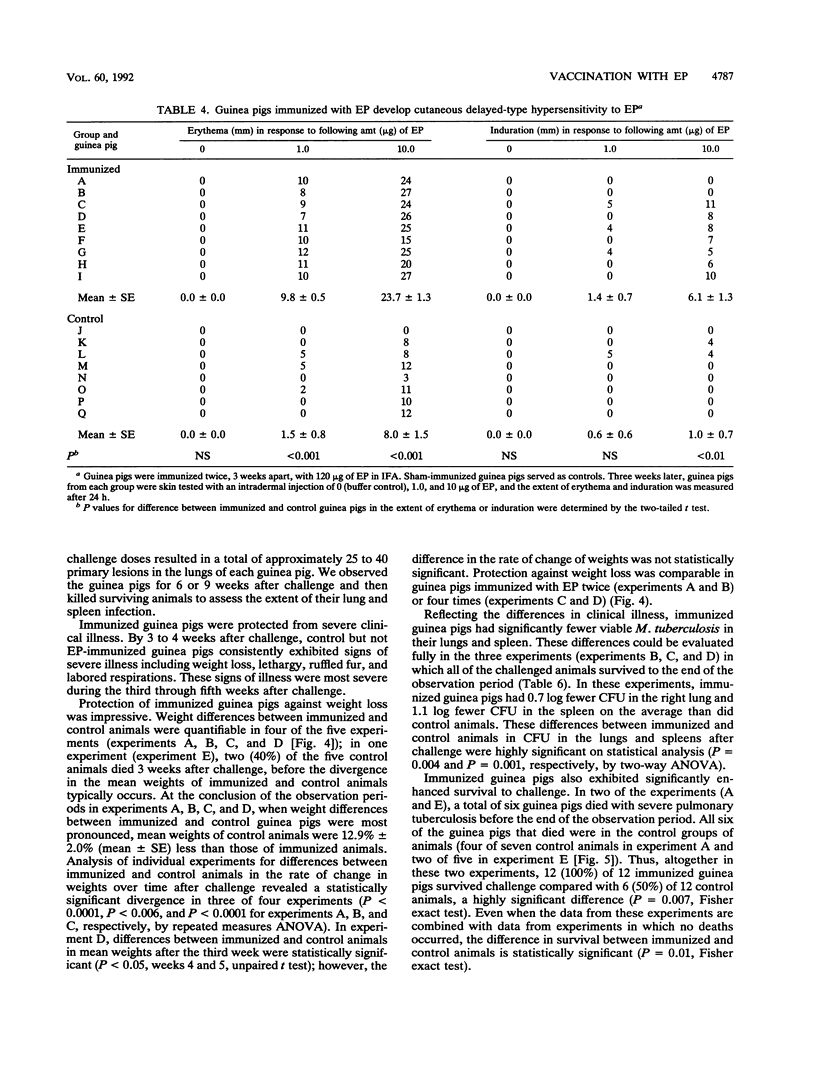


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersen A. B., Yuan Z. L., Hasløv K., Vergmann B., Bennedsen J. Interspecies reactivity of five monoclonal antibodies to Mycobacterium tuberculosis as examined by immunoblotting and enzyme-linked immunosorbent assay. J Clin Microbiol. 1986 Mar;23(3):446–451. doi: 10.1128/jcm.23.3.446-451.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blander S. J., Horwitz M. A. Vaccination with the major secretory protein of Legionella pneumophila induces cell-mediated and protective immunity in a guinea pig model of Legionnaires' disease. J Exp Med. 1989 Mar 1;169(3):691–705. doi: 10.1084/jem.169.3.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breiman R. F., Horwitz M. A. Guinea pigs sublethally infected with aerosolized Legionella pneumophila develop humoral and cell-mediated immune responses and are protected against lethal aerosol challenge. A model for studying host defense against lung infections caused by intracellular pathogens. J Exp Med. 1987 Mar 1;165(3):799–811. doi: 10.1084/jem.165.3.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coates A. R., Hewitt J., Allen B. W., Ivanyi J., Mitchison D. A. Antigenic diversity of Mycobacterium tuberculosis and Mycobacterium bovis detected by means of monoclonal antibodies. Lancet. 1981 Jul 25;2(8239):167–169. doi: 10.1016/s0140-6736(81)90355-x. [DOI] [PubMed] [Google Scholar]
- Cohn M. L., Waggoner R. F., McClatchy J. K. The 7H11 medium for the cultivation of mycobacteria. Am Rev Respir Dis. 1968 Aug;98(2):295–296. doi: 10.1164/arrd.1968.98.2.295. [DOI] [PubMed] [Google Scholar]
- Collins F. M., Lamb J. R., Young D. B. Biological activity of protein antigens isolated from Mycobacterium tuberculosis culture filtrate. Infect Immun. 1988 May;56(5):1260–1266. doi: 10.1128/iai.56.5.1260-1266.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DUBOS R. J., SCHAEFER W. B. Antituberculous immunity induced in mice by virulent primary infection; its inhibition by chemoprophylaxis. Am Rev Tuberc. 1956 Oct;74(4):541–551. doi: 10.1164/artpd.1956.74.4.541. [DOI] [PubMed] [Google Scholar]
- De Bruyn J., Bosmans R., Turneer M., Weckx M., Nyabenda J., Van Vooren J. P., Falmagne P., Wiker H. G., Harboe M. Purification, partial characterization, and identification of a skin-reactive protein antigen of Mycobacterium bovis BCG. Infect Immun. 1987 Jan;55(1):245–252. doi: 10.1128/iai.55.1.245-252.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fine P. E. The BCG story: lessons from the past and implications for the future. Rev Infect Dis. 1989 Mar-Apr;11 (Suppl 2):S353–S359. doi: 10.1093/clinids/11.supplement_2.s353. [DOI] [PubMed] [Google Scholar]
- Forget A., Benoit J. C., Turcotte R., Gusew-Chartrand N. Enhancement activity of anti-mycobacterial sera in experimental Mycobacterium bovis (BCG) infection in mice. Infect Immun. 1976 May;13(5):1301–1306. doi: 10.1128/iai.13.5.1301-1306.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefford M. J. Transfer of adoptive immunity to tuberculosis in mice. Infect Immun. 1975 Jun;11(6):1174–1181. doi: 10.1128/iai.11.6.1174-1181.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leveton C., Barnass S., Champion B., Lucas S., De Souza B., Nicol M., Banerjee D., Rook G. T-cell-mediated protection of mice against virulent Mycobacterium tuberculosis. Infect Immun. 1989 Feb;57(2):390–395. doi: 10.1128/iai.57.2.390-395.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMaster P. R., Tsang V. C., Wong K. H., Feeley J. C., Gann D. S. Comparative adjuvant activities of Legionella pneumophila and Mycobacterium tuberculosis. Int Arch Allergy Appl Immunol. 1984;73(4):357–362. doi: 10.1159/000233498. [DOI] [PubMed] [Google Scholar]
- Orme I. M. Characteristics and specificity of acquired immunologic memory to Mycobacterium tuberculosis infection. J Immunol. 1988 May 15;140(10):3589–3593. [PubMed] [Google Scholar]
- Orme I. M., Collins F. M. Adoptive protection of the Mycobacterium tuberculosis-infected lung. Dissociation between cells that passively transfer protective immunity and those that transfer delayed-type hypersensitivity to tuberculin. Cell Immunol. 1984 Mar;84(1):113–120. doi: 10.1016/0008-8749(84)90082-0. [DOI] [PubMed] [Google Scholar]
- Orme I. M., Collins F. M. Protection against Mycobacterium tuberculosis infection by adoptive immunotherapy. Requirement for T cell-deficient recipients. J Exp Med. 1983 Jul 1;158(1):74–83. doi: 10.1084/jem.158.1.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orme I. M. Induction of nonspecific acquired resistance and delayed-type hypersensitivity, but not specific acquired resistance in mice inoculated with killed mycobacterial vaccines. Infect Immun. 1988 Dec;56(12):3310–3312. doi: 10.1128/iai.56.12.3310-3312.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn T. C. Interactions of the human immunodeficiency virus and tuberculosis and the implications for BCG vaccination. Rev Infect Dis. 1989 Mar-Apr;11 (Suppl 2):S379–S384. doi: 10.1093/clinids/11.supplement_2.s379. [DOI] [PubMed] [Google Scholar]
- Reggiardo Z., Middlebrook G. Failure of passive serum transfer of immunity against aerogenic tuberculosis in rabbits. Proc Soc Exp Biol Med. 1974 Jan;145(1):173–175. doi: 10.3181/00379727-145-37771. [DOI] [PubMed] [Google Scholar]
- Research towards global control and prevention of tuberculosis with an emphasis on vaccine development. A Fogarty International Center Workshop, Bethesda, Maryland, 3-5 November 1987. Proceedings. Rev Infect Dis. 1989 Mar-Apr;11 (Suppl 2):S335–S490. [PubMed] [Google Scholar]
- Ribi E., Meyer T. J., Azuma I., Parker R., Brehmer W. Biologically active components from mycobacterial cell walls. IV. Protection of mice against aerosol infection with virulent mycobacterium tuberculosis. Cell Immunol. 1975 Mar;16(1):1–10. doi: 10.1016/0008-8749(75)90180-x. [DOI] [PubMed] [Google Scholar]
- Rieder H. L., Cauthen G. M., Kelly G. D., Bloch A. B., Snider D. E., Jr Tuberculosis in the United States. JAMA. 1989 Jul 21;262(3):385–389. [PubMed] [Google Scholar]
- Schlesinger L. S., Bellinger-Kawahara C. G., Payne N. R., Horwitz M. A. Phagocytosis of Mycobacterium tuberculosis is mediated by human monocyte complement receptors and complement component C3. J Immunol. 1990 Apr 1;144(7):2771–2780. [PubMed] [Google Scholar]
- Smith D. W., McMurray D. N., Wiegeshaus E. H., Grover A. A., Harding G. E. Host-parasite relationships in experimental airborne tuberculosis. IV. Early events in the course of infection in vaccinated and nonvaccinated guinea pigs. Am Rev Respir Dis. 1970 Dec;102(6):937–949. doi: 10.1164/arrd.1970.102.6.937. [DOI] [PubMed] [Google Scholar]
- WEISS D. W. Antituberculous vaccination in the guinea pig with non-living vaccines. Am Rev Tuberc. 1958 Apr;77(4):719–724. doi: 10.1164/artpd.1958.77.4.719. [DOI] [PubMed] [Google Scholar]
- Wiegeshaus E. H., McMurray D. N., Grover A. A., Harding G. E., Smith D. W. Host-parasite relationships in experimental airborne tuberculosis. 3. Relevance of microbial enumeration to acquired resistance in guinea pigs. Am Rev Respir Dis. 1970 Sep;102(3):422–429. doi: 10.1164/arrd.1970.102.3.422. [DOI] [PubMed] [Google Scholar]