Abstract
Syncytin-2 is an envelope gene from the human endogenous retrovirus FRD (HERV-FRD) co-opted by an ancestral primate host, conserved in evolution over >40 Myr, specifically expressed in the placenta, and with a cell–cell fusogenic activity likely contributing to placenta morphogenesis. Here, using the GeneBridge4 human/Chinese hamster radiation hybrid panel, we mapped and identified the human receptor for syncytin-2. This receptor—namely Major Facilitator Superfamily Domain Containing 2 (MFSD2)—belongs to a large family of presumptive carbohydrate transporters with 10–12 membrane-spanning domains, is located at chromosomal position 1p34.2, and is conserved in evolution. An expression vector for MFSD2 confers fusogenicity to otherwise insusceptible cells upon trans-fection of syncytin-2. It also confers infectivity to syncytin-2 pseudotypes, consistent with this protein being the receptor for the ancestrally acquired HERV-FRD family of endogenous retroviruses. At variance with the human gene, neither mouse nor rat MFSD2 can mediate membrane fusion, which is consistent with the fact that the envelope-derived syncytin genes co-opted by rodents during evolution are not orthologous to the human syncytin genes. Remarkably, a real-time quantitative RT-PCR analysis of MFSD2 in various human tissues demonstrates specific expression in the placenta, as well as in the human BeWo choriocarcinoma cell line, which discloses enhancement of receptor expression upon induction by forskolin of cell–cell fusion and syncytium formation. In situ hybridization of human placental tissue using an MFSD2-specific probe further unambiguously demonstrates receptor expression at the level of the syncytiotrophoblast, again consistent with a role in placenta morphogenesis.
Keywords: envelope protein, human endogenous retrovirus (HERV), major facilitator superfamily domain containing 2 (MFSD2), syncytiotrophoblast
The placenta is an autonomous and transient organ essentially aimed at mediating nutrient and gas exchange between mother and fetus during intrauterine life. In several mammalian species with a hemochorial placenta—including human—a key process of placental morphogenesis is the fusion of trophoblastic cells into a multinucleated layer called syncytiotrophoblast, which constitutes the main materno–fetal barrier in direct contact with maternal blood and fulfils essential trophic exchange functions (1–3).
Little is known about the molecular mechanisms involved in trophoblastic differentiation. However, a major advance has been made by the identification of envelope (Env) proteins encoded by endogenous retroviruses (ERVs) and likely involved in the formation of the syncytiotrophoblast (4–8). The mammalian genomes indeed harbor thousands of ERV elements that display a structure close to that of the integrated proviral form of exogenous retroviruses (gag, pol, and env-related regions flanked by 2 long terminal repeats) and most probably are the remnants of past infections of the germ line by ancestral retroviruses (9, 10). Although the vast majority of these elements are defective, a few of them still contain intact ORFs, notably in env genes. During the retroviral life cycle, Env glycoproteins, which are anchored in the lipid bilayer of viral surface envelopes, are involved first in cell surface receptor recognition and subsequently in viral entry by driving the fusion of the viral envelope with the cell membrane. Expression of Env proteins at the cell surface can also trigger cell–cell fusion provided that their cognate receptors are exposed at the surface of neighboring cells. A systematic search through the human genome sequence has identified 18 env genes encoding a full-length ORF whose products may potentially have a function (4, 11–13). Among them, the syncytin-1 and -2 proteins are specifically expressed within the placenta at the cytotrophoblast-syncytiotrophoblast interface and have been reported to induce cell–cell fusion ex vivo by interacting with distinct receptors (4, 5, 7, 14, 15). Syncytin-1 was shown to be involved in the differentiation and fusion of human cytotrophoblasts in primary cultures (5, 7, 14). In addition, the functionality of these 2 genes has been conserved in evolution since the time of their insertion into the primate genome (some 25 Myr ago for syncytin-1 and >40 Myr for syncytin-2), and they currently display remarkably little polymorphism in the human population (4, 16, 17), 2 strong signs of purifying selection. The host has most probably co-opted these genes of retroviral origin for its own benefit, more specifically, for syncytiotrophoblast morphogenesis. Understanding the physiology of syncytiotrophoblast formation and the role of both syncytins in the fusion process requires identification of their cognate receptors and their tissue distribution in the developing placenta. A receptor for syncytin-1 has been identified as the RD114/mammalian type D retrovirus receptor, variously referred to as RDR/ASCT2/ATB0/SLC1A5 (5). It is an amino acid transporter ubiquitously expressed in most human tissues, including the placenta, where it is expressed mainly in the cytotrophoblasts (18–20). Here, we report on the identification of the syncytin-2 receptor via the use of a human/hamster radiation hybrid panel (21, see also 22–24). The identified gene is a receptor with multiple membrane-spanning domains that can mediate cell–cell fusion and infection by retroviral pseudotypes bearing syncytin-2. Remarkably, real-time RT-PCR on RNA from a large panel of human tissues demonstrates that its expression is placenta-specific and, more precisely, in the syncytiotrophoblast as revealed by in situ hybridization. A model is presented illustrating the primary role of syncytin-2 and its newly identified cognate receptor in syncytiotrophoblast formation.
Results
Identification of the Syncytin-2 Receptor.
We had shown that syncytin-2, as a bona fide retroviral Env protein, can be incorporated into env-defective lentiviral virions [e.g., simian immunodeficiency virus (SIV)] to generate pseudotypes that are infectious for a subset of target cells (25). These target cells included several human cell lines but not mouse 3T3 cells or CHO cells that were resistant to infection. Using the same pseudotyping assay, we first checked that the Chinese hamster fibroblast A23 cell line, which had been used to generate the GeneBridge4 human/hamster radiation hybrid (RH) panel (21, 26), is indeed resistant to infection (see control “A” in Fig. 1B), thus allowing the RH panel to be used to tentatively map the syncytin-2 receptor. Therefore, we screened the 93 GeneBridge4 cell lines for infection by using a lacZ expression cassette packaged into SIV particles pseudotyped with syncytin-2 (Fig. 1 A and B). The infection results for the 93 ordered cell lines were:
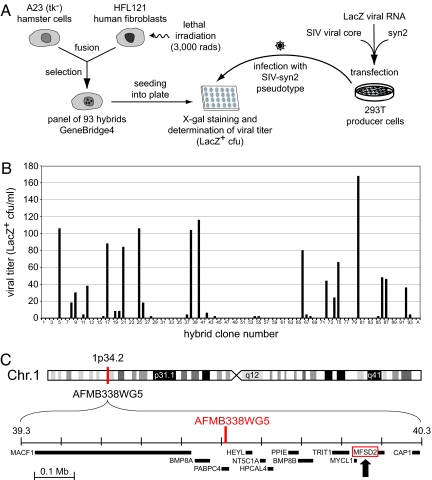
Fig. 1.
Rationale of the retroviral pseudotype assay for the screening of the GeneBridge4 human/Chinese hamster Radiation Hybrid panel and mapping of the human syncytin-2 receptor MFSD2. (A) The 93 hybrid clones of the GeneBridge4 panel (21) generated as schematized (Left) were seeded and infected with viral pseudotypes produced by co-transfection of human 293T cells with expression vectors for the SIV core, the syncytin-2 Env protein, and a lacZ-containing retroviral transcript (Right). Viral titers were determined 3 days after infection by X-gal staining of the cells. (B) Pseudotype viral titers obtained in a representative experiment involving the simultaneous assay of the 93 clones of the panel (and control A23 cells, “A” at the right end of the abscissa axis), with each cell clone infected in duplicate and examined for the number of lacZ+ foci. The resulting matrix was derived and data were analyzed by using the RH map program. (C) Localization of the identified genetic marker (AFMb3385) on chromosome 1, with position of the genes present within 1 Mb, as determined by using the UCSC Genome Browser. The MFSD2 gene is arrowed.
000010011001000010111000110000200000010100000000000000000000000001000001011000010000011000010 [1 scored as positive infection (> 6 cfu/ml, set as a threshold), 0 scored as uninfected, and 2 indicates that the cell line was unavailable for analysis]. The data were analyzed by using the RH map program (27). We found the susceptibility locus for syncytin-2-mediated infection to map on chromosome 1p34.2, near marker RH2550 (AFMb338wg5), with a highly significant logarithm of odds (LOD) score (log10 of the likelihood ratio) of 11.65. Then we used the University of California Santa Cruz (UCSC) Genome Browser (http://genome.ucsc.edu/) to identify the genes present within 1 Mb of the identified marker (Fig. 1C). Among the 12 candidate genes, one was found to encode a putative transmembrane protein, namely Major Facilitator Superfamily Domain Containing 2 (MFSD2), and therefore was a good candidate as a retrovirus receptor (reviewed in refs. 28 and 29). A complete cDNA clone was obtained from Invitrogen and introduced into a CMV-driven expression vector. A23 and HeLa cells were then transiently transfected with this expression vector (as well as with an empty vector or an expression vector for the syncytin-1 receptor ASCT2 used as controls) and assayed for susceptibility to syncytin-2 pseudotypes. As illustrated in Fig. 2A, MFSD2 clearly conferred on transfected A23 and HeLa cells susceptibility to infection by the syncytin-2 pseudotypes, and the effect was specific for MFSD2 as it was not observed with the control ASCT2-transfected cells—the latter gaining instead susceptibility to syncytin-1 pseudotypes. Infection of the MFSD2-transfected cells also clearly depended on the syncytin-2 protein because it was not observed with control pseudotypes without Env nor with constructs pseudotyped with syncytin-1 (Fig. 2A). Altogether, the data strongly suggest that MFSD2 is indeed the receptor for the ancestral HERV-FRD ERVs. Its definite ability to mediate syncytin-2-dependent cell–cell fusion was finally demonstrated by the experiments illustrated in Fig. 2B in which distinct pools of A23 cells, transfected either with MFSD2 or with syncytin-2 (supplemented with a beta-galactosidase expression vector), were mixed and assayed for cell–cell fusion. As illustrated in this figure, cell–cell fusion (as revealed by the presence of large LacZ+ syncytia) could be observed with the syncytin-2 and MFSD2 pair (as well as with the ASCT2 and syncytin-1 pair as a positive control) but not with any of the other combinations.
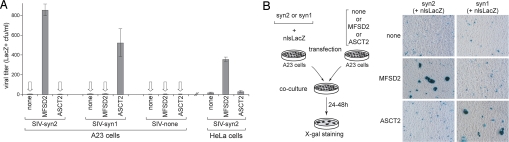
Fig. 2.
MFSD2-transduced cells can be infected by syncytin-2 retroviral pseudotypes (A) and mediate cell–cell fusion upon coculture with syncytin-2-expressing cells (B). (A) Chinese hamster A23 or human HeLa cells were transiently transfected with an expression vector for MFSD2, the syncytin-1 receptor ASCT2, or an empty vector (none), and 2 days after transfection were infected with SIV particles generated as in Fig. 1 and pseudotyped with either syncytin-1 (SIV-syn1), syncytin-2 (SIV-syn2), or no Env (SIV-none). Viral titers were determined by X-gal staining of the cells 3 days after infection; arrows indicate undetectable viral titers (no lacZ+ cells). (B) Cell–cell fusion was assayed (Left) upon independent transfection of A23 cells with an expression vector for either syncytin-1 (syn1) or syncytin-2 (syn2) together with an expression vector for a nuclear-located nlsLacZ gene, or with an expression vector for MFSD2, for the syncytin-1 receptor ASCT2, or an empty vector (none). One day after transfection, cells were resuspended and cocultured as indicated for 1–2 days, fixed, and X-gal stained (Right). Syncytia can be easily detected via the accumulation of lacZ+ nuclei, for both the syn1/ASCT2 and the syn2/MFSD2 pairs, with only mononucleated cells visible in the other cases.
Characterization of the Transmembrane MFSD2 Receptor.
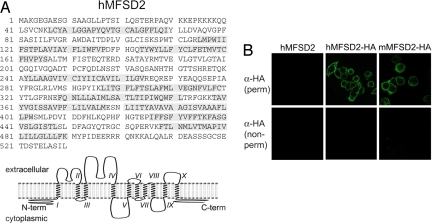
MFSD2 is an uncharacterized member of a large family of integral membrane proteins (30, 31). Its primary sequence and the predicted structure of the protein [transmembrane helices based on a hidden Markov model (TMHMM), http://www.cbs.dtu.dk/services/TMHMM; Phobius, http://phobius.sbc.su.se] is shown in Fig. 3A. It is a 530 amino acid protein with 10 putative membrane-spanning hydrophobic domains and N- and C-terminal ends predicted to be intracellular. A conserved domain shared by members of the MFS superfamily and entitled “Sugar_tr Superfamily” (accession number cl09119 in the Conserved Domain Database http://www.ncbi.nlm.nih.gov/Structure/cdd/cdd.shtml) is located between positions 39 and 510. The cellular localization of the receptor and its orientation within the membrane was further assessed by immunofluorescence analysis of cells transduced with MFSD2 expression vectors after having introduced (or not) an HA-tag at the C terminus of the cloned receptor. As illustrated by the confocal images in Fig. 3B by using an anti-HA monoclonal antibody, labeling was observed at the cell membrane of the MFSD2-HA cells (and not of the control MFSD2 cells), and only under conditions of cell permeabilization, which is consistent with the HA-tagged C terminus being exposed on the cytoplasmic face of the cell membrane, as predicted. As also illustrated in the figure, a similar result was obtained with one of the mammalian MFSD2 orthologs, namely mouse MFSD2, that we have cloned and assayed.
Fig. 3.
Sequence and transmembrane organization of the MFSD2 receptor. (A) Primary sequence of the 530 amino acid-long human MFSD2 protein, with the 10 membrane-spanning domains predicted by the TMHMM program indicated in gray on the sequence, together with the schematic structure of the protein. (B) Immunofluorescence analysis of the human (hMFSD2-HA) or murine (mMFSD2-HA) C-terminally HA-tagged MFSD2 proteins using as a control the untagged hMFSD2 protein. The tagged proteins were verified to be still functional in a pseudotype assay as in Fig. 2 (data not shown). HeLa cells transduced with the corresponding expression vector were either fixed, permeabilized and stained with an anti-HA antibody (whole-cell staining, “perm”) or observed directly after staining without prior fixation or permeabilization (cell surface staining, “nonperm”). Observations were made with confocal microscopy by using a FITC-conjugated secondary antibody which demonstrated cell surface localization of the human and murine MFSD2 proteins with the HA-tag only accessible after membrane permeabilization.
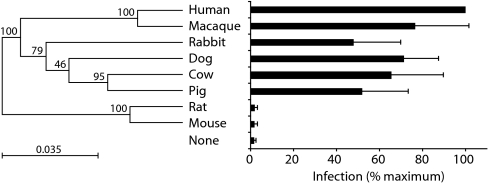
MFSD2 is a highly conserved gene that can be identified in all mammals whose genomic sequence is available. This conservation is consistent with its putative role as a carbohydrate transporter. Sequence comparison discloses 84–95% amino acid similarities among the mammal species shown in Fig. 4 with rodents (namely rat and mouse) being the most distant with notably a 5 amino acid insertion within the helix II to III loop. It is noteworthy that the cloning of the cDNA of the orthologous MFSD2 genes for this series of mammals and assay for infectivity of the corresponding transduced HeLa cells demonstrates susceptibility to the syncytin-2 pseudotypes by using the same assay as above, except for the murine and rat MFSD2 genes (Fig. 4). This negative result is consistent with the lack of fusogenicity that we observed for syncytin-2 by using murine (and rat) cells (data not shown) (4) and with syncytin-2 having entered the primate genome but not that of rodents (see Discussion). Neither did the mouse syncytin genes (namely syncytin-A and -B) (6)—which are not orthologous to the human genes—induce cell–cell fusion in an assay similar to that in Fig. 2B, by using both mouse and human MFSD2-transduced cells (data not shown).
Fig. 4.
The indicated mammalian orthologs of the human MFSD2 gene (or no gene in “none”) were cloned as cDNAs into a CMV-driven expression vector and assayed for SIV-syncytin-2 pseudotype infection as in Fig. 2A. Infection (lacZ+ cfu/ml) is expressed as a percentage of that observed with human MFSD2 (Right). The molecular phylogenetic tree (Left) was generated on alignment of the MFSD2 amino acid sequences by using the CLC Sequence Viewer 4 program (http://www.clcbio.com/index.php?id = 28). The most distantly related proteins (namely the human and the mouse sequences) still disclose 84% identity.
Expression of MFSD2 Is Placenta-Specific and Associated with Trophoblast Cells.
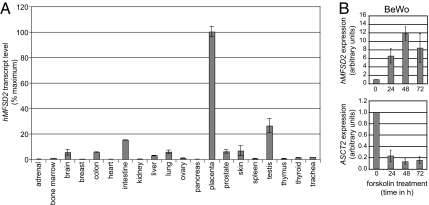
To further characterize the identified MFSD2 gene and its possible involvement in placenta physiology, we investigated its expression profile by quantitative real-time RT-PCR analysis of its transcript level by using a large panel of human tissues. As observed in Fig. 5A, a remarkable property of MFSD2 is its high-level expression specifically in the placenta, with expression in all other tested tissues at least 10-fold lower—with the exception of the testis in which it is only 4-fold lower. Noteworthily, this placenta-specific expression profile is closely related to that of the syncytins (reviewed in ref. 9) and is a rather surprising result because metabolite transporters are generally rather ubiquitously expressed (as is, for instance, the ASCT2 syncytin-1 receptor) (18). Interestingly, analysis of the expression of the orthologous MFSD2 genes in the mouse, rat, and rabbit discloses a similar profile, again with specific expression in the placenta—although at a lower level for the mouse and rat (data not shown). This is consistent with MFSD2 having a placenta-specific transport function, independent of its “secondary” function as a receptor for the primate syncytin-2.
Fig. 5.
Real-time RT-PCR analysis of MFSD2 transcripts in human tissues (A) and BeWo trophoblast cells (B). (A) MFSD2 mRNA expression in a panel of 20 healthy human tissues as determined by real-time qRT-PCR. Transcript levels were normalized relative to the amount of 18S mRNA and are expressed relative to that in the placenta (same order of magnitude as that of the syncytin-2 gene, data not shown). (B) MFSD2 and ASCT2 expression in BeWo cells upon forskolin induction of cell differentiation and fusion. Cells were treated with forskolin (50 μM) and RNA was extracted at the indicated times after induction of fusion. MFSD2 and ASCT2 mRNA levels were determined by real-time qRT-PCR analysis. They were normalized relative to the amount of 18S mRNA and are expressed relative to that before induction (level taken as unity).
In relation to the possible involvement of MFSD2 in syncytin-2-mediated cell–cell fusion in placental development, we also analyzed its expression by real-time RT-PCR in the BeWo cell line. This is a choriocarcinoma cell line often used as a model for trophoblast cells and syncytial formation because it can be induced to undergo cell–cell fusion and form multinucleated syncytia by forskolin treatment. As illustrated in Fig. 5B, MFSD2 is indeed expressed in BeWo cells and, remarkably, forskolin treatment results in a >10-fold induction of MFSD2, concomitant with cell fusion. Noteworthily, the opposite regulation is observed for the ASCT2 syncytin-1 receptor, which discloses conversely a markedly reduced expression.
In Situ Hybridization Analysis of MFSD2 Expression.
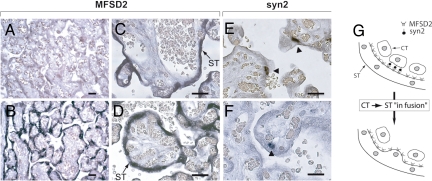
To further investigate MFSD2 expression, we carried out in situ hybridization experiments on paraffin sections of human term placenta. Specific digoxigenin-marked antisense probes were synthesized for both MFSD2 and syncytin-2, as well as the corresponding sense probes as controls. As observed in Fig. 6 A–D, specific labeling was observed with the antisense MFSD2 probe (Fig. 6 B–D; not observed with the control, Fig. 6A) at the level of the trophoblast cells, at the periphery of the placenta floating villi. Labeling was never observed at the level of the mesenchymal and endothelial cells. Close examination of the labeled cells strongly suggests that labeling is at the level of the syncytiotrophoblast. This is further strengthened by in situ hybridization using the syncytin-2 antisense probe (Fig. 6 E and F) which discloses only discrete labeled cells, most probably corresponding to cytotrophoblasts (they are the only cells that have been demonstrated to express syncytin-2 by using an anti-syncytin2 monoclonal antibody and immuno-histochemistry, with no expression in the syncytiotrophoblast) (15, 32). Although it remains difficult to ensure that MFSD2 is not expressed in the cytotrophoblasts because of the very high level of expression of MFSD2 in the syncytiotrophoblast, the observed localization is consistent with the BeWo data demonstrating induction of MFSD2 upon induction of syncytia formation. A scenario can be proposed (Fig. 6 G and Discussion) in which the specific—and exclusive—expression of syncytin-2 and MFSD2 in the mononucleated cytotrophoblasts and the syncytiotrophoblast, respectively, would permit the continuous “in-fusion” of the proliferating cytotrophoblasts into the syncytiotrophoblast, thus allowing its “maintenance.”
Fig. 6.
In situ hybridization of human term placenta for MFSD2 and syncytin-2 expression, and model for the “in-fusion” of cytotrophoblasts into the syncytiotrophoblast. (A–F) In situ hybridization of sections of the villous layer of human term placenta with digoxigenin-labeled MFSD2 sense (A, negative control), MFSD2 antisense (B–D), and syncytin-2 antisense riboprobes (E and F), revealed with an alkaline phosphatase-conjugated anti-digoxigenin antibody. The enlarged views show syncytiotrophoblast (ST) labeling for MFSD2 (B–D) and sparse cytotrophoblast labeling for syncytin-2 (arrowhead, E and F). In E, syncytial “knots” corresponding to detaching syncytial fragments on their way to be released into maternal blood can be observed (adjacent to the labeled cytotrophoblasts). (Scale bars, 50 μm.) (G) A model for the “in-fusion” of cytotrophoblasts (CT) into the syncytiotrophoblast, with the syncytin-2 protein expressed on a fraction of the cytotrophoblasts and MFSD2 expressed on the syncytiotrophoblast allowing controlled fusion and maintenance of the syncytiotrophoblast layer.
Discussion
Retrovirus receptors have been identified by using a variety of approaches. These included the biochemical purification of envelope-binding molecules, the generation of antibodies to cell surface proteins that block infection, and the introduction of genomic or cDNA libraries from susceptible into resistant cells, followed by infection with the virus—or pseudotypes—of interest, engineered to encode an identifiable reporter gene. Although such techniques are extremely powerful, the identification of some virus receptors has been refractory to these methods, and an alternative approach with radiation hybrid panels has been used in 3 instances, namely for the identification of the Jaagsiekte sheep retrovirus (JSRV), mouse mammary tumor virus, and Mus caroli ERV receptors (22–24). In the case of syncytin-2, because of the only low viral titers that could be obtained with pseudotypes (precluding the use of expression libraries) and the absence of any hint that the receptor could be an already known retroviral receptor as was the case for syncytin-1 (which uses the type D retrovirus receptor ASCT2) (5), we chose the latter method to tentatively isolate its receptor. The method proved again its efficacy, and the MFSD2 gene was mapped and demonstrated to be sufficient to confer both cell–cell fusogenicity—in the presence of syncytin-2—to otherwise resistant cells, as well as infectivity to pseudotypes. MFSD2 is a member of a large family of 10–12 transmembrane spanning domain proteins and adds to the list of presently identified retrovirus receptors which already includes a number of ion channels and transporters of small molecules, with a few exceptions including the JSRV receptor Hyal2 (28, 29, 33). In this respect, the identified receptor would fit with the nature of “classical” receptors for retroviruses, again consistent with the retroviral origin of syncytin-2 and the HERV-FRD family of elements. Database analysis of MFSD2 and homologs suggests that it could be a carbohydrate transporter although no definite proof for this function is available. Actually, our present analysis of the expression profile of the human, mouse, rat, and rabbit MFSD2 orthologs, which disclose related patterns with placenta-specific expression, together with the in situ hybridization analyses carried on the human placenta, suggest that MFSD2 primary function is indeed a transport function, most probably acting at the level of the syncytiotrophoblast. This would be consistent with this cell layer being essential in materno–fetal exchanges (2, 3) and could suggest that MFSD2 is specifically devoted to the transport of molecules required in placenta homeostasis. A “secondary” function of MFSD2, as demonstrated in the present study, would be to act as a transmembrane receptor for syncytin-2, thus, mediating cell fusion. Noteworthily, we show that this function is not fully conserved among MFSD2 orthologs, with the mouse and rat gene products not acting as receptors. Besides the fact that this property should allow the identification of the domains and amino acids involved in the receptor function per se (via the construction of human/mouse MFSD2 chimeras), it illustrates the exquisite interactions that exist between hosts and parasites. Indeed, the fact that the murine syncytins are not the orthologs of the human syncytins but correspond to distinct ERV elements (6) is consistent with the lack of receptor activity of the murine and rat MFSD2. In this respect, it will be interesting to determine whether other mammals, in which the MFSD2 protein is a receptor for syncytin-2, possess endogenous elements with an envelope closely related to that of HERV-FRD. Finally, a surprising property of MFSD2 is its specific expression in the placental trophoblast, a rather uncommon feature for a carbohydrate transporter but a very “adequate” property for a refined modulation of cytotrophoblast fusion. This is all the more remarkable as there is a priori no specific selection pressure favoring this localization for retrovirus “endogenization” which only requires entry of the retroviral genome into an ancestral host germ line (in this respect, it may be significant that some MFSD2 expression is detected in the testis although it remains to be demonstrated that it originates from the germ line cells). Specific expression in the syncytiotrophoblast is therefore most likely a property of the host gene which has been “selected” for a refined regulation of cell fusion in a process where the 2 partners involved (namely the Env protein and the receptor) are each finely regulated, being not expressed concomitantly on the same cell type, thus allowing specific fusion between distinct cellular structures (namely the cytotrophoblasts and the syncytiotrophoblast). This property should, in principle, restrict fusion to “in-fusion” of the mononucleated cytotrophoblast into the syncytiotrophoblast, thus allowing the growth and maintenance of the latter structure under conditions where the underlying cytotrophoblasts do not undergo a “self-fusion” process that would otherwise prevent an orderly syncytiotrophoblast organization. In this respect, it will be of interest to unravel the regulatory sequences (and the corresponding transcription factors binding to them) responsible for their antagonistic but complementary cell type-dependent regulations (in both the syncytin-2 and MFSD2 genes). It will also be important to determine whether the MFSD2 gene discloses polymorphisms among the human population that could result in the impairment of its syncytin-2 receptor function (as observed for the mouse and rat orthologs). They could be involved, in a symmetrical manner with respect to syncytin-2, in pathological processes that are encountered in human placentation, possibly associated, for instance, with preeclampsia and intrauterine fetal growth retardation (2, 3).
Materials and Methods
Cells.
HeLa, 293T, and A23 cells (26) were grown in DMEM with 10% FCS (Invitrogen). The A23-derived GeneBridge4 RH clones (contributed by J. Weissenbach) (21, 26) were grown with HAT (100 μM hypoxanthine, 0.4 μM aminopterin, and 16 μM thymidine). BeWo human choriocarcinoma cells were grown in F12-K medium with 15% FCS and 2 mM l-glutamine. BeWo cell fusion was induced with 50 μM forskolin (Sigma) in DMSO added at 50% confluence (day 0) and forskolin-supplemented medium changed on each subsequent day (a parallel treatment was performed with equal volumes of DMSO for the control).
Pseudotyping Assays and GeneBridge4 RH Panel Screening.
SIV virions pseudotyped with syncytin-2 were produced by cotransfecting 106 293T cells with: 2.25 μg pSIV3+ (encoding SIV retroviral proteins except Env) (34); 2.25 μg R9SA (a LacZ-marked defective SIV retroviral vector) (35); and 0.5 μg of syncytin-2 expression vector using the modified bovine serum (MBS) transfection kit (Stratagene). Supernatants from the transfected cells were harvested 48 h after transfection, filtered through 0.45 μm pore-size PVDF membranes, supplemented with Polybrene (4 μg/ml), transferred to the RH clones seeded in 24-well plates (2 × 104 cells per well) the day before infection, followed by spinoculation at 1,200 × g for 2 h 30 min at 25°C. Viral titers were determined 3 days later by in situ X-Gal staining and counting LacZ+ cfu. A threshold value for positive infection of >6 cfu/ml gave the best LOD scores and gene localization results on analysis of the data by using the RH map program (27).
Plasmids.
The human MFSD2 cDNA (Invitrogen) was recloned into pCMV-SPORT6 vector (Invitrogen) after PCR amplification (5′-ATCACCGGTCATGGCCAAAGGAGAAGG-3′, 5′-ATCCGCTCGAGCTAGAGGATGCTAGCCAGCTC-3′) and restriction with AgeI/XhoI. MFSD2 cDNA from other species (Open Biosystems) were recloned into pCMV-SPORT6 as above. For retroviral transductions, the AgeI/XhoI MFSD2 fragments were inserted into the pDFG MLV-derived retroviral vector (36, 37). For C-terminal tagging with the HA epitope, human (SnaBI/NheI) and murine (SnaBI/AccI + AccI/XbaI) MFSD2-containing fragments were inserted in frame into a HA-containing pCMV4 plasmid (gift of M. Malim, King's College, London). The MFSD2-HA fragments (AgeI/SmaI) were then inserted into the pDFG MLV-derived retroviral vector (see above). The phCMV-syn1, phCMV-syn2, pGagPol MLV, and phCMV-VSV-G plasmids have been described (4, 25).
Cell Fusion and Infection Assays.
For cell fusion assays, A23 cells seeded at 5 × 105 cells per 60-mm dish were transfected by using the Lipofectamine LTX kit (Invitrogen) with 3 μg of either MFSD2 or ASCT2 or an empty vector, or with 1.5 μg of either syn1 or syn2 expression vector supplemented with 1.5 μg R9SA. One day after transfection, 3.5 × 105 cells from each group of transfected cells were cocultured in 6-well plates. Syncytia were visualized by X-Gal staining. For infection assays on transiently transfected cells, A23 or HeLa cells were first transfected as above with 3 μg MFSD2, ASCT2, or an empty vector, seeded in 24-well plates (104 cells per well for A23 and 4 × 104 for HeLa cells), and infected 1 day later with SIV-syn1 or -syn2 pseudotypes, as above. Viral titers were determined 3 days after infection by in situ X-Gal staining. Infection was also performed on stable MFSD2-transduced cells generated as follows: MLV pseudotypes were first produced by cotransfection of 106 293T cells with 2.25 μg pGagPol MLV, 2.25 μg pDFG-MFSD2 (expressing the MFSD2 cDNA and an hygromycin gene), and 0.5 μg phCMV-VSV-G (MBS transfection kit). Supernatants were then used to infect HeLa cells and the MFSD2-expressing cells were isolated by hygromycin selection.
Immunofluorescence Microscopy.
Stably transduced HeLa cells expressing the human or murine MFSD2 tagged (or not) with the HA epitope were grown on 14-mm glass coverslips for 24 h. Cells were then fixed in PBS-4% paraformaldehyde and permeabilized in PBS-0.1% SDS at 4°C. After blocking in PBS-3% BSA, cells were immunostained with a rat anti-HA monoclonal antibody (3F10; Roche Applied Science) and a goat FITC-conjugated anti-rat IgG secondary antibody (AbD Serotec). For MFSD2-HA detection only at the cell surface, incubation with the antibodies was performed at 4°C without prior fixation and permeabilization of the cells. Cells were then fixed as above.
Real-Time RT-PCR.
Total RNA was extracted from Bewo cells and frozen organs from mouse and rabbit (RNeasy RNA isolation kit, Qiagen). Human and rat tissue total RNA were obtained from Clontech and Zyagen. Reverse transcription was performed with 1 μg of DNase-treated RNA as in ref. 12. Real-time qPCR was with 5 μl of diluted (1:25) cDNA in a final volume of 25 μl by using SYBR Green PCR Master Mix (or TaqMan Universal PCR Master Mix for 18S rRNA detection) (Applied Biosystems). Amplifications were as in ref. 12 with primers: 5′-CTCCTGGCCATCATGCTCTC-3′ and 5′-GGCCACCAAGATGAGAAA-3′ for MFSD2, and 5′-GGCTTGGTAGTGTTTGCCAT-3′ and 5′-GGGCAAAGAGTAAACCCACA-3′ for ASCT2. To normalize for any minor variations in the amount of total cDNA, amplification using primers for the18S rRNA was performed as an internal control.
In Situ Hybridization Assays.
The full-length (1,592 bp) MFSD2 ORF and a PCR-amplified 1,038 bp syncytin-2 fragment (primers 5′-ATGGGCCTGCTCCTGCTG-3′ and 5′-GGGCAACGGGGAATTCC-3′) were cloned into PGEM-Teasy (Promega) for in vitro synthesis of the antisense and sense riboprobes, generated with T7 or SP6 RNA polymerase and digoxygenin-11-UTP (Roche Applied Science). Paraffin-embedded human term placenta tissue sections (Zyagen) were processed, hybridized at 42°C overnight with the riboprobes, and incubated further at 25°C for 2 h with alkaline phosphatase-conjugated, anti-digoxigenin antibody Fab fragments (Roche Applied Science). Sections were stained by using the nitroblue tetrazolium and 5-bromo-4-chloro-3-indolyl phosphate alkaline phosphate substrates as indicated by the manufacturer (Roche Applied Science).
Acknowledgments.
We thank A. Dupressoir, O. Heidmann, and D. Evain-Brion for helpful discussions. This work was supported by the Centre National de la Recherche Scientifique (CNRS), a grant from the Ligue Nationale contre le Cancer (Equipe labellisée), and fellowships from the CNRS (to S.P.), the Association pour la Recherche sur le Cancer (to D.R.), and the Fondation pour la Recherche Médicale (to C.V.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
References
- 1.Leiser R, Kaufmann P. Placental structure: In a comparative aspect. Exp Clin Endocrinol. 1994;102:122–134. doi: 10.1055/s-0029-1211275. [DOI] [PubMed] [Google Scholar]
- 2.Moffett A, Loke C. Immunology of placentation in eutherian mammals. Nat Rev Immunol. 2006;6:584–594. doi: 10.1038/nri1897. [DOI] [PubMed] [Google Scholar]
- 3.Bischof P, Irminger-Finger I. The human cytotrophoblastic cell, a mononuclear chameleon. Int J Biochem Cell Biol. 2005;37:1–16. doi: 10.1016/j.biocel.2004.05.014. [DOI] [PubMed] [Google Scholar]
- 4.Blaise S, de Parseval N, Bénit L, Heidmann T. Genomewide screening for fusogenic human endogenous retrovirus envelopes identifies syncytin 2, a gene conserved on primate evolution. Proc Natl Acad Sci USA. 2003;100:13013–13018. doi: 10.1073/pnas.2132646100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blond JL, et al. An envelope glycoprotein of the human endogenous retrovirus HERV-W is expressed in the human placenta and fuses cells expressing the type D mammalian retrovirus receptor. J Virol. 2000;74:3321–3329. doi: 10.1128/jvi.74.7.3321-3329.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dupressoir A, et al. Syncytin-A end syncytin-B, two fusogenic placenta-specific murine envelope genes of retroviral origin conserved in Muridae. Proc Natl Acad Sci USA. 2005;102:725–730. doi: 10.1073/pnas.0406509102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mi S, et al. Syncytin is a captive retroviral envelope protein involved in human placental morphogenesis. Nature. 2000;403:785–789. doi: 10.1038/35001608. [DOI] [PubMed] [Google Scholar]
- 8.Dunlap KA, et al. Endogenous retroviruses regulate periimplantation placental growth and differentiation. Proc Natl Acad Sci USA. 2006;103:14390–14395. doi: 10.1073/pnas.0603836103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Parseval N, Heidmann T. Human endogenous retroviruses: From infectious elements to human genes. Cytogenet Genome Res. 2005;110:318–332. doi: 10.1159/000084964. [DOI] [PubMed] [Google Scholar]
- 10.Bannert N, Kurth R. The evolutionary dynamics of human endogenous retroviral families. Annu Rev Genomics Hum Genet. 2006;7:149–173. doi: 10.1146/annurev.genom.7.080505.115700. [DOI] [PubMed] [Google Scholar]
- 11.Blaise S, de Parseval N, Heidmann T. Functional characterization of two newly identified Human Endogenous Retrovirus coding envelope genes. Retrovirology. 2005;2:19. doi: 10.1186/1742-4690-2-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Parseval N, Lazar V, Bénit L, Casella J, Heidmann T. Survey of human genes of retroviral origin: Identification and transcriptome of the genes with coding capacity for complete envelope proteins. J Virol. 2003;77:10414–10422. doi: 10.1128/JVI.77.19.10414-10422.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Villesen P, Aagaard L, Wiuf C, Pedersen FS. Identification of endogenous retroviral reading frames in the human genome. Retrovirology. 2004;1:32. doi: 10.1186/1742-4690-1-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Frendo JL, et al. Direct involvement of HERV-W Env glycoprotein in human trophoblast cell fusion and differentiation. Mol Cell Biol. 2003;23:3566–3574. doi: 10.1128/MCB.23.10.3566-3574.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Malassine A, et al. Expression of the fusogenic HERV-FRD Env glycoprotein (syncytin 2) in human placenta is restricted to villous cytotrophoblastic cells. Placenta. 2007;28:185–191. doi: 10.1016/j.placenta.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 16.de Parseval N, et al. Comprehensive search for intra- and inter-specific sequence polymorphisms among coding envelope genes of retroviral origin found in the human genome: Genes and pseudogenes. BMC Genomics. 2005;6:117. doi: 10.1186/1471-2164-6-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mallet F, et al. The endogenous retroviral locus ERVWE1 is a bona fide gene involved in hominoid placental physiology. Proc Natl Acad Sci USA. 2004;101:1731–1736. doi: 10.1073/pnas.0305763101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Green BJ, Lee CS, Rasko JE. Biodistribution of the RD114/mammalian type D retrovirus receptor, RDR. J Gene Med. 2004;6:249–259. doi: 10.1002/jgm.517. [DOI] [PubMed] [Google Scholar]
- 19.Rasko JE, Battini JL, Gottschalk RJ, Mazo I, Miller AD. The RD114/simian type D retrovirus receptor is a neutral amino acid transporter. Proc Natl Acad Sci USA. 1999;96:2129–2134. doi: 10.1073/pnas.96.5.2129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hayward MD, Potgens AJ, Drewlo S, Kaufmann P, Rasko JE. Distribution of human endogenous retrovirus type W receptor in normal human villous placenta. Pathology. 2007;39:406–412. doi: 10.1080/00313020701444572. [DOI] [PubMed] [Google Scholar]
- 21.Gyapay G, et al. A radiation hybrid map of the human genome. Hum Mol Genet. 1996;5:339–346. doi: 10.1093/hmg/5.3.339. [DOI] [PubMed] [Google Scholar]
- 22.Miller AD, Bergholz U, Ziegler M, Stocking C. Identification of the myelin protein plasmolipin as the cell entry receptor for Mus caroli endogenous retrovirus. J Virol. 2008;82:6862–6868. doi: 10.1128/JVI.00397-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rai SK, DeMartini JC, Miller AD. Retrovirus vectors bearing jaagsiekte sheep retrovirus Env transduce human cells by using a new receptor localized to chromosome 3p21.3. J Virol. 2000;74:4698–4704. doi: 10.1128/jvi.74.10.4698-4704.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ross SR, Schofield JJ, Farr CJ, Bucan M. Mouse transferrin receptor 1 is the cell entry receptor for mouse mammary tumor virus. Proc Natl Acad Sci USA. 2002;99:12386–12390. doi: 10.1073/pnas.192360099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Blaise S, Ruggieri A, Dewannieux M, Cosset F-L, Heidmann T. Identification of an envelope protein from the FRD family of Human Endogenous Retroviruses (HERV-FRD) conferring infectivity on retroviral particles and functional conservation among simians. J Virol. 2004;78:1050–1054. doi: 10.1128/JVI.78.2.1050-1054.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Walter MA, Spillett DJ, Thomas P, Weissenbach J, Goodfellow PN. A method for constructing radiation hybrid maps of whole genomes. Nat Genet. 1994;7:22–28. doi: 10.1038/ng0594-22. [DOI] [PubMed] [Google Scholar]
- 27.Boehnke M, Lange K, Cox DR. Statistical methods for multipoint radiation hybrid mapping. Am J Hum Genet. 1991;49:1174–1188. [PMC free article] [PubMed] [Google Scholar]
- 28.Sommerfelt MA. Retrovirus receptors. J Gen Virol. 1999;80:3049–3064. doi: 10.1099/0022-1317-80-12-3049. [DOI] [PubMed] [Google Scholar]
- 29.Overbaugh J, Miller AD, Eiden MV. Receptors and entry cofactors for retroviruses include single and multiple transmembrane-spanning proteins as well as newly described glycophosphatidylinositol-anchored and secreted proteins. Microbiol Mol Biol Rev. 2001;65:371–389. doi: 10.1128/MMBR.65.3.371-389.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pao SS, Paulsen IT, Saier MH., Jr Major facilitator superfamily. Microbiol Mol Biol Rev. 1998;62:1–34. doi: 10.1128/mmbr.62.1.1-34.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Law CJ, Maloney PC, Wang DN. Ins and Outs of Major Facilitator Superfamily Antiporters. Annu Rev Microbiol. 2008;62:289–305. doi: 10.1146/annurev.micro.61.080706.093329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Malassine A, et al. Human endogenous retrovirus-FRD envelope protein (syncytin 2) expression in normal and trisomy 21-affected placenta. Retrovirology. 2008;5:6. doi: 10.1186/1742-4690-5-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miller AD. Hyaluronidase 2 and its intriguing role as a cell-entry receptor for oncogenic sheep retroviruses. Semin Cancer Biol. 2008;18:296–301. doi: 10.1016/j.semcancer.2008.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Negre D, et al. Characterization of novel safe lentiviral vectors derived from simian immunodeficiency virus (SIVmac251) that efficiently transduce mature human dendritic cells. Gene Ther. 2000;7:1613–1623. doi: 10.1038/sj.gt.3301292. [DOI] [PubMed] [Google Scholar]
- 35.Negre D, Cosset FL. Vectors derived from simian immunodeficiency virus (SIV) Biochimie. 2002;84:1161–1171. doi: 10.1016/s0300-9084(02)00036-6. [DOI] [PubMed] [Google Scholar]
- 36.Suzuki T, et al. Viral interleukin 10 (IL-10), the human herpes virus 4 cellular IL-10 homologue, induces local anergy to allogeneic and syngeneic tumors. J Exp Med. 1995;182:477–486. doi: 10.1084/jem.182.2.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mangeney M, Heidmann T. Tumor cells expressing a retroviral envelope escape immune rejection in vivo. Proc Natl Acad Sci USA. 1998;95:14920–14925. doi: 10.1073/pnas.95.25.14920. [DOI] [PMC free article] [PubMed] [Google Scholar]