Abstract
Fatigue is a debilitating symptom or side effect experienced by many patients on long-term dialysis. Fatigue has a considerable effect on patients’ health-related quality of life and is viewed as being more important than survival by some patients. There are many challenges renal providers face when attempting to reduce fatigue in dialysis patients. The lack of a reliable, valid, and sensitive fatigue scale complicates the accurate identification of this symptom. Symptoms of daytime sleepiness and depression overlap with fatigue, making it difficult to target specific therapies. Moreover, many chronic health conditions common in the long-term dialysis population may lead to the development of fatigue and contribute to the day-to-day and diurnal variation of fatigue in patients. Key to improving the assessment and treatment of fatigue is improving our understanding of potential mediators, as well as potential therapies. Cytokines have emerged as an important mediator of fatigue and have been studied extensively in cancer related fatigue. In addition, although erythropoietin stimulating agents (ESA) have been shown to mitigate fatigue, the recent controversy regarding ESA dosing in chronic kidney disease (CKD) suggests that ESA therapy may not serve as the sole therapy to improve fatigue in this population. In conclusion, fatigue is an important and often under-recognized symptom in the dialysis population. Possible interventions for minimizing fatigue in patients on long-term dialysis should be aimed at improving health care provider awareness, developing improved methods of measurement, better understanding of the pathogenesis, as well as management of known contributing factors.
Keywords: Fatigue, end stage renal disease, quality of life, psychometrics, cytokines, post dialysis fatigue
Introduction
Fatigue is one of the most frequent complaints of dialysis patients and is associated with impaired health-related quality of life (HRQOL). The prevalence of fatigue ranges from 60% to as high as 97% in patients on long-term renal replacement therapy. 1–7 The importance of fatigue to patients with kidney disease is underscored by the observation that 94% of hemodialysis patients endorsed a willingness to undergo more frequent dialysis if there would be an associated increase in energy level. 8 Despite the importance of fatigue to patients, health care providers remain largely unaware of both the presence and severity of fatigue among dialysis patients. 9
Although the clinical assessment of fatigue in dialysis patients has proven difficult for physicians, it is important to recognize fatigue since there are a number of treatable causes. The recognition of fatigue may be difficult since the recovery from fatigue has great inter-patient variability. 10 After recognizing fatigue and assessing its severity, the physician should first consider the general physiologic and psychological etiologies for fatigue such as hypothyroidism and depression. In addition, there are dialysis-related causes of fatigue and some of the factors that may lead to fatigue in ESRD patients include uremia, anemia, sleep disorders and psychosocial distress, which may be amenable to intervention.
This report aims to examine the current definitions and theories of fatigue, instruments to measure fatigue, the underlying physiologic and psychological correlates of fatigue and possible interventions to improve fatigue in ESRD patients. This review also examines the role of inflammation in the development of fatigue and explores how inflammation may act as a common mediator of fatigue for both physiologic and psychological causes. Finally, potential pharmacological and behavioral interventions to improve fatigue in patients with ESRD are outlined.
Definition of Fatigue
Fatigue is a subjective sense of weakness, lack of energy, and tiredness. 11 Lee et al. proposed that it can be conceptualized as located on a continuum of exhaustion and tiredness on one end, with energy and vitality at the opposite end of the continuum. 12 This position has been supported by the National Institutes of Health Patient-Reported Outcomes Measurement Information System (PROMIS) initiative where the items from the SF-36 vitality scale measure fatigue and energy level at the upper end of a fatigue scale. 14 Ream and Richardson described fatigue as “a subjective, unpleasant symptom which incorporates total body feelings ranging from tiredness to exhaustion creating an unrelenting overall condition which interferes with individuals’ ability to function to their normal capacity.” 13 Similar to Lee et al, it is our belief that fatigue is on a continuum with weakness, lack of energy, and tiredness on one end and energy, vitality, and pep at the other extreme.
The theoretical frameworks for understanding fatigue include the theories of (1) unpleasant symptoms, (2) peripheral and central fatigue, and (3) a multi-dimensional fatigue experience in ESRD. According to the theory of unpleasant symptoms by Lenz et al., factors contributing to fatigue can be categorized as physiological, psychological and socio-demographic, all of which have multiple complex and reciprocal interactions with fatigue. There are a variety of interactions among the contributing factors and various symptoms resulting in a synergistic impact on the performance variables, which in turn reciprocally influence the symptoms and contributing factors. 15 Chaudhuri and Behan introduced the concept of central versus peripheral fatigue. Central fatigue is defined as “the failure to initiate and/or sustain attention tasks (mental fatigue) and physical activities (physical fatigue) requiring self motivation”. Peripheral or motor fatigue is due to fatigue in either the muscle itself or due to brain control over the muscle. 16 Lee et al. categorized the multi-dimensional fatigue experience of hemodialysis (HD) patients in Taiwan into three inextricably linked domains – physical, affective and cognitive.17 These theoretical frameworks underscore the multi-dimensional aspects of fatigue and suggest that physiological, psychological and socio-demographic factors contribute to fatigue.
One approach to this multi-dimensional symptom has been to operationalize this complex set of symptoms by developing and subsequently applying criteria to identify patients with clinically important fatigue. Criteria proposed by Cella et al. 18 for defining cancer-related fatigue could be extrapolated to develop criteria specific for ESRD-related fatigue. The criteria for cancer-related fatigue include the presence of significant fatigue every day or nearly every day during the same 2-week period in the past month. In addition, there is the presence of five or more of following symptoms - generalized weakness or limb heaviness, diminished concentration, decreased interest in engaging in usual activities, insomnia or hypersomnia, unrefreshing sleep, perceived need to struggle to overcome inactivity, marked emotional reactivity to feeling fatigued, difficulty completing daily tasks, perceived problems with short-term memory and post-exertional malaise lasting several hours. 18 The use of criteria to define clinically important fatigue would help to better understand the prevalence and predictors of fatigue in the ESRD population.
Measurement of Fatigue
There are a number of choices when selecting a brief assessment tool for fatigue in patients with ESRD. The most widely used instrument in the dialysis population is the vitality scale of the SF-36. 19, 20 The SF-36 vitality subscale, which consists of 4 items, is considered to be at one end of a spectrum of fatigue. The vitality construct captures a mild reduction in energy level but fails to capture the negative aspects of fatigue such as weakness, lack of motivation, and difficulty with concentration. In addition to the SF-36, a number of symptom indices use single items to measure the presence and severity of fatigue.7, 21 Fatigue scales vary in brevity, reliability, and responsiveness to interventions, and most of them have not been validated in the CKD population. Though, the Revised-Piper Fatigue Scale, comprised of 22 items, has been shown to be reliable. 22, 23 The Multi-dimensional Fatigue Inventory (MFI-20) has also been used to capture overall fatigue. 24 However, the HD population demonstrated difficulty with comprehension of this instrument and there was poor internal-consistency reliability. 25 Multiple aspects of fatigue and its impact on daily life are measured by the Functional Assessment of Chronic Illness Therapy Fatigue (FACIT-F). In patients with cancer and rheumatoid arthritis, the FACIT fatigue scale has shown excellent reliability and strong association with the vitality scales of the SF-36. 26–29
While most fatigue instruments measure the overall experience of fatigue over a period of time ranging from weeks to months, dialysis patients also experience day-to-day and diurnal variation in fatigue. Fatigue assessments using traditional instruments may fail to capture this variability due to recall bias. Ecological momentary assessment (EMA) provides an important measurement tool to assess subjective fatigue repeatedly and reliably while avoiding recall bias. EMA incorporates repeated real-time measurement of phenomena such as symptoms, behaviors, or physiological processes, as they occur in naturalistic settings. 30–32 It may be that a real-time or experiential assessment of fatigue would provide additional information on the experience of dialysis patients, leading to improved treatment of severe fatigue.
Contributors to Fatigue in ESRD
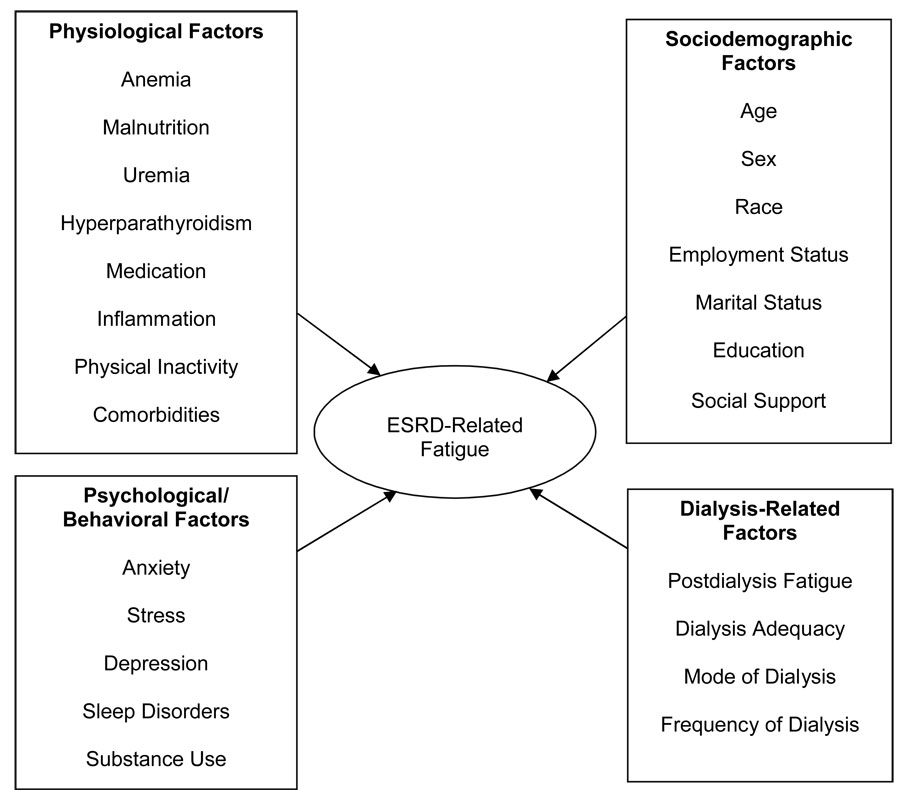
In the dialysis population, physiologic, behavioral, treatment-related, and individual characteristics may be correlated with fatigue (Figure 1). Physiologic etiologies include anemia, malnutrition, uremia, dialysis inadequacy, hyperparathyroidism, coexisting chronic illnesses, sleep disorders, depression and side effects of medications. Dietary and fluid restriction may also play a role. 33 Physical inactivity has been associated with higher levels of fatigue. 34 Socio-demographic factors including age, sex, race, educational, marital, and vocational status may also play a role in the experience of fatigue in dialysis patients. 35, 36 In a study among HD patients in Taiwan, higher levels of fatigue were reported by female, older and unemployed patients. 35 It is also important to note that pro-inflammatory cytokines have emerged as potential mediators of fatigue, providing a common biological pathway for physiologic, behavioral, and treatment-related factors to cause fatigue in the dialysis population.
Figure 1.
Inflammation and Fatigue
Multiple clinical and animal studies have demonstrated a relationship between ‘sickness behavior,’ which includes a constellation of symptoms such as fatigue, and changes in pro-inflammatory cytokines.37 ESRD is an inflammatory state characterized by elevated circulating levels of pro-inflammatory cytokines. 38–41 Although the causes of elevated cytokines in these patients are not fully understood, it has been suggested that ESRD patients have overproduction of cytokines by peripheral blood mononuclear cells (PBMCs) secondary to chronic activation by interaction with dialysis membranes. 42 43, 44 Moreover, in this complex pathological condition, the possibility of intrinsic alterations of signaling pathways and immune defects cannot be excluded. 45 Interleukin (IL)-6, C-Reactive Protein (CRP) and Tumor Necrosis Factor (TNF)-α have been associated with mortality, decreased muscle strength and ‘vital exhaustion’ in the elderly and post-myocardial infarction patients. 46–49 Table 1 delineates a number of human studies that have linked inflammatory cytokines to fatigue in both aging and chronic health conditions such as cancer and chronic fatigue syndrome. 50–52 Interestingly, elevated levels of pro-inflammatory cytokines have been linked to an increase in energy expenditure, mortality and lower functional status in HD patients. 53–55 In a recent study among 30 HD patients, higher levels of IL-6 were associated with significantly higher levels of resting energy expenditure,54 which has been previously associated with higher mortality in HD and peritoneal dialysis (PD). 53–57
Table 1.
Role of cytokines and inflammation in fatigue among populations with chronic health conditions
| Study | Subjects | Cytokines | Fatigue Related Outcomes | Results |
|---|---|---|---|---|
| Kamimura et al. (2007) 54 | 30 HD and 11 controls | IL-6 | Resting energy expenditure | IL-6 is positively associated with resting energy expenditure (which is positively associated with higher mortality in HD and PD patients 127) |
| Janszky et al. (2005) 48 | 235 women survivors after acute myocardial infarction | IL-6, hsCRP, IL-1ra (IL-1 receptor antagonist) | Self rated health Vital exhaustion Depression | Positive correlation between IL-6 and hsCRP and vital exhaustion and poor self rated health |
| Schubert et al. (2007) 128 | Meta analysis of 18 studies (1037 subjects) on cancer related fatigue | Multiple cytokines | Fatigue in cancer pts | Significant positive correlation between fatigue and IL-6, IL-1ra and neopterin No significant correlation with IL-1β or TNF- α |
| Cesari et al. (2004) 46 | 1020 elderly (≥ 65 yrs) enrolled in InCHIANTI study | IL-6, IL-6sR, IL-1ra, IL-10, IL-1β, TNF- α, CRP | Physical performance and muscle strength | High levels of IL-6, IL-1ra, CRP are associated with poor physical performance and muscle strength in older persons |
| Collado-Hidalgo et al. (2006) 129 | 50 breast cancer survivors ≥ 2 yrs after successful primary therapy | IL-6, TNF- α production following lipopolysaccharide stimulation, IL-1ra, sIL-6 receptor | Fatigue in breast cancer survivors | Increased production of IL-6, TNF- α following lipopolysaccharide stimulation and higher IL-1ra and soluble IL-6 receptor levels in fatigued pts |
| Meyers et al. (2005) 130 | 54 pts with AML/MDS | IL-6, IL-1RA, IL-8 and TNF- α | Cognitive function Fatigue Quality of life | Higher IL-6, IL-1RA and TNF- α are associated with fatigue |
Cytokines might contribute to fatigue by directly activating the central nervous system, hypothalamic pituitary and adrenal axis or indirectly triggering multi-system deregulation due to chronic inflammation. 56 For example, Interferon (IFN) produces neurasthenia, a neurological fatigue suggestive of frontal lobe changes manifesting as lack of motivation. 57 Cytokines such as IL-1, IL-6, TNF-α suppress erythropoesis and have been hypothesized to be contributing to anemia and fatigue in cancer patients. 51 Cytokines (IL-6, TNF), trigger hyperresponsiveness of muscular ergoreceptors, which sense fatigue or the work performed by the muscle, and thus contribute to fatigue. 58 Cytokine mediated malnutrition and hypoalbuminemia may also contribute to fatigue. Animal studies have suggested that IL-6 decreases hepatic albumin synthesis, and serum IL-6 levels have been shown to be inversely related to albumin levels in lymphoma patients. 52 IL-6 also induces protein catabolism, lipolysis and insulin resistance and has been shown to have a strong negative correlation with serum albumin in patients undergoing HD.42, 59 Cytokines may also mimic leptin and target hypothalamic neurons regulating food intake and energy expenditure resulting in decreased appetite and hypermetabolic state. 51 In addition to their direct effects on muscle and the nervous system, cytokines are also associated with sleep disorders, depression, anxiety, and physical inactivity and may mediate fatigue through these conditions. 60–64
Anemia and Fatigue
The use of ESA to correct anemia in dialysis patients has been shown to improve HRQOL, fatigue, exercise tolerance and work capacity. 65, 66 A systematic review of the ESA therapy in patients with renal insufficiency and cancer revealed a consistently positive relationship between HRQOL and hematocrit levels, with the strongest effect on the energy/fatigue domains. 67 These findings were confirmed by a meta-analysis of the impact of epoetin alfa in patients with chronic kidney disease. 68 More recently, two studies of pre-dialysis CKD patients, the Correction of Hemoglobin Outcomes in Renal Insufficiency (CHOIR) study and the Cardiovascular Risk Reduction by Early Anemia Treatment with Epoetin Beta (CREATE) study, compared the HRQOL in patients with higher versus lower target hemoglobin levels. 69, 70 The CREATE study reported significantly improved fatigue symptoms in patients with higher hemoglobin, whereas the CHOIR study did not reveal any significant differences between the two groups. However, the lack of association between anemia and fatigue in recent studies may relate to the relatively higher targeted hemoglobin levels for the control groups in the post-erythropoietin era. 26 Although anemia, resulting from reduced erythropoietin production, has been cited as an important contributor to fatigue in both the dialysis population and other chronic conditions, 71 the optimal hemoglobin target remains unclear and may vary among individuals depending on the severity of fatigue.
Biochemical markers and Fatigue
While it is thought that the uremic syndrome may manifest as fatigue and weakness, the association between fatigue and biochemical markers such as albumin, creatinine, Kt/V, urea reduction ratio, phosphate, calcium and fatigue has been inconsistent. 25, 71, 72 Uremia may lead to protein and energy malnutrition, nausea, and loss of appetite, which can all contribute to fatigue. 73 However, studies have shown no significant associations among fatigue and biochemical variables, including serum albumin level. 25, 71 Metabolic derangements in uremia may cause carnitine deficiency, which is required for energy generation by skeletal muscles. 74 Intravenous L-carnitine supplementation has been shown to significantly improve fatigue in a small randomized trial in HD patients. 75
The treatment of uremia by dialysis may also influence fatigue, as the mode and frequency of dialysis are associated with fatigue. The potential impact of dialysis modality was shown in the Choices for Health Outcomes in Caring for ESRD (CHOICE) study comparing the HRQOL in conventional HD and PD patients. There was no significant difference in the vitality scores among HD and PD patients at the initiation of dialysis therapy; however patients on PD experienced significantly lower vitality at one year. 76 The stimuli for inflammatory response in PD patients include fluid overload, decreased cytokine clearance, presence of uremia-modified proteins, presence of chronic infections and metabolic disturbances (including hyperglycemia). 77, 78, 2 Lastly, PD patients often suffer from nocturnal pruritus and 'difficulties finding a comfortable sleeping position,' resulting in impaired sleep quality, which contributes to daytime sleepiness and fatigue. 79 Fatigue is an important outcome for quotidian dialysis trials since the frequency of dialysis may also affect fatigue in HD patients, with some studies demonstrating that compared to conventional therapy, quotidian dialysis significantly improves perceived energy level, uremic symptoms, cognitive functioning and overall HRQOL. 80, 81, 82
Post-dialysis Fatigue
Post-hemodialysis fatigue is a common, often incapacitating symptom 17, 83, 84 and may be improved with more frequent treatment. Lindsay et. al looked at post-dialysis fatigue in 45 subjects and found a positive association between “time to recover (minutes) from HD” and fatigue; patients with longer recovery time tended to have greater levels of fatigue. Also, the relationship between recovery time and fatigue was strongest immediately after dialysis and weakened progressively during the time between sessions. In this study, the time to recover from HD also showed a significant positive association with the total dialysis stress score, which encompasses an array of physical signs and symptoms that can arise during HD procedure. 10 Ultrafiltration, diffusion, osmotic disequilibrium, changes in blood pressure, blood membrane interactions, higher levels of tumor necrosis factor and psychological factors like depression have all been implicated in the pathogenesis of post-dialysis fatigue. 83–85 Post-dialysis fatigue has been shown to be less prevalent in daily HD patients. Lindsay et al. reported that daily HD patients required significantly less time to recover fully after dialysis compared to controls (p<0.001). 86 In another study, the minutes to recovery decreased from 397 ± 395 in conventional thrice-weekly HD to 30 ± 44 in quotidian HD at 18 months of follow up. 87 Interestingly, longer post-dialysis fatigue has been associated with shorter survival. 88, 89 This suggests that patients with longer recovery time may have a greater degree of underlying inflammation, which could contribute to a higher incidence of coronary artery disease and mortality. 90 Further studies are needed to assess the impact of frequent and novel dialysis techniques on post-dialysis fatigue.
Sleep and Fatigue
Sleep disorders have been hypothesized to be associated with fatigue through two mechanisms, the disturbance of sleep resulting in daytime sleepiness and the separate underlying biological pathways associated with a variety of sleep disorders. Dialysis patients have high rates of sleep apnea, insomnia, restless legs syndrome and excessive daytime sleepiness. 91, 92 Impaired sleep initiation, maintenance, and adequacy are associated with significantly lower vitality in both HD and PD patients. 25, 79, 93 Sleep apnea has been associated with lower HRQOL in patients on HD, and those without sleep apnea experience significantly better vitality, social functioning and emotional and mental health. 94 Other symptoms, such as restless legs, which are common in dialysis patients and affect sleep quality, may also impact vitality. In a study of 894 dialysis patients, symptoms of restless legs were significantly associated with lower physical and mental well-being, lower vitality, higher bodily pain, and lower sleep quality. 92
The relationship of sleep disorders to increased levels of inflammatory cytokines may help explain the association of sleep disorders with fatigue in this population. In a recent study among 57 PD patients, higher levels of IL-18 were associated with poor sleep quality. 64 In healthy people, the administration of TNF-α and IL-1β increase the amount of non-rapid eye movement (NREM) sleep and decrease REM sleep.95 IL-6 is associated with the amount and depth of sleep, and higher levels are associated with poor sleep. 96, 97 IL-1α and TNF-α have also been associated with sleep disordered breathing in dialysis patients. 98 In healthy people, elevated levels of TNF-α and IL-6 have been associated with circadian rhythm disruption and obstructive sleep apnea (OSA) independent of obesity. 99, 100, 96 Further research is warranted to investigate the possible shared biological mechanisms associated with sleep disorders and fatigue.
Depression and Fatigue
Fatigue and depression are closely interrelated and depression may manifest as feelings of tiredness and lack of energy. Depression has also been shown to correlate strongly with overall symptom burden and severity including fatigue in dialysis patients. 7 Depression is the most common psychiatric illness in patients with ESRD, with prevalence rates ranging from 15% to 69%. 101–103 Depression has been found to be associated with changes in cellular and humoral immunity including decreased T lymphocyte proliferation, NK cell activity as well as increased production of IL-1 , IL-6, and IFN-gamma. 63, 104 High levels of IL-6, IL-8 and TNF- α have been associated with an increased prevalence of major depression in older people and healthy women. 61, 63, 105, 106 Some anti-inflammatory drugs including infliximab ameliorate depressive symptoms and improve HRQOL. 107 Specific to patients with ESRD, Lee and colleagues found that treatment with antidepressants resulted in decreased levels of IL-1 β, independent of the response to treatment. For patients who responded to treatment with selective serotonin re-uptake inhibitors, IL-6 was found to be lower when compared to those patients who did not respond to treatment. 108 Thus, although evidence demonstrating a causal association between depression and altered level of cytokines is lacking, depression may contribute to fatigue through inflammatory pathways.
Physical Inactivity and Fatigue
Physical inactivity is associated with higher levels of fatigue in ESRD patients. 34 In addition, obesity, which has been described as a chronic inflammatory state, may also mediate alterations in levels of certain cytokines leading to fatigue. 109 Acute exercise results in an inflammatory response (e.g., increases in white blood cell counts, IL-1, and CRP), whereas regular exercise has an anti-inflammatory effect and reduces the level of pro-inflammatory cytokines. 62, 110, 111 However, the effect of physical activity on the immune system may be different in HD patients than in healthy adults. 112 There is also evidence that muscle catabolism is increased in dialysis patients, which may be due to insulin resistance, acidosis or inflammation. This may lead to muscular fatigue and further physical inactivity.113, 114 Dialysis patients have severe exercise limitations which have been attributed to muscle atrophy and weakness, presence of abnormal mitochondria and impaired oxidative capacity.115 Muscle fatigue, defined as the reduction in force with repeated or sustained contractions can lead to manifestations of myopathy. Contributors of excessive muscle fatigue in dialysis patients include poor oxidative metabolism, greater accumulation of metabolic by-products, central activation failure and impaired neuromuscular propagation.116 Endurance training has been shown to increase muscle strength, power, peak work rate, VO(2)peak, fatigability and physical function.117 In addition, exercise rehabilitation programs may have morphological and metabolic benefits in the skeletal muscles and improve work capacity.118
Interventions to Reduce Fatigue
Due to the complexity of fatigue, a multi-disciplinary approach to treatment should be adopted by nephrologists (Table 2). In order to address the level of fatigue, this symptom first needs to be recognized and accurately measured by health care providers. All renal providers should receive training on identifying and addressing the issue of fatigue. Developing improved methods of defining and measuring fatigue, including real-time or ecological momentary assessment, will help to identify patterns in the severity of fatigue. Because there is no widely accepted tool for screening fatigue in the ESRD population, health care providers should consider screening for a sense of fatigue and tiredness that has a substantial impact on patients’ functional abilities. Given the high rate of sleep disorders, practitioners should clarify if the patient is sleepy or drowsy rather than weak and lacking in energy. If the patient reports that fatigue leads to functional impairment, providers should actively consider common causes such as worsening heart failure, chronic fatigue syndrome, hypothyroidism, liver disease, depression, sleep disorders, autoimmune diseases as well as the kidney-disease related factors outlined in this review.
Table 2.
Potential multi-disciplinary approaches to improving fatigue in ESRD patients
| Targeted area | Interventions |
|---|---|
| Increase health care provider awareness | • Education of prevalence, importance and severity of fatigue |
| • Training at identifying symptoms of fatigue | |
| Improve measurement of fatigue | • Development of criteria for defining fatigue |
| • Development of improved fatigue scales specific for this population | |
| • Use of ecological momentary assessment for measurement of day-to-day and diurnal variation in fatigue | |
| • Development of improved survey modalities such as telephone interview, computer-assisted interview, and proxy administration of interviews to reduce selection bias | |
| • Frequent screening for fatigue | |
| Address gaps in understanding pathogenesis of fatigue | • Role of cytokines |
| • Mode of dialysis | |
| • Frequency of dialysis | |
| • Thermoneutral hemodialysis | |
| Test potential therapies for fatigue in ESRD | Non-pharmacological |
| • Nutritional therapy | |
| • Sleep therapy and sleep hygiene | |
| • Exercise | |
| • Stress management | |
| • Cognitive-behavioral treatment of depression | |
| • Energy Conservation | |
| • Acupressure | |
| • Treatment of substance abuse and dependence | |
| Pharmacological | |
| • Hematopoeitics | |
| • Antidepressants | |
| • Anxiolytics | |
| • Levocarnitine | |
| • Human growth hormone | |
| Improving social support for patients with fatigue | • Family members and care providers education and training |
| • Addressing caregiver fatigue | |
A better understanding of the pathogenesis of fatigue, particularly the role of cytokines may help in designing interventions aimed at reducing inflammation and fatigue. Management of factors such as anemia and sleep disorders is fundamental. Treatment of depression, anxiety, stress, substance abuse, obesity, and malnutrition may be helpful, although studies substantiating the role of these interventions are lacking.
There is also a need for further research to test potential therapies for fatigue. Non-pharmacological interventions targeting nutrition, sleep hygiene, stress management, and treatment of depression may potentially decrease fatigue. Some small studies indicate that acupressure may help to improve fatigue, depression and sleep quality in dialysis patients. 119, 120 Exercise and yoga have also been studied as effective measures in improving fatigue. 117, 121 Whether this is due to the direct effect of muscle strengthening or indirect effect on the cytokines (or both) is unknown. Energy conservation strategies, such as those used for multiple sclerosis patients may similarly improve fatigue in ESRD patients.122 Overall, the bulk of evidence in the ESRD population as potential non-pharmacologic intervention consists of small trials assessing the impact of rehabilitation, exercise and more frequent hemodialysis.
Among the pharmacological measures to improve fatigue, there is existing evidence for the use of ESA to reduce fatigue as outlined above. 67, 68 Some small trials suggest that human growth hormone (HGH) may improve fatigue and overall HRQOL in dialysis patients – possible mechanisms being improvement in the nutritional status (lean body mass, albumin) or changes in levels of certain inflammatory mediators (decrease in TNF-α level). 123, 124 Intravenous levocarnitine infusion has also been shown to effect fatigue.75 Psychostimulants such as methylphenidate have shown significant improvement in cancer related fatigue and may be useful in ESRD patients, although evidence supporting this is lacking.125 Since many patients with ESRD have been shown to have mood disorders, the physician should consider screening for depression and implement treatment as appropriate.
Lastly, improving social support for the patient with severe fatigue is crucial and helps the patient cope with the disabling symptoms. Fatigue and tiredness may extend to the caregiver, who may provide support for home dialysis or the care of a child with ESRD. A recent review and meta-ethnography outlined fatigue and tiredness as major concerns of caregivers of children with ESRD.126 Therefore, the care for patients with ESRD includes education for the family as well as addressing fatigue issues in the caregiver.
Conclusion
Fatigue is a common and complex phenomenon that significantly decreases HRQOL among dialysis patients. Although highly prevalent, fatigue is often an unrecognized and under-treated symptom in the dialysis population. The lack of adequate methods for measurement, lack of provider awareness, and the complex pathogenesis of fatigue have confounded the development of effective interventions to treat fatigue. Thus, there is a strong need to develop improved assessment methods and investigate the role of other contributing factors, especially inflammatory mediators in fatigue in ESRD. A better appreciation of these may provide potential targets for therapeutic interventions in the future. Further research is needed to evaluate the effectiveness and impact of these interventions in the optimization of patient-centered quality of life in the dialysis population.
Acknowledgements
Support: Dr. Unruh was supported by grants DK66006 and DK77785 from the National Institute of Diabetes and Digestive and Kidney Diseases, by the Paul Teschan Research Fund and by the Fresenius Medical Care Young Investigator Grant of the National Kidney Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosure: None.
References
- 1.Cardenas DD, Kutner NG. The problem of fatigue in dialysis patients. Nephron. 1982;30(4):336–340. doi: 10.1159/000182512. [DOI] [PubMed] [Google Scholar]
- 2.Chang WK, Hung KY, Huang JW, Wu KD, Tsai TJ. Chronic fatigue in long-term peritoneal dialysis patients. Am J Nephrol. 2001;21(6):479–485. doi: 10.1159/000046652. [DOI] [PubMed] [Google Scholar]
- 3.Laupacis A, Muirhead N, Keown P, Wong C. A disease-specific questionnaire for assessing quality of life in patients on hemodialysis. Nephron. 1992;60(3):302–306. doi: 10.1159/000186769. [DOI] [PubMed] [Google Scholar]
- 4.Murtagh FE, Addington-Hall J, Higginson IJ. The prevalence of symptoms in end-stage renal disease: a systematic review. Adv Chronic Kidney Dis. 2007;14(1):82–99. doi: 10.1053/j.ackd.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 5.Parfrey PS, Vavasour HM, Henry S, Bullock M, Gault MH. Clinical features and severity of nonspecific symptoms in dialysis patients. Nephron. 1988;50(2):121–128. doi: 10.1159/000185141. [DOI] [PubMed] [Google Scholar]
- 6.Unruh M, Benz R, Greene T, et al. Effects of hemodialysis dose and membrane flux on health-related quality of life in the HEMO Study. Kidney Int. 2004;66(1):355–366. doi: 10.1111/j.1523-1755.2004.00738.x. [DOI] [PubMed] [Google Scholar]
- 7.Weisbord SD, Fried LF, Arnold RM, et al. Prevalence, severity, and importance of physical and emotional symptoms in chronic hemodialysis patients. J Am Soc Nephrol. 2005;16(8):2487–2494. doi: 10.1681/ASN.2005020157. [DOI] [PubMed] [Google Scholar]
- 8.Ramkumar N, Beddhu S, Eggers P, Pappas LM, Cheung AK. Patient preferences for in-center intense hemodialysis. Hemodial Int. 2005;9(3):281–295. doi: 10.1111/j.1492-7535.2005.01143.x. [DOI] [PubMed] [Google Scholar]
- 9.Weisbord SD, Fried LF, Mor MK, et al. Renal provider recognition of symptoms in patients on maintenance hemodialysis. Clin J Am Soc Nephrol. 2007;2(5):960–967. doi: 10.2215/CJN.00990207. [DOI] [PubMed] [Google Scholar]
- 10.Lindsay RM, Heidenheim PA, Nesrallah G, Garg AX, Suri R. Minutes to recovery after a hemodialysis session: a simple health-related quality of life question that is reliable, valid, and sensitive to change. Clin J Am Soc Nephrol. 2006;1(5):952–959. doi: 10.2215/CJN.00040106. [DOI] [PubMed] [Google Scholar]
- 11.Stone P, Richards M, Hardy J. Fatigue in patients with cancer. Eur J Cancer. 1998;34(11):1670–1676. doi: 10.1016/s0959-8049(98)00167-1. [DOI] [PubMed] [Google Scholar]
- 12.Lee KA, Hicks G, Nino-Murcia G. Validity and reliability of a scale to assess fatigue. Psychiatry Res. 1991;36(3):291–298. doi: 10.1016/0165-1781(91)90027-m. [DOI] [PubMed] [Google Scholar]
- 13.Ream E, Richardson A. Fatigue: a concept analysis. Int J Nurs Stud. 1996;33(5):519–529. doi: 10.1016/0020-7489(96)00004-1. [DOI] [PubMed] [Google Scholar]
- 14. [Accessed March 3, 2008]; at www.nihpromis.org.
- 15.Lenz ER, Pugh LC, Milligan RA, Gift A, Suppe F. The middle-range theory of unpleasant symptoms: an update. ANS Adv Nurs Sci. 1997;19(3):14–27. doi: 10.1097/00012272-199703000-00003. [DOI] [PubMed] [Google Scholar]
- 16.Chaudhuri A, Behan PO. Fatigue and basal ganglia. J Neurol Sci. 2000;179 S 1–2:34–42. doi: 10.1016/s0022-510x(00)00411-1. [DOI] [PubMed] [Google Scholar]
- 17.Lee BO, Lin CC, Chaboyer W, Chiang CL, Hung CC. The fatigue experience of haemodialysis patients in Taiwan. Journal of clinical nursing. 2007;16(2):407–413. doi: 10.1111/j.1365-2702.2005.01409.x. [DOI] [PubMed] [Google Scholar]
- 18.Cella D, Peterman A, Passik S, Jacobsen P, Breitbart W. Progress toward guidelines for the management of fatigue. Oncology (Williston Park) 1998;12(11A):369–377. [PubMed] [Google Scholar]
- 19.Kalantar-Zadeh K, Unruh M. Health related quality of life in patients with chronic kidney disease. Int Urol Nephrol. 2005;37(2):367–378. doi: 10.1007/s11255-004-0012-4. [DOI] [PubMed] [Google Scholar]
- 20.Unruh ML, Weisbord SD, Kimmel PL. Health-related quality of life in nephrology research and clinical practice. Seminars in dialysis. 2005;18(2):82–90. doi: 10.1111/j.1525-139X.2005.18206.x. [DOI] [PubMed] [Google Scholar]
- 21.Davison SN, Jhangri GS, Johnson JA. Cross-sectional validity of a modified Edmonton symptom assessment system in dialysis patients: a simple assessment of symptom burden. Kidney Int. 2006;69(9):1621–1625. doi: 10.1038/sj.ki.5000184. [DOI] [PubMed] [Google Scholar]
- 22.Dagnelie PC, Pijls-Johannesma MC, Pijpe A, et al. Psychometric properties of the revised Piper Fatigue Scale in Dutch cancer patients were satisfactory. J Clin Epidemiol. 2006;59(6):642–649. doi: 10.1016/j.jclinepi.2005.09.015. [DOI] [PubMed] [Google Scholar]
- 23.Piper BF, Dibble SL, Dodd MJ, Weiss MC, Slaughter RE, Paul SM. The revised Piper Fatigue Scale: psychometric evaluation in women with breast cancer. Oncol Nurs Forum. 1998;25(4):677–684. [PubMed] [Google Scholar]
- 24.Smets EM, Garssen B, Bonke B, De Haes JC. The Multidimensional Fatigue Inventory (MFI) psychometric qualities of an instrument to assess fatigue. J Psychosom Res. 1995;39(3):315–325. doi: 10.1016/0022-3999(94)00125-o. [DOI] [PubMed] [Google Scholar]
- 25.McCann K, Boore JR. Fatigue in persons with renal failure who require maintenance haemodialysis. J Adv Nurs. 2000;32(5):1132–1142. doi: 10.1046/j.1365-2648.2000.01584.x. [DOI] [PubMed] [Google Scholar]
- 26.Chandran V, Bhella S, Schentag C, Gladman DD. Functional assessment of chronic illness therapy-fatigue scale is valid in patients with psoriatic arthritis. Ann Rheum Dis. 2007;66(7):936–939. doi: 10.1136/ard.2006.065763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tyring S, Gottlieb A, Papp K, et al. Etanercept and clinical outcomes, fatigue, and depression in psoriasis: double-blind placebo-controlled randomised phase III trial. Lancet. 2006;367(9504):29–35. doi: 10.1016/S0140-6736(05)67763-X. [DOI] [PubMed] [Google Scholar]
- 28.Wadler S, Brain C, Catalano P, Einzig AI, Cella D, Benson AB., 3rd Randomized phase II trial of either fluorouracil, parenteral hydroxyurea, interferon-alpha-2a, and filgrastim or doxorubicin/docetaxel in patients with advanced gastric cancer with quality-of-life assessment: eastern cooperative oncology group study E6296. Cancer J. 2002;8(3):282–286. doi: 10.1097/00130404-200205000-00013. [DOI] [PubMed] [Google Scholar]
- 29.Yellen SB, Cella DF, Webster K, Blendowski C, Kaplan E. Measuring fatigue and other anemia-related symptoms with the Functional Assessment of Cancer Therapy (FACT) measurement system. J Pain Symptom Manage. 1997;13(2):63–74. doi: 10.1016/s0885-3924(96)00274-6. [DOI] [PubMed] [Google Scholar]
- 30.Curran SL, Beacham AO, Andrykowski MA. Ecological momentary assessment of fatigue following breast cancer treatment. Journal of behavioral medicine. 2004;27(5):425–444. doi: 10.1023/b:jobm.0000047608.03692.0c. [DOI] [PubMed] [Google Scholar]
- 31.Hacker ED, Ferrans CE. Ecological momentary assessment of fatigue in patients receiving intensive cancer therapy. J Pain Symptom Manage. 2007;33(3):267–275. doi: 10.1016/j.jpainsymman.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 32.Moskowitz DS, Young SN. Ecological momentary assessment: what it is and why it is a method of the future in clinical psychopharmacology. J Psychiatry Neurosci. 2006;31(1):13–20. [PMC free article] [PubMed] [Google Scholar]
- 33.Pagels A, Heiwe S, Hylander B. Nutritional status of pre-dialysis patients. Edtna Erca J. 2006;32(3):162–166. doi: 10.1111/j.1755-6686.2006.tb00009.x. [DOI] [PubMed] [Google Scholar]
- 34.Brunier GM, Graydon J. The influence of physical activity on fatigue in patients with ESRD on hemodialysis. Anna J. 1993;20(4):457–461. discussion 62, 521. [PubMed] [Google Scholar]
- 35.Liu HE. Fatigue and associated factors in hemodialysis patients in Taiwan. Res Nurs Health. 2006;29(1):40–50. doi: 10.1002/nur.20109. [DOI] [PubMed] [Google Scholar]
- 36.Unruh M, Miskulin D, Yan G, et al. Racial differences in health-related quality of life among hemodialysis patients. Kidney Int. 2004;65(4):1482–1491. doi: 10.1111/j.1523-1755.2004.00529.x. [DOI] [PubMed] [Google Scholar]
- 37.Dantzer R, Kelley KW. Twenty years of research on cytokine-induced sickness behavior. Brain Behav Immun. 2007;21(2):153–160. doi: 10.1016/j.bbi.2006.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ganz PA, Bower JE. Cancer related fatigue: a focus on breast cancer and Hodgkin's disease survivors. Acta Oncol. 2007;46(4):474–479. doi: 10.1080/02841860701367845. [DOI] [PubMed] [Google Scholar]
- 39.Rao M, Wong C, Kanetsky P, et al. Cytokine gene polymorphism and progression of renal and cardiovascular diseases. Kidney Int. 2007 doi: 10.1038/sj.ki.5002391. [DOI] [PubMed] [Google Scholar]
- 40.Bergstrom J, Lindholm B, Lacson E, Jr, et al. What are the causes and consequences of the chronic inflammatory state in chronic dialysis patients? Seminars in dialysis. 2000;13(3):163–175. doi: 10.1046/j.1525-139x.2000.00044.x. [DOI] [PubMed] [Google Scholar]
- 41.Herbelin A, Nguyen AT, Zingraff J, Urena P, Descamps-Latscha B. Influence of uremia and hemodialysis on circulating interleukin-1 and tumor necrosis factor alpha. Kidney Int. 1990;37(1):116–125. doi: 10.1038/ki.1990.16. [DOI] [PubMed] [Google Scholar]
- 42.Memoli B, Minutolo R, Bisesti V, et al. Changes of serum albumin and C-reactive protein are related to changes of interleukin-6 release by peripheral blood mononuclear cells in hemodialysis patients treated with different membranes. Am J Kidney Dis. 2002;39(2):266–273. doi: 10.1053/ajkd.2002.30545. [DOI] [PubMed] [Google Scholar]
- 43.Girndt M, Sester U, Kaul H, Kohler H. Production of proinflammatory and regulatory monokines in hemodialysis patients shown at a single-cell level. J Am Soc Nephrol. 1998;9(9):1689–1696. doi: 10.1681/ASN.V991689. [DOI] [PubMed] [Google Scholar]
- 44.Memoli B, Libetta C, Rampino T, et al. Hemodialysis related induction of interleukin-6 production by peripheral blood mononuclear cells. Kidney Int. 1992;42(2):320–326. doi: 10.1038/ki.1992.292. [DOI] [PubMed] [Google Scholar]
- 45.Le Meur Y, Lorgeot V, Aldigier JC, Wijdenes J, Leroux-Robert C, Praloran V. Whole blood production of monocytic cytokines (IL-1 beta, IL-6, TNF-alpha, sIL-6R, IL-1Ra) in haemodialysed patients. Nephrol Dial Transplant. 1999;14(10):2420–2426. doi: 10.1093/ndt/14.10.2420. [DOI] [PubMed] [Google Scholar]
- 46.Cesari M, Penninx BW, Pahor M, et al. Inflammatory markers and physical performance in older persons: the InCHIANTI study. J Gerontol A Biol Sci Med Sci. 2004;59(3):242–248. doi: 10.1093/gerona/59.3.m242. [DOI] [PubMed] [Google Scholar]
- 47.Harris TB, Ferrucci L, Tracy RP, et al. Associations of elevated interleukin-6 and C-reactive protein levels with mortality in the elderly. Am J Med. 1999;106(5):506–512. doi: 10.1016/s0002-9343(99)00066-2. [DOI] [PubMed] [Google Scholar]
- 48.Janszky I, Lekander M, Blom M, Georgiades A, Ahnve S. Self-rated health and vital exhaustion, but not depression, is related to inflammation in women with coronary heart disease. Brain Behav Immun. 2005;19(6):555–563. doi: 10.1016/j.bbi.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 49.Visser M, Pahor M, Taaffe DR, et al. Relationship of interleukin-6 and tumor necrosis factor-alpha with muscle mass and muscle strength in elderly men and women: the Health ABC Study. J Gerontol A Biol Sci Med Sci. 2002;57(5):M326–M332. doi: 10.1093/gerona/57.5.m326. [DOI] [PubMed] [Google Scholar]
- 50.Buchwald D, Wener MH, Pearlman T, Kith P. Markers of inflammation and immune activation in chronic fatigue and chronic fatigue syndrome. J Rheumatol. 1997;24(2):372–376. [PubMed] [Google Scholar]
- 51.Kurzrock R. The role of cytokines in cancer-related fatigue. Cancer. 2001;92(6 Suppl):1684–1688. doi: 10.1002/1097-0142(20010915)92:6+<1684::aid-cncr1497>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 52.Seymour JF, Talpaz M, Cabanillas F, Wetzler M, Kurzrock R. Serum interleukin-6 levels correlate with prognosis in diffuse large-cell lymphoma. J Clin Oncol. 1995;13(3):575–582. doi: 10.1200/JCO.1995.13.3.575. [DOI] [PubMed] [Google Scholar]
- 53.Balakrishnan VS, Guo D, Rao M, et al. Cytokine gene polymorphisms in hemodialysis patients: association with comorbidity, functionality, and serum albumin. Kidney Int. 2004;65(4):1449–1460. doi: 10.1111/j.1523-1755.2004.00531.x. [DOI] [PubMed] [Google Scholar]
- 54.Kamimura MA, Draibe SA, Dalboni MA, et al. Serum and cellular interleukin-6 in haemodialysis patients: relationship with energy expenditure. Nephrol Dial Transplant. 2007;22(3):839–844. doi: 10.1093/ndt/gfl705. [DOI] [PubMed] [Google Scholar]
- 55.Kimmel PL, Phillips TM, Simmens SJ, et al. Immunologic function and survival in hemodialysis patients. Kidney Int. 1998;54(1):236–244. doi: 10.1046/j.1523-1755.1998.00981.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hopkins SJ. Central nervous system recognition of peripheral inflammation: a neural, hormonal collaboration. Acta Biomed. 2007;78 Suppl 1:231–247. [PubMed] [Google Scholar]
- 57.Adams F, Quesada JR, Gutterman JU. Neuropsychiatric manifestations of human leukocyte interferon therapy in patients with cancer. Jama. 1984;252(7):938–941. [PubMed] [Google Scholar]
- 58.Piepoli M, Clark AL, Volterrani M, Adamopoulos S, Sleight P, Coats AJ. Contribution of muscle afferents to the hemodynamic, autonomic, and ventilatory responses to exercise in patients with chronic heart failure: effects of physical training. Circulation. 1996;93(5):940–952. doi: 10.1161/01.cir.93.5.940. [DOI] [PubMed] [Google Scholar]
- 59.Bologa RM, Levine DM, Parker TS, et al. Interleukin-6 predicts hypoalbuminemia, hypocholesterolemia, and mortality in hemodialysis patients. Am J Kidney Dis. 1998;32(1):107–114. doi: 10.1053/ajkd.1998.v32.pm9669431. [DOI] [PubMed] [Google Scholar]
- 60.Brambilla F, Bellodi L, Perna G, Bertani A, Panerai A, Sacerdote P. Plasma interleukin-1 beta concentrations in panic disorder. Psychiatry Res. 1994;54(2):135–142. doi: 10.1016/0165-1781(94)90002-7. [DOI] [PubMed] [Google Scholar]
- 61.Bremmer MA, Beekman AT, Deeg DJ, et al. Inflammatory markers in late-life depression: Results from a population-based study. J Affect Disord. 2007 doi: 10.1016/j.jad.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 62.Kasapis C, Thompson PD. The effects of physical activity on serum C-reactive protein and inflammatory markers: a systematic review. J Am Coll Cardiol. 2005;45(10):1563–1569. doi: 10.1016/j.jacc.2004.12.077. [DOI] [PubMed] [Google Scholar]
- 63.Trzonkowski P, Mysliwska J, Godlewska B, et al. Immune consequences of the spontaneous pro-inflammatory status in depressed elderly patients. Brain Behav Immun. 2004;18(2):135–148. doi: 10.1016/S0889-1591(03)00111-9. [DOI] [PubMed] [Google Scholar]
- 64.Yang JY, Huang JW, Chiang CK, et al. Higher plasma interleukin-18 levels associated with poor quality of sleep in peritoneal dialysis patients. Nephrol Dial Transplant. 2007 doi: 10.1093/ndt/gfm231. [DOI] [PubMed] [Google Scholar]
- 65.Moreno F, Sanz-Guajardo D, Lopez-Gomez JM, Jofre R, Valderrabano F. Increasing the hematocrit has a beneficial effect on quality of life and is safe in selected hemodialysis patients. Spanish Cooperative Renal Patients Quality of Life Study Group of the Spanish Society of Nephrology. J Am Soc Nephrol. 2000;11(2):335–342. doi: 10.1681/ASN.V112335. [DOI] [PubMed] [Google Scholar]
- 66.Mann JF. What are the short-term and long-term consequences of anaemia in CRF patients? Nephrol Dial Transplant. 1999;14 Suppl 2:29–36. doi: 10.1093/ndt/14.suppl_2.29. [DOI] [PubMed] [Google Scholar]
- 67.Ross SD, Fahrbach K, Frame D, Scheye R, Connelly JE, Glaspy J. The effect of anemia treatment on selected health-related quality-of-life domains: a systematic review. Clin Ther. 2003;25(6):1786–1805. doi: 10.1016/s0149-2918(03)80170-4. [DOI] [PubMed] [Google Scholar]
- 68.Jones M, Ibels L, Schenkel B, Zagari M. Impact of epoetin alfa on clinical end points in patients with chronic renal failure: a meta-analysis. Kidney Int. 2004;65(3):757–767. doi: 10.1111/j.1523-1755.2004.00450.x. [DOI] [PubMed] [Google Scholar]
- 69.Drueke TB, Locatelli F, Clyne N, et al. Normalization of hemoglobin level in patients with chronic kidney disease and anemia. N Engl J Med. 2006;355(20):2071–2084. doi: 10.1056/NEJMoa062276. [DOI] [PubMed] [Google Scholar]
- 70.Singh AK, Szczech L, Tang KL, et al. Correction of anemia with epoetin alfa in chronic kidney disease. N Engl J Med. 2006;355(20):2085–2098. doi: 10.1056/NEJMoa065485. [DOI] [PubMed] [Google Scholar]
- 71.Ossareh S, Roozbeh J, Krishnan M, Liakopoulos V, Bargman JM, Oreopoulos DG. Fatigue in chronic peritoneal dialysis patients. Int Urol Nephrol. 2003;35(4):535–541. doi: 10.1023/b:urol.0000025610.67447.b9. [DOI] [PubMed] [Google Scholar]
- 72.Chikotas N, Gunderman A, Oman T. Uremic syndrome and end-stage renal disease: physical manifestations and beyond. J Am Acad Nurse Pract. 2006;18(5):195–202. doi: 10.1111/j.1745-7599.2006.00123.x. [DOI] [PubMed] [Google Scholar]
- 73.Pupim LB, Cuppari L, Ikizler TA. Nutrition and metabolism in kidney disease. Semin Nephrol. 2006;26(2):134–157. doi: 10.1016/j.semnephrol.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 74.Schreiber B. Levocarnitine and dialysis: a review. Nutr Clin Pract. 2005;20(2):218–243. doi: 10.1177/0115426505020002218. [DOI] [PubMed] [Google Scholar]
- 75.Brass EP, Adler S, Sietsema KE, Hiatt WR, Orlando AM, Amato A. Intravenous L-carnitine increases plasma carnitine, reduces fatigue, and may preserve exercise capacity in hemodialysis patients. Am J Kidney Dis. 2001;37(5):1018–1028. doi: 10.1016/s0272-6386(05)80019-8. [DOI] [PubMed] [Google Scholar]
- 76.Wu AW, Fink NE, Marsh-Manzi JV, et al. Changes in quality of life during hemodialysis and peritoneal dialysis treatment: generic and disease specific measures. J Am Soc Nephrol. 2004;15(3):743–753. doi: 10.1097/01.asn.0000113315.81448.ca. [DOI] [PubMed] [Google Scholar]
- 77.Cueto-Manzano AM, Gonzalez-Espinoza L, Martin del Campo F, Fortes PC, Pecoits-Filho R. Inflammation in peritoneal dialysis: a Latin-American perspective. Perit Dial Int. 2007;27(3):347–352. [PubMed] [Google Scholar]
- 78.Pecoits-Filho R, Stenvinkel P, Wang AY, Heimburger O, Lindholm B. Chronic inflammation in peritoneal dialysis: the search for the holy grail? Perit Dial Int. 2004;24(4):327–339. [PubMed] [Google Scholar]
- 79.Yngman-Uhlin P, Edell-Gustafsson U. Self-reported subjective sleep quality and fatigue in patients with peritoneal dialysis treatment at home. Int J Nurs Pract. 2006;12(3):143–152. doi: 10.1111/j.1440-172X.2006.00566.x. [DOI] [PubMed] [Google Scholar]
- 80.Kutner NG. Quality of life and daily hemodialysis. Seminars in dialysis. 2004;17(2):92–98. doi: 10.1111/j.0894-0959.2004.17203.x. [DOI] [PubMed] [Google Scholar]
- 81.Suri RS, Nesrallah GE, Mainra R, et al. Daily hemodialysis: a systematic review. Clin J Am Soc Nephrol. 2006;1(1):33–42. doi: 10.2215/CJN.00340705. [DOI] [PubMed] [Google Scholar]
- 82.Suri RS, Garg AX, Chertow GM, et al. Frequent Hemodialysis Network (FHN) randomized trials: study design. Kidney Int. 2007;71(4):349–359. doi: 10.1038/sj.ki.5002032. [DOI] [PubMed] [Google Scholar]
- 83.Sklar A, Newman N, Scott R, Semenyuk L, Schultz J, Fiacco V. Identification of factors responsible for postdialysis fatigue. Am J Kidney Dis. 1999;34(3):464–470. doi: 10.1016/s0272-6386(99)70073-9. [DOI] [PubMed] [Google Scholar]
- 84.Sklar AH, Riesenberg LA, Silber AK, Ahmed W, Ali A. Postdialysis fatigue. Am J Kidney Dis. 1996;28(5):732–736. doi: 10.1016/s0272-6386(96)90256-5. [DOI] [PubMed] [Google Scholar]
- 85.Dreisbach AW, Hendrickson T, Beezhold D, Riesenberg LA, Sklar AH. Elevated levels of tumor necrosis factor alpha in postdialysis fatigue. Int J Artif Organs. 1998;21(2):83–86. [PubMed] [Google Scholar]
- 86.Lindsay RM, Kortas C. Hemeral (daily) hemodialysis. Advances in renal replacement therapy. 2001;8(4):236–249. doi: 10.1053/jarr.2001.27593. [DOI] [PubMed] [Google Scholar]
- 87.Heidenheim AP, Muirhead N, Moist L, Lindsay RM. Patient quality of life on quotidian hemodialysis. Am J Kidney Dis. 2003;42(1 Suppl):36–41. doi: 10.1016/s0272-6386(03)00536-5. [DOI] [PubMed] [Google Scholar]
- 88.Kutner NG, Brogan D. Fielding B. Physical and psychosocial resource variables related to long-term survival in older dialysis patients. Geriatric nephrology and urology. 1997;7(1):23–28. doi: 10.1023/a:1008204311582. [DOI] [PubMed] [Google Scholar]
- 89.Kutner NG, Lin LS, Fielding B, Brogan D, Hall WD. Continued survival of older hemodialysis patients: investigation of psychosocial predictors. Am J Kidney Dis. 1994;24(1):42–49. doi: 10.1016/s0272-6386(12)80158-2. [DOI] [PubMed] [Google Scholar]
- 90.Aukrust P, Yndestad A, Smith C, Ueland T, Gullestad L, Damas JK. Chemokines in cardiovascular risk prediction. Thromb Haemost. 2007;97(5):748–754. [PubMed] [Google Scholar]
- 91.Merlino G, Piani A, Dolso P, et al. Sleep disorders in patients with end-stage renal disease undergoing dialysis therapy. Nephrol Dial Transplant. 2006;21(1):184–190. doi: 10.1093/ndt/gfi144. [DOI] [PubMed] [Google Scholar]
- 92.Unruh ML, Levey AS, D'Ambrosio C, Fink NE, Powe NR, Meyer KB. Restless legs symptoms among incident dialysis patients: association with lower quality of life and shorter survival. Am J Kidney Dis. 2004;43(5):900–909. doi: 10.1053/j.ajkd.2004.01.013. [DOI] [PubMed] [Google Scholar]
- 93.Unruh ML, Buysse DJ, Dew MA, et al. Sleep quality and its correlates in the first year of dialysis. Clin J Am Soc Nephrol. 2006;1(4):802–810. doi: 10.2215/CJN.00710206. [DOI] [PubMed] [Google Scholar]
- 94.Sanner BM, Tepel M, Esser M, et al. Sleep-related breathing disorders impair quality of life in haemodialysis recipients. Nephrol Dial Transplant. 2002;17(7):1260–1265. doi: 10.1093/ndt/17.7.1260. [DOI] [PubMed] [Google Scholar]
- 95.Krueger JM, Fang J, Taishi P, Chen Z, Kushikata T, Gardi J. Sleep. A physiologic role for IL-1 beta and TNF-alpha. Ann N Y Acad Sci. 1998;856:148–159. doi: 10.1111/j.1749-6632.1998.tb08323.x. [DOI] [PubMed] [Google Scholar]
- 96.Mills PJ, Dimsdale JE. Sleep apnea: a model for studying cytokines, sleep, and sleep disruption. Brain Behav Immun. 2004;18(4):298–303. doi: 10.1016/j.bbi.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 97.Vgontzas AN, Zoumakis M, Bixler EO, et al. Impaired nighttime sleep in healthy old versus young adults is associated with elevated plasma interleukin-6 and cortisol levels: physiologic and therapeutic implications. J Clin Endocrinol Metab. 2003;88(5):2087–2095. doi: 10.1210/jc.2002-021176. [DOI] [PubMed] [Google Scholar]
- 98.Gul A, Aoun N, Trayner EM., Jr Why do patients sleep on dialysis? Seminars in dialysis. 2006;19(2):152–157. doi: 10.1111/j.1525-139X.2006.00142.x. [DOI] [PubMed] [Google Scholar]
- 99.Entzian P, Linnemann K, Schlaak M, Zabel P. Obstructive sleep apnea syndrome and circadian rhythms of hormones and cytokines. Am J Respir Crit Care Med. 1996;153(3):1080–1086. doi: 10.1164/ajrccm.153.3.8630548. [DOI] [PubMed] [Google Scholar]
- 100.Vgontzas AN, Papanicolaou DA, Bixler EO, Kales A, Tyson K, Chrousos GP. Elevation of plasma cytokines in disorders of excessive daytime sleepiness: role of sleep disturbance and obesity. J Clin Endocrinol Metab. 1997;82(5):1313–1316. doi: 10.1210/jcem.82.5.3950. [DOI] [PubMed] [Google Scholar]
- 101.Hedayati SS, Bosworth HB, Kuchibhatla M, Kimmel PL, Szczech LA. The predictive value of self-report scales compared with physician diagnosis of depression in hemodialysis patients. Kidney Int. 2006;69(9):1662–1668. doi: 10.1038/sj.ki.5000308. [DOI] [PubMed] [Google Scholar]
- 102.Kimmel PL, Cukor D, Cohen SD, Peterson RA. Depression in end-stage renal disease patients: a critical review. Adv Chronic Kidney Dis. 2007;14(4):328–334. doi: 10.1053/j.ackd.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 103.Watnick S, Kirwin P, Mahnensmith R, Concato J. The prevalence and treatment of depression among patients starting dialysis. Am J Kidney Dis. 2003;41(1):105–110. doi: 10.1053/ajkd.2003.50029. [DOI] [PubMed] [Google Scholar]
- 104.Bosker FJ, Westerink BH, Cremers TI, et al. Future antidepressants: what is in the pipeline and what is missing? CNS Drugs. 2004;18(11):705–732. doi: 10.2165/00023210-200418110-00002. [DOI] [PubMed] [Google Scholar]
- 105.Penninx BW, Kritchevsky SB, Yaffe K, et al. Inflammatory markers and depressed mood in older persons: results from the Health, Aging and Body Composition study. Biol Psychiatry. 2003;54(5):566–572. doi: 10.1016/s0006-3223(02)01811-5. [DOI] [PubMed] [Google Scholar]
- 106.Suarez EC, Lewis JG, Krishnan RR, Young KH. Enhanced expression of cytokines and chemokines by blood monocytes to in vitro lipopolysaccharide stimulation are associated with hostility and severity of depressive symptoms in healthy women. Psychoneuroendocrinology. 2004;29(9):1119–1128. doi: 10.1016/j.psyneuen.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 107.Lichtenstein GR, Bala M, Han C, DeWoody K, Schaible T. Infliximab improves quality of life in patients with Crohn's disease. Inflamm Bowel Dis. 2002;8(4):237–243. doi: 10.1097/00054725-200207000-00001. [DOI] [PubMed] [Google Scholar]
- 108.Lee SK, Lee HS, Lee TB, et al. The effects of antidepressant treatment on serum cytokines and nutritional status in hemodialysis patients. J Korean Med Sci. 2004;19(3):384–389. doi: 10.3346/jkms.2004.19.3.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Vgontzas AN, Bixler EO, Chrousos GP. Obesity-related sleepiness and fatigue: the role of the stress system and cytokines. Ann N Y Acad Sci. 2006;1083:329–344. doi: 10.1196/annals.1367.023. [DOI] [PubMed] [Google Scholar]
- 110.Nicklas BJ, Mychaleckyj J, Kritchevsky S, et al. Physical function and its response to exercise: associations with cytokine gene variation in older adults with knee osteoarthritis. J Gerontol A Biol Sci Med Sci. 2005;60(10):1292–1298. doi: 10.1093/gerona/60.10.1292. [DOI] [PubMed] [Google Scholar]
- 111.Wilund KR. Is the anti-inflammatory effect of regular exercise responsible for reduced cardiovascular disease? Clin Sci (Lond) 2007;112(11):543–555. doi: 10.1042/CS20060368. [DOI] [PubMed] [Google Scholar]
- 112.Hung AM, Chertow GM, Young BS, Carey S, Johansen KL. Inflammatory markers are unrelated to physical activity, performance, and functioning in hemodialysis. J Ren Nutr. 2002;12(3):170–176. doi: 10.1053/jren.2002.33513. [DOI] [PubMed] [Google Scholar]
- 113.Lee SW, Park GH, Lee SW, Song JH, Hong KC, Kim MJ. Insulin resistance and muscle wasting in non-diabetic end-stage renal disease patients. Nephrol Dial Transplant. 2007;22(9):2554–2562. doi: 10.1093/ndt/gfm204. [DOI] [PubMed] [Google Scholar]
- 114.Rajan VR, Mitch WE. Muscle wasting in chronic kidney disease: the role of the ubiquitin proteasome system and its clinical impact. Pediatr Nephrol. 2007 doi: 10.1007/s00467-007-0594-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Fitts RH. Cellular mechanisms of muscle fatigue. Physiol Rev. 1994;74(1):49–94. doi: 10.1152/physrev.1994.74.1.49. [DOI] [PubMed] [Google Scholar]
- 116.Johansen KL, Doyle J, Sakkas GK, Kent-Braun JA. Neural and metabolic mechanisms of excessive muscle fatigue in maintenance hemodialysis patients. Am J Physiol Regul Integr Comp Physiol. 2005;289(3):R805–R813. doi: 10.1152/ajpregu.00187.2005. [DOI] [PubMed] [Google Scholar]
- 117.Storer TW, Casaburi R, Sawelson S, Kopple JD. Endurance exercise training during haemodialysis improves strength, power, fatigability and physical performance in maintenance haemodialysis patients. Nephrol Dial Transplant. 2005;20(7):1429–1437. doi: 10.1093/ndt/gfh784. [DOI] [PubMed] [Google Scholar]
- 118.Deligiannis A. Exercise rehabilitation and skeletal muscle benefits in hemodialysis patients. Clin Nephrol. 2004;61 Suppl 1:S46–S50. [PubMed] [Google Scholar]
- 119.Tsay SL. Acupressure and fatigue in patients with end-stage renal disease-a randomized controlled trial. Int J Nurs Stud. 2004;41(1):99–106. doi: 10.1016/s0020-7489(03)00079-8. [DOI] [PubMed] [Google Scholar]
- 120.Tsay SL, Cho YC, Chen ML. Acupressure and Transcutaneous Electrical Acupoint Stimulation in improving fatigue, sleep quality and depression in hemodialysis patients. Am J Chin Med. 2004;32(3):407–416. doi: 10.1142/S0192415X04002065. [DOI] [PubMed] [Google Scholar]
- 121.Yurtkuran M, Alp A, Yurtkuran M, Dilek K. A modified yoga-based exercise program in hemodialysis patients: a randomized controlled study. Complement Ther Med. 2007;15(3):164–171. doi: 10.1016/j.ctim.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 122.Mathiowetz VG, Finlayson ML, Matuska KM, Chen HY, Luo P. Randomized controlled trial of an energy conservation course for persons with multiple sclerosis. Mult Scler. 2005;11(5):592–601. doi: 10.1191/1352458505ms1198oa. [DOI] [PubMed] [Google Scholar]
- 123.Feldt-Rasmussen B, Lange M, Sulowicz W, et al. Growth hormone treatment during hemodialysis in a randomized trial improves nutrition, quality of life, and cardiovascular risk. J Am Soc Nephrol. 2007;18(7):2161–2171. doi: 10.1681/ASN.2006111207. [DOI] [PubMed] [Google Scholar]
- 124.Kotzmann H, Yilmaz N, Lercher P, et al. Differential effects of growth hormone therapy in malnourished hemodialysis patients. Kidney Int. 2001;60(4):1578–1585. doi: 10.1046/j.1523-1755.2001.00971.x. [DOI] [PubMed] [Google Scholar]
- 125.Minton O, Stone P, Richardson A, Sharpe M, Hotopf M. Drug therapy for the management of cancer related fatigue. Cochrane Database Syst Rev. 2008;(1):CD006704. doi: 10.1002/14651858.CD006704.pub2. [DOI] [PubMed] [Google Scholar]
- 126.Tong A, Lowe A, Sainsbury P, Craig JC. Experiences of parents who have children with chronic kidney disease: a systematic review of qualitative studies. Pediatrics. 2008;121(2):349–360. doi: 10.1542/peds.2006-3470. [DOI] [PubMed] [Google Scholar]
- 127.Wang AY, Sea MM, Tang N, et al. Resting energy expenditure and subsequent mortality risk in peritoneal dialysis patients. J Am Soc Nephrol. 2004;15(12):3134–3143. doi: 10.1097/01.ASN.0000144206.29951.B2. [DOI] [PubMed] [Google Scholar]
- 128.Schubert C, Hong S, Natarajan L, Mills PJ, Dimsdale JE. The association between fatigue and inflammatory marker levels in cancer patients: a quantitative review. Brain Behav Immun. 2007;21(4):413–427. doi: 10.1016/j.bbi.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 129.Collado-Hidalgo A, Bower JE, Ganz PA, Cole SW, Irwin MR. Inflammatory biomarkers for persistent fatigue in breast cancer survivors. Clin Cancer Res. 2006;12(9):2759–2766. doi: 10.1158/1078-0432.CCR-05-2398. [DOI] [PubMed] [Google Scholar]
- 130.Meyers CA, Albitar M, Estey E. Cognitive impairment, fatigue, and cytokine levels in patients with acute myelogenous leukemia or myelodysplastic syndrome. Cancer. 2005;104(4):788–793. doi: 10.1002/cncr.21234. [DOI] [PubMed] [Google Scholar]