Abstract
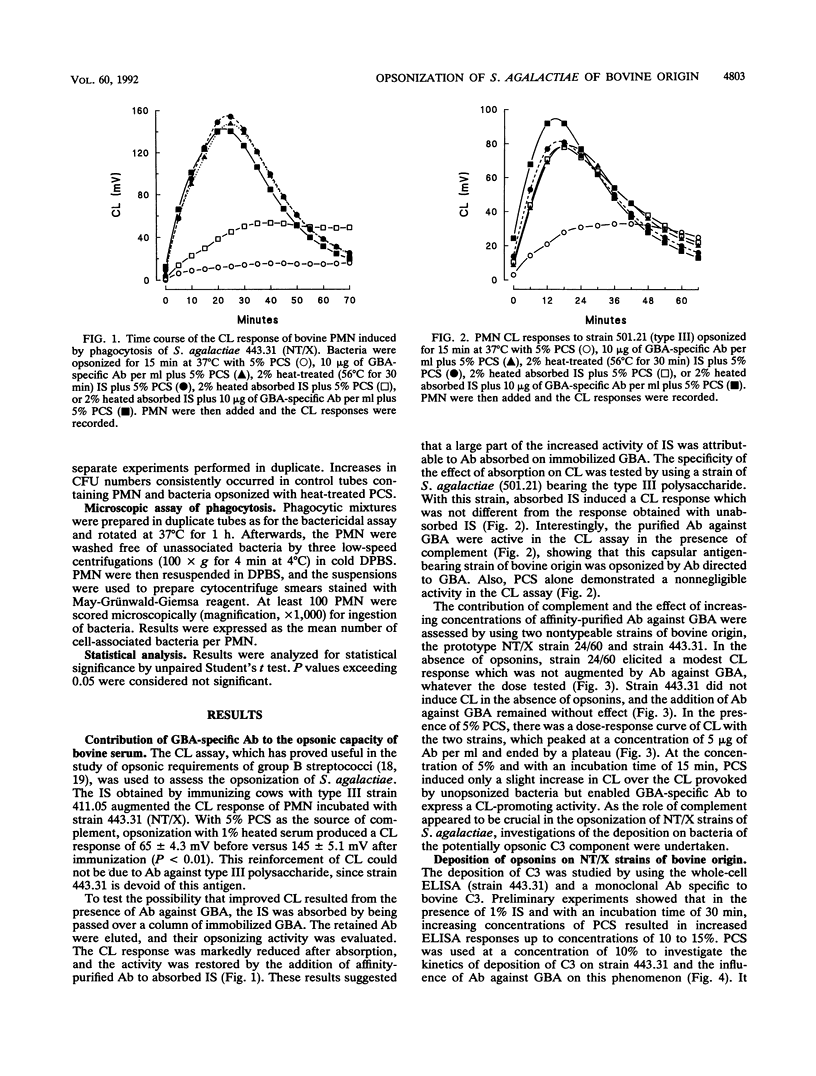
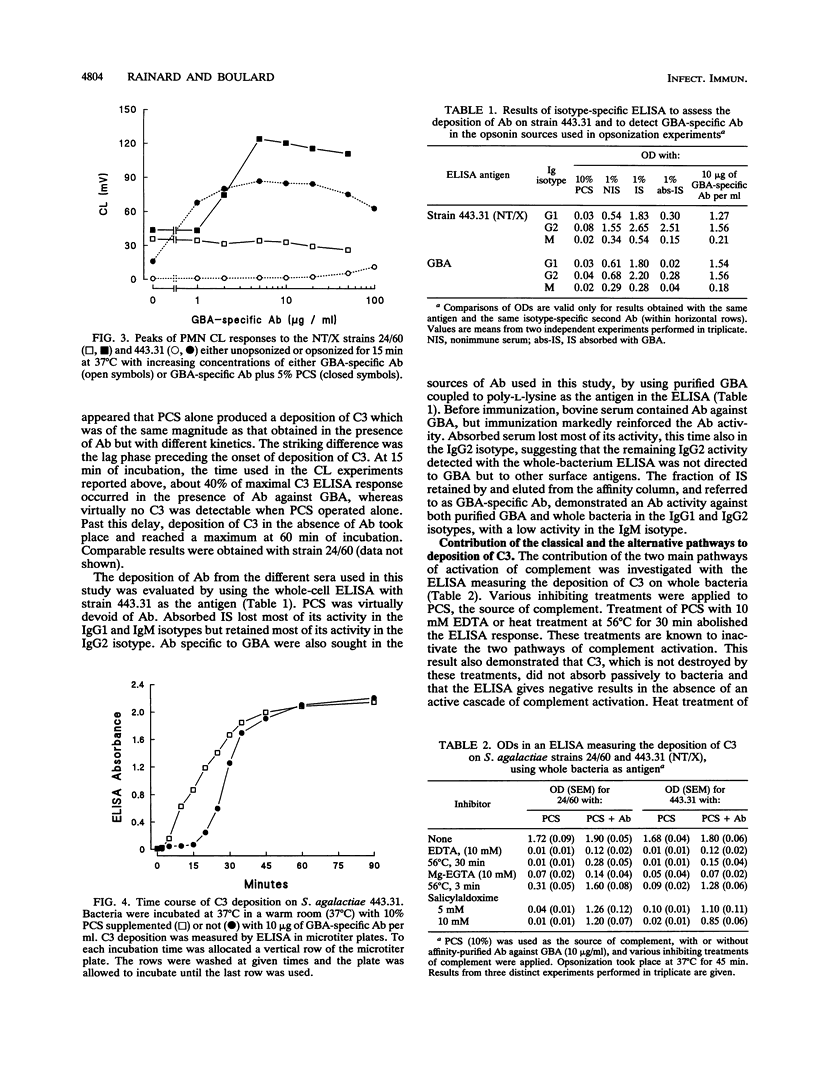
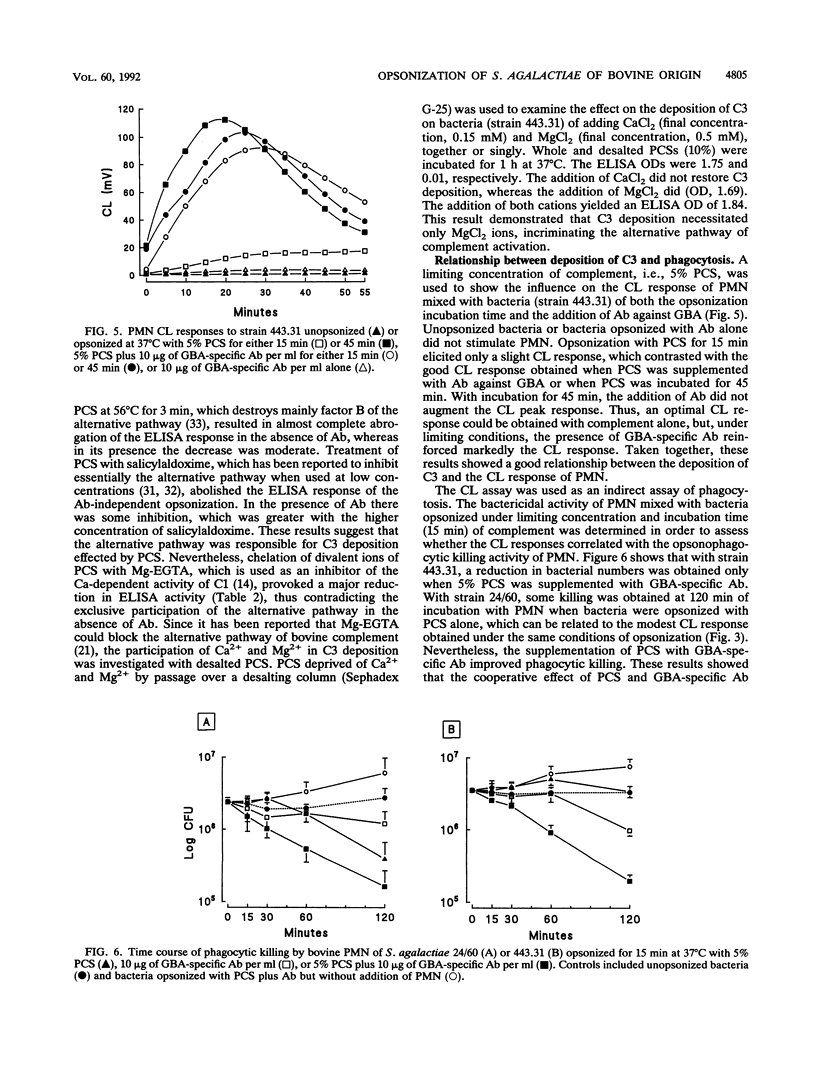
The contribution of bovine complement and antibodies (Ab) against the group B polysaccharidic antigen (GBA) to the opsonization of Streptococcus agalactiae isolated from bovine mastitis cases was investigated by using affinity-purified Ab. GBA-specific Ab were not opsonic by themselves, but in the presence of complement (precolostral calf serum) with an opsonization time of 15 min, they exhibited a dose-dependent opsonic activity in a polymorphonuclear leukocyte chemiluminescence assay. Kinetic studies of the deposition of complement component C3 on protein X-bearing nontypeable (NT/X) strains with an enzyme-linked immunosorbent assay showed that C3 was deposited on bacteria in the absence of Ab but that GBA-specific Ab markedly accelerated the process by reducing the lag phase, which extended up to 15 min when Ab were absent. In the absence of Ab, C3 deposition was inhibited by 5 mM salicylaldoxime or heat treatment at 56 degrees C for 3 min and necessitated Mg2+ ions but not Ca2+ ions, suggesting that activation of complement was effected by the alternative pathway only. When GBA-specific Ab were added to complement, the inhibitory treatments lost much of their efficacy, suggesting that the classical pathway was recruited. Deposition of C3 on NT/X strains in the absence of Ab induced chemiluminescence and phagocytic killing. With the addition of GBA-specific Ab, the numbers of surviving bacteria were halved (P < 0.05) compared with killing in the presence of complement alone. It can be concluded that NT/X strains are activators of the alternative pathway of complement and that GBA-specific Ab reinforce the opsonic efficiency of serum by recruiting the classical pathway and slightly enhancing phagocytic killing.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker C. J., Edwards M. S., Webb B. J., Kasper D. L. Antibody-independent classical pathway-mediated opsonophagocytosis of type Ia, group B streptococcus. J Clin Invest. 1982 Feb;69(2):394–404. doi: 10.1172/JCI110463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker C. J. Group B streptococcal infections. Adv Intern Med. 1980;25:475–501. [PubMed] [Google Scholar]
- Baker C. J., Kasper D. L. Group B streptococcal vaccines. Rev Infect Dis. 1985 Jul-Aug;7(4):458–467. doi: 10.1093/clinids/7.4.458. [DOI] [PubMed] [Google Scholar]
- Baltimore R. S., Kasper D. L., Baker C. J., Goroff D. K. Antigenic specificity of opsonophagocytic antibodies in rabbit anti-sera to group B streptococci. J Immunol. 1977 Feb;118(2):673–678. [PubMed] [Google Scholar]
- Boulard C. Degradation of bovine C3 by serine proteases from parasites Hypoderma lineatum (Diptera, Oestridae). Vet Immunol Immunopathol. 1989 Mar;20(4):387–398. doi: 10.1016/0165-2427(89)90083-4. [DOI] [PubMed] [Google Scholar]
- Carlson G. P., Kaneko J. J. Isolation of leukocytes from bovine peripheral blood. Proc Soc Exp Biol Med. 1973 Mar;142(3):853–856. doi: 10.3181/00379727-142-37131. [DOI] [PubMed] [Google Scholar]
- De Cueninck B. J. C142 complement activity and conglutinogen in bovine milk. Int Arch Allergy Appl Immunol. 1979;59(3):323–327. doi: 10.1159/000232276. [DOI] [PubMed] [Google Scholar]
- De Cueninck B. J., Shockman G. D., Swenson R. M. Group B, type III streptococcal cell wall: composition and structural aspects revealed through endo-N-acetylmuramidase-catalyzed hydrolysis. Infect Immun. 1982 Feb;35(2):572–581. doi: 10.1128/iai.35.2.572-581.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobbins C. N., Jr Mastitis losses. J Am Vet Med Assoc. 1977 May 15;170(10 Pt 2):1129–1132. [PubMed] [Google Scholar]
- Duncan J. R., Wilkie B. N., Hiestand F., Winter A. J. The serum and secretory immunoglobulins of cattle: characterization and quantitation. J Immunol. 1972 Apr;108(4):965–976. [PubMed] [Google Scholar]
- Eads M. E., Levy N. J., Kasper D. L., Baker C. J., Nicholson-Weller A. Antibody-independent activation of C1 by type Ia group B streptococci. J Infect Dis. 1982 Nov;146(5):665–672. doi: 10.1093/infdis/146.5.665. [DOI] [PubMed] [Google Scholar]
- Edwards M. S., Nicholson-Weller A., Baker C. J., Kasper D. L. The role of specific antibody in alternative complement pathway-mediated opsonophagocytosis of type III, group B Streptococcus. J Exp Med. 1980 May 1;151(5):1275–1287. doi: 10.1084/jem.151.5.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fine D. P., Marney S. R., Jr, Colley D. G., Sergent J. S., Des Prez R. M. C3 shunt activation in human serum chelated with EGTA. J Immunol. 1972 Oct;109(4):807–809. [PubMed] [Google Scholar]
- Fong J. S., Muschel L. H., Good R. A. Kinetics of bovine complement. I. Formation of a lytic intermediate. J Immunol. 1971 Jul;107(1):28–33. [PubMed] [Google Scholar]
- Gray B. M. ELISA methodology for polysaccharide antigens: protein coupling of polysaccharides for adsorption to plastic tubes. J Immunol Methods. 1979;28(1-2):187–192. doi: 10.1016/0022-1759(79)90340-5. [DOI] [PubMed] [Google Scholar]
- Hahn G., Tolle A. Vergleichende Untersuchungen zur Charakterisierung von aus Mensch und Rind isolierten Gruppe B-Streptokokken (Sc. agalactiae) mit Hilfe eines Bakterizidie-Tests. Zentralbl Bakteriol A. 1981 Mar;249(1):15–23. [PubMed] [Google Scholar]
- Hastings M. J., Easmon C. S. Variations in the opsonic requirements of group B streptococcus type III. Br J Exp Pathol. 1981 Oct;62(5):519–525. [PMC free article] [PubMed] [Google Scholar]
- Hemming V. G., Hall R. T., Rhodes P. G., Shigeoka A. O., Hill H. R. Assessment of group B streptococcal opsonins in human and rabbit serum by neutrophil chemiluminescence. J Clin Invest. 1976 Dec;58(6):1379–1387. doi: 10.1172/JCI108593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard C. J. Variation in the susceptibility of bovine mycoplasmas to killing by the alternative complement pathway in bovine serum. Immunology. 1980 Nov;41(3):561–568. [PMC free article] [PubMed] [Google Scholar]
- Jensen N. E. Production and evaluation of antisera for serological type determination of group-B streptococci by double diffusion in agarose gel. Acta Pathol Microbiol Scand B. 1979 Apr;87B(2):77–83. doi: 10.1111/j.1699-0463.1979.tb02407.x. [DOI] [PubMed] [Google Scholar]
- Kasper D. L. Bacterial capsule--old dogmas and new tricks. J Infect Dis. 1986 Mar;153(3):407–415. doi: 10.1093/infdis/153.3.407. [DOI] [PubMed] [Google Scholar]
- Lancefield R. C., McCarty M., Everly W. N. Multiple mouse-protective antibodies directed against group B streptococci. Special reference to antibodies effective against protein antigens. J Exp Med. 1975 Jul 1;142(1):165–179. doi: 10.1084/jem.142.1.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackie D. P., Pollock D. A., Logan E. F. In vitro bactericidal assay of bovine polymorphonuclear leucocytes against a group B streptococcus. Res Vet Sci. 1982 Sep;33(2):240–242. [PubMed] [Google Scholar]
- Mackie D. P., Pollock D. A., Logan E. F. The opsonic activity of whey and sera from heifers experimentally infected with Streptococcus agalactiae. Br Vet J. 1985 Jul-Aug;141(4):349–354. doi: 10.1016/0007-1935(85)90083-1. [DOI] [PubMed] [Google Scholar]
- McGuire T. C., Musoke A. J., Kurtti T. Functional properties of bovine IgG1 and IgG2: interaction with complement, macrophages, neutrophils and skin. Immunology. 1979 Oct;38(2):249–256. [PMC free article] [PubMed] [Google Scholar]
- Morrison J. R., Wright C. L. Streptococcus agalactiae serotypes in the south west of Scotland. Vet Rec. 1984 Oct 27;115(17):439–439. doi: 10.1136/vr.115.17.439. [DOI] [PubMed] [Google Scholar]
- Mueller R., Carroll E. J., Panico L. Complement C 3 levels and haemolytic activity in normal and mastitic whey. Zentralbl Veterinarmed B. 1982 Mar;29(2):99–106. doi: 10.1111/j.1439-0450.1982.tb01204.x. [DOI] [PubMed] [Google Scholar]
- Oliver S. P., Mitchell B. A. Prevalence of mastitis pathogens in herds participating in a mastitis control program. J Dairy Sci. 1984 Oct;67(10):2436–2440. doi: 10.3168/jds.S0022-0302(84)81592-1. [DOI] [PubMed] [Google Scholar]
- Osler A. G., Sandberg A. L. Alternate complement pathways. Prog Allergy. 1973;17(0):51–92. [PubMed] [Google Scholar]
- Pang A. S., Aston W. P. Alternative complement pathway in bovine serum: lysis of human erythrocytes. Am J Vet Res. 1977 Mar;38(3):355–359. [PubMed] [Google Scholar]
- Pangburn M. K., Schreiber R. D., Müller-Eberhard H. J. C3b deposition during activation of the alternative complement pathway and the effect of deposition on the activating surface. J Immunol. 1983 Oct;131(4):1930–1935. [PubMed] [Google Scholar]
- Raff H. V., Siscoe P. J., Wolff E. A., Maloney G., Shuford W. Human monoclonal antibodies to group B streptococcus. Reactivity and in vivo protection against multiple serotypes. J Exp Med. 1988 Sep 1;168(3):905–917. doi: 10.1084/jem.168.3.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rainard P. Isotype antibody response in cows to Streptococcus agalactiae group B polysaccharide-ovalbumin conjugate. J Clin Microbiol. 1992 Jul;30(7):1856–1862. doi: 10.1128/jcm.30.7.1856-1862.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rainard P., Lautrou Y., Poutrel B. Ingestion and killing of Streptococcus agalactiae by bovine granulocytes in the presence of natural opsonins. Vet Microbiol. 1988 Sep;18(1):41–50. doi: 10.1016/0378-1135(88)90114-9. [DOI] [PubMed] [Google Scholar]
- Rainard P., Lautrou Y., Sarradin P., Poutrel B. Protein X of Streptococcus agalactiae induces opsonic antibodies in cows. J Clin Microbiol. 1991 Sep;29(9):1842–1846. doi: 10.1128/jcm.29.9.1842-1846.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rainard P., Poutrel B., Caffin J. P. Assessment of hemolytic and bactericidal complement activities in normal and mastitic bovine milk. J Dairy Sci. 1984 Mar;67(3):614–619. doi: 10.3168/jds.S0022-0302(84)81346-6. [DOI] [PubMed] [Google Scholar]
- Reite B., Oram J. D. Bacterial inhibitors in milk and other biological fluids. Nature. 1967 Oct 28;216(5113):328–330. doi: 10.1038/216328a0. [DOI] [PubMed] [Google Scholar]
- Schalm O. W., Lasmanis J., Carroll E. J. Experimental Streptococcus agalactiae mastitis in cattle: attempts to superimpose the organism in lactating glands harboring unrelated bacterial infections and in glands with experimentally induced sterile inflammation. Am J Vet Res. 1967 May;28(124):685–695. [PubMed] [Google Scholar]
- Wibawan I. W., Lämmler C. Properties of group B streptococci with protein surface antigens X and R. J Clin Microbiol. 1990 Dec;28(12):2834–2836. doi: 10.1128/jcm.28.12.2834-2836.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson C. D., Richards M. S. A survey of mastitis in the British dairy herd. Vet Rec. 1980 May 24;106(21):431–435. doi: 10.1136/vr.106.21.431. [DOI] [PubMed] [Google Scholar]
- van Zaane D., Ijzerman J. Monoclonal antibodies against bovine immunoglobulins and their use in isotype-specific ELISAs for rotavirus antibody. J Immunol Methods. 1984 Sep 4;72(2):427–441. doi: 10.1016/0022-1759(84)90011-5. [DOI] [PMC free article] [PubMed] [Google Scholar]



