Abstract
Introduction
The optimum duration of cardiopulmonary resuscitation (CPR) prior to first rescue shock is unknown. Clinical trials have used 90s and 180s. Neither of these durations may be optimal. We sought to determine the optimum duration of CPR prior to first defibrillation attempt and whether this varied depending on the duration of ventricular fibrillation (VF). In this porcine model of basic life support, our outcomes were rates of return of spontaneous circulation (ROSC), survival, and coronary perfusion pressure (CPP).
Methods
We anesthetized and instrumented 45 swine and then induced VF. After 5 or 8 minutes of untreated VF, we randomized the swine to mechanical CPR for 90, 180, or 300s. A single rescue shock (150J biphasic) was then administered. If this shock failed, 2 minutes of mechanical CPR were completed prior to the next rescue shock. CPP was calculated for each 30 second epoch. ROSC was defined as a blood pressure >80mmHg sustained for 60s. Survival was defined as sustained ROSC for 20 minutes. Data were analyzed with descriptive statistics, Fisher’s exact test, and ANOVA.
Results
In the 5 minute VF cohort, the rate of ROSC did not differ between the three groups (90s: 25%; 180s: 38%; 300s: 38%, p>.05). Survival rates did not differ (90s: 25%; 180s: 25%; 300s: 25%, p>0.05). In the 8 minute VF cohort, no animals experienced ROSC or survival. CPP were calculated by 30 second epoch and did not differ between the three groups (p>0.05). CPPs decline after 180s of CPR.
Conclusions
ROSC and survival were equivalent regardless of VF duration and CPR duration. When CPR begins late, CPPs are low, stressing the importance of early CPR. We do not recommend 300s of CPR unless a defibrillator is unavailable.
Keywords: Cardiopulmonary resuscitation (CPR), Resuscitation, Heart Arrest, Drugs
Introduction
The optimum duration of cardiopulmonary resuscitation (CPR) prior to first rescue shock in prolonged ventricular fibrillation (VF) cardiac arrest is not known. High-quality CPR improves the chances for survival. [1, 2] The American Heart Association Emergency Cardiac Care Guidelines (AHA ECC) recommends five cycles of chest compressions (~ 2 minutes) prior to rescue countershock in cases of unwitnessed cardiac arrest. [1] Prior animal literature has demonstrated that increasing ratios of compressions to ventilations in short-duration VF cardiac arrest results in increased survival. [3, 4] Clinical trials by Cobb et al. examined 90s of CPR prior to countershock while Wik et al. examined 180s of CPR prior to countershock. [5, 6] Clinical studies have demonstrated that providing continuous chest compressions (CCC) may improve outcomes in selected cohorts. [7, 8] Each of these studies note improvement in cohorts experiencing short-duration VF arrest. We sought to determine the optimum duration of CPR prior to the first defibrillation attempt. We also sought to determine whether this optimum amount of CPR varied depending on the duration of VF. In this porcine model of basic life support (BLS), our outcomes were rates of return of spontaneous circulation (ROSC), survival, and coronary perfusion pressure (CPP). This BLS model simulates an out-of-hospital cardiac arrest being treated by minimally trained first responders and/or basic emergency medical technicians, equipped with an automated external defibrillator (AED). These caregivers do not have the capability to administer drugs.
Methods
The University of Pittsburgh Institutional Animal Care and Use Committee approved this study.
Animal Preparation
All animals were prepared in a standardized fashion. We sedated the animals with intramuscular ketamine (10.0 mg/kg) and xylazine (4.0 mg/kg). We gained intravenous (IV) access via a peripheral ear vein. We established a surgical plane of anesthesia using a rapid IV infusion of alpha-chloralose (50 mg/kg), and maintained this with a continuous infusion of the same (10 mg/kg/hr).
We intubated the swine with a 5-0 cuffed endotracheal tube via direct laryngoscopy, and ventilated them with a FiO2 of 21% using an Ohmeda 7000 ventilator (Ohmeda, BOC Health Care, Madison, WI). Ventilation was begun at a tidal volume of 15-18 cc/kg, a ventilatory rate of 12 breaths per minute, and an inspiration: expiration ratio of 50%. Ventilation was adjusted to maintain eucapnea (end-tidal carbon dioxide 35-45 torr), which we measured with a side-steam capnometer (LifePak 12, Medtronic Physio-Control, Inc., Redmond, Washington). We measured core body temperature by placing an esophageal probe (Bi-Temp Temperature Monitor, Respiratory Supply Products, Inc., Irvine, California) into the animals’ esophagus. We placed three surface electrodes configured to correspond to a standard Lead II electrocardiogram (ECG), and monitored this continuously. After establishing a surgical depth plane anesthesia, we paralyzed the animals with pancuronium (4 mg initial bolus IV with additional 2 mg boluses as needed).
We then placed arterial and venous introducers (9 Fr) in the right femoral artery and vein and passed 7 Fr micro-manometer tipped pressure catheters (Mikro-Tip Catheter Transducers SPR-471A and SPC-370-S, Millar Instruments, Houston, Texas) into the ascending aorta and right atrium. Arterial and venous pressures were monitored continuously with the same data acquisition system used to record the ECG. These data were acquired digitally at a sampling rate of 1000 points/second with a commercially available software package (Chart, v.5.3, ADInstruments, Castle Hill, Australia). Coronary perfusion pressure (CPP) was calculated as the aortic pressure minus the right atrial pressure, measured at the end of the relaxation phase of the duty cycle. We analyzed an arterial blood gas (ABG) as soon as arterial access was established (i-STAT Portable Clinical Analyzer, Heska Corporation, Waukesha, WI). We repeated this any time ventilator settings were changed. We induced VF by delivering a three second, 60 Hz, 100 mA AC current externally across the thorax. We recorded the anesthesia time, which we defined as the time from the induction of anesthesia to the time VF was induced.
Experimental Design
After we induced VF, all animals were randomized to either 5 or 8 minutes without treatment of any kind. After 5 or 8 minutes, resuscitation was begun using a programmable oxygen-powered mechanical resuscitation device (Thumper, Model 1007, Michigan Instruments, Grand Rapids, MI). We randomized the swine to mechanical CPR for 90s, 180s, or 300s. We delivered chest compressions with the animals supine in a plexiglass v-board, in the antero-posterior direction, at a depth of 38 mm, a rate of 100 compressions per minute. The duty cycle was 50%, and we used a compression to ventilation ratio of 15:1 (this was the maximum available from this machine at the time). All ventilations were delivered by one investigator (DS) using a conventional bag-valve technique to simulate BLS resuscitation. All animals had their abdomens bound with one frontal and two lateral pads, a technique we have long employed in this model. Adjustment of the chest compressions to the proper depth took approximately 15 seconds. The device was not adjusted at any time after this initial adjustment.
Our laboratory uses an impedance-compensating, truncated exponential biphasic defibrillation waveform (LifePak 12, Medtronic-Physio-Control, Redmond, Washington) with a fixed dose of energy of 150J. All countershocks were manually administered by one investigator (JJM) to eliminate intra-user variability. If the rescue shock resulted in ROSC (defined here as a systolic blood pressure of 80 mmHg sustained for at least one minute continuously), the animal was ventilated and survived for 20 minutes, simulating arrival at the hospital. If this shock failed, 2 minutes of mechanical CPR were completed prior to the next rescue shock. This study was designed to represent a BLS crew with AED. Therefore, no drugs were administered.
Figure 1 depicts the experimental timeline. Animals in which a pulse was not restored and maintained had resuscitative interventions continued for 20 minutes beyond the start of the resuscitation (i.e. 25-28 minutes after the induction of VF). Any animal surviving for the 20-minute endpoint was euthanized with a rapid IV injection of 40 mEq of KCl. Thus, the experimental endpoints were either 20-minute survival after attaining ROSC, or 20 minutes of failed resuscitation. If the animal refibrillated, CPR was continued for 2 minutes and rescue shock was delivered if rhythm-appropriate (i.e. the ECG rhythm was VT or VF).
Figure 1.
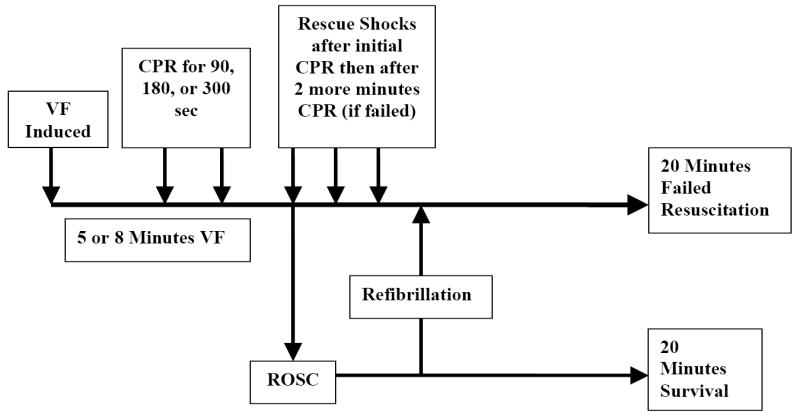
Experimental timeline.
The primary dependent variables for this study were CPP, ROSC, and 20-minute survival. We also measured the peak aortic and venous pressures during CPR as a surrogate for vascular tone. CPP, peak aortic and peak venous pressures were calculated using compressions 6-10 in each 30 second epoch. We measured the middle 5 compressions of the cycles because we have observed that they most accurately represent the average for the epoch.
Statistical Analyses
We calculated descriptive statistics (reported as means and standard deviations, and proportion) for all baseline characteristics. We compared dichotomous variables with two-tailed Fisher’s Exact Test. We analyzed continuous variables with repeated measures ANOVA. We used an alpha error rate of 0.05 as a criterion for determining statistical significance. We performed statistical analyses using a commercially available software package (Stata version 9.0, College Station, Texas).
Results
The baseline characteristics for the two groups were similar (Table 1).
Table 1.
Baseline characteristics of 5 and 8 minute VF groups. Reported as number (SD), except as noted below.
| 5 minute VF (N=24) | 8 minute VF (N=21) | |
|---|---|---|
| pH | 7.47 (0.04) | 7.47 (0.04) |
| pCO2 | 37.1 (3.56) | 38.0 (2.36) |
| pO2 | 99.4 (50) | 85.9 (13) |
| Glucose | 117 (22) | 108 (20.7) |
| Anesthesia time (min) | 35.0 (6.5) | 32.4 (9.1) |
| Hb | 9.3 (1.42) | 9.8 (0.94) |
| Hct | 27.5 (4.2) | 28.8 (2.6) |
| Ketamine (mg) | 300 (0) | 329 (99) |
| Weight (kg) | 26.2 (2.55) | 27.4 (2.15) |
| % male | 38 | 52 |
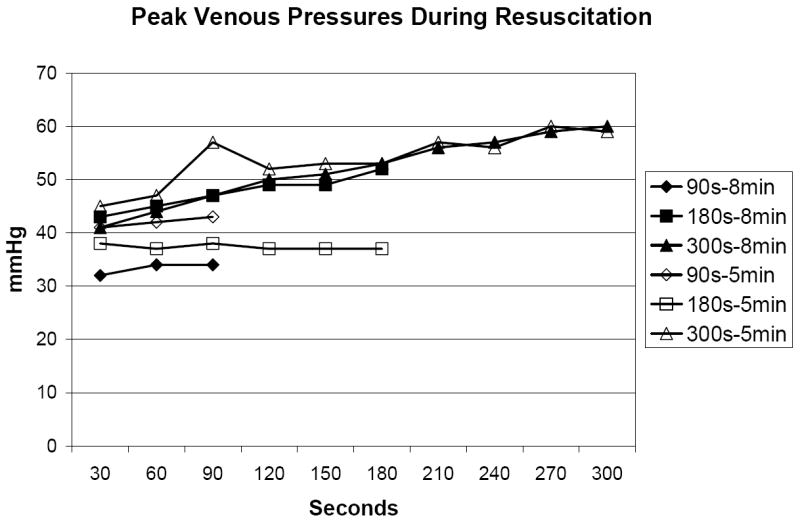
In the 5 minute VF group, the rate of ROSC did not differ between the three groups (90s: 25%; 180s: 38%; 300s: 38%, p>0.05). Survival rates did not differ (90s: 25%; 180s: 25%; 300s: 25%, p>0.05). In the 8 minute VF group, no animals experienced ROSC or survival. CPPs are presented by 30 second epoch in Figure 2 and did not differ between the three groups (p>0.05). CPP’s declined after 180s of CPR. Peak aortic pressure and peak venous pressure are depicted in Figures 3A and 3B. Peak aortic pressure increased during the first 210s of CPR and then began to decrease. Peak venous pressures generally increased throughout the duration of CPR and closely approximated peak aortic pressure after 300s of CPR.
Figure 2.
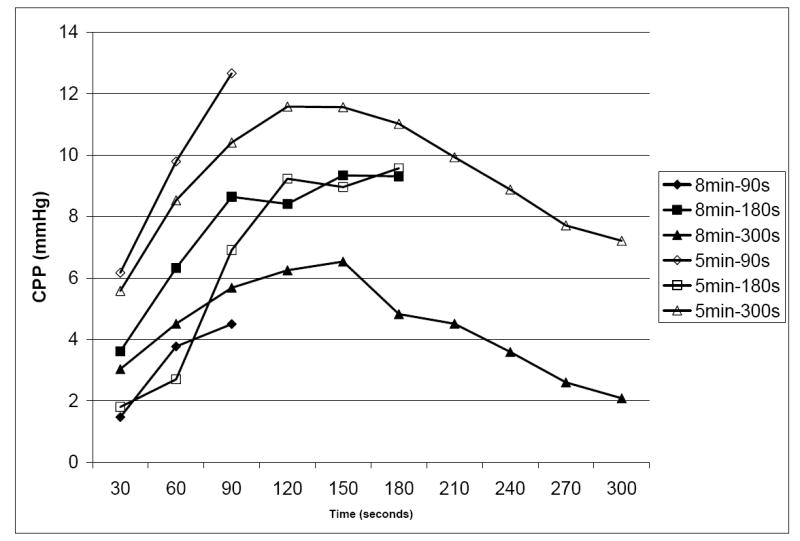
Coronary perfusion pressure by VF duration and by duration of CPR.
Figure 3.
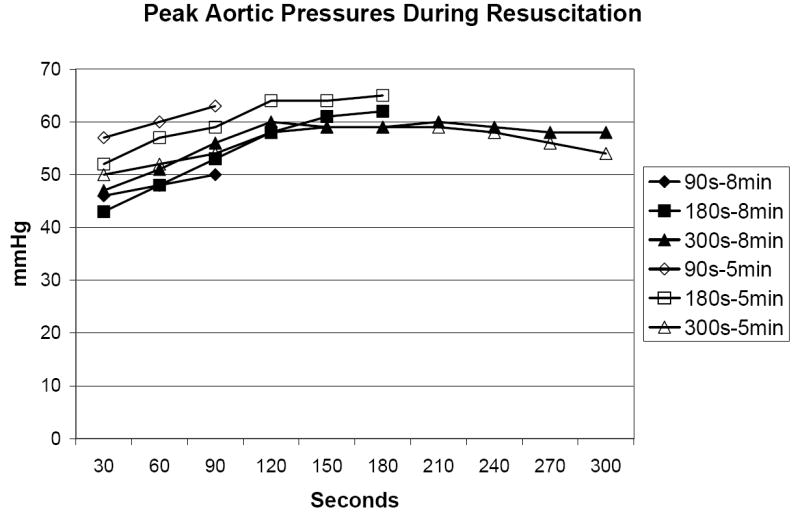
A. Peak aortic pressure during resuscitation.
B. Peak venous pressure during resuscitation.
Discussion
In this swine model of prolonged VF, increasing the duration of CPR prior to rescue countershock did not improve ROSC or survival. Of note, in the 8 minute VF cohort, no animal achieved ROSC or survival. The results in the 8 minute cohort are similar to our prior work where animals were randomized to a scaling exponent value of 1.3 prior to immediate countershock. [9] This scaling exponent value correlates to 8 minutes of untreated VF. In other studies of prolonged VF, we achieved higher rates of ROSC and survival through the use of a drug cocktail that includes vasopressors during resuscitation. [9-15] Similar models used by Berg et al. and Niemann et al. also note higher rates of ROSC in a 7.5 to 8 minute VF cohort. [16, 17]
The 5 minute VF cohort demonstrated improved results, however, rates of ROSC and survival are lower than our prior data where animals received immediate countershock following 5 minutes of untreated VF. [9] Our survival rate is lower than noted by Berg et al. who incorporated vasopressors in a model with similar VF duration and noted rates of ROSC >50%. [16] However, our rates of ROSC are within the 95% CI cited by Niemann et al. in a similar 5 minute VF model. [18] Our study supports the present ECC recommendations of 2 minutes of CPR prior to defibrillation. However, these results suggest that increasing the duration of CPR prior to defibrillation alone will not improve rates of ROSC or survival in prolonged VF.
The low rate of ROSC reported in this experiment is not without precedent. We recently reported a meta-analysis on the rates of ROSC during experiments in our lab. [19] In this analysis of 271 swine, a regression model was used to determine expected rates of ROSC based on time to first intervention. Based on this model, we would anticipate an 11% rate of ROSC in the 8 minute VF cohort that received 90s of CPR prior to first defibrillation, 7% ROSC in the 180s cohort and 3% ROSC in the 300s cohort.
Our results are also similar to those expected in the 5 minute VF cohort. Based on our prior work, we would anticipate a 27% rate of ROSC in the 90s group, 18% ROSC in the 180s group and 10% ROSC in the 300s group.
One possible reason for the low rates of ROSC and survival is the low CPPs generated in these animals. Prior research in a series of 100 humans has demonstrated that a minimal threshold of 15 mmHg is necessary for ROSC to occur, though this did not guarantee ROSC. [20] It is postulated that this level of CPP is necessary to perfuse the myocardium and reverse the metabolic abnormalities that accompany VF (such as high-energy phosphates depletion and extracellular potassium release). Prior work demonstrated higher CPP values with late vasopressor administration when CPR is initiated earlier. [21-24] Our results demonstrate that after 5-8 minutes of untreated VF, a loss of vascular tone has occurred that does not respond well to CPR alone. This is shown by the decrease in aortic peak pressure and equilibration of venous and arterial pressures.
These results support the value of early bystander CPR. They also have significant implications for our municipality, as time to arrival of emergency medical services is 6 minutes. [25] Given these results, we do not recommend 300s of CPR prior to countershock in prolonged VF unless a defibrillator is unavailable. There are two major reasons for this recommendation. First, as depicted in Figure 2, CPP markedly decreases after 180s of CPR. In the 8 minute cohort, the CPPs generated at 300s are 2 mmHg and well below those necessary to generate ROSC. Second, prior research has demonstrated rescuer fatigue after 1 minute of CPR. [26, 27]
Limitations
This study has several limitations. First, the animals used were young and sexually immature animals. The cardiovascular physiology of these animals may be different than that of many people who experience out-of-hospital cardiac arrest. Second, VF was electrically induced and not preceded by an ischemic insult. Thus, this model may represent VF of a dysrhythmic nature, but not ischemically-induced VF. Third, the outcomes assessed are ROSC and short-term survival. Most animal studies do not provide information on neurologically-intact survival, which is the most relevant outcome from the perspective of the patient. We note that previous studies showing short-term benefits have frequently failed to translate to long-term survival. Fourth, we did chest compressions with a mechanical device. Performing chest compressions is strenuous and rescuers may fatigue during longer bouts of CPR in the clinical setting. The chest compressions done here would have been consistent in force and depth throughout the entire episode of CPR, regardless of duration. This may not happen in the clinical setting with manual CPR. However, this would again favor one of the shorter durations (90 or 180s) of CPR if rates of ROSC are the same.
Conclusions
Increasing the duration of CPR prior to rescue countershock does not result in increased rates of ROSC or survival in a swine model of prolonged VF. We do not recommend 300s of CPR prior to countershock unless a defibrillator is not available. Vasopressors may be required to augment CPP in prolonged VF cardiac arrest.
Supplementary Material
Acknowledgments
None.
Funding Sources Dr. Rittenberger is supported by Grant Number 1 KL2 RR024154-02 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), and NIH Roadmap for Medical Research. Dr. Rittenberger is also supported by an unrestricted grant from the National Association of EMS Physicians/Zoll EMS Resuscitation Research Fellowship. Drs. Suffoletto and Menegazzi are supported by the Clinical Research Skills Development Core of the Resuscitation Outcomes Consortium through the National Heart, Lung and Blood Institute 5U01 HL077871-02. Dr. Menegazzi is also supported by the National Heart, Lung and Blood institute 5R01 HL080483-2. Drs. Menegazzi and Suffoletto are supported by the Society for Academic Emergency Medicine Institutional Research Training Grant. This study was supported through an unrestricted grant from the Pittsburgh Emergency Medicine Foundation.
Footnotes
Conflict of Interest Statement The authors have no conflicts of interest to report.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.2005 American Heart Association guidelines for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation. 2005;112(Supp):IV–19. IV–34. doi: 10.1161/CIRCULATIONAHA.105.166550. [DOI] [PubMed] [Google Scholar]
- 2.Bossaert L, Van Hoeyweghen R. Cerebral Resuscitation Study Group. Bystander Cardiopulmonary Resuscitation (CPR) in out-of-hospital cardiac arrest. Resuscitation. 1989;17:S55–S69. doi: 10.1016/0300-9572(89)90091-9. [DOI] [PubMed] [Google Scholar]
- 3.Berg RA, Sanders AB, Kern KB, Hilwig RW, Heidenreich JW, Porter ME, Ewy GA. Adverse hemodynamic effects of interrupting chest compressions for rescue breathing during cardiopulmonary resuscitation for ventricular fibrillation cardiac arrest. Circulation. 2001;104:2465–2470. doi: 10.1161/hc4501.098926. [DOI] [PubMed] [Google Scholar]
- 4.Sanders AB, Kern KB, Berg RA, Hilwig RW, Heidenrich J, Ewy GA. Survival and neurologic outcome after cardiopulmonary resuscitation with four different chest compression-ventilation ratios. Ann Emerg Med. 2002;40:553–562. doi: 10.1067/mem.2002.129507. [DOI] [PubMed] [Google Scholar]
- 5.Cobb LA, Fahrenbruch CE, Walsh TR, Copass MK, Olsufka M, Breskin M, Hallstrom AP. Influence of cardiopulmonary resuscitation prior to defibrillation in patients with out-of-hospital ventricular fibrillation. JAMA. 1999;281:1182–1188. doi: 10.1001/jama.281.13.1182. [DOI] [PubMed] [Google Scholar]
- 6.Wik L, Hansen TB, Fylling F, Steen T, Vaagenes P, Auestad BH, Steen PA. Delaying defibrillation to give basic cardiopulmonary resuscitation to patients with out-of-hospital ventricular fibrillation. JAMA. 2003;289:1389–1395. doi: 10.1001/jama.289.11.1389. [DOI] [PubMed] [Google Scholar]
- 7.Hallstrom A, Cobb L, Johnson E, Copass M. Cardiopulmonary resuscitation by chest compression alone or with mouth-to-mouth ventilation. New Engl J Med. 2000;342:1546–1553. doi: 10.1056/NEJM200005253422101. [DOI] [PubMed] [Google Scholar]
- 8.SOS-KANTO study group. Cardiopulmonary resuscitation by bystanders with chest compression only (SOS-KANTO): an observational study. Lancet. 2007;369:920–926. doi: 10.1016/S0140-6736(07)60451-6. [DOI] [PubMed] [Google Scholar]
- 9.Menegazzi JJ, Callaway CW, Sherman LD, Hostler DP, Wang HE, Fertig KC, Logue ES. Ventricular fibrillation scaling exponent can guide timing of defibrillation and other therapies. Circulation. 2004;109:926–931. doi: 10.1161/01.CIR.0000112606.41127.D2. [DOI] [PubMed] [Google Scholar]
- 10.Mader TJ, Menegazzi JJ, Rittenberger JC, Suffoletto BS, Callaway CW, Salcido DD, Logue ES. The effect of adenosine A1 receptor antagonism on return of spontaneous circulation and short-term survival in prolonged ventricular fibrillation. Prehosp Emerg Care. 2008;12 doi: 10.1080/10903120802101223. in press. [DOI] [PubMed] [Google Scholar]
- 11.Betz AE, Menegazzi JJ, Logue ES, Callaway CW, Wang HE. A randomized comparison of manual, mechanical, and high-impulse chest compression in a porcine model of prolonged ventricular fibrillation. Resuscitation. 2006;69:495–501. doi: 10.1016/j.resuscitation.2005.09.026. [DOI] [PubMed] [Google Scholar]
- 12.Menegazzi JJ, Salcido DD, Menegazzi MT, Rittenberger JC, Suffoletto BP, Logue ES, Mader TJ. Effects of an impedance threshold device on hemodynamics and restoration of spontaneous circulation in prolonged porcine ventricular fibrillation. Prehosp Emerg Care. 2007;11:179–185. doi: 10.1080/10903120701206073. [DOI] [PubMed] [Google Scholar]
- 13.Seaberg DC, Menegazzi JJ, Check B, MacLeod BA, Yealy DM. Use of a cardiocerebral-protective drug cocktail prior to countershock in a porcine model of prolonged ventricular fibrillation. Resuscitation. 2001;51:301–308. doi: 10.1016/s0300-9572(01)00426-9. [DOI] [PubMed] [Google Scholar]
- 14.Menegazzi JJ, Seaberg DC, Yealy DM, Davis EA, MacLeod BA. Combination pharmacotherapy with delayed countershock vs. standard advanced cardiac life support after prolonged ventricular fibrillation. Prehosp Emerg Care. 2000;4:31–37. doi: 10.1080/10903120090941614. [DOI] [PubMed] [Google Scholar]
- 15.Menegazzi JJ, Davis EA, Yealy DM, Molner RL, Nicklas KA, Hosack GM, Honingford EA, Klain MM. An experimental algorithm versus standard advanced cardiac life support in a swine model of out-of-hospital cardiac arrest. Ann Emerg Med. 1993;22:235–239. doi: 10.1016/s0196-0644(05)80211-2. [DOI] [PubMed] [Google Scholar]
- 16.Berg RA, Hilwig RW, Ewy GA, Kern KB. Precountershock cardiopulmonary resuscitation improves initial response to defibrillation from prolonged ventricular fibrillation: a randomized, controlled swine study. Crit Care Med. 2004;32:1352–1357. doi: 10.1097/01.ccm.0000127780.01362.e5. [DOI] [PubMed] [Google Scholar]
- 17.Niemann JT, Cairns CB, Sharma J, Lewis RJ. Treatment of prolonged ventricular fibrillation. Immediate countershock versus high-dose epinephrine and CPR preceding countershock. Circulation. 1992;85:281–287. doi: 10.1161/01.cir.85.1.281. [DOI] [PubMed] [Google Scholar]
- 18.Niemann JT, Cruz B, Garner D, Lewis RJ. Immediate countershock versus cardiopulmonary resuscitation before countershock in a 5-minute swine model of ventricular fibrillation arrest. Ann Emerg Med. 2000;36:543–546. doi: 10.1067/mem.2000.109441. [DOI] [PubMed] [Google Scholar]
- 19.Rittenberger JC, Menegazzi JJ, Callaway CW. Association of delay to first intervention with return of spontaneous circulation in a swine model of cardiac arrest. Resuscitation. 2007;73:154–160. doi: 10.1016/j.resuscitation.2006.07.029. [DOI] [PubMed] [Google Scholar]
- 20.Paradis NA, Martin GB, Rivers EP, Goetting MG, Appleton TJ, Feingold M, Nowak RM. Coronary perfusion pressure and the return of spontaneous circulation in human cardiopulmonary resuscitation. JAMA. 1990;263:1106–1113. [PubMed] [Google Scholar]
- 21.Stadlbauer KH, Wagner-Berger HG, Wenzel V, Voelckel WG, Krismer AC, Klima G, Rheinberger K, Pechlaner S, Mayr VK, Lindner KH. Survival with full neurologic recovery after prolonged cardiopulmonary resuscitation with a combination of vasopressin and epinephrine in pigs. Anesth Analg. 2003;96:1743–1749. doi: 10.1213/01.ANE.0000066017.66951.7F. [DOI] [PubMed] [Google Scholar]
- 22.Mayr VD, Raedler C, Wenzel V, Lindner KH, Strohmenger H. A comparison of epinephrine and vasopressin in a porcine model of cardiac arrest after rapid intravenous injection of bupivicaine. Anesth Analg. 2004;98:1426–1431. doi: 10.1213/01.ane.0000108488.05900.a8. [DOI] [PubMed] [Google Scholar]
- 23.Babar SI, Berg RA, Hilwig RW, Kern KB, Ewy GA. Vasopressin versus epinephrine during cardiopulmonary resuscitation: a randomized swine outcome study. Resuscitation. 1999;41:185–192. doi: 10.1016/s0300-9572(99)00071-4. [DOI] [PubMed] [Google Scholar]
- 24.Sanders AB, Kern KB, Berg RA, Hilwig RW, Heidenrich J, Ewy GA. Survival and neurologic outcome after cardiopulmonary resuscitation with four different chest compression-ventilation ratios. Ann Emerg Med. 2002;40:553–562. doi: 10.1067/mem.2002.129507. [DOI] [PubMed] [Google Scholar]
- 25.Wang HE, Min A, Hostler D, Chuang C, Callaway CW. Differential effects of out-of-hospital interventions on short- and long-term survival after cardiopulmonary arrest. Resuscitation. 2005;67:69–74. doi: 10.1016/j.resuscitation.2005.05.008. [DOI] [PubMed] [Google Scholar]
- 26.Hightower D, Thomas SH, Stone CK, Dunn K, March JA. Decay in quality of closed-chest compressions over time. Ann Emerg Med. 1995;26:300–303. doi: 10.1016/s0196-0644(95)70076-5. [DOI] [PubMed] [Google Scholar]
- 27.Ochoa FJ, Ramalle-Gomara E, Visa V, Saralegui I. The effect of rescuer fatigue on the quality of chest compressions. Resuscitation. 2003;37:149–152. doi: 10.1016/s0300-9572(98)00057-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


