Summary
Sleep changes markedly across the life span and complaints about insomnia are prevalent in older people [1]. Whether age-related alterations in sleep are due to modifications in social factors, circadian physiology, homeostatic drive or the ability to sleep remains unresolved. We assessed habitual sleep duration at home and then quantified daytime sleep propensity, sleep duration and sleep structure in an inpatient protocol that included extended sleep opportunities covering 2/3 of the circadian cycle (12 hours at night and 4 hours in the afternoon) for 3-7 days in 18 older and 35 younger healthy men and women. At baseline, older subjects had less daytime sleep propensity than younger subjects. Total daily sleep duration, which was initially longer than habitual sleep duration, declined during the experiment to asymptotic values that were 1.5 hour shorter in older (7.4 ± 0.4 s.e.m., hour) than younger subjects (8.9 ± 0.4)., Rapid-eye movement sleep and non-rapid eye movement sleep contributed about equally to this reduction. Thus, in the absence of social and circadian constraints, both daytime sleep propensity and the maximal capacity for sleep are reduced in older people. These data have important implications for understanding age-related insomnia.
Keywords: aging, sleep, homeostasis, recovery, sleep duration, insomnia
Results and Discussion
To assess age-related changes in maximal capacity for sleep and in daytime sleep propensity, we studied 18 older subjects (12 males, 6 females; age range 60-76 years; mean ± s.d.: 67.8 ± 4.3 years) and 35 younger subjects (17 males, 18 females; 18-32 years; 21.9 ± 3.3 years). The subjects were healthy and had no sleep complaints or clinically significant sleep disorders as assessed by a physical exam, medical history, hematology, biochemistry and a clinical polysomnogram.
Habitual sleep timing in social environment
We first assessed sleep patterns while the subjects were living at home in their habitual social environment (Figure 1). Older subjects showed an increased ‘morning’ diurnal preference (“Owl-Lark” scores: older: 60.5 ± 11.3 s.d. vs. younger: 51.3 ± 8.5; p<0.001), earlier bedtimes (22:48 ± 1:00 vs. 0:24 ±1:12; p<0.001) and earlier wake times (6:54 ± 1:00 vs. 8:48 ± 1:18; p<0.001). Habitual Sleep Duration (HSD) (derived from self-report and verified using actigraphy) varied considerably among individuals: the observed range was 7.0-9.0 hours in older subjects and 6.1-10.3 hours in the younger (4 with HSD < 7.0 hrs; 18 with 7.0 hrs ≤ HSD ≤ 9.0 hrs (the same range as the older subjects); 13 with HSD > 9.0 hrs) There was no significant difference in mean HSD between the groups: 8.1 ± 0.6 hours for older subjects and 8.5 ± 1.0 hours for younger subjects.
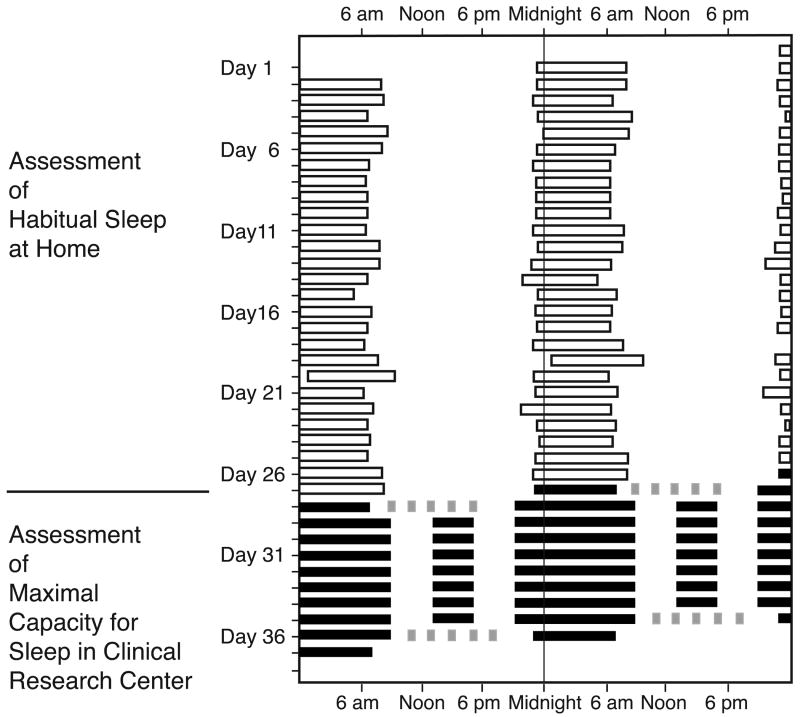
Figure 1.
Protocol and sleep times from one subject. Data are plotted in raster format in which time in hours is plotted horizontally and day number is plotted vertically; two consecutive days are presented on each row. The timing of sleep, as derived from actigraphy and self-report while the subject was at home, is indicated by open boxes. Inpatient sleep episodes are indicated by black boxes and Multiple Sleep Latency Tests by gray boxes. In the present report, we include the data collected through the sleep extension segment of the protocol; data from the second set of MSLTs and following sleep episode are not included.
Baseline sleep physiology and daytime sleep propensity
We next assessed the physiological characteristics of sleep using polysomnographic recordings in our inpatient facility (Figure 1). For baseline sleep, bedtimes were scheduled at the same time and duration as each subject's assessed at-home schedule. In accordance with previous studies, older subjects had significantly less non-Rapid Eye Movement (NREM) sleep Stages 2 and 4, less Rapid-Eye Movement (REM) sleep, lower Total Sleep Time (TST), and significantly more Wake (Table in Supplemental materials). These age-related changes were also observed when we compared those young and older people for whom HSD could be matched, i.e. comparing all older subjects with the subset of younger subjects for whom HSD fell between 7.0 and 9.0 hours.
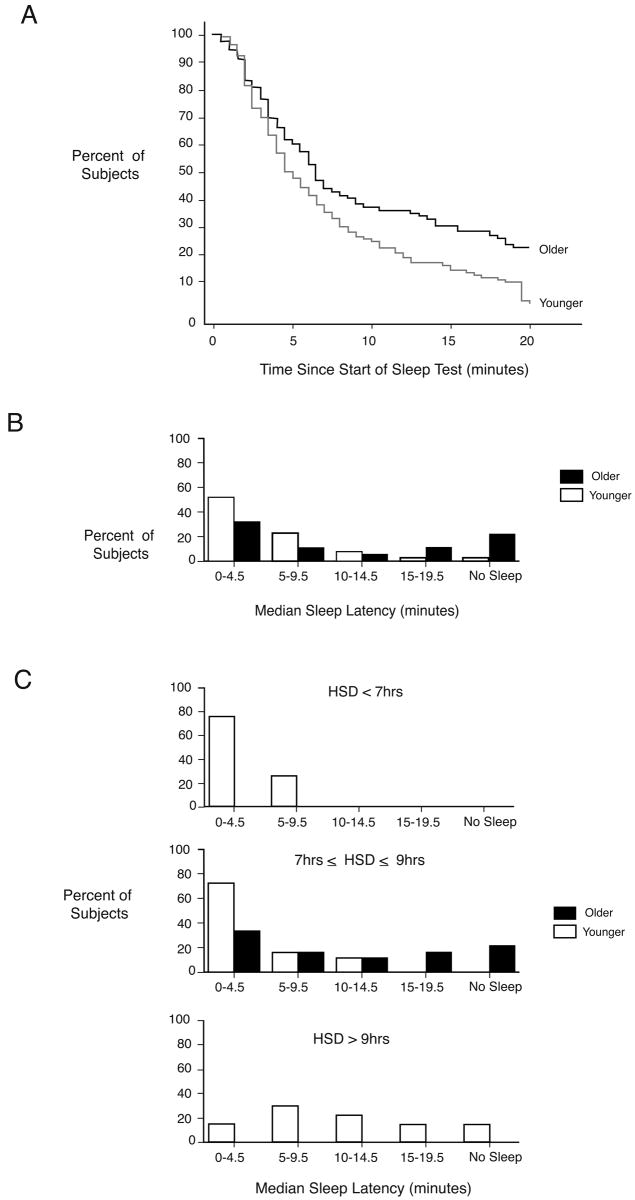
To assess whether these age-related reductions in sleep duration and sleep consolidation are accompanied by an increase in daytime sleep propensity, we used the Multiple Sleep Latency Test (MSLT), a validated tool widely used in clinical practice [2]. Survivor-based statistical analysis revealed that older subjects were less likely to fall asleep and if they did fall asleep, took more time to do so than younger subjects (p=0.009)(Figure 2a). The distribution of median MSLT results was skewed to longer sleep latency times for older subjects (Figure 2b) (p<0.001). Since daytime sleep propensity is affected by nighttime sleep duration [3-5], we also compared the MSLT values for younger individuals whose HSD was >9 hours to those whose HSD was <7 hours (Figure 2c). The longer sleepers had lower sleep propensity during the day than the shorter sleepers.
Figure 2.
Daytime sleep propensity as assessed by the Multiple Sleep Latency Test. (a) Survival curves of sleep latency, i.e. the percentage of subjects who are awake for that wake duration or shorter is plotted against elapsed time during 20-minute nap opportunities. Data of all five nap opportunities for each subject are included. (b) Histograms of percentage of older and younger subjects by median sleep latency. (c) Histograms of percentage of subjects by median sleep latency separately for subjects with a Habitual Sleep Duration less than 7 hours, between 7 and 9 hours and greater than 9 hours.
Thus, in older subjects, who have less deep Stage 4 sleep and experience more awakenings during nighttime sleep, daytime sleep propensity is lower than in young subjects who experience a much better polysomnographically determined quality sleep. These data are contrary to the commonly held belief that the reduced nighttime sleep quality in older people would cause increased daytime sleep propensity. Other studies have reported increased sleep latencies (reflecting reduced daytime sleep propensity) during the MSLT in older people [6,7]. The current MSLT data may be interpreted as either reflecting a reduced homeostatic drive for sleep in older people or age-related reduction in the ability to initiate sleep. “Sleep need” relates to a homeostatic process with a set point or equilibrium value. We assume that an individual has a set sleep need and if that need is not met (condition of a sleep debt), then (1) the person will sleep more given additional sleep opportunity and (2) the person will sleep until the sleep debt is expressed and the person is at the homeostatic sleep equilibrium; (3) sleep length is approximately constant at homeostatic sleep equilibrium; and (4) at this equilibrium, daytime sleep propensity and waking function are stable. A reduced sleep ability implies that changes in processes related to sleep initiation and maintenance (e.g. increased arousal [8], or neuroanatomic changes [9,10]) prevent the individual from meeting their sleep need. Although it is very difficult to distinguish between these two interpretations, we further addressed this issue by quantifying the time course of total sleep time (TST) during extended daytime and nighttime sleep opportunities for several days. Subjects had 16 hours of bedrest per 24 hours for 3-7 days (Figure 1) in the absence of time-of-day information: 12-hours at night and 4-hours in the afternoon, thereby including the habitual time of afternoon naps, when the circadian timing system has been reported to permit or facilitate sleep in particular in older people. This protocol, with polysomnographically recorded sleep in darkness in a sound attenuated room and in the absence of time-of-day information, is designed to unmask any endogenous sleep drive and thereby assesses any sleep debt as well as the maximal capacity for sleep.
Sleep, subjective sleepiness and objective waking performance
During the first day with extended sleep opportunity, both younger and older subjects slept more than during the baseline sleep episode and more than their HSD. This confirms that sleep duration is constrained by social or lifestyle factors. The initial rise, followed by a decline in TST across days of extended sleep opportunity, is evidence of the presence of sleep debt in young and older individuals. This interpretation is supported by the changes in waking subjective alertness and objective waking performance. After the first nighttime 12-hour sleep episode, there were increases in self-report of “Alert” (p=0.0001) and “Fresh” (p=0.0001) and decreases in the Karolinska Sleepiness Scale (p<0.0001) in younger and older subjects. For objective measures, there was significant and similar improvement in older and younger subjects after the first nighttime 12 hour sleep episode on two performance tasks: increased number of calculations attempted (p<0.0001) and scores on the Digit Symbol Substitution Test (p<0.0001), but no changes in lapses in attention or median reaction time on the Psychomotor Vigilance Test or other mood ratings (data not shown). These data are consistent with the view that self-selected HSD is less than optimal for daytime alertness and functioning.
Asymptotic duration of sleep, NREM sleep and REM sleep
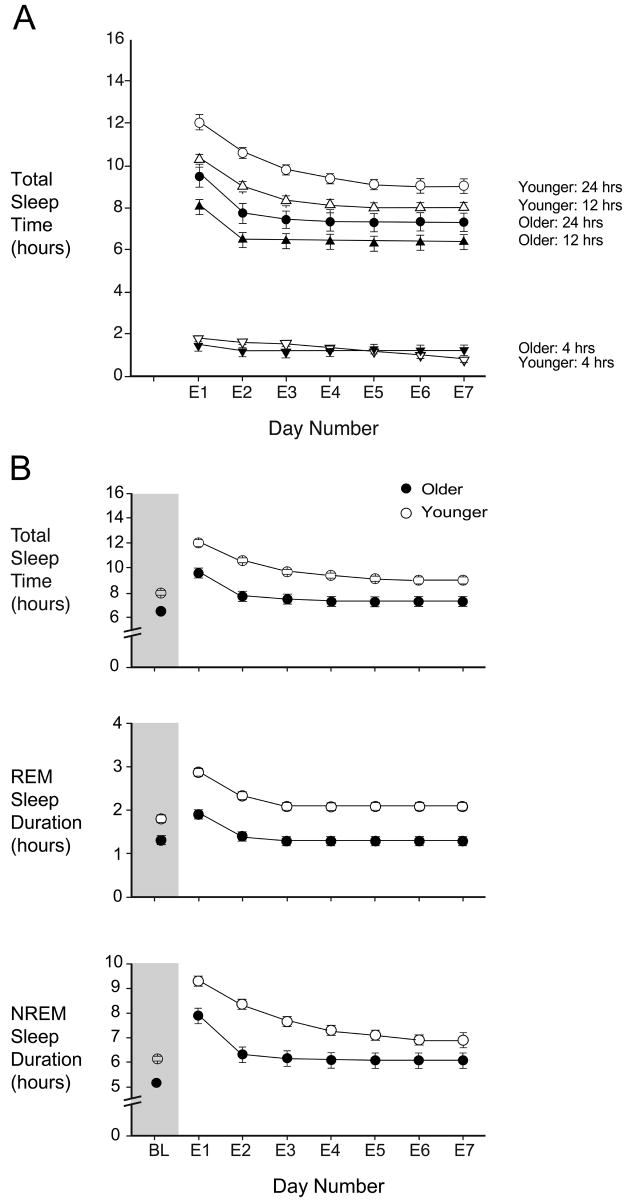
Older people slept less than young people every day of the extended sleep opportunities (Table S2 available online). To investigate whether the two age groups would continue to differ if the experiment were to be continued indefinitely, the dynamics and asymptotic values of sleep duration and sleep structure were estimated by mixed model exponential-equation regression. The asymptotic level for TST for older subjects was 7.4 hours (95% C.I. 6.6-8.3 hours) and was achieved during the second 24 hours of extended sleep opportunity. The asymptotic value for TST for younger subjects was 8.9 hours (C.I. 8.1-9.7 hours) (p=0.009 vs. older) and was not achieved until approximately the fifth 24-hour of extended sleep opportunity. For younger subjects with HSD of the same ranges as the older subjects, the asymptote was 9.2 hours (C.I. 8.3–10.1 hours) (p=0.005 vs. older). Asymptotic TST for the 12-hour nighttime sleep episodes also differed between the age groups, with older persons having less nighttime sleep (Figure 3a, Table 1). We also assessed the asymptotic duration for the various sleep stages. The 1.5 hour difference in TST for nighttime plus daytime sleep was composed of ∼0.8 hour difference in REM sleep and ∼0.8 hour difference in NREM sleep. For the nighttime sleep opportunities, the difference was also approximately equally composed of changes in REM sleep and NREM sleep, although the time to reach asymptotic level was longer for NREM sleep than for REM sleep, the significance of which is not known.
Figure 3.
a) Total Sleep Time (TST) during each day (E1-E7) of increased sleep opportunity, and during the 12-hour nighttime and the 4-hour daytime sleep opportunity within each day. b) TST, Rapid-Eye Movement (REM) sleep and non-REM (NREM) sleep during the baseline sleep episode (Day BL, shaded area) and during days E1-E7 for older (filled symbols) and younger (open symbols) subjects. The data are plotted as mean and standard errors estimated by the fitted exponential function.
Table 1.
Asymptotic duration (± standard error) of sleep measures in young and older subjects during extended sleep opportunities. Data were fit to the equation: Sleep Measure=Asymptote + b*exp(-(time constant)*day).
| Sleep measure | Older All HSD | Younger All HSD | Younger Matched HSD range |
|---|---|---|---|
| HSD range (hr) | 7.0 – 9.0 | 6.1-10.3 | 7.0 – 9.0 |
| Per 24 hours | |||
| Total Sleep Time (hr) | 7.4 (0.4) | 8.9 (0.4)** | 9.2 (0.4)** |
| REM sleep (hr) | 1.3 (0.1) | 2.1 (0.1)*** | 2.0 (0.1)*** |
| All NREM Sleep (hr) | 6.1 (0.3) | 6.7 (0.4) | 7.1 (0.4) * |
| NREM sleep Stage 1 (hr) | 1.2 (0.2) | N/A | 1.4 (0.1) |
| NREM sleep Stage 2 (hr) | 4.1 (0.4) | 4.4 (0.4) | 4.6 (0.4) |
| NREM sleep Stage 3 (hr) | 0.6 (0.1) | 0.5 (0.1) | 0.5 (0.1) |
| NREM sleep Stage 4 (hr) | 0.2 (0.1) | 0.6 (0.1)*** | 0.5 (0.1)** |
| SWS (hr) | 0.8 (0.1) | 1.1 (0.1)* | 1.1(0.1) |
| Wake (hr) | 8.5 (0.4) | 7.0 (0.4)** | 6.7 (0.4) |
| Nighttime (12-hour) only | |||
| Total Sleep Time (hr) | 6.4 (0.3) | 7.9 (0.2)*** | 8.1(0.3)*** |
| REM sleep (hr) | 1.2 (0.1) | 1.9 (0.1)*** | 1.9 (0.1)*** |
| All NREM Sleep (hr) | 5.1 (0.2) | 5.9 (0.2) ** | 6.2 (0.2) *** |
| NREM sleep Stage 1 (hr) | 1.0 (0.1) | 1.1 (0.1) | 1.1 (0.1). |
| NREM sleep Stage 2 (hr) | 3.5 (0.2) | 3.9 (0.3) | 4.1 (0.2) |
| NREM sleep Stage 3 (hr) | 0.5 (0.1) | 0.4 (0.0) | 0.4 (0.1) |
| NREM sleep Stage 4 (hr) | 0.1 (0.1) | 0.5 (0.1)*** | 0.4 (0.1)*** |
| SWS (hr) | 0.7 (0.1) | 0.9 (0.1)* | 0.9 (0.1) |
| Wake (hr) | 5.5 (0.3) | 4.0 (0.2)*** | 3.8 (0.3)*** |
| Daytime (4-hour) only | |||
| Total Sleep Time (hr) | 1.2 (0.2) | N/A | N/A |
p≤0.05
p≤0.01
p≤0.001 for comparisons versus Older.
N/A indicates no asymptote was statistically fit.
Discussion
The amount of sleep required for maintaining alertness, performance and good health is a question asked by people of all ages. Many older persons complain of problems related to the amount of sleep achieved, including problems falling asleep at night, remaining asleep and awakening too early in the morning [11]. Studies have documented marked changes across the life span with respect to the quantity and timing of sleep, including self-reported decreased amounts of nighttime sleep, polysomnographically assessed reductions in Slow Wave Sleep (NREM Sleep stages 3 and 4, SWS) and increased daytime sleep [12,13]. Despite these well documented age-related changes in sleep, their interpretation continues to be discussed. Current conceptual and physiological models of sleep highlight the contribution of circadian processes, homeostatic factors as well as social and lifestyle factors to sleep duration [14,15]. Identifying which interpretation best reflects the processes underlying age-related phenomenological changes in sleep is important because it will have an impact on the approach taken to cope with and/or treat these changes. For example, if sleep need does not decline with age, the older person who only sleeps for 6.5 hours per night is probably sleep deprived. This is important because there is increasing evidence that inadequate sleep is detrimental not only for alertness and performance, but also for safety, immune and metabolic function, learning, and other health outcomes (e.g., [16-22]). Alternatively, if sleep need declines with age, 6.5 hours of polysomnographically confirmed sleep, which corresponds to approximately 7-7.5 hours time in bed, may be adequate for a 70 year old healthy individual. Not recognizing these age-related changes in sleep need may lead to self-selecting a time-in-bed in excess of age-appropriate required sleep duration which in turn may contribute to complaints of insomnia and difficulties falling asleep, remaining asleep or awakening too early.
The primary objective of our study was to assess changes in sleep in healthy older people under conditions which allowed for the assessment of maximal capacity for sleep in the absence of lifestyle and circadian and environmental factors that might compromise such an assessment. Many previous studies did not adequately assess the health (including the presence of sleep disorders or medical conditions or medications that may affect sleep) of their older subjects and many studies used a standard 8-hour nighttime sleep opportunity to quantify the age-related changes in sleep. Most previous studies used protocols in which social and circadian constraints on sleep were not minimized to the extent of our current protocol.
In accordance with many previous studies [23-26], our baseline data show that healthy aging is accompanied by changes in diurnal preferences, sleep timing, sleep duration, NREM sleep and REM sleep. Furthermore, baseline daytime sleep propensity was reduced in older people: when matched for HSD, younger people were more sleepy on the MSLT. There are several possible reasons why older people have longer sleep latencies than younger people during the MSLT: (1) Older subjects might not be able to sleep during the circadian day [6,27] due to a “circadian wakeup” signal; therefore, daytime sleep propensity should not be used as a proxy for sleep drive or sleep ‘need’. However, the fact that older subjects had significant amounts of sleep during the 4-hour sleep opportunities in this study argues against this hypothesis. (2) Older subjects may have a lower sleep need; therefore, despite a reduced nighttime sleep duration, daytime sleep drive is lower in older than in younger individuals. This latter interpretation is supported by the observed reduction of asymptotic sleep duration in the older people in this study. (3) Older subjects may have decreased sleep ability, observed as more trouble initiating sleep or remaining asleep, because of increased arousal, similar to what has been reported in insomnia [8], or because of neuroanatomic changes in relevant brain areas with aging [9,10]. Although our data do not allow us to distinguish between the latter two interpretations, the observation that in both age groups alertness improved to a similar extent during the sleep extension phase is in accordance with an age-related reduction in sleep need.
The initial increase in sleep duration when given extended opportunity and the subsequent decline demonstrates that both younger and older people carry a ‘sleep debt’. This sleep debt dissipates with excess sleep and its duration reaches asymptotic levels of 8.9 hours in the younger subjects and 7.4 hours in older subjects. Because the reported time spent in bed (HSD) while living in their normal environment was 8.5 hours in the young individuals, it follows that young subjects do not allow themselves enough time to meet their asymptotic sleep need. In the older subjects HSD was 8.1 hours which is in excess of the 7.4 asymptotic total sleep duration; however, our baseline PSG data demonstrated that older subjects only sleep approximately 6.5 hours during 8.1 hours time in bed, and thereby also incur a sleep debt.
Previous studies have shown increased sleep, and improved subjective alertness and objectively assessed performance given increased opportunity in adolescents [28], college students [29] and other young adults [30,31], [30]. These previous reports and our current data are remarkably consistent with respect to asymptotic sleep duration (>8 hours) and all suggest habitual sleep durations obtained in the presence of social and life-style constraints are less than the physiological set point and that many individuals, young people in particular, live with a ‘sleep debt’.
Our study is the first to compare the asymptotic sleep duration in younger and older adults who are given both nighttime and daytime sleep opportunities with only minimal external constraints on sleep duration. Under these conditions, there was a reduction in both NREM and REM sleep in older persons compared to young. This contrasts with results of studies in which the sleep of young and older people was recorded while time in bed was limited to 8 hours at night. These previous studies have emphasized that aging primarily affects SWS only. Longer sleep opportunities or sleep at other circadian phases [24,25] may be necessary to observe changes in both NREM and REM sleep. To what extent this change in both NREM and REM sleep is associated with other age-related changes such as neural plasticity and cognition, remains to be established. However, recognition that the maximal capacity for sleep changes even during healthy aging may lead to novel approaches to the treatment of sleep complaints associated with aging.
Experimental Procedures
Subjects
Healthy older (target 60-80 years) and younger (target 18-30 years) men and women were recruited to the study. None were recruited by reported HSD. All were healthy by history, physical exam, ECG, and laboratory tests of blood and urine, and all passed a clinical sleep screen to verify the absence of the clinical sleep disorders sleep apnea and nocturnal myoclonus. None were using any prescription or non-prescription medications. None had worked night or rotating shifts in the past three years nor crossed more than one time zone in the previous three months.
Procedures
The inpatient portion of the study was conducted in the Brigham and Women's Hospital General Clinical Research Center. The environment was free of external time cues such as windows, clocks, radio, internet access, TV, or non-staff visitors. The protocol was approved by the Partners Healthcare Institutional Review Board.
For three weeks prior to admission, study volunteers were instructed to abstain from all medication, health food supplements, caffeine, tobacco, and alcohol. Subjects were tested for the presence of these prohibited substances at least once during screening and again at inpatient admission. For two weeks, all self-selected sleep-wake times were reported in a daily diary and telephone call-ins to a time-stamped machine. Though very few naps were reported, they were included in further calculations. These self-selected sleep-wake times were averaged to produce the schedule for the following week, which was also the week before admission and which included only nighttime sleep episodes. The actual sleep and wake times, as determined by self-report, telephone call-in times, and wrist actigraphy, during this last week were used to compute Habitual Sleep Duration (HSD), which was then used to schedule the timing and duration of the first nighttime (baseline) sleep episode after admission. Note that in this report, HSD refers to the time spent in bed; any wakefulness during this period is included. Whereas it may be more accurate to refer to this measure as Habitual Bedrest Duration, we use the term HSD; most epidemiology studies use the term sleep duration and in our protocol bedrest refers to extended sleep opportunities. To be eligible for the inpatient portion of the study, subjects needed to maintain the scheduled sleep time and wake time within 30 minutes; subjects were allowed a maximum of two minor deviations from this schedule (out of a total of 14 sleep and wake times in the last week before inpatient study) and no naps for the last week before admission.
All inpatient events were scheduled relative to the subject's HSD and habitual bed and wake time. All inpatient sleep was recorded polysomnographically. Subjects completed performance and alertness testing every 0.5-2 hours while awake. For their first inpatient night (BL), all subjects were scheduled to sleep at the same time and of a duration equal to their HSD. On the second inpatient day, subjects underwent a Multiple Sleep Latency Test (MSLT) protocol beginning two hours after awakening [32]. During each of five tests scheduled two hours apart within the MSLT, a subject is instructed to lie quietly with eyes closed and try to fall asleep: latency to any stage of sleep is reported. The subject is awakened when specific sleep criteria are met. If the subject is still awake after 20 minutes, the test is terminated. Subjects then began a schedule of 24-hour days with 16 hours of sleep opportunity: 12 hours centered at mid-habitual nighttime sleep episode and 4 hours centered 12 hours opposite. By randomization, subjects participated in three 24-hours day of this schedule (9 younger subjects, 4 older); four 24-hour days of this schedule (17 younger, 10 older); or seven 24-hour days of this schedule (9 younger, 4 older).
Supplementary Material
Table 1: Mean (standard deviation) for polysomnographic sleep measures during Baseline sleep, which was scheduled according to each subject's habitual sleep schedule. For the younger subjects, data are provided for both the entire group and for the subset of younger subjects with HSD between 7 and 9 hours (to match the observed HSD range of all the older subjects).
Table 2: Mean (standard error) for polysomnographic sleep measures for each day (12-hour nighttime plus 4-hour daytime episodes) of extended sleep opportunity.
Acknowledgments
This study was funded by grants from the National Institutes of Health P01-AG09975, K02-HD045459 (EBK) and NCRR-GCRC-M01-RR02635 (to the BWH GCRC). DJD is supported by the BBSRC and Wellcome Trust. We thank Dr. Wei Wang for statistical advice and the staff of the GCRC for the conduct of the study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Shochat T, Pillar G, Malhotra A. Sleep and Aging. Int J Sleep Disorders. 2007;3:92–102. [Google Scholar]
- 2.Littner MR, Kushida C, Wise M, Davila DG, Morgenthaler T, Lee-Chiong T, et al. Practice parameters for clinical use of the multiple sleep latency test and the maintenance of wakefulness test. Sleep. 2005;28:113–121. doi: 10.1093/sleep/28.1.113. [DOI] [PubMed] [Google Scholar]
- 3.Carskadon MA, Dement WC. Effects of total sleep loss on sleep tendency. Percept Mot Skills. 1979;48:495–506. doi: 10.2466/pms.1979.48.2.495. [DOI] [PubMed] [Google Scholar]
- 4.Carskadon MA, Dement WC. Sleep loss in elderly volunteers. Sleep. 1985;8:207–221. doi: 10.1093/sleep/8.3.207. [DOI] [PubMed] [Google Scholar]
- 5.Rosenthal L, Roehrs TA, Rosen A, Roth T. Level of sleepiness and total sleep time following various time in bed conditions. Sleep. 1993;16:226–232. doi: 10.1093/sleep/16.3.226. [DOI] [PubMed] [Google Scholar]
- 6.Dijk D, Groeger J, Deacon S, Stanley N. Age related reduction in daytime sleep propensity. J Sleep Research. 2006;15(Supplement 1) [Google Scholar]
- 7.Arand D, Bonnet M, Hurwitz T, Mitler M, Rosa R, Sangal RB. The clinical use of the MSLT and MWT. Sleep. 2005;28:183–184. doi: 10.1093/sleep/28.1.123. [DOI] [PubMed] [Google Scholar]
- 8.Edinger JD, Glenn DM, Bastian LA, Marsh GR, Dailey D, Hope TV, et al. Daytime testing after laboratory or home-based polysomnography: comparisons of middle-aged insomnia sufferers and normal sleepers. J Sleep Res. 2003;12:43–52. doi: 10.1046/j.1365-2869.2003.00335.x. [DOI] [PubMed] [Google Scholar]
- 9.Allen LS, Hines M, Shryne JE, Gorski RA. Two sexually dimorphic cell groups in the human brain. J Neurosci. 1989;9:497–506. doi: 10.1523/JNEUROSCI.09-02-00497.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hofman MA, Swaab DF. The sexually dimorphic nucleus of the preoptic area in the human brain: a comparative morphometric study. J Anat. 1989;164:55–72. [PMC free article] [PubMed] [Google Scholar]
- 11.Bliwise DL. Normal Aging. In: Kryger MH, Roth T, Dement WC, editors. Principles and Practice of Sleep Medicine. Philadelphia: Elsevier Saunders; 2005. pp. 24–38. [Google Scholar]
- 12.National Sleep Foundation. Executive summary of the 2003 “Sleep in America” Poll. 2003. [Google Scholar]
- 13.Monk TH, Thompson WK, Buysse DJ, Hall M, Nofzinger EA, Reynolds CF., III Sleep in healthy seniors: a diary study of the relation between bedtime and the amount of sleep obtained. J Sleep Res. 2006;15:256–260. doi: 10.1111/j.1365-2869.2006.00534.x. [DOI] [PubMed] [Google Scholar]
- 14.Dijk DJ, Lockley SW. Integration of human sleep-wake regulation and circadian rhythmicity. J Appl Physiol. 2002;92:852–862. doi: 10.1152/japplphysiol.00924.2001. [DOI] [PubMed] [Google Scholar]
- 15.Saper CB, Cano G, Scammell TE. Homeostatic, circadian, and emotional regulation of sleep. J Comp Neurol. 2005;493:92–98. doi: 10.1002/cne.20770. [DOI] [PubMed] [Google Scholar]
- 16.Van Dongen HPA, Maislin G, Mullington JM, Dinges DF. The cumulative cost of additional wakefulness: Dose-response effects on neurobehavioral functions and sleep physiology from chronic sleep restriction and total sleep deprivation. Sleep. 2003;26:117–126. doi: 10.1093/sleep/26.2.117. [DOI] [PubMed] [Google Scholar]
- 17.Belenky G, Wesensten NJ, Thorne DR, Thomas ML, Sing HC, Redmond DP, et al. Patterns of performance degradation and restoration during sleep restriction and subsequent recovery: A sleep dose-response study. J Sleep Res. 2003;12:1–12. doi: 10.1046/j.1365-2869.2003.00337.x. [DOI] [PubMed] [Google Scholar]
- 18.Spiegel K, Leproult R, Van Cauter E. Impact of sleep debt on metabolic and endocrine function. Lancet. 1999;354:1435–1439. doi: 10.1016/S0140-6736(99)01376-8. [DOI] [PubMed] [Google Scholar]
- 19.Barger LK, Cade BE, Ayas NT, Cronin JW, Rosner B, Speizer FE, et al. Extended work shifts and the risk of motor vehicle crashes among interns. N Engl J Med. 2005;352:125–134. doi: 10.1056/NEJMoa041401. [DOI] [PubMed] [Google Scholar]
- 20.Peberdy MA, Ornato JP, Larkin GL, Braithwaite RS, Kashner TM, Carey SM, et al. Survival from in-hospital cardiac arrest during nights and weekends. JAMA. 2008;299:785–792. doi: 10.1001/jama.299.7.785. [DOI] [PubMed] [Google Scholar]
- 21.Ferrie JE, Shipley MJ, Cappuccio FP, Brunner E, Miller MA, Kumari M, et al. A prospective study of change in sleep duration: associations with mortality in the Whitehall II cohort. Sleep. 2007;30:1659–1666. doi: 10.1093/sleep/30.12.1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Colten HR, Alteveogt BM, editors. Institute of Medicine. Sleep disorders and sleep deprivation: An unmet public health problem. Washington, D.C.: National Academies Press; 2006. pp. 1–500. [PubMed] [Google Scholar]
- 23.Roenneberg T, Kuehnle T, Pramstaller PP, Ricken J, Havel M, Guth A, et al. A marker for the end of adolescence. Curr Biol. 2004;14:R1038–R1039. doi: 10.1016/j.cub.2004.11.039. [DOI] [PubMed] [Google Scholar]
- 24.Dijk DJ, Duffy JF, Riel E, Shanahan TL, Czeisler CA. Ageing and the circadian and homeostatic regulation of human sleep during forced desynchrony of rest, melatonin and temperature rhythms. J Physiol (Lond) 1999;516.2:611–627. doi: 10.1111/j.1469-7793.1999.0611v.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Buysse DJ, Monk TH, Carrier J, Begley A. Circadian patterns of sleep, sleepiness, and performance in older and younger adults. Sleep. 2005;28:1365–1376. doi: 10.1093/sleep/28.11.1365. [DOI] [PubMed] [Google Scholar]
- 26.Duffy JF, Dijk DJ, Hall EF, Czeisler CA. Relationship of endogenous circadian melatonin and temperature rhythms to self-reported preference for morning or evening activity in young and older people. J Investig Med. 1999;47:141–150. [PMC free article] [PubMed] [Google Scholar]
- 27.Dijk DJ, Duffy JF, Czeisler CA. Contribution of circadian physiology and sleep homeostasis to age-related changes in human sleep. Chronobiol Int. 2000;17:285–311. doi: 10.1081/cbi-100101049. [DOI] [PubMed] [Google Scholar]
- 28.Carskadon MA, Acebo C, Seifer R. Extended nights, sleep loss, and recovery sleep in adolescents. Arch Ital Biol. 2001;139:301–312. [PubMed] [Google Scholar]
- 29.Kamdar BB, Kaplan KA, Kezirian EJ, Dement WC. The impact of extended sleep on daytime alertness, vigilance, and mood. Sleep Med. 2004;5:441–448. doi: 10.1016/j.sleep.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 30.Wehr TA, Moul DE, Barbato G, Giesen HA, Seidel JA, Barker C, et al. Conservation of photoperiod-responsive mechanisms in humans. Am J Physiol. 1993;265:R846–R857. doi: 10.1152/ajpregu.1993.265.4.R846. [DOI] [PubMed] [Google Scholar]
- 31.Rajaratnam SM, Middleton B, Stone BM, Arendt J, Dijk DJ. Melatonin advances the circadian timing of EEG sleep and directly facilitates sleep without altering its duration in extended sleep opportunities in humans. J Physiol. 2004;561:339–351. doi: 10.1113/jphysiol.2004.073742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Carskadon MA, Dement WC, Mitler MM, Roth T, Westbrook PR, Keenan S. Guidelines for the multiple sleep latency test (MSLT): a standard measure of sleepiness. Sleep. 1986;9:519–524. doi: 10.1093/sleep/9.4.519. [DOI] [PubMed] [Google Scholar]
- 33.Rechtschaffen A, Kales A. A manual of standardized terminology, techniques and scoring system for sleep stages of human Subjects. Washington, D.C.: U.S. Government Printing Office; 1968. [Google Scholar]
- 34.Bond A, Lader M. The use of analogue scales in rating subjective feelings. Br J Med Psychol. 1974;47:211–218. [Google Scholar]
- 35.Wyatt JK, Ritz-De Cecco A, Czeisler CA, Dijk DJ. Circadian temperature and melatonin rhythms, sleep, and neurobehavioral function in humans living on a 20-h day. Am J Physiol Regul Integr Comp Physiol. 1999;277:R1152–R1163. doi: 10.1152/ajpregu.1999.277.4.r1152. [DOI] [PubMed] [Google Scholar]
- 36.Klein KE, Wegmann HM, Bruner H. Circadian rhythm in indices of human performance, physical fitness and stress resistance. Aerospace Med. 1968;39:512–518. [PubMed] [Google Scholar]
- 37.Wyatt JK, Cajochen C, Ritz-De Cecco A, Czeisler CA, Dijk DJ. Low-dose, repeated caffeine administration for circadian-phase-dependent performance degradation during extended wakefulness. Sleep. 2004;27:374–381. doi: 10.1093/sleep/27.3.374. [DOI] [PubMed] [Google Scholar]
- 38.Dinges DF, Powell JW. Microcomputer analyses of performance on a portable, simple visual RT task during sustained operations. Behavior Research Methods, Instruments & Computers. 1985;17:652–655. [Google Scholar]
- 39.Klerman EB, Dijk DJ. Interindividual variation in sleep duration and its association with sleep debt in young adults. Sleep. 2005;28:1253–1259. doi: 10.1093/sleep/28.10.1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table 1: Mean (standard deviation) for polysomnographic sleep measures during Baseline sleep, which was scheduled according to each subject's habitual sleep schedule. For the younger subjects, data are provided for both the entire group and for the subset of younger subjects with HSD between 7 and 9 hours (to match the observed HSD range of all the older subjects).
Table 2: Mean (standard error) for polysomnographic sleep measures for each day (12-hour nighttime plus 4-hour daytime episodes) of extended sleep opportunity.