Abstract
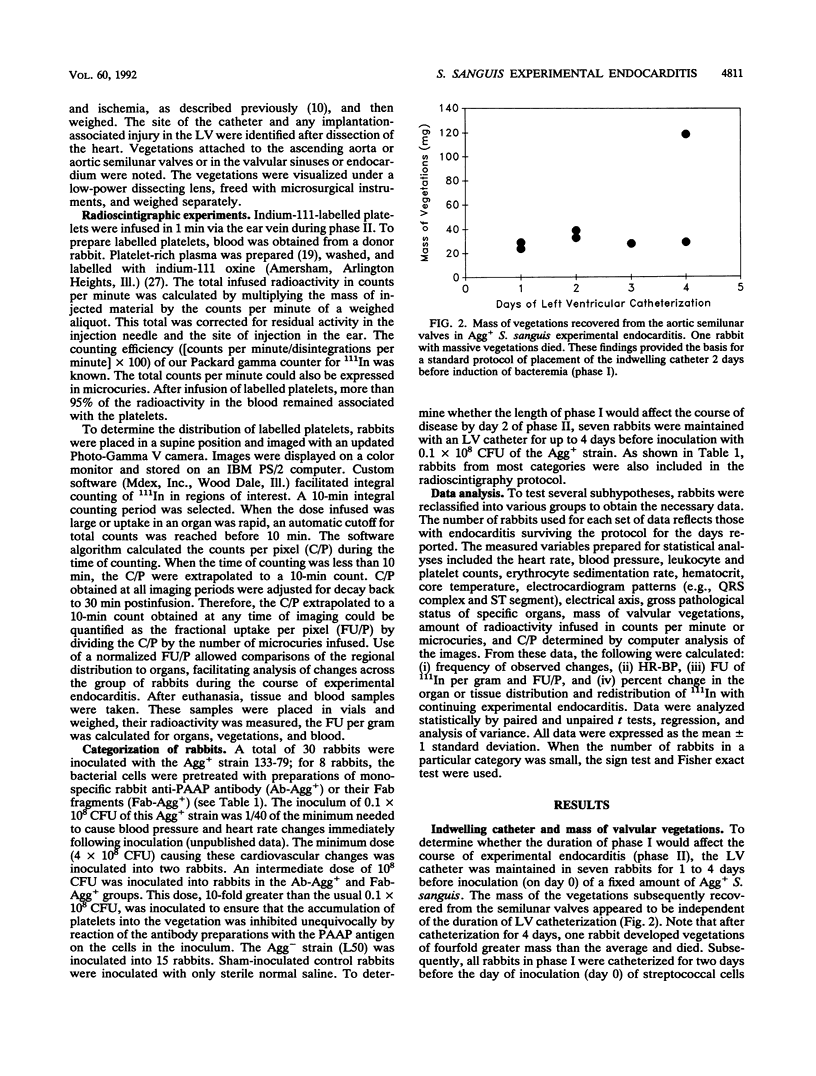
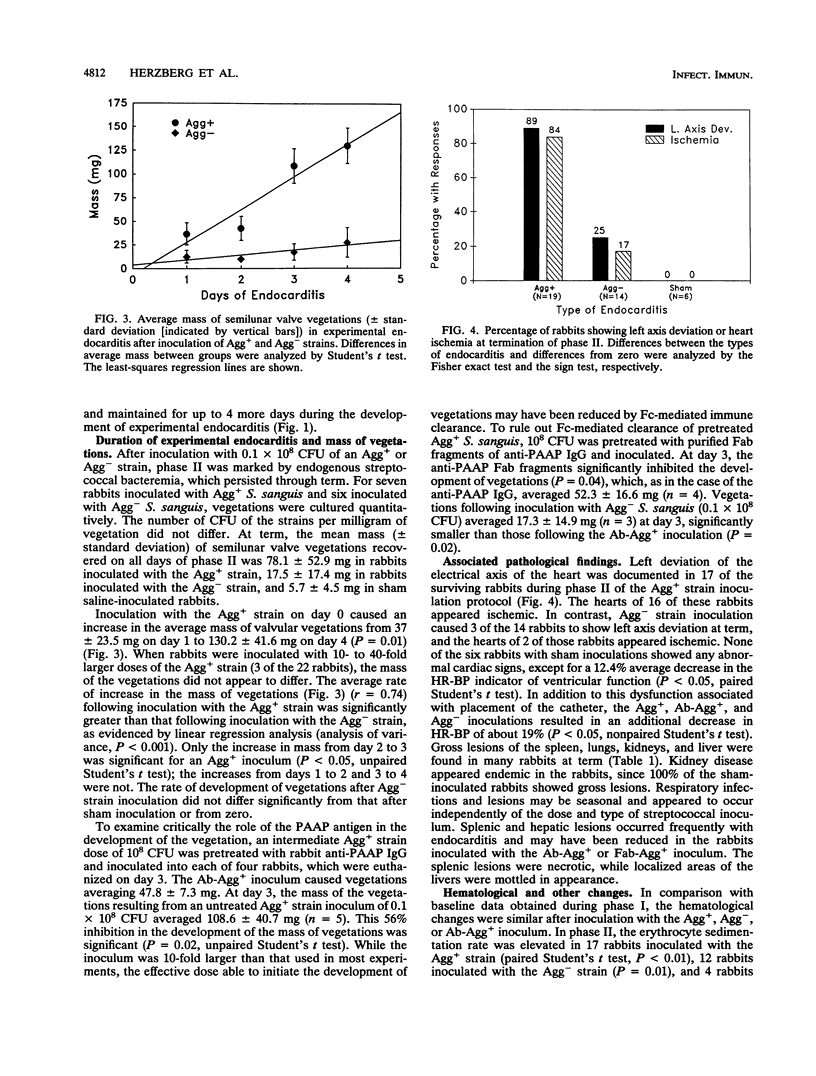
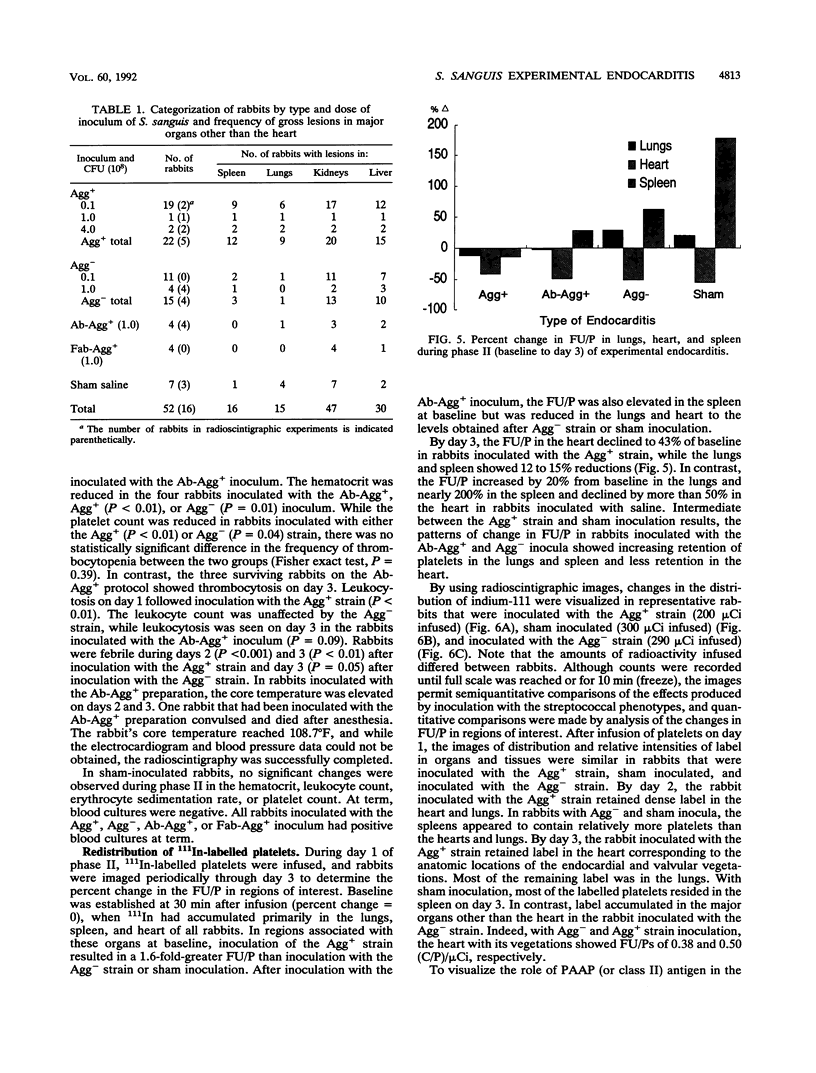
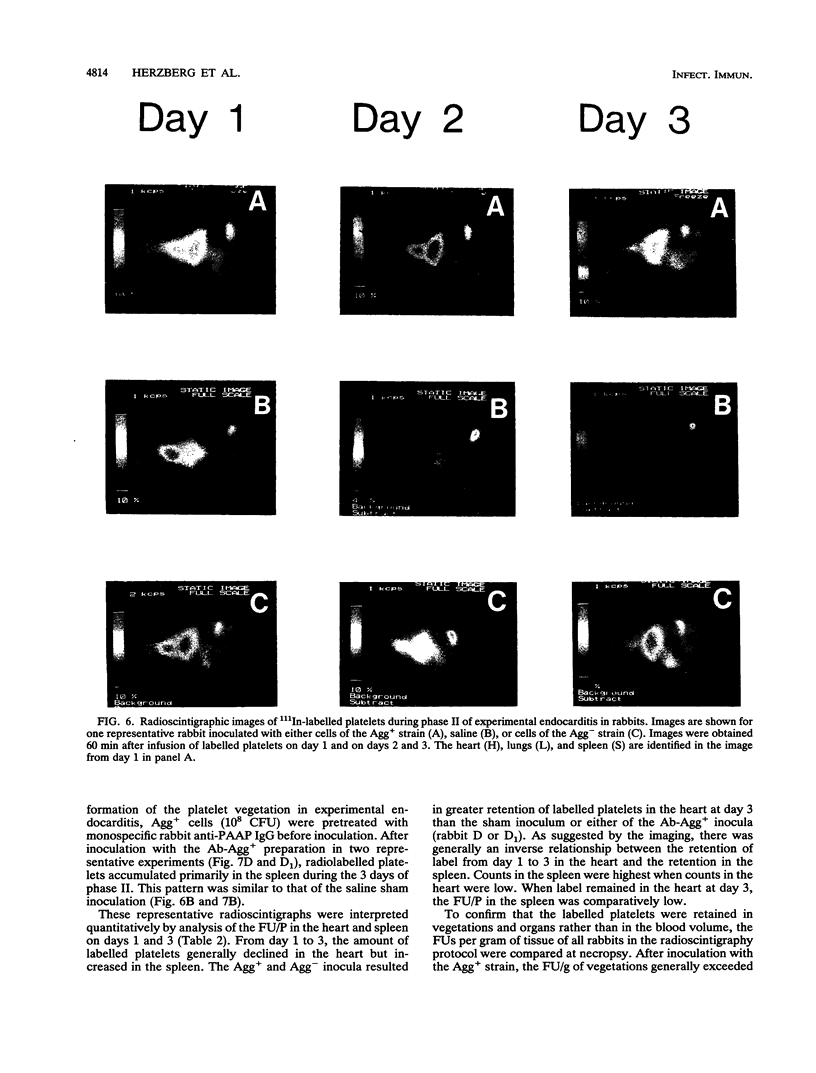
A strain of Streptococcus sanguis that induced rabbit platelets to aggregate in vitro (Agg+ phenotype) was hypothesized to be a more virulent pathogen than an Agg- strain in experimental endocarditis in rabbits. A left ventricular catheter was implanted, and then an Agg+ or Agg- strain was inoculated intravenously. Vegetations formed on the aortic semilunar valves but were unaffected by the duration of implantation of the catheter. Vegetations enlarged by accumulating platelets and their mass increased directly with the duration of endocarditis. Inoculation of the Agg+ strain consistently caused endocarditis with significantly larger vegetations, a more severe clinical course (including febrile episodes, hematological changes, and signs of myocardial ischemia), more gross lesions in major organs, and greater mortality than inoculation with the Agg- strain, saline, or the Agg+ strain pretreated with monospecific rabbit immunoglobulin G or Fab fragments against its platelet aggregation-associated protein (PAAP; class II). In experimental endocarditis, PAAP expressed by Agg+ S. sanguis appeared to be an important virulence factor.
Full text
PDF

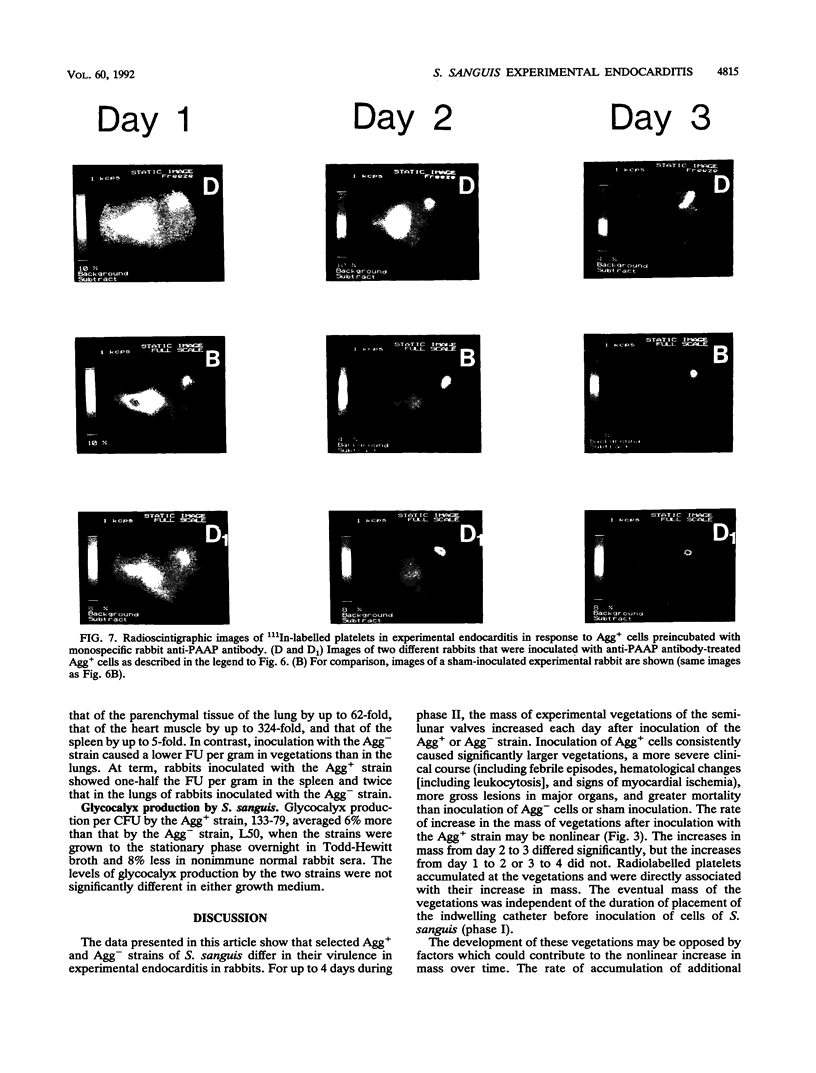



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baddour L. M., Christensen G. D., Lowrance J. H., Simpson W. A. Pathogenesis of experimental endocarditis. Rev Infect Dis. 1989 May-Jun;11(3):452–463. doi: 10.1093/clinids/11.3.452. [DOI] [PubMed] [Google Scholar]
- Buiting A. G., Thompson J., van der Keur D., Schmal-Bauer W. C., Bertina R. M. Procoagulant activity of endocardial vegetations and blood monocytes in rabbits with Streptococcus sanguis endocarditis. Thromb Haemost. 1989 Nov 24;62(3):1029–1033. [PubMed] [Google Scholar]
- Carroll G. C., Sebor R. J. Dental flossing and its relationship to transient bacteremia. J Periodontol. 1980 Dec;51(12):691–692. doi: 10.1902/jop.1980.51.12.691. [DOI] [PubMed] [Google Scholar]
- Dajani A. S., Bisno A. L., Chung K. J., Durack D. T., Freed M., Gerber M. A., Karchmer A. W., Millard H. D., Rahimtoola S., Shulman S. T. Prevention of bacterial endocarditis. Recommendations by the American Heart Association. JAMA. 1990 Dec 12;264(22):2919–2922. [PubMed] [Google Scholar]
- Dall L. H., Herndon B. L. Association of cell-adherent glycocalyx and endocarditis production by viridans group streptococci. J Clin Microbiol. 1990 Aug;28(8):1698–1700. doi: 10.1128/jcm.28.8.1698-1700.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dall L., Herndon B. Quantitative assay of glycocalyx produced by viridans group streptococci that cause endocarditis. J Clin Microbiol. 1989 Sep;27(9):2039–2041. doi: 10.1128/jcm.27.9.2039-2041.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dall L., Keilhofner M., Herndon B., Barnes W., Lane J. Clindamycin effect on glycocalyx production in experimental viridans streptococcal endocarditis. J Infect Dis. 1990 Jun;161(6):1221–1224. doi: 10.1093/infdis/161.6.1221. [DOI] [PubMed] [Google Scholar]
- DiNubile M. J., Calderwood S. B., Steinhaus D. M., Karchmer A. W. Cardiac conduction abnormalities complicating native valve active infective endocarditis. Am J Cardiol. 1986 Dec 1;58(13):1213–1217. doi: 10.1016/0002-9149(86)90384-x. [DOI] [PubMed] [Google Scholar]
- Durack D. T., Beeson P. B. Experimental bacterial endocarditis. I. Colonization of a sterile vegetation. Br J Exp Pathol. 1972 Feb;53(1):44–49. [PMC free article] [PubMed] [Google Scholar]
- Durack D. T., Beeson P. B., Petersdorf R. G. Experimental bacterial endocarditis. 3. Production and progress of the disease in rabbits. Br J Exp Pathol. 1973 Apr;54(2):142–151. [PMC free article] [PubMed] [Google Scholar]
- Erickson P. R., Herzberg M. C. A collagen-like immunodeterminant on the surface of Streptococcus sanguis induces platelet aggregation. J Immunol. 1987 May 15;138(10):3360–3366. [PubMed] [Google Scholar]
- Erickson P. R., Herzberg M. C. Purification and partial characterization of a 65-kDa platelet aggregation-associated protein antigen from the surface of Streptococcus sanguis. J Biol Chem. 1990 Aug 25;265(24):14080–14087. [PubMed] [Google Scholar]
- Erickson P. R., Herzberg M. C., Tierney G. Cross-reactive immunodeterminants on Streptococcus sanguis and collagen. Predicting a structural motif of platelet-interactive domains. J Biol Chem. 1992 May 15;267(14):10018–10023. [PubMed] [Google Scholar]
- Garrison P. K., Freedman L. R. Experimental endocarditis I. Staphylococcal endocarditis in rabbits resulting from placement of a polyethylene catheter in the right side of the heart. Yale J Biol Med. 1970 Jun;42(6):394–410. [PMC free article] [PubMed] [Google Scholar]
- Hamill R. J. Role of fibronectin in infective endocarditis. Rev Infect Dis. 1987 Jul-Aug;9 (Suppl 4):S360–S371. doi: 10.1093/clinids/9.supplement_4.s360. [DOI] [PubMed] [Google Scholar]
- Herzberg A. J., Bossen E. H., Walther P. J. Adenoid cystic carcinoma of the breast metastatic to the kidney. A clinically symptomatic lesion requiring surgical management. Cancer. 1991 Sep 1;68(5):1015–1020. doi: 10.1002/1097-0142(19910901)68:5<1015::aid-cncr2820680518>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Herzberg M. C., Brintzenhofe K. L., Clawson C. C. Aggregation of human platelets and adhesion of Streptococcus sanguis. Infect Immun. 1983 Mar;39(3):1457–1469. doi: 10.1128/iai.39.3.1457-1469.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzberg M. C., Brintzenhofe K. L., Clawson C. C. Cell-free released components of Streptococcus sanguis inhibit human platelet aggregation. Infect Immun. 1983 Oct;42(1):394–401. doi: 10.1128/iai.42.1.394-401.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzberg M. C., Gong K., MacFarlane G. D., Erickson P. R., Soberay A. H., Krebsbach P. H., Manjula G., Schilling K., Bowen W. H. Phenotypic characterization of Streptococcus sanguis virulence factors associated with bacterial endocarditis. Infect Immun. 1990 Feb;58(2):515–522. doi: 10.1128/iai.58.2.515-522.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hockett R. N., Loesche W. J., Sodeman T. M. Bacteraemia in asymptomatic human subjects. Arch Oral Biol. 1977;22(2):91–98. doi: 10.1016/0003-9969(77)90084-x. [DOI] [PubMed] [Google Scholar]
- Kessler C. M., Nussbaum E., Tuazon C. U. In vitro correlation of platelet aggregation with occurrence of disseminated intravascular coagulation and subacute bacterial endocarditis. J Lab Clin Med. 1987 Jun;109(6):647–652. [PubMed] [Google Scholar]
- Lerner P. I., Weinstein L. Infective endocarditis in the antibiotic era. N Engl J Med. 1966 Feb 3;274(5):259–contd. doi: 10.1056/NEJM196602032740506. [DOI] [PubMed] [Google Scholar]
- Lowrance J. H., Baddour L. M., Simpson W. A. The role of fibronectin binding in the rat model of experimental endocarditis caused by Streptococcus sanguis. J Clin Invest. 1990 Jul;86(1):7–13. doi: 10.1172/JCI114717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mage M. G. Preparation of Fab fragments from IgGs of different animal species. Methods Enzymol. 1980;70(A):142–150. doi: 10.1016/s0076-6879(80)70045-9. [DOI] [PubMed] [Google Scholar]
- Overholser C. D., Moreillon P., Glauser M. P. Experimental bacterial endocarditis after dental extractions in rats with periodontitis. J Infect Dis. 1987 Jan;155(1):107–112. doi: 10.1093/infdis/155.1.107. [DOI] [PubMed] [Google Scholar]
- Riba A. L., Thakur M. L., Gottschalk A., Andriole V. T., Zaret B. L. Imaging experimental infective endocarditis with indium-111-labeled blood cellular components. Circulation. 1979 Feb;59(2):336–343. doi: 10.1161/01.cir.59.2.336. [DOI] [PubMed] [Google Scholar]
- Sullam P. M., Valone F. H., Mills J. Mechanisms of platelet aggregation by viridans group streptococci. Infect Immun. 1987 Aug;55(8):1743–1750. doi: 10.1128/iai.55.8.1743-1750.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sussman J. I., Baron E. J., Tenenbaum M. J., Kaplan M. H., Greenspan J., Facklam R. R., Tyburski M. B., Goldman M. A., Kanzer B. F., Pizzarello R. A. Viridans streptococcal endocarditis: clinical, microbiological, and echocardiographic correlations. J Infect Dis. 1986 Oct;154(4):597–603. doi: 10.1093/infdis/154.4.597. [DOI] [PubMed] [Google Scholar]
- Verhaaren H., Claeys G., Verschraegen G., de Niel C., Leroy J., Clement D. Endocarditis from a dental focus. Importance of oral hygiene in valvar heart disease. Int J Cardiol. 1989 Jun;23(3):343–347. doi: 10.1016/0167-5273(89)90194-0. [DOI] [PubMed] [Google Scholar]
- Weinberger I., Rotenberg Z., Zacharovitch D., Fuchs J., Davidson E., Agmon J. Native valve infective endocarditis in the 1970s versus the 1980s: underlying cardiac lesions and infecting organisms. Clin Cardiol. 1990 Feb;13(2):94–98. doi: 10.1002/clc.4960130206. [DOI] [PubMed] [Google Scholar]





