Abstract
Aim
To assess the expression level of fms-like tyrosine kinase 3 (FLT3), the incidence of FLT3/internal tandem duplications (ITD) mutation, and prognostic value of FLT3 changes in different types of adult leukemia.
Methods
Bone marrow mononuclear cells were isolated from 147 adult patients with leukemia. Reverse transcriptase polymerase chain reaction (PCR) was used to screen FLT3/ITD mutation and quantitative PCR was performed to evaluate the expression of the FLT3 transcript. Flow cytometry was used for detection of FLT3 receptor protein expression on bone marrow mononuclear cells. Pearson correlation analysis was performed to estimate the significance of FLT3.
Results
FLT3 expression was higher in acute myeloid leukemia and B-acute lymphoid leukemia than in T-acute lymphoid leukemia (P = 0.006, P = 0.001) and chronic myelogenous leukemia (P < 0.001). In chronic myelogenous leukemia, FLT3 expression in blast transformation phase was higher than in acceleration phase (P = 0.023). Surface expression of FLT3 protein was correlated with high percentage of bone marrow blasts and with FLT3 mRNA expression (r = 0.366, P < 0.001) in acute leukemia. FLT3/ITDs in the juxtamembrane domain were found in 25% of patients with acute myeloid leukemia and 7% of patients with acute lymphoid leukemia. FLT3/ITD positive sequences had 36, 42, and 57 nucleotides. FLT3/ITD mutation was associated with a higher white blood cell count, higher marrow blast percentage, and elevated serum lactate dehydrogenase (P = 0.045, P = 0.014, P < 0.001, respectively) and not associated with a higher FLT3 mRNA and FLT3 protein expression, and lower complete remission (P = 0.091, P = 0.060, P = 0.270, respectively).
Conclusion
FLT3 expression levels differed in different types of adult leukemia. Overexpression of FLT3 and presence of a positive FLT3/ITD mutation in acute leukemia were associated with unfavorable clinical characteristics and poor prognosis.
The fms-like tyrosine kinase 3 (FLT3) gene belongs to the class III receptor tyrosine kinases and is predominantly expressed on hematopoietic progenitor cells in the bone marrow, thymus, and lymph nodes (1). An abnormality in the FLT3 gene is implicated in the pathogenesis of acute myeloid leukemia (2-4). Approximately 25% of patients with adult acute myeloid leukemia harbor internal tandem duplications (ITD) within the juxtamembrane domain of the FLT3 gene (5,6). FLT3/ITDs cause structural changes in the juxtamembrane and this disrupts the autoinhibitory conformation of the receptor (7) by promoting constitutive activation of both receptor (8-10) and downstream effectors, which all leads to a bad prognosis (11,12). In the last decade, FLT3/ITD mutations have been reported in 13-32% of adult patients with acute myeloid leukemia and in a small number of patients with acute lymphoid leukemia (13-16). Patients with this abnormality have increased incidence of leukocytosis and decreased overall survival in comparison with patients without this abnormality. These findings indicate that FLT3/ITDs not only play an important role in the pathogenesis mechanism of leukemia but also have a prognostic value.
A previous study has demonstrated that high levels of FLT3 were expressed in leukemia and lymphoma cell lines including pre-B, myeloid, and monocytic cell lines (17). Also, several studies have shown that high levels of FLT3 were expressed in 70-100% of patients with acute myeloid leukemia and B-acute lymphoid leukemia and in about 30% of patients with T-acute lymphoid leukemia (18,19). Likewise, a small number of chronic myelogenous leukemia blast crisis and chronic lymphocytic leukemia cells has been shown to express FLT3 (18,20). These data indicate that FLT3 expression may play a role in the survival or proliferation of leukemic blasts. Using Western blotting, Carow et al (18) found no FLT3 expression in the normal bone marrow, but identified FLT3 protein in 14 of 14 B-cell acute lymphoid leukemia cases, 36 of 41 acute myeloid leukemia cases, and 1 of 4 T-cell acute lymphoid leukemia cases. Though FLT3 expression in leukemia and its clinical significance have been widely investigated, little is known about FLT3 expression level and its clinical significance in Chinese patients with adult leukemia. Most of the studies have used Western blot assay as FLT3 protein assay, whereas flow cytometry on intact leukemic cell surface has been rarely used. We used flow cytometry on cell surface to investigate the expression of FLT3 receptor and quantitative polymerase chain reaction (PCR) to investigate FLT3 mRNA expression, as well as performed identification of FLT3/ITDs in different types of adult leukemia.
Patients and methods
The study included 120 patients with newly diagnosed acute leukemia – 60 with acute myeloid leukemia, 30 with B-acute lymphoid leukemia, 30 with T-acute lymphoid leukemia; 27 with chronic myeloid leukemia; and 30 controls (Table 1). Diagnosis was based on May-Grunwald-Giemsa-stained bone marrow smears and cytochemistry performed according to the French-American-British (FAB) group criteria (21). Leukocyte differentiation antigens were analyzed by immunofluorescent method for some cases. Complete remission was defined as normocellular bone marrow containing less than 5% blasts and showing evidence of normal maturation of other bone marrow elements. Patients and controls provided informed consent to use their samples for this study.
Table 1.
Clinical and demographic characteristics of 147 Chinese patients and controls with different types of adult leukemia*
| Type of adult leukemia | No. of patients (male/female) | Age (years; median, range) |
|---|---|---|
| Controls | 30 (17/13) | 48 (18-76) |
| Idiopathic thrombocytopenic purpura | 5 (0/5) | 40 (18-60) |
| Iron deficiency anemia | 15 (9/6) | 50 (25-76) |
| Normal | 10 (8/2) | 49 (20-72) |
| Chronic myelogenous leukemia: | 27 (15/12) | 52 (23-77) |
| chronic phase | 10 (6/4) | 49 (23-73) |
| acceleration phase | 7 (4/3) | 51 (30-71) |
| lymphoid blast | 3 (2/1) | 56 (35-77) |
| blast crisis | 7 (3/4) | 53 (32-76) |
| Acute myeloid leukemia (FAB type): | 60 (36/24) | 47 (15-75) |
| M2 | 20 (12/8) | 45 (21-69) |
| M3 | 15 (8/7) | 45 (15-60) |
| M4 | 7 (4/3) | 53 (25-71) |
| M5 | 15 (10/5) | 48 (19-75) |
| M6 | 3 (2/1) | 52 (35-69) |
| Acute lymphoid leukemia | 60 (30/30) | 45 (17-77) |
| T acute lymphoid leukemia | 30 (16/14) | 46 (19-77) |
| B-acute lymphoid leukemia | 30 (14/16) | 44 (17-70) |
*Abbreviations: M2 – acute myeloblastic leukemia with maturation; M3 – acute promyelocytic leukemia; M4 – acute myelomonocytic leukemia; M5 – acute monocytic leukemia; M6 – acute erythroid leukemia; FAB – French-American-British classification of acute myeloid leukemia (21).
mRNA expression analysis
Patients and controls’ bone marrow mononuclear cells were separated on a Ficoll-Hypaque density gradient. Total RNA was extracted from bone marrow mononuclear cells by using Trizol Kit (Invitrogen, Carlsbad, CA, USA). cDNA was synthesized from each RNA by a random primer and Moloney murine leukemia virus reverse transcriptase (Promage, Madison, WI, USA) according to the manufacturer’s recommendations. FLT3 gene expression was assessed by reverse transcriptase PCR (RT-PCR) and quantitative PCR. The primer pairs of FLT3 for RT-PCR were 5′-TGTCGAGCAGTACTCTAAACA-3′ and 5′-ATCCTAGTACCTTCCCAAACTC-3′ (22). The β-actin gene, with primers 5′-TGACGGGGTCACCCACAC-3′ and 5′-CTAGAAGCATTTGCGGTGGA-3′, served as an internal control. Reagents consisted of Taq polymerase, dNTP, buffer, and MgCl2. Reaction was performed in three steps as follows: denaturation at 94°C, annealing at 50°C, and elongation at 72°C. After 35 cycles, PCR products were fractionated through 2.5% agarose gels and viewed under UV illumination after ethidium bromide staining.
Expression of FLT3 mRNA was analyzed with quantitative PCR in 20 controls and 50 patients with acute myeloid leukemia (16 acute myeloblastic leukemia with maturation; 12 acute promyelocytic leukemia; 5 acute myelomonocytic leukemia; 15 acute monocytic leukemia; 2 acute erythroid leukemia), 20 patients with B-acute lymphoid leukemia, 20 patients with T-acute lymphoid leukemia, and 27 patients with chronic myelogenous leukemia. Quantitative PCR was done by Quantitect SYBR green PCR reagent (Qiagen, Miami, FL, USA) following the manufacturer’s recommendations and using a quantitative PCR instrument RotorGene 6000 (Corbett Research, Mortlake, Australia). The primer sequences of FLT3 were forward – 5′-TCAAGTGCTGTGCATACAATTCCC-3′ and reverse – 5′-CACCTGTACCATCTGTAGCTGGCT-3′; glyceraldehyde 3-phosphate dehydrogenase (GAPDH) served as a reference with the following sequences: forward – 5′-CCAAAATCAAGTGGGGCGATG-3′ and reverse – 5′-AAAGGTGGAGGAGTGGGTGTCG-3′ (23). Thermal cycling for FLT3 was performed as follows: step 1 – at 95°C for 15 minutes; step 2 – at 95°C for 15 seconds; step 3 – at 50°C for 30 seconds, step 4 – at 72°C for 1 minute. Steps 2-4 were repeated for 35 cycles. The copy number of each sample was calculated by relative quantification. A dilution series of 5 different concentrations was used to generate two standard curves, one for FLT3 and another for GAPDH. FLT3 and GAPDH were simultaneously amplified and the threshold cycle (Ct) of each gene was obtained. Ct values of the sample and the corresponding standard curve were used to calculate the amounts of GAPDH and FLT3 mRNA. FLT3 mRNA expression was then presented as ratio of FLT3/GAPDH mRNA in the sample, as the expression of GAPDH was constant across samples.
Screening for mutation of FLT3/ITD
Amplified products were resolved on 2.5% agarose gels. FLT3/ITD mutations were identified as bands migrating above the expected 366 bp size of the wild-type FLT3 fragment. Amplified cDNA fragments were purified and sequence analysis performed by ABI BigDye (Applied Biosystems, Foster City, CA, USA).
Flow cytometry assay of FLT3 receptor protein expression
Bone marrow mononuclear cells were washed twice with phosphate buffered saline (PBS) and incubated at 37°C for 1 hour with concentration of 100 μg/mL goat antihuman FLT3 antibody (R&D System, Inc, Minneapolis, MN, USA). The cells were washed with PBS three times and incubated with fluorescence isothiocyanate-conjugated rabbit anti-goat immunoglobulin antibody for 1 hour. Flow cytometry analysis was performed by gating all cells acquired after Ficoll separation. The percentages of positive cells were determined using isotype controls. The correlation between the amounts of FLT3-positive cells and corresponding percentage of blasts (according to morphology classification in the bone marrow) was analyzed in acute leukemia.
Statistical analysis
Difference in median values of age, peripheral white blood cell counts, copy number, and lactate dehydrogenase between two groups were analyzed with the Mann-Whiney U test and among three or more groups with student Newman-Keuls test for distribution. The analysis of the distribution between two variables was performed by Pearson correlation test. Analysis of the frequencies was performed by Fisher exact test for 2 × 2 tables. Statistical analyses were performed with Statistical Package for the Social Sciences (SPSS Inc., Chicago, IL, USA). All P values were two-tailed and considered statistically significant if P < 0.05.
Results
Expression of FLT3 transcript in leukemia
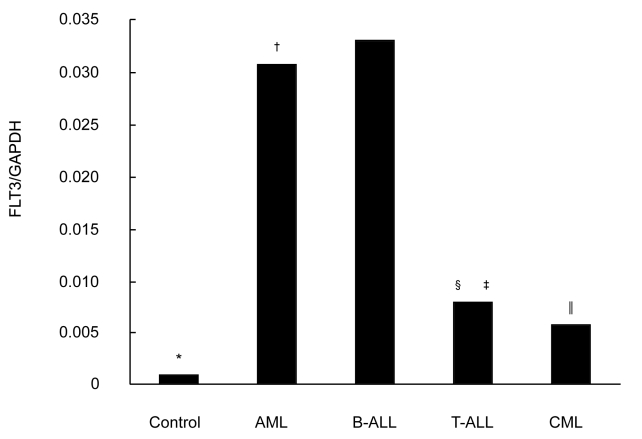
RT-PCR combined with gel-image analysis system was used to determine the expression of FLT3 transcript in 147 patients with leukemia and 30 controls. Of 147 patients, there were 120 patients with acute leukemia and 27 patients with chronic myelogenous leukemia. Of these, 138 (93.9%) showed FLT3 mRNA expression (117 patients with acute leukemia and 21 with chronic myelogenous leukemia). Quantitative PCR showed that FLT3 mRNA was expressed in 90 patients with acute leukemia, 27 patients with chronic myelogenous leukemia, and 20 controls (Figure 1). There was a significant difference between patients with acute myeloid leukemia and patients with chronic myelogenous leukemia in FLT3 expression (Figure 1). In patients with acute myeloid leukemia, B-acute lymphoid leukemia, and T-acute lymphoid leukemia, mean percentage of blasts was 61.0%, 56.5%, and 55.8%, respectively, and the expression level in acute myeloid leukemia and B-acute lymphoid leukemia was significantly higher than in T-acute lymphoid leukemia. In chronic myelogenous leukemia, the expression of FLT3 transcript was associated with disease progression of transformation (Table 2).
Figure 1.
Analysis of fms-like tyrosine kinase 3 (FLT3) mRNA expression levels in controls and patients with acute myeloid leukemia (AML), B-acute lymphoid leukemia (B-ALL), T-acute lymphoid leukemia (T-ALL), and chronic myelogenous leukemia (CML). The data were calculated from the ratio of FLT3 to glyceraldehyde 3-phosphate dehydrogenase (GAPDH, internal control) by quantitative polymerase chain reaction. Ratios for controls, AML, B-ALL, T-ALL, CML were 0.001, 0.031, 0.033, 0.008, and 0.006, respectively. Asterisk indicates P < 0.001, control vs leukemia; dagger indicates P = 0.091, AML vs B-ALL; double dagger indicates P = 0.006, AML vs T-ALL; section mark indicates P = 0.001, B-ALL vs T-ALL; parallel mark indicates P < 0.001, AML vs CML (Student Newman-Keuls test).
Table 2.
Expression level of the fms-like tyrosine kinase 3 (FLT3) transcript and clinical characteristics in chronic myelogenous leukemia (CML) used by quantitative polymerase chain reaction*
| Characteristics | CML chronic phase (n = 10) | CML acceleration phase (n = 7) | CML blast crisis (n = 10) | P† |
|---|---|---|---|---|
| PBWBC count ( × 109/L, mean±SD) |
41.0 ± 7.5 |
80.6 ± 8.1 |
113.2 ± 46.4 |
0.001‡ |
| 0.089§ | ||||
| Percentage of blasts in the bone marrow |
0.030 ± 0.009 |
0.15 ± 0.03 |
0.642 ± 0.182 |
0.001‡ |
| 0.001§ | ||||
| Expression level of FLT3 mRNA (FLT3/GAPDH) |
0.002 |
0.004 |
0.017 |
0.307‡ |
| 0.023§ | ||||
| Surface expression of FLT3 receptor protein | 0.067 ± 0.019 | 0.10 ± 0.01 | 0.158 ± 0.030 | 0.002‡ |
| 0.001§ |
*Abbreviations: PBWBC – peripheral blood white blood cell; GAPDH – glyceraldehyde 3-phosphate dehydrogenase.
†Student Newman-Keuls test.
‡CML chronic phase in comparison with CML acceleration phase.
§CML acceleration phase in comparison with CML blast crisis.
Incidence of FLT3/ITD mutation in acute leukemia
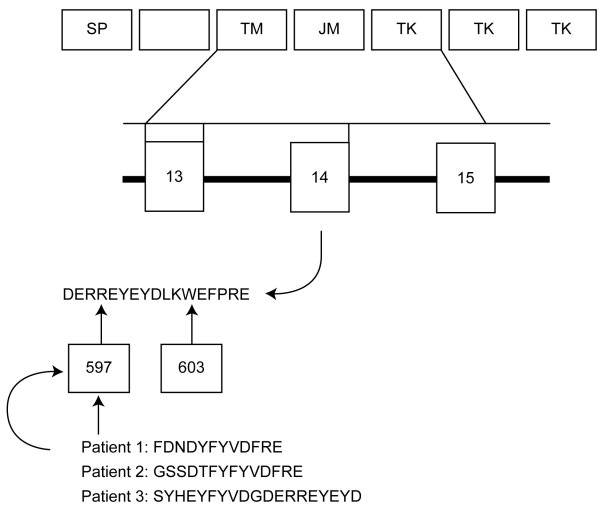
Of 60 patients with acute myeloid leukemia, 15 had FLT3/ITD mutation. Patients with acute monocytic leukemia had higher FLT3/ITD mutation rate than patients with other FAB types (Table 3). Of 60 patients with acute lymphoid leukemia, 4 had FLT3/ITD mutations. No FLT3/ITD mutation was found in chronic myelogenous leukemia. Sequence analysis performed in 3 FLT3/ITD mutations showed that mutations involved exon 14, the sizes of the inserting nucleotides were 36, 42, and 57 pairs, and all had tandem duplication. The location of each mutation also varied, with the ITD in two patients starting at codon 597 and in another patient at codon 603. The deduced amino acid sequences for FLT3/ITD mutation are shown in Figure 2.
Table 3.
Distribution of fms-like tyrosine kinase 3/internal tandem duplications (FLT3/ITD) mutations in acute myeloid leukemia
| FAB type* | No. of FLT3/ITD+ patients |
|---|---|
| M2 (n = 20) | 3 |
| M3 (n = 15) | 3 |
| M4 (n = 7) | 2 |
| M5 (n = 15) | 6 |
| M6 (n = 3) | 1 |
*Abbreviations: M2 – acute myeloblastic leukemia with maturation; M3 – acute promyelocytic leukemia; M4 – acute myelomonocytic leukemia; M5 – acute monocytic leukemia; M – acute erythroid leukemia; FAB – French-American-British Classification of acute myeloid leukemia (21).
Figure 2.
Sequence analysis results of fms-like tyrosine kinase 3/internal tandem duplications (FLT3/ITD) mutations in three patients with acute leukemia and deduced amino acid sequences for mutation region. Abbreviations: SP – signal peptide; TM – trans-membrane domain; JM – juxtamembrane domain; TK – tyrosine kinase domain.
Characteristics of patients with FLT3/ITD mutation
Nineteen of 120 patients with acute leukemia had FLT3/ITD mutation (Table 4). The presence of the mutation was not related to sex or age. However, it was associated with a higher peripheral white blood cell count and a higher percentage of blasts in the bone marrow at diagnosis. Patients with acute leukemia with FLT3/ITD had significantly higher serum LDH concentration than patients with acute leukemia without FLT3/ITD. As to the expression level, FLT3 transcript in 12 patients with tandem duplication was up-regulated, but the difference was not significant. The expressive signal of FLT3 protein in patients with acute leukemia with FLT3/ITD mutation was stronger than in patients with acute leukemia without FLT3/ITD mutation.
Table 4.
Expression levels and clinical characteristics of patients with positive and negative fms-like tyrosine kinase 3/internal tandem duplications (FLT3/ITD+ and FLT3/ITD-) acute leukemia analyzed by reverse transcriptase-polymerase chain reaction and flow cytometry
| No. of patients |
|||
|---|---|---|---|
| Characteristic | FLT3/ITD+ AL | FLT3/ITD- AL | P |
| Age (years; median, range)* | 44 (21-70) | 47 (15-77) | 0.813‡ |
| Age >60 y | 6 | 29 | |
| Sex:* | 0.821‡ | ||
| male | 10 | 56 | |
| female | 9 | 45 | |
| Expression level of FLT3 mRNA† | 0.062 | 0.034 | 0.091§ |
| Surface expression level of FLT3 protein (%)* | 22.0 | 16.0 | 0.060§ |
| Peripheral blood white blood cell count ( × 109/L)* | 41.6 | 29.5 | 0.045§ |
| Blasts in the bone marrow (%)* | 79 | 56 | 0.014§ |
| Serum lactate dehydrogenase concentration (μ/L)* | 996.8 | 213.6 | <0.001§ |
| Outcome:* | 0.270‡ | ||
| complete remission | 14 | 85 | |
| failure | 5 | 16 | |
| complete remission rate | 73.7% | 84.2% | |
*19 FLT3/ITD- and 101 FLT3/ITD+ cases.
†12 FLT3/ITD- and 78 FLT3/ITD+ cases.
‡Fisher exact test.
§Mann-Whiney U test.
The complete remission rate for 120 patients with acute leukemia in this study was 82.5%. Patients with FLT3/ITD mutation had lower complete remission rate than patients without FLT3/ITD mutation (73.68% vs 84.16%), although the relationship was not significant.
FLT3 receptor expression and its correlation with bone marrow blasts
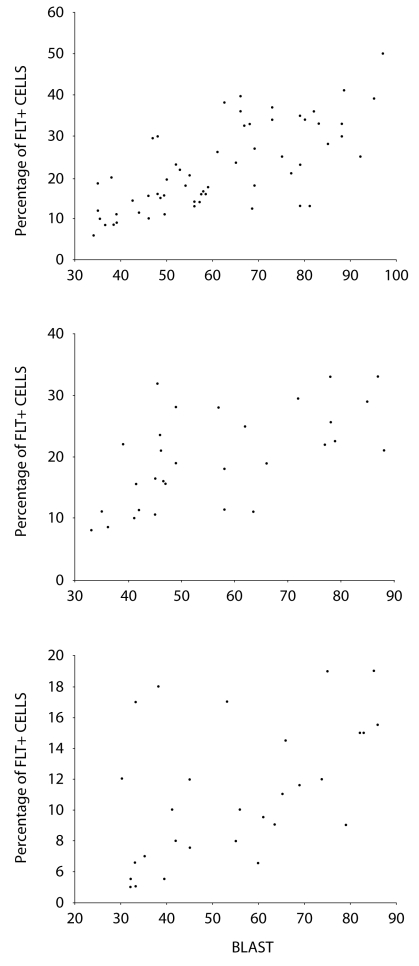
Mean expression level (percentage of FLT3 positive cells) of FLT3 protein in controls was 2.20 ± 1.06%, which was significantly lower than in patients with leukemia (17.38 ± 9.45%, P < 0.001) (Table 2). The expression of FLT3 was significantly higher in patients with acute leukemia (18.82 ± 9.66%) than in patients with chronic myelogenous leukemia in chronic (6.74 ± 1.85%, P < 0.001) and acceleration phase (10.11% ± 1.92, P = 0.019), but no significant difference was observed in comparison with patients in the blast transformation phase (15.81% ± 3.02, P = 0.307). In the acute leukemia group, Pearson correlation analysis showed that the percentage of FLT3-positive cells was significantly related to blast count (Figure 3). The expression of FLT3 transcript was correlated with that of FLT3 receptor protein (Figure 4).
Figure 3.
Surface expression level of fms-like tyrosine kinase 3 (FLT3) analyzed by flow cytometry according to the percentage of bone marrow blasts, FLT3 expression levels increase with the number of bone marrow blasts in acute leukemia. (A) Correlation of FLT3 expression with blasts in bone marrow in acute myeloid leukemia (r = 0.716; P < 0.001); (B) Correlation of FLT3 expression with blasts in bone marrow in B-acute lymphoid leukemia (r = 0.607; P < 0.001); (C) Correlation of FLT3 expression with blasts in bone marrow in T-acute lymphoid leukemia (r = 0.524; P = 0.003).
Figure 4.
Correlation between surface expression of fms-like tyrosine kinase 3 (FLT3) protein and mRNA expression levels. Correlation of surface expression of FLT3 with FLT3/glyceraldehyde 3-phosphate dehydrogenase (GAPDH) in 90 patients with acute leukemia analyzed by quantitative polymerase chain reaction. Pearson correlation shows a significant correlation between receptor expression and mRNA expression (r = 0.366, P < 0.001).
Discussion
Our study showed that FLT3 mRNA was expressed in all patients with B- acute lymphoid leukemia and acute myeloid leukemia and 90% of patients with T-acute lymphoid leukemia. Compared with controls, the mean expression level of the FLT3 transcript in acute lymphoid leukemia and acute myeloid leukemia was evidently increased. This is in close agreement with previously published results (18,20). Due to a limited number of acute myeloid leukemia cells available for this analysis, we did not investigate the correlations with FAB type and cytogenetics, which had previously been demonstrated as important factors for outcome of acute myeloid leukemia (5,6,11,15). Larger scale investigations on the associations between FLT3 expression level and cytogenetics should be performed in future research.
We found FLT3 mRNA and protein expression in chronic myelogenous leukemia, which is consistent with the results by Carow et al (18). Compared with the chronic phase of chronic myelogenous leukemia, 7 patients in the acceleration phase had significantly increased white blood cell count and number of blasts in the bone marrow, but not the expression level of the FLT3 transcript. Also, patients in the acceleration phase had significantly higher surface expression of FLT3 receptor protein than patients in the chronic phase. In chronic myelogenous leukemia, FLT3 protein expression increased more than mRNA expression. This was different than in acute leukemia, probably because FLT3 mRNA level is associated with cell types and stage of blast maturation and perhaps has an unknown biological cause. Although larger scale analysis is required to clarify the clinical significance of increased FLT3 expression, we believe that FLT3 expression may contribute to chronic myelogenous leukemia disease progression and that its estimation may be helpful for identification of chronic myelogenous leukemia blast transformation. This is in accordance with the study by Lin et al (24), who detected FLT3 gene mutations in chronic myeloproliferative diseases and found FLT3 mutations correlated with an increased number of blasts. FLT3 may participate in blast transformation in chronic myeloproliferative diseases, including chronic myelogenous leukemia.
No data have been available on the expression of FLT3 protein in acute leukemia. We found that the surface expression of FLT3 receptor protein in bone marrow mononuclear cells was related to FLT3 mRNA. This is in line with the findings of Kuchenbauer et al (25), who reported that CD135 receptor expression was correlated with FLT3 mRNA. On the other hand, Ozeki et al (26) found that surface expression level of FLT3 was not related to the expression of the FLT3 transcript. This might be due to the fact that they analyzed a limited number of acute myeloid leukemia samples. Kuchenbauer et al (27) reported higher Spearman rank correlation than we did, probably because they used direct conjugated antibody. As to the mechanism of FLT3 protein overexpression, they suggested an autocrine stimulatory mechanism of FLT3 receptor-ligand (FL). FLT3-FL loop, which is expressed in all cell lines, plays an important role in the pathobiology of leukemia. Combined with various cytokines, FL has synergistic or additive mitogenic effect, which leads to significant anti-apoptotic effects on primary acute myeloid leukemia cells (27). FLT3 proteins on the cell surface were internalized when exogenous FL stimulation was administered (3). Our results showed that an increased FLT3 protein levels in bone marrow mononuclear cells were related to the number of blast cells in the bone marrow, suggesting that FLT3 protein assay may be a useful biomarker for making leukemia prognosis.
In our cohort, the frequency of FLT3/ITD mutation was 25% in acute myeloid leukemia and 7% in acute lymphoid leukemia, which is consistent with findings of a Japanese study (23%) and Kottaridis et al (27%) (14,15). Our initial findings showed that Chinese patients with leukemia shared the same gene alternation as patients from different genetic backgrounds, suggesting a common mechanism for the pathogenesis of acute leukemia. We found a significant association between the presence of the FLT3/ITD mutation, a higher white blood cell count, a higher marrow blast percentage, and elevated serum LDH. This suggests that FLT3/ITD is an independent prognostic factor in acute leukemia. Other studies have also reported that high FLT3 expression levels were associated with an unfavorable prognosis (26,28).
In our study, the expression of FLT3 transcript in patients with tandem duplication was increased, but the increase was not significant. Ozeki et al (26) also reported the association between FLT3/ITD mutation and expression of FLT. The explanation for such an association is that expression levels of FLT3/ITD depend on the co-expression of the wild type and the mutant alleles. It has been detected that FLT3 expression levels were related to the relative proportions of wild type and mutant FLT3 (25). Future analysis of FLT3 expression levels in patients with FLT3/ITD mutation should examine the relative fragments of wild type and mutant-FLT3.
To further assess the prognostic significance of FLT3/ITD mutation, we analyzed whether FLT3/ITD mutation influenced the complete remission rate of leukemia. The results showed that patients with FLT3/ITD had a little lower complete remission rate than patients without FLT3/ITD mutation, but the difference was not significant, which is in accordance with other studies (14,15). Lack of effect of FLT3/ITD mutation on the complete remission rate might be explained by a lack of effect of FLT3/ITD mutation on chemosensitivity of leukemic cells at diagnosis. Though it did not affect complete remission rate, FLT3 mutation has been found to predict relapse rate and overall survival (14,29). In fact, the presence of FLT3/ITD mutation has been suggested to have a major impact on long-term outcome (15).
Recently, Stirewalt et al (30) has reported that the size of FLT3/ITD has prognostic significance in patients with acute myeloid leukemia. They showed that increased ITD size was associated with decreased overall survival and relapse free survival. We sequenced three PCR products of patients with acute leukemia with FLT3/ITD mutation, detecting the insertion sizes of 36 bp, 42 bp, and 57 bp. Insertion nucleotides >40 bp were regarded as large; two patients in our group had large size ITD mutation. The patient with 42 bp insertion died three months after two courses of chemotherapy. The patient with 57 bp insertion died 5 months after diagnosis. The third patient, with a secondary acute monocytic type of leukemia, experienced myelodysplastic syndrome with spontaneous splenic rupture and died 10 months later. It seems that larger ITD size is related to poorer clinical outcomes. However, more studies and patients are needed to confirm the effect of FLT3/ITD mutation size on prognosis.
In summary, our results suggest that FLT3 expression levels exhibit significant difference in various types of adult leukemia and that the presence of FLT3/ITD mutation is an independent prognostic factor for acute leukemia. Such results may provide a new target for leukemia therapy.
Acknowledgment
This work was supported in part by a grant-in-aid from Chinese Medical Board, USA (No. 99-698).
References
- 1.Rosnet O, Schiff C, Pebusque MJ, Marchetto S, Tonnelle C, Toiron Y, et al. Human FLT3/FLK2 gene: cDNA cloning and expression in hematopoietic cells. Blood. 1993;82:1110–9. [PubMed] [Google Scholar]
- 2.Drexler HG. Expression of FLT3 receptor and response to FLT3 ligand by leukemic cells. Leukemia. 1996;10:588–99. [PubMed] [Google Scholar]
- 3.Fenski R, Flesch K, Serve S, Mizuki M, Oelmann E, Kratz-Albers K, et al. Constitutive activation of FLT3 in acute myeloid leukaemia and its consequences for growth of 32D cells. Br J Haematol. 2000;108:322–30. doi: 10.1046/j.1365-2141.2000.01831.x. [DOI] [PubMed] [Google Scholar]
- 4.Hayakawa F, Towatari M, Kiyoi H, Tanimoto M, Kitamura T, Saito H, et al. Tandem-duplicated FLT3 constitutively activates STAT5 and MAP kinase and introduces autonomous cell growth in IL-3-dependent cell lines. Oncogene. 2000;19:624–31. doi: 10.1038/sj.onc.1203354. [DOI] [PubMed] [Google Scholar]
- 5.Schnittger S, Schoch C, Dugas M, Kern W, Staib P, Wuchter C, et al. Analysis of FLT3 length mutations in 1003 patients with acute myeloid leukemia: correlation to cytogenetics, FAB subtype, and prognosis in the AMLCG study and usefulness as a marker for the detection of minimal residual disease. Blood. 2002;100:59–66. doi: 10.1182/blood.V100.1.59. [DOI] [PubMed] [Google Scholar]
- 6.Thiede C, Steudel C, Mohr B, Schaich M, Schakel U, Platzbecker U, et al. Analysis of FLT3-activating mutations in 979 patients with acute myelogenous leukemia: association with FAB subtypes and identification of subgroups with poor prognosis. Blood. 2002;99:4326–35. doi: 10.1182/blood.V99.12.4326. [DOI] [PubMed] [Google Scholar]
- 7.Griffith J, Black J, Faerman C, Swenson L, Wynn M, Lu F, et al. The structural basis for autoinhibition of FLT3 by the juxtamembrane domain. Mol Cell. 2004;13:169–78. doi: 10.1016/S1097-2765(03)00505-7. [DOI] [PubMed] [Google Scholar]
- 8.Tse KF, Mukherjee G, Small D. Constitutive activation of FLT3 stimulates multiple intracellular signal transducers and results in transformation. Leukemia. 2000;14:1766–76. doi: 10.1038/sj.leu.2401905. [DOI] [PubMed] [Google Scholar]
- 9.Kiyoi H, Ohno R, Ueda R, Saito H, Naoe T. Mechanism of constitutive activation of FLT3 with internal tandem duplication in the juxtamembrane domain. Oncogene. 2002;21:2555–63. doi: 10.1038/sj.onc.1205332. [DOI] [PubMed] [Google Scholar]
- 10.Yamamoto Y, Kiyoi H, Nakano Y, Suzuki R, Kodera Y, Miyawaki S, et al. Activating mutation of D835 within the activation loop of FLT3 in human hematologic malignancies. Blood. 2001;97:2434–9. doi: 10.1182/blood.V97.8.2434. [DOI] [PubMed] [Google Scholar]
- 11.Frohling S, Schlenk RF, Breitruck J, Benner A, Kreitmeier S, Tobis K, et al. Prognostic significance of activating FLT3 mutations in younger adults (16 to 60 years) with acute myeloid leukemia and normal cytogenetics: a study of the AML Study Group Ulm. Blood. 2002;100:4372–80. doi: 10.1182/blood-2002-05-1440. [DOI] [PubMed] [Google Scholar]
- 12.Zhang GS, Dai CW, Peng HL, Xu YX, Pei MF. Myelodysplastic syndrome with transformation to acute monocytic leukemia with FLT3-ITD mutation following orthotopic liver transplantation. Leuk Res. 2006;30:908–10. doi: 10.1016/j.leukres.2005.11.025. [DOI] [PubMed] [Google Scholar]
- 13.Abu-Duhier FM, Goodeve AC, Wilson GA, Gari MA, Peake IR, Rees DC, et al. FLT3 internal tandem duplication mutations in adult acute myeloid leukaemia define a high-risk group. Br J Haematol. 2000;111:190–5. doi: 10.1046/j.1365-2141.2000.02317.x. [DOI] [PubMed] [Google Scholar]
- 14.Kiyoi H, Naoe T, Nakano Y, Yokota S, Minami S, Miyawaki S, et al. Prognostic implication of FLT3 and N-RAS gene mutations in acute myeloid leukemia. Blood. 1999;93:3074–80. [PubMed] [Google Scholar]
- 15.Kottaridis PD, Gale RE, Frew ME, Harrison G, Langabeer SE, Belton AA, et al. The presence of a FLT3 internal tandem duplication in patients with acute myeloid leukemia (AML) adds important prognostic information to cytogenetic risk group and response to the first cycle of chemotherapy: analysis of 854 patients from the United Kingdom Medical Research Council AML 10 and 12 trials. Blood. 2001;98:1752–9. doi: 10.1182/blood.V98.6.1752. [DOI] [PubMed] [Google Scholar]
- 16.Yokota S, Kiyoi H, Nakao M, Iwai T, Misawa S, Okuda T, et al. Internal tandem duplication of the FLT3 gene is preferentially seen in acute myeloid leukemia and myelodysplastic syndrome among various hematological malignancies. A study on a large series of patients and cell lines. Leukemia. 1997;11:1605–9. doi: 10.1038/sj.leu.2400812. [DOI] [PubMed] [Google Scholar]
- 17.Meierhoff G, Dehmel U, Gruss HJ, Rosnet O, Birnbaum D, Quentmeier H, et al. Expression of FLT3 receptor and FLT3-ligand in human leukemia-lymphoma cell lines. Leukemia. 1995;9:1368–72. [PubMed] [Google Scholar]
- 18.Carow CE, Levenstein M, Kaufmann SH, Chen J, Amin S, Rockwell P, et al. Expression of the hematopoietic growth factor receptor FLT3 (STK-1/Flk2) in human leukemias. Blood. 1996;87:1089–96. [PubMed] [Google Scholar]
- 19.Stacchini A, Fubini L, Severino A, Sanavio F, Aglietta M, Piacibello W. Expression of type III receptor tyrosine kinases FLT3 and KIT and responses to their ligands by acute myeloid leukemia blasts. Leukemia. 1996;10:1584–91. [PubMed] [Google Scholar]
- 20.Birg F, Courcoul M, Rosnet O, Bardin F, Pebusque MJ, Marchetto S, et al. Expression of the FMS/KIT-like gene FLT3 in human acute leukemias of the myeloid and lymphoid lineages. Blood. 1992;80:2584–93. [PubMed] [Google Scholar]
- 21.Bennett JM, Catovsky D, Daniel MT, Flandrin G, Galton DA, Gralnick HR, et al. Proposed revised criteria for the classification of acute myeloid leukemia. A report of the French-American-British Cooperative Group. Ann Intern Med. 1985;103:620–5. doi: 10.7326/0003-4819-103-4-620. [DOI] [PubMed] [Google Scholar]
- 22.Levis M, Tse KF, Smith BD, Garrett E, Small DA. FLT3 tyrosine kinase inhibitor is selectively cytotoxic to acute myeloid leukemia blasts harboring FLT3 internal tandem duplication mutations. Blood. 2001;98:885–7. doi: 10.1182/blood.V98.3.885. [DOI] [PubMed] [Google Scholar]
- 23.Takahashi S, Harigae H, Ishii KK, Inomata M, Fujiwara T, Yokoyama H, et al. Over-expression of FLT3 induces NF-kappaB pathway and increases the expression of IL-6. Leuk Res. 2005;29:893–9. doi: 10.1016/j.leukres.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 24.Lin P, Jones D, Medeiros LJ, Chen W, Vega-Vazquez F, Luthra R. Activating FLT3 mutations are detectable in chronic and blast phase of chronic myeloproliferative disorders other than chronic myeloid leukemia. Am J Clin Pathol. 2006;126:530–3. doi: 10.1309/JT5BE2L1FGG8P8Y6. [DOI] [PubMed] [Google Scholar]
- 25.Kuchenbauer F, Kern W, Schoch C, Kohlmann A, Hiddemann W, Haferlach T, et al. Detailed analysis of FLT3 expression levels in acute myeloid leukemia. Haematologica. 2005;90:1617–25. [PubMed] [Google Scholar]
- 26.Ozeki K, Kiyoi H, Hirose Y, Iwai M, Ninomiya M, Kodera Y, et al. Biologic and clinical significance of the FLT3 transcript level in acute myeloid leukemia. Blood. 2004;103:1901–8. doi: 10.1182/blood-2003-06-1845. [DOI] [PubMed] [Google Scholar]
- 27.Drexler HG, Meyer C, Quentmeier H. Effects of FLT3 ligand on proliferation and survival of myeloid leukemia cells. Leuk Lymphoma. 1999;33:83–91. doi: 10.3109/10428199909093728. [DOI] [PubMed] [Google Scholar]
- 28.Bullinger L, Dohner K, Bair E, Frohling S, Schlenk RF, Tibshirani R, et al. Use of gene-expression profiling to identify prognostic subclasses in adult acute myeloid leukemia. N Engl J Med. 2004;350:1605–16. doi: 10.1056/NEJMoa031046. [DOI] [PubMed] [Google Scholar]
- 29.Rombouts WJ, Blokland I, Lowenberg B, Ploemacher RE. Biological characteristics and prognosis of adult acute myeloid leukemia with internal tandem duplications in the Flt3 gene. Leukemia. 2000;14:675–83. doi: 10.1038/sj.leu.2401731. [DOI] [PubMed] [Google Scholar]
- 30.Stirewalt DL, Kopecky KJ, Meshinchi S, Engel JH, Pogosova-Agadjanyan EL, Linsley J, et al. Size of FLT3 internal tandem duplication has prognostic significance in patients with acute myeloid leukemia. Blood. 2006;107:3724–6. doi: 10.1182/blood-2005-08-3453. [DOI] [PMC free article] [PubMed] [Google Scholar]