Abstract
Cyclooxygenases (COXs) catalyze the conversion of arachidonic acid to prostaglandins (PGs), thromboxanes, and hydroxyeicosatetraenoic acids. In the present study, we investigated several dietary bioflavonoids for their ability to modulate the catalytic activity of COX I and II in vitro and also in cultured cells. We found that some of them are the most powerful direct stimulators of the catalytic activity of COX I and II known to date, increasing the formation of prostaglandin products in vitro by up to 11-fold over the controls. This stimulatory effect of bioflavonoids is enzyme specific because none of them stimulates the catalytic activity of a number of lipooxygenases tested. Compared with phenol, a prototypical COX stimulator commonly used in vitro, the naturally occurring bioflavonoids are up to 29 times more efficacious in stimulating the COX activity. Additional studies using intact cells in culture showed that some of the dietary compounds that were active in the biochemical assays also activated the formation of PGE2 (a representative PG) when they were present at 0.01 to 1 μM concentrations. The stimulatory effect of dietary compounds on COX-mediated PG formation is far more potent in intact cells than in the in vitro assays. Mechanistically, bioflavonoids mainly acted to slow down the suicidal inactivation of the COX enzymes, but they did not appear to reactivate the inactivated enzymes. The finding of this study suggests that some of the bioflavonoids likely will serve as the naturally occurring cofactors for the COX enzymes in humans.
Keywords: Cyclooxygenase activation, prostaglandins
Arachidonic acid (AA) is metabolized in the body by cyclooxygenases (COXs) to form various prostaglandins (PGs), thromboxanes (TXs), and hydroxyeicosatetraenoic acids (HETEs) (1–4). Many of these autacoids exert a whole host of biological actions in the body through activation of specific membrane receptors (5–7). COXs are intracellular enzymes with two distinct catalytic activities: one for cyclooxygenation that converts AA to PGG2, and one for peroxidation that further transforms PGG2 to PGH2 (1). In humans, two COX isoforms, namely, COX I and COX II, have been identified (2, 8–15). They share approximately 60% overall sequence similarity, although the sequence homology in the catalytically important regions is much higher (11–15). At present, it is widely accepted the view that COX II, which is often induced in the presence of various mitogens, plays a more important role in mediating inflammation, whereas COX I, whose cellular levels vary over a smaller range, may largely function as a house-keeping enzyme (8).
Earlier studies showed that phenol, a nonphysiological chemical with antioxidant activity, has a direct stimulatory effect on the catalytic activity of COX I and II in vitro (16, 17). Because of this unique property, this organic chemical has been commonly added to the reaction mixture to optimize the catalytic activity of COX I and II in vitro. It is generally thought that phenol functions like a supporting cofactor for the COX I- and II-mediated metabolism of AA in vitro.
Notably, there are many phenolic/polyphenolic compounds that are naturally occurring and abundantly present in our daily diet, such as bioflavonoids. Bioflavonoids are among the most powerful antioxidants and free radical-scavengers present in our food. Historically, some members of the bioflavonoid family were once considered as vitamin-like chemicals (18), because of their unique biological property in reducing capillary fragility and permeability. In the past half century, a great deal of research has been conducted to better understand a variety of biological actions associated with bioflavonoids as well as other dietary polyphenolic compounds (19–26).
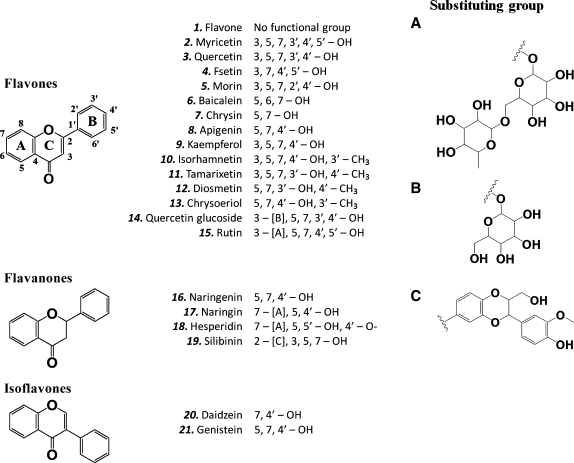
At present, however, little is known about whether there are naturally occurring phenolic compounds that are used in the body to serve as physiological cofactors to support the COX-mediated formation of PG products. In our search for answers, we examined various bioflavonoids (structures shown in Fig. 1) for their ability to modulate the catalytic activity of COX I and II in vitro, and their activity was compared with phenol, a prototypical activator of the COX activity. We report here our finding that some of the bioflavonoids represent a group of most powerful direct stimulators of the catalytic activity of COX I and II known to date, resulting in an increase in the formation of various AA metabolites in vitro and also in intact cells in culture. These data suggest a new possibility that one of the important biological functions of bioflavonoids in the human body likely is to serve as the naturally occurring cofactors for the COX enzymes.
Fig. 1.
Chemical structures of bioflavonoids and other compounds used in this study.
MATERIALS AND METHODS
Chemicals
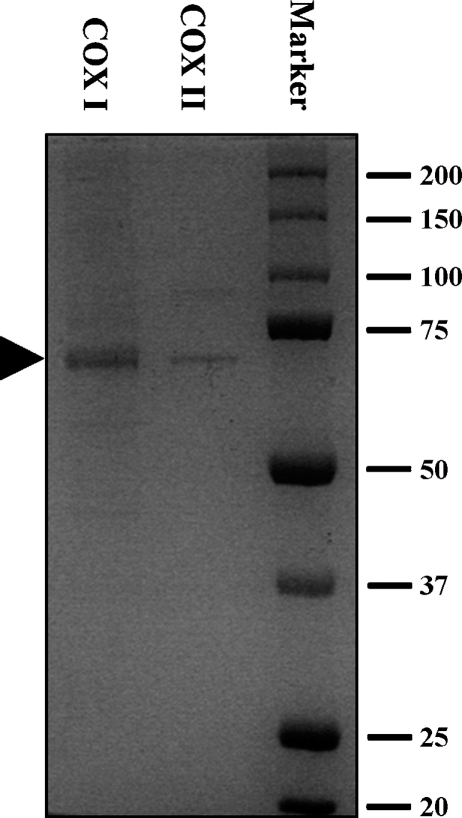
[14C]Arachidonic acid ([14C]AA, specific radioactivity of 53 Ci/mol) was purchased from PerkinElmer (Boston, MA). Indomethacin, NS-398, hematin, flavone, myricetin, quercetin, fisetin, morin, chrysin, kaempferol, baicalein, daidzein, genistein, silibinin, apigenin, and lipopolysaccharide (LPS; from Escherichia coli, serotype 055:b5) were purchased from Sigma Co. (St. Louis, MO). Chrysoeriol, isorhamnetin, diosmetin, and tamarixetin were purchased from Extrasynthese (Cedex, France). COX I, COX II, 12-lipoxygenase (12-LOX), 15-LOX, PGI2, PGG2, TXB2, PGE2, 6-keto-PGF1α, PGF2α, PGH2, PGD2, 5-hydroxy-5Z,8Z,11Z,13E-eicosatetraenoic acid (5-HETE), 11-hydroxy-5Z,8Z,11Z,13E-eicosatetraenoic acid (11-HETE), 12-hydroxy-5Z,8Z,11Z,13E-eicosatetraenoic acid (12-HETE), 15-hydroxy-5Z,8Z,11Z,13E-eicosatetraenoic acid (15-HETE), 12-hydroxy-5Z,8E,10E-heptadecatrienoic acid (12-HHT) and the enzymatic immunoassay kits for PGE2 were purchased from Cayman Co. (Ann Arbor, MI). According to the supplier, the COX I and COX II preparations used in our present study had a purity of approximately 95% and 70%, respectively, which was confirmed by our SDA-PAGE analysis (Fig. 2).
Fig. 2.
SDS-PAGE analysis (with Coomassie brilliant blue staining) of the relative purity of the cyclooxygenase (COX) I and II enzymes used in the present study. The arrow indicates the location of COX I and II protein bands.
Assay of cyclooxygenase and lipoxygenase activity
For assaying the catalytic activity of COX I and II in vitro, the incubation mixtures (added to an Eppendrof tube) consisted of 20 μM [14C]AA (0.2 μCi) as substrate, COX I or COX II as the enzyme (0.5 or 0.97 μg/ml, respectively), 10 mM ethylenediaminetetraacetic acid (EDTA), 1 mM reduced glutathione, 1 μM hematin, and a bioflavonoid in 200 μl Tris-HCl buffer (100 mM, pH 7.4). Similarly, for measuring 12-LOX and 15-LOX activities, the incubation mixtures included 20 μM [14C]AA (0.2 μCi) as substrate, 12-LOX or 15-LOX as the enzyme (at 72 μg/ml or 75 ng/ml, respectively), and a bioflavonoid in a final volume of 200 μl Tris-HCl buffer (100 mM, pH 7.4). The bioflavonoids or other test compounds were initially dissolved in pure ethanol and then diluted with the reaction buffer. The reaction was incubated at 37°C for 5 min and terminated by adding 15 μl of 0.5 N HCl to each tube. Ethyl acetate (600 μl) was added immediately for extraction. The dried extracts were redissolved in acetonitrile and analyzed by HPLC for determining the metabolite compositions.
The HPLC system consisted of a Waters 2690 solvent delivery system, a Waters 484 UV-detector, and an IN/US β-RAM radioflow detector, coupled with a C18 column (Atlantis, 4.6 × 150 mm). For COX metabolites, they were eluted with a linear gradient from 93% solvent A (25% acetonitrile in water containing 0.01% acetic acid) and 7% solvent B (100% acetonitrile containing 0.01% acetic acid) to 14% A and 86% B over 27 min. The gradient was then changed to 100% A over a 3-min period at a flow rate of 1 ml/min. For LOX metabolites, they were eluted with a linear gradient from 93% A and 7% B to 32% A and 68% B over 10 min. The gradient was then changed to 100% A over a 2-min period at a flow rate of 1 ml/min. The radioactive fractions were detected using a radioflow detector, while the nonradioactive coeluting standards were detected at 230 nm (wavelength) for HHT and various HETEs, and at 200 nm for other PG products.
Mass spectrometric analysis of various AA metabolites formed
We used the liquid chromatography-mass spectrometry (LC-MS/MS) for structural identification of the AA metabolites formed. The HPLC system consisted of a Shimadzu SIL-20AC autosampler, a pair of LC-20AD pumps, a DGU-20A3 degasser, and a SCL-10AVP system controller (Shimadzu, Tokyo, Japan). The mass spectrometer was a Waters Quattro Premier triple quadrupole instrument with an electrospray interface source (Waters, Milford, MA). The entire LC-MS/MS system is controlled by MassLynx 4.0 software. Thirty percent of the HPLC column effluent was introduced into an electrospray interface operated in the negative ionization mode. The interface used nitrogen desolvation gas at 650 L/hour, 400°C. The instrument was operated in the multiple reaction monitoring mode, and each standard molecule was individually tested for optimization of various parameters such as cone voltage and collision energy. For optimization of the cone voltages and collision energies during the method development, a solution containing each analyte was infused into the electrospray ionization source at 10 μL/min using a syringe pump (Pump 11, Harvard Apparatus, Holliston, MA). The mass spectra for various product ions (daughter ions) were recorded using the continuum averaging mode of operation.
Determination of the kinetic parameters (Km, Vmax, and Vmax/Km) for COX I and II
To determine the kinetic parameters (Km and Vmax) for COX I- and II-mediated formation of PGF2α, PGE2, PGD2, and 12-HHT, we used various concentrations of [14C]AA as substrate in the absence or presence of a bioflavonoid. The x-axis of the Michaelis-Menten curves was the varying concentrations of [14C]AA and the y-axis was the corresponding rate of formation of various AA products. The curves were analyzed using the SigmaPlot 8.0 software to determine the Km (μM) and Vmax (nmol/μg protein/5 min) values.
Cell culture experiments
The murine macrophage RAW264.7 cell line was obtained from American Type Culture Collection (Rockville, MD) and maintained in DMEM containing L-glutamine, glucose, and sodium bicarbonate. To determine the effect of myricetin (a representative dietary bioflavonoid) on the expression of COX I and II proteins in cultured RAW264.6 cells, these cells were treated with myricetin alone (0–100 μM) or in combination with LPS. The COX I and COX II protein levels were analyzed using 10% SDS-PAGE in a Mini-Protein system (BioRad, Hercules, CA). After electrophoresis, the protein bands on the gel were transferred onto the PVDF membrane (BioRad, Hercules, CA) for Western blot analysis. The membrane was first blocked with 5% nonfat dried milk powder in Tris-HCl-buffered saline containing 0.1% Tween-20 (the blocking solution), and then it was probed with polyclonal rabbit antibodies (Upstate, Lake Placid, NY) against COX I, or the polyclonal goat antibodies (Upstate, Lake Placid, NY) against COX II. The primary antibody-antigen complexes were detected using the goat anti-rabbit IgG for COX I and the rabbit anti-goat for COX II conjugated to horseradish peroxidase (Invitrogen, Carlsbad, CA) and developed according to procedures supplied by the Amersham ECL Plus (Piscataway, NJ).
To determine the effect of bioflavonoids on the formation of PGE2 (a representative PG) by LPS-pretreated RAW264.7 cells, the cells were first stimulated with 1 μg/ml LPS for 2 h to induce COX II expression, and then the medium was removed and replaced with 300 μL of serum-free DMEM with or without the dietary compound at concentrations of 0.01, 0.1 1, 10, and 100 μM. NS-398 (a COX II specific inhibitor, at 10 μM) and indomethacin (a nonspecific inhibitor of COX I and COX II, 10 μM) were used for comparison. After additional 2-h incubation, the culture media were collected and PGE2 levels in the culture medium were measured by using the EIA kit.
RESULTS
Characterization of AA metabolites formed by COX I and II
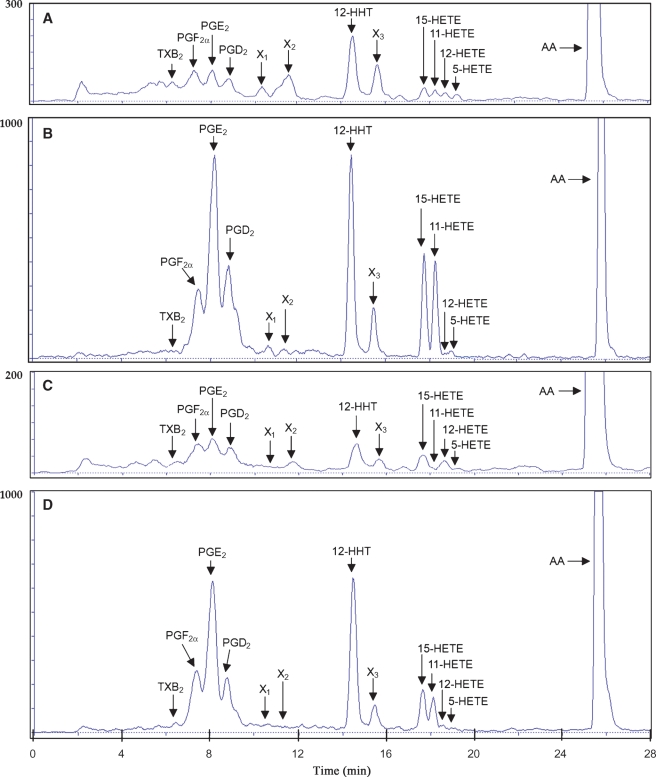
Incubations of COX I or COX II with 20 μM [14C]AA produced several radioactive AA metabolites, which included PGF2α, PGE2, PGD2, 12-HHT, several HETE derivatives (5-HETE, 11-HETE, 12-HETE, and 15-HETE), plus a few unidentified metabolites (Fig. 3). The overall AA metabolite profiles formed by COX I and II were similar. 12-HHT was the most abundant product formed in COX I and II-mediated reactions. PGG2 and PGH2, two well-known putative intermediates in AA metabolism, were not detected in these in vitro reactions. It is likely that these intermediates might be present transiently and then transformed to other stable PG products. TXB2 was formed in small quantity. In this study, we have mostly focused on the formation of four major COX products, namely, PGF2α, PGE2, PGD2, and 12-HHT, when we evaluated the effect of bioflavonoids on AA metabolism.
Fig. 3.
The HPLC chromatographs showing the separation of the [14C]AA metabolites formed by COX I- and II-mediated reactions. The incubation mixture (in an Eppendrof tube) consisted of 20 μM [14C]AA (0.2 μCi) as substrate, COX I or COX II as the enzyme (0.5 or 0.97 μg/ml, respectively), 10 mM ethylenediaminetetraacetic acid (EDTA), 1 mM reduced glutathione, 1 μM hematin, and a stimulator chemical in 200 μl of 100 mM Tris-HCl buffer, pH 7.4. A, B: The radioactive AA metabolites formed by COX II in the absence or presence of 100 μM myricetin as a stimulator, respectively. C, D: The radioactive AA metabolites formed by COX I in the absence or presence of 100 μM myricetin as a stimulator, respectively. Each data point is the mean of duplicate determinations. HETE, hydroxyeicosatetraenoic acid.
The identity of various AA metabolites was first probed by comparing their HPLC retention times with those of authentic standards. Then LC-MS/MS was used to confirm the structural identity of the AA metabolites detected. To do so, the metabolites were deprotonated to form their corresponding [M-H] precursor ions in the electrospray ionization source and then were detected in the negative ion mode. The MS/MS detector was used to fragment the precursor ions to form specific product ions. The fragmentation pattern of each AA metabolite produced in vitro was then matched against a library of authentic standards. A complete match of the retention time of a given metabolite peak and also its mass fragmentation pattern was needed for confirmation of the identity of the metabolite formed. Using this method, we unequivocally confirmed the identities of PGF2α, PGE2, PGD2, 12-HHT, 15-HETE, 11-HETE, 12-HETE, and 5-HETE.
Stimulation of COX I and II catalytic activity in vitro by bioflavonoids
Optimization of AA metabolism conditions
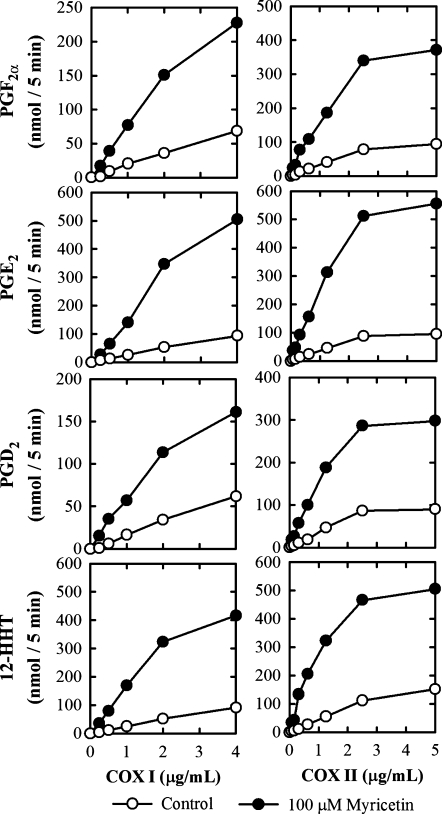
To quantify the COX I- and II-mediated metabolism of [14C]AA in vitro, we first conducted a series of assays to determine the suitable concentrations of the enzymes and the substrate, as well as the incubation time for the metabolic reactions. The formation of several AA metabolites, such as PGF2α, PGE2, PGD2, and 12-HHT, was increased with COX I protein concentrations from 0 to 4 μg/ml and with COX II from 0 to 5 μg/ml either in the presence or absence of myricetin, a representative bioflavonoid stimulator (Fig. 4). The Km values for the COX II-mediated formation of PGF2α, PGE2, PGD2, and 12-HHT were all below 2 μM (Table 1). Accordingly, a saturating substrate concentration of 20 μM [14C]AA was used in all in vitro metabolism experiments reported here unless indicated otherwise. Notably, it was reported in several earlier studies that the COX-mediated reaction for the formation of various PG products reached a plateau very rapidly, within 1 min (16, 17, 27). Our data also showed that the enzymatic reactions reached plateaus usually within 1 min of incubation. A uniform 5-min incubation time was used in all experiments presented in this study.
Fig. 4.
Effect of the COX concentrations on the formation of several quantitatively-predominant AA metabolites [PGF2α, PGE2, PGD2, and 12-HHT] in the absence (open circle) or presence of 100 μM myricetin (filled circle). The incubation mixtures consisted of 20 μM [14C]AA (0.2 μCi) as substrate, different concentrations of COX I or COX II as indicated, 10 mM EDTA, 1 mM reduced glutathione, 1 μM hematin, and with or without 100 μM myricetin in 200 μl of 100 mM Tris-HCl buffer, pH 7.4. The incubations were carried out at 37°C for 5 min. Each data point is the mean of duplicate determinations.
TABLE 1.
Kinetic parameters (Km and VMAX values) for the COX-mediated formation of PGF2α, PGE2, PGD2, and 12-hydroxy-5Z,8E,10E-heptadecatrienoic acid (12-HHT)
| COX II
|
COX I
|
||||||
|---|---|---|---|---|---|---|---|
| Product | Myricetin | Km (μM) | Vmax (nmol/μg/5 min) | Vmax/Km | Km (μM) | Vmax (nmol/μg/5 min) | Vmax/Km |
| PGF2α | 0 μM | 1.8 | 38 | 20.8 | 4.0 | 23 | 5.8 |
| 100 μM | 9.9 | 236 | 23.8 | 4.5 | 90 | 20 | |
| PGE2 | 0 μM | 1.2 | 41 | 34.2 | 1.9 | 29 | 15.3 |
| 100 μM | 5.5 | 457 | 83.0 | 2.2 | 160 | 72.7 | |
| PGD2 | 0 μM | 0.9 | 30 | 33.3 | 5.8 | 19 | 3.3 |
| 100 μM | 2.4 | 179 | 74.6 | 5.3 | 72 | 13.6 | |
| 12-HHT | 0 μM | 1.0 | 44 | 43.5 | 1.5 | 26 | 17.3 |
| 100 μM | 2.6 | 375 | 144.0 | 1.8 | 190 | 105.6 | |
| 5-HETE | 0 μM | 4.2 | 7 | 1.7 | 4.2 | 4 | 1 |
| 100 μM | 13 | 7 | 0.5 | 6.4 | 2 | 0.3 | |
| 11-HETE | 0 μM | 9.7 | 14 | 1.4 | 4.6 | 5 | 1.1 |
| 100 μM | 15.4 | 142 | 9.2 | 4.8 | 35.2 | 7.3 | |
| 12-HETE | 0 μM | 8.4 | 11 | 1.3 | 5 | 13 | 2.6 |
| 100 μM | 15.6 | 7 | 0.4 | 6.6 | 2 | 0.3 | |
| 15-HETE | 0 μM | 8.2 | 16 | 2.0 | 261.3 | 222 | 0.8 |
| 100 μM | 35.2 | 168 | 4.8 | 126.1 | 230 | 1.8 | |
COX, cyclooxygenase; HETE, hydroxyeicosatetraenoic acid.
Effect on AA metabolite profiles
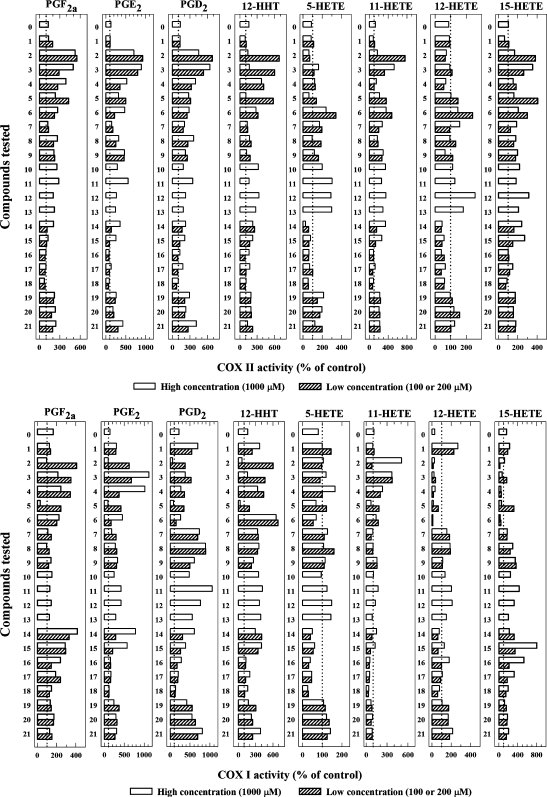
A total of some 20 naturally occurring bioflavonoids were tested in the present study for their ability to modulate the catalytic activity of COX I and II in vitro, and the data were summarized in Fig. 5. The profiles of stimulation of the COX I- and II-mediated formation of different AA metabolites by different bioflavonoids were different. For COX II, myricetin (at 200 μM) had the highest efficacy, increasing the formation of PGF2α, PGE2, PGD2, and 12-HHT by 549%, 924%, 677%, and 680%, respectively (Fig. 5, upper panels). Quercetin, fisetin, morin, and tamarixetin also had high efficacy for stimulating the formation of some of the AA metabolites. Among the various AA metabolites formed by COX II, PGE2 was increased with many of the bioflavonoids tested. Tamarixetin, baicalein, kaempferol, genistein, quercetin glucoside, apigenein, isorhamnetin, diosmetin, silibinin, rutin, diadzein, chrysoeriol, and chrysin had moderate activity in stimulating the formation of major AA metabolites. Naringin, naringenin, hesperidin, and flavone had either weak or no appreciable effect on the COX II-mediated formation of major AA metabolites (Fig. 5, upper panels).
Fig. 5.
Effect of bioflavonoids on the catalytic activity of COX II (upper panels) and COX I (lower panels) in vitro. The incubation mixtures consisted of 20 μM [14C]AA (0.2 μCi) as substrate, COX II (at 0.97 μg/ml) or COX I (at 0.5 μg/ml) as the enzyme, a test compound, 10 mM EDTA, 1 mM reduced glutathione, and 1 μM hematin in 200 μl of 100 mM Tris-HCl buffer, pH 7.4. The incubations were carried out at 37°C for 5 min. Note that for most test compounds, the high concentration (open bar) used was 1 mM, and the low concentration (filled bar) was either 100 μM (compounds 14, 15, 16, 17, and 18) or 200 μM (compounds 1, 2, 3, 4, 5, 6, 7, 8, 9, 19, 20, and 21). For some of the test compounds, only one concentration (1 mM) was tested. Compound 0 is phenol. The structures of all test compounds are listed in Fig. 1. Each data point is the mean of duplicate determinations. The dotted line denotes the control activity in the absence of any test compound.
When COX I was the enzyme, quercetin and fisetin were the two most efficacious stimulators of PGE2 formation (Fig. 5, lower panels). Notably, several other bioflavonoids (such as tamarixetin, genistein, chrysoeriol, diosmetin, kaempferol, apigenin, daidzein) had a preferential stimulation of the COX I-mediated formation of PGD2 (by up to 10-fold) over other AA metabolites (PGF2α, PGE2, and 12-HHT). Interestingly, flavone (a man-made flavonoid without any hydroxyl groups) also exerted a strong preferential efficacy in stimulating the COX I activity for the formation of PGD2 (692%), but it only had a weak stimulatory effect on the COX II activity. Resveratrol also inhibited the COX I activity.
For comparison, we have also tested the effect of phenol, the prototypical activator of the catalytic activity of COX I and II under the same conditions. The presence of phenol at 2 mM (a concentration used in many published studies) showed only a modest stimulation of the COX I- and II-mediated conversion of AA to various PG products (Fig. 5). When phenol is compared with some of the bioflavonoids (such as quercetin and myricetin) for their ability to stimulate COX I and II-mediated formation of PGE2, the naturally occurring compounds had up to 22- to 29-fold higher efficacy than phenol.
Lastly, it is also of note that the degree of stimulation of COX I and II by various bioflavonoids did not show a meaningful degree of correlation, except for the formation of PGE2 (r2 = 0.458) and 12-HHT (r2 = 0.299). This result suggests that although the AA metabolite profiles formed by COX I and II are similar, their sensitivity to the stimulation by various bioflavonoids as well as the underlying mechanisms of their stimulation are likely different.
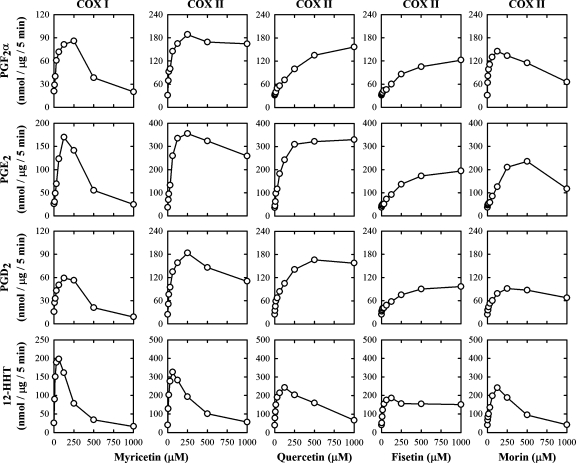
Concentration dependence of bioflavonoids
Using myricetin as an example, its stimulatory effect on COX I-mediated formation of various AA metabolites, such as PGF2α, PGE2, PGD2, and 12-HHT, was clearly concentration dependent (Fig. 6). The curve had a biphasic pattern: at lower concentrations (⩽125 μM), it strongly stimulated the formation of most AA metabolites in a concentration-dependent manner and reached a plateau when 125–250 μM of myricetin was present. At concentrations (⩾250 μM), myricetin started to show a concentration-dependent reduction of its stimulatory effect, and it almost returned to the basal levels (or even slightly below that) when 1,000 μM of myricetin was present. The maximal stimulation of COX I-mediated formation of PGF2α, PGE2, PGD2, and 12-HHT by myricetin was approximately 4-, 7-, 4-, and 8-fold, respectively. Myricetin also had a strong stimulation of the COX II-mediated formation of PGF2α, PGE2, PGD2, and 12-HHT (approximately 6-, 10-, 8-, and 8-fold, respectively, of the controls) (Fig. 6). Notably, myricetin's biphasic pattern of regulation of COX II-mediated formation of PGF2α, PGE2, and PGD2 was somewhat less pronounced compared with COX I, with a reduced self-inhibition when it was present at higher concentrations (Fig. 6). In addition, we have also compared the concentration-dependent effect of quercetin, fisetin, and morin on the catalytic activity of COX II in vitro, and similar curve patterns were observed (Fig. 6).
Fig. 6.
Effect of myricetin, quercetin, fisetin, and morin on the COX activity in vitro. The incubation mixtures consisted of nine different concentrations (0, 7.8, 15.6, 31.3, 62.5, 125, 250, 500, and 1,000 μM) of myricetin, quercetin, fisetin, or morin as the stimulator, 20 μM [14C]AA (0.2 μCi) as substrate, COX I or COX II as the enzyme (0.5 or 0.97 μg/ml, respectively), 10 mM EDTA, 1 mM reduced glutathione, and 1 μM hematin in 200 μl of 100 mM Tris-HCl buffer, pH 7.4. The incubations were carried out at 37°C for 5 min. Each data point is the mean of duplicate determinations.
Mechanisms for the stimulation of COX II activity by bioflavonoids
Enzyme kinetics
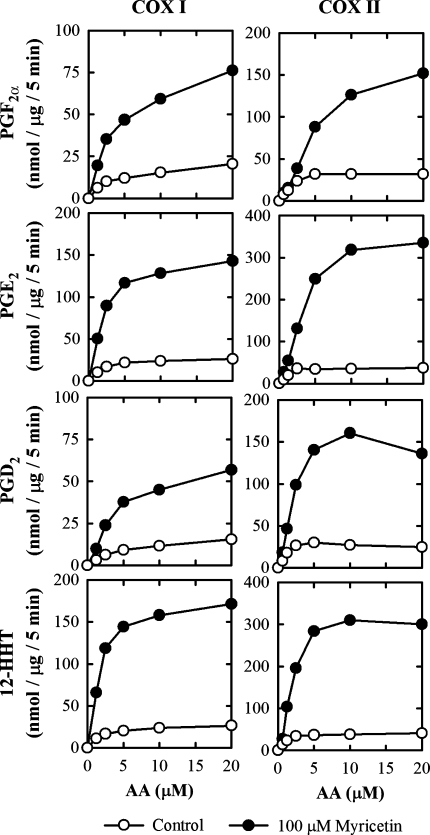
To evaluate the enzyme kinetics for the stimulation of COX I and II activity by dietary polyphenols, several representative bioflavonoids (myricetin, quercetin, fisetin, and morin) were chosen for further studies. We focused on analyzing the formation of PGF2α, PGE2, PGD2, and 12-HHT as major products. The Michaelis-Menten curves for the COX I- and II-mediated formation of representative AA metabolites in the presence of myricetin is shown in Fig. 7. All kinetic parameters (Km, Vmax, and Vmax/Km) for COX I- and II-mediated formation of PGF2α, PGE2, PGD2, 12-HHT, 5-HETE, 11-HETE, 12-HETE, and 15-HETE in the presence or absence of different bioflavonoids were calculated using curve regression analyses and were summarized in Table 1 for comparison. The presence of a bioflavonoid greatly increased the Vmax values (up to 11-fold), although the apparent the Km value for the formation of PGE2 was also increased (approximately 2.5-fold). In most cases, the Vmax/Km values were significantly increased, which shows an increased catalytic efficiency for AA metabolism by COX I and II in vitro. It is of note that while 15-HETE was a quantitatively major metabolite formed by COX I and II, its apparent Km values were unusually high compared with all other AA metabolites characterized (Table 1).
Fig. 7.
The dependence of AA concentrations on the formation of several quantitatively predominant AA metabolites (PGF2α, PGE2, PGD2, and 12-HHT) when 100 μM myricetin of present (filled circle) or absence (open circle). The incubation mixture consisted of indicated [14C]AA (0.2 μCi) as substrate, COX I or COX II as the enzyme (0.5 or 0.97 μg/ml, respectively), 10 mM EDTA, 1 mM reduced glutathione, 1 μM hematin, and with (filled circle) or without (open circle) 100 μM myricetin in 200 μl of 100 mM Tris-HCl buffer, pH 7.4. The incubations were carried out at 37°C for 5 min. Each data point is the mean of duplicate determinations. The kinetic paramters (Km, Vmax and Vmax/Km) for all AA metabolites were summarized in Table 1.
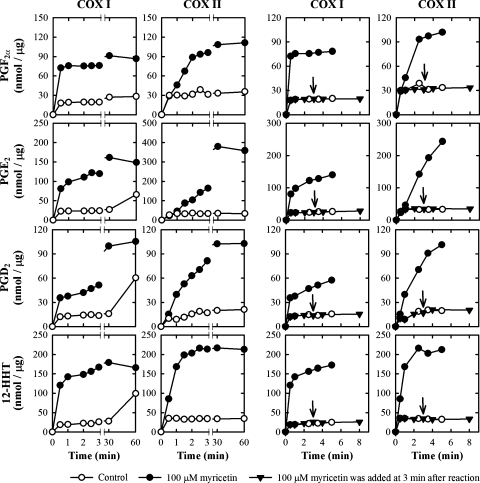
Time course of the stimulation
In the absence of any bioflavonoids, the COX II-mediated formation of PGE2 and other AA metabolites reached a plateau within 1 min (sometimes as short as 20 to 30 s). However, in the presence of 100 μM myricetin, the amount of PGs formed by COX II increased continuously for nearly 5 min (Fig. 8). When myricetin was added to the reaction mixtures after a 3-min incubation at 37°C, the formation of PGs was not further increased (Fig. 8). This observation suggests that the presence of myricetin only slows down the self-inactivation of the COX enzyme, but it does not restore the enzyme molecules that are already inactivated.
Fig. 8.
Effect of incubation time on the formation of several quantitatively predominant AA metabolites (PGF2α, PGE2, PGD2, and 12-HHT) when 100 μM myricetin was present (filled circle) or absence (open circle). The incubation mixture consisted of 20 μM [14C]AA (0.2 μCi) as substrate, COX I or COX II as the enzyme (0.5 or 0.97 μg/ml, respectively), 10 mM EDTA, 1 mM reduced glutathione, 1 μM hematin, and with or without 100 μM myricetin in 200 μl of 100 mM Tris-HCl buffer, pH 7.4. The incubations were carried out at 37°C for indicated time. Each data point is the mean of duplicate determinations. An arrow indicates the addition of myricetin at 3 min after incubation.
Effect of bioflavonoids on PGE2 formation in RAW264.7 cells
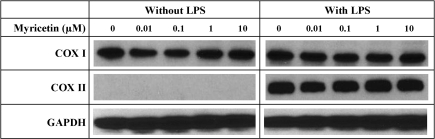
We conducted a number of experiments to determine the modulating effect of representative dietary bioflavonoids on PG formation in cultured RAW264.7 cells that were pretreated with LPS for 2 h to induce COX II expression. Western blot analysis showed that treatment of murine RAW264.7 cells with LPS increased the protein levels of COX II, but not COX I (Fig. 9). In comparison, treatment with myricetin alone or in combination with LPS did not appreciably alter COX II protein levels in these cells (Fig. 9). These data showed that pretreatment of RAW264.7 cells with LPS significantly and selectively induced the levels of COX II protein but not the COX I protein.
Fig. 9.
Effect of lipopolysaccharide (LPS) and myricetin on COX I and COX II protein levels in RAW264.7 cells in culture. After treatment with 1 μg/ml LPS for 2 h, RAW264.7 cells were incubated with different concentrations of myricetin for an additional 2 h. Western blot analysis of cell lysates was performed with antibodies specific for COX I or COX II, coupled with a secondary antibody conjugated with horseradish peroxidase. A total of three experiments were conducted, and a representative data set from one of the experiments was shown.
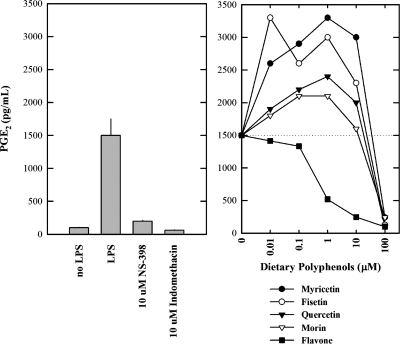
Next we have determined the effect of these bioflavonoids on the levels of PGE2 (a representative PG) in the culture media of LPS-pretreated RAW264.7 cells using an EIA kit. Cells pretreated with LPS had a markedly increased formation of PGE2, and the formation of PGE2 was almost completely inhibited when 10 μM NS-398 (a specific COX II inhibitor) or 10 μM indomethacin (a nonselective COX inhibitor) was present (Fig. 10A). These data suggest that the PGE2 formed by LPS-pretreated RAW264.7 cells was mainly catalyzed by the COX II enzyme that was selectively induced during LPS pretreatment. Addition of myricetin, quercetin, fisetin, or morin to LPS-pretreated RAW264.7 cells exhibited a dual pattern of concentration-dependent stimulation and inhibition of PGE2 formation (Fig. 10B). At lower concentrations (⩽1 μM), the formation of PGE2 was stimulated in a concentration-dependent manner by the presence of these dietary compounds, and the stimulation reached a plateau when the concentration reached approximately 1 μM. However, at higher concentrations (>10 μM), these dietary compounds showed a concentration-dependent inhibition of PGE2 formation (Fig. 10B). In comparison, flavone (which does not have a stimulatory effect on COX II-mediated formation of PGs in vitro; see Fig. 5) did not have a stimulatory effect on the formation of PGE2 in LPS-pretreated RAW264.7 cells. Instead it inhibited PGE2 formation in a concentration-dependent manner, which is consistent with the finding from in vitro enzymatic assays.
Fig. 10.
Effect of representative dietary bioflavonoids on the release of PGE2 from LPS-pretreated RAW264.7 cells in culture. Cells were pretreated with 1 μg/ml LPS for 2 h to induce COX II expression, and then the culture media were removed and replaced with 300 μL serum-free medium with or with the test compound (myricetin, fisetin, quercetin, or morin) for an additional 2 h. The following concentrations of the test compounds were used: 0.01, 0.1 1, 10, and 100 μM. NS-398 (a COX II specific inhibitor, at 10 μM) and indomethacin (a nonspecific COX inhibitor, at 10 μM) were also tested for comparison. The levels of PGE2 were measured using an EIA (Cayman, Ann Arbor, MI). Each point was the mean of duplicate determinations.
Effect of bioflavonoids on the in vitro catalytic activity of LOXs
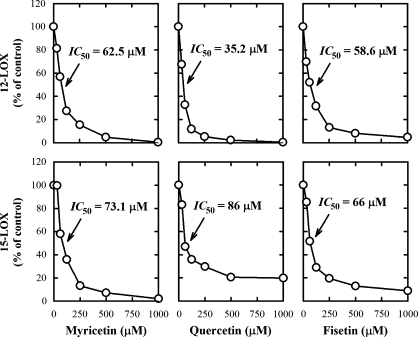
To determine whether the observed direct stimulation of COX activity by certain bioflavonoids is an enzyme-specific phenomenon, we have compared their effect on the LOX-mediated AA metabolism. The formation of 12-HETE by 12-LOX and the formation of 15-HETE by 15-LOX was uniformly inhibited by all bioflavonoids tested (Fig. 11). Notably, when COX I and II were used as the enzymes, the formation of small amounts of various HETEs was also detected. Interestingly, some of the bioflavonoids stimulated the COX-mediated formation of HETEs (particularly 11-HETE and 15-HETE) in a similar way as they stimulated the COX-mediated formation of PGs (Fig. 3).
Fig. 11.
The inhibitory effect of myricetin, quercetin, and fisetin on the catalytic activity of 12-LOX and 15-LOX in vitro. The incubation mixture consisted of seven different concentrations (0, 31.3, 62.5, 125, 250, 500, and 1,000 μM) of quercetin, fisetin, or morin, 20 μM [14C]AA (0.2 μCi) as substrate, and 12-LOX or 15-LOX as the enzyme (72 μg/ml or 75 ng/ml, respectively) in a final volume of 200 μl of 100 mM Tris-HCl buffer, pH 7.4. The incubations were carried out at 37°C for 5 min. Each data point is the mean of duplicate determinations.
DISCUSSION
Direct stimulation of the catalytic activity of COX I and II by bioflavonoids
In 1980, Baumann, von Bruchhausen, and Wurm (28) first reported that some of the dietary polyphenols, such as galangin and luteolin, inhibited the COX-mediated AA peroxidation when present at high concentrations. Since then, several researchers have also reported that other dietary polyphenols, including many of the common bioflavonoids tested in the present study, inhibited the catalytic activity of COX I and II in vitro and in vivo when present at high concentrations (29, 30). In addition, bioflavonoids have been reported to inhibit the LOX activity (31). The effect of polyphenols on the catalytic activity of 5-LOX and 12-LOX has been studied extensively in the past in an effort to better understand their anti-inflammatory properties (32). This inhibitory effect has often been used as a mechanistic explanation for their chemopreventive effect against certain types of cancer (33).
In the present study, we have evaluated some 20 naturally occurring bioflavonoids for their ability to modulate the catalytic activity of COX I and II in vitro. To our surprise, some of the common bioflavonoids, such as myricetin, quercetin, and fisetin, were found to have a powerful, direct stimulatory effect (up to 11-fold increase) on the COX I- and II-mediated formation of PGE2 and other PG products. This stimulatory effect of bioflavonoids was enzyme specific because none of them stimulated the catalytic activity of LOXs. Compared with phenol, a prototypical COX stimulator in vitro, the naturally occurring bioflavonoids are up to 29 times more efficacious in stimulating the COX activity. To the best of our knowledge, these natural compounds are the most powerful direct stimulators of the in vitro COX I and II catalytic activity known to date.
In addition, we have also conducted experiments using cultured cells, which clearly showed that these bioflavonoids could strongly stimulate COX-mediated formation of PGE2 (a representative PGs) in intact cells, and a strong stimulatory effect was observed at physiologically achievable concentrations (10 to 100 nM). These data provide support for the novel concept that these dietary bioflavonoids are physiologically relevant activators of the COX I and II activity in vivo.
Here it should be noted that the potency of these dietary compounds in intact cells (Fig. 10) is much higher than what was seen in the in vitro enzymatic assays (Figs. 5 and 6). This discrepancy may lie in the difference between the in vitro reaction conditions, which may not be best suited for studying the interactions of the highly lipophilic COX enzymes with the water-soluble dietary compounds. In partial support of this explanation, we noticed that the concentration-dependent dual stimulation and inhibition was also observed in almost exactly the same pattern in both in vitro biochemical assays and intact cells, although the COX enzymes in intact live cells were far more sensitive to the actions of these dietary compounds than the COX enzymes used in the in vitro biochemical assays.
The exact molecular mechanism by which bioflavonoids stimulate the catalytic activity of COX I and II is not precisely understood at present. As observed in the present study and also in several other studies (34–37), COX I and II rapidly undergo suicidal inactivation, which likely is mediated by chemically reactive radical intermediates formed during enzymatic catalysis of cyclooxidation and peroxidation. Data from our present study showed that bioflavonoids mainly act to slow down the suicidal inactivation of the COX enzymes, but they cannot reactivate the inactivated enzymes.
Bioflavonoids are known to have strong radical-scavenging activity as well as antioxidant activity (38). Earlier structure-activity relationship studies have shown that the presence of a 3-hydroxyl group in the heterocyclic C-ring, a catechol group in the B-ring, and a C2-C3 double bond of a bioflavonoid favored its antioxidant and free radical-scavenging activity (38–41). The stimulatory effect of bioflavonoids on COX-mediated AA metabolism may be contributed, in part, by their radical-scavenging properties. It is possible that the hydroxyl groups of bioflavonoids may bind to the enzymes to act as electron donors, accelerating their peroxidative activity to convert PGG2 to PGH2. However, the free radical-scavenging activity of various bioflavonoids is not believed to be a major mechanism of their actions because the degree of stimulation of COX activity by bioflavonoids did not correlate with the number of hydroxyl groups present at their B-ring. Notably, flavone (a synthetic compound with no hydroxyl groups attached to it) also showed strong, selective activity in stimulating the formation of certain AA metabolites. This observation is rather intriguing, and it sheds light on the mechanisms of the stimulatory actions of these bioflavonoids.
Physiological/pathophysiological implications
In the body, the COX I- and II-mediated metabolism of AA results in the formation of various PGs, TXs, and HETEs (1–4). These autacoids exert a whole host of biological actions in the body through activation of specific membrane receptors (5–7). One of the best known functions of PGs is their ability to mediate an inflammatory response (42). It is expected that persistent alterations, either increase or decrease, of the COX I- and II-mediated biosynthesis of autacoids in vivo will have consequential health effects. Preclinical studies have shown that inhibition of the COX I and II activity in vivo may inhibit the intestinal tumorigenesis in the APCmin/+ mice (33). However, long-term inhibition of COX I and/or COX II by effective COX inhibitors has also been closely linked to a number of notorious side effects including gastrointestinal ulceration and bleeding as well as increased risk for heart attacks (43). The gastrointestinal toxicity appears to be largely related to COX I inhibition (44). In comparison, selective COX II inhibition (caused by the use of Celebrex or Vioxx) would decrease the production of vascular prostacyclin (PGI2), thereby affecting the balance between the levels and functions of the prothrombotic and antithrombotic eicosanoids (45, 46). These clinical observations suggest that lower basal levels of the COX I and II activity are not always better or beneficial for optimal health; on the contrary, it is believed that a balanced COX activity is required for the normal physiological functions in vivo.
Bioflavonoids are phytochemicals produced by various plants in large quantities (47). They are among the most potent antioxidants present in our diet, and have been a subject of a great deal of research in the past several decades because of their many health-promoting benefits and broad pharmacological effects (16–22). A number of recent studies have shown that many of these dietary polyphenols can be effectively absorbed in the digestive system in humans and are present in circulation and tissues at physiologically or pharmacologically relevant concentrations (48–53). Historically, some members of the bioflavonoid family were once considered to be a class of vitamin-like compounds (18), because of their unique biological property in reducing capillary fragility and permeability. Nowadays, bioflavonoids are not considered as vitamins because they have not been shown unequivocally to be essential dietary constituents required for normal physiological functions. Mechanistically, it is generally believed that their potent antioxidant and free radical-scavenging activity (54–59) contributes importantly to many of their health-promoting effects. However, the results of our present study raise an exciting possibility that some of the unique vitamin-like actions of bioflavonoids may be attributable to their strong stimulatory effect on the COX I and II catalytic activity in vivo. Stated differently, we hypothesize that bioflavonoids may be a group of naturally occurring cofactors that are used in the body by COX I and II to support their catalytic activity. If this assumption proves to be correct, then this would naturally lead to the suggestion that the original proposal of nearly half a century ago concerning bioflavonoids as vitamin-like compounds may still be valid.
CONCLUSION
In the present study, we have tested some 20 naturally occurring bioflavonoids for their ability to stimulate the catalytic activity of COX I and II in PG biosynthesis in vitro. We found that some of the bioflavonoids, such as myricetin, quercetin, fisetin, and morin, have a powerful direct stimulatory effect on the COX I and II activity, and they are perhaps the most efficacious stimulators of the COX I and II catalytic activity known to date. Given the large amount of preexisting information in the literature concerning the inhibitory effect of dietary polyphenols on the COX activity, our finding is rather intriguing, and it suggests that bioflavonoids may represent a unique class of physiological, high-efficacy stimulators of the COX I and II activity in vivo.
Acknowledgments
The authors would like to thank Dr. Gregory A. Reed at the University of Kansas Medical Center for helpful discussion.
Abbreviations
AA, arachidonic acid
COX, cyclooxygenase
EDTA, ethylenediaminetetraacetic acid
HETE, hydroxyeicosatetraenoic acid
HHT, heptadecatrienoic acid
LC-MS/MS, liquid chromatography-mass spectrometry
LOX, lipoxygenase
LPS, lipopolysaccharide
PG, prostaglandin
TX, thromboxane
Published, JLR Papers in Press, July 26, 2008.
Footnotes
This study was supported, in part, by a grant from the NIH (ES 015242).
References
- 1.Hamberg M., and B. Samuelsson. 1967. Oxygenation of unsaturated fatty acids by the vesicular gland of sheep. J. Biol. Chem. 242 5344–5354. [PubMed] [Google Scholar]
- 2.Miyamoto T., N. Ogino, S. Yamamoto, and O. Hayaishi. 1976. Purification of prostaglandin endoperoxide synthetase from bovine vesicular gland microsomes. J. Biol. Chem. 251 2629–2636. [PubMed] [Google Scholar]
- 3.Marnett L. J. 2000. Cyclooxygenase mechanisms. Curr. Opin. Chem. Biol. 4 545–552. [DOI] [PubMed] [Google Scholar]
- 4.Kurumbail R. G., J. R. Kiefer, and L. J. Marnett. 2001. Cyclooxygenase enzymes: catalysis and inhibition. Curr. Opin. Struct. Biol. 22 752–760. [DOI] [PubMed] [Google Scholar]
- 5.Regan J. W. 2003. EP2 and EP4 prostanoid receptor signaling. Life Sci. 74 143–153. [DOI] [PubMed] [Google Scholar]
- 6.Lee J. L., A. Kim, L. Kopelovich, D. R. Bickers, and M. Athar. 2005. Differential expression of E prostanoid receptors in murine and human non-melanoma skin cancer. J. Invest. Dermatol. 125 818–825. [DOI] [PubMed] [Google Scholar]
- 7.Sung Y. M., G. He, and S. M. Fischer. 2005. Lack of expression of the EP2 but not EP3 receptor for prostaglandin E2 results in suppression of skin tumor development. Cancer Res. 65 9304–9311. [DOI] [PubMed] [Google Scholar]
- 8.Smith W. L., D. L. DeWitt, and R. M. Garavito. 2000. Cyclooxygenases: structural, cellular, and molecular biology. Annu. Rev. Biochem. 69 145–182. [DOI] [PubMed] [Google Scholar]
- 9.Hemler M., and W. E. Lands. 1976. Purification of the cyclooxygenase that forms prostaglandins. Demonstration of two forms of iron in the holoenzyme. J. Biol. Chem. 251 5575–5579. [PubMed] [Google Scholar]
- 10.van der Ouderaa F. J., M. Buytenhek, D. H. Nugteren, and D. A. Van Dorp. 1997. Purification and characterisation of prostaglandin endoperoxide synthetase from sheep vesicular glands. Biochim. Biophys. Acta. 487 315–331. [DOI] [PubMed] [Google Scholar]
- 11.Xie W. L., J. G. Chipman, D. L. Robertson, R. L. Erikson, and D. L. Simmons. 1991. Expression of a mitogen-responsive gene encoding prostaglandin synthase is regulated by mRNA splicing. Proc. Natl. Acad. Sci. USA. 88 2692–2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kujubu D. A., B. S. Fletcher, B. C. Varnum, R. W. Lim, and H. R. Herschman. 1991. TIS10, a phorbol ester tumor promoter-inducible mRNA from Swiss 3T3 cells, encodes a novel prostaglandin synthase/cyclooxygenase homologue. J. Biol. Chem. 266 12866–12872. [PubMed] [Google Scholar]
- 13.O'Banion M. K., H. B. Sadowski, V. Winn, and D. A. Young. 1991. A serum- and glucocorticoid-regulated 4-kilobase mRNA encodes a cyclooxygenase-related protein. J. Biol. Chem. 266 23261–23267. [PubMed] [Google Scholar]
- 14.Sirois J., and J. S. Richards. 1992. Purification and characterization of a novel, distinct isoform of prostaglandin endoperoxide synthase induced by human chorionic gonadotropin in granulosa cells of rat preovulatory follicles. J. Biol. Chem. 267 6382–6388. [PubMed] [Google Scholar]
- 15.Hla T., and K. Neilson. 1992. Human cyclooxygenase-2 cDNA. Proc. Natl. Acad. Sci. USA. 89 7384–7388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.William L. S., and E. M. L. William. 1971. Stimulation and blockade of prostaglandin biosynthesis. J. Biol. Chem. 246 6700–6702. [PubMed] [Google Scholar]
- 17.Yuchiong H., and H. B. Dunford. 1992. Prostaglandin H synthase kinetics. J. Biol. Chem. 267 17649–17657. [PubMed] [Google Scholar]
- 18.Samuel B., and B. Barishaw. 1949. The use of hesperidin-C in the treatment of abnormal capillary fragility. Exp. Med. Surg. 7 358–365. [PubMed] [Google Scholar]
- 19.Terao J., M. Piskula, and Q. Yao. 1994. Protective effect of epicatechin, epicatechin gallate, and quercetin on lipid peroxidation in phospholipid bilayers. Arch. Biochem. Biophys. 308 278–284. [DOI] [PubMed] [Google Scholar]
- 20.Deschner E. E., J. Ruperto, G. Wong, and H. L. Newmark. 1991. Quercetin and rutin as inhibitors of azoxymethanol-induced colonic neoplasia. Carcinogenesis. 12 1193–1196. [DOI] [PubMed] [Google Scholar]
- 21.Tzeng S. H., W. C. Ko, F. N. Ko, and C. M. Teng. 1991. Inhibition of platelet aggregation by some flavonoids. Thromb. Res. 64 91–100. [DOI] [PubMed] [Google Scholar]
- 22.Rump A. F., M. Schussler, D. Acar, A. Cordes, R. Ratke, M. Theisohn, R. Rosen, W. Klaus, and U. Fricke. 1995. Effects of different inotropes with antioxidant properties on acute regional myocardial ischemia in isolated rabbit hearts. Gen. Pharmacol. 26 603–611. [DOI] [PubMed] [Google Scholar]
- 23.Gil B., M. J. Sanz, M. C. Terencio, M. L. Ferrandiz, G. Bustos, M. Paya, R. Gunasegaran, and M. J. Alcaraz. 1994. Effects of flavonoids on Naja naja and human recombinant synovial phospholipases A2 and inflammatory responses in mice. Life Sci. 54 333–338. [DOI] [PubMed] [Google Scholar]
- 24.Ferrandiz M. L., and M. J. Alcaraz. 1991. Anti-inflammatory activity and inhibition of arachidonic acid metabolism by flavonoids. Agents Actions. 32 283–288. [DOI] [PubMed] [Google Scholar]
- 25.Middleton E., Jr., and C. Kandaswami. 1992. Effects of flavonoids on immune and inflammatory cell functions. Biochem. Pharmacol. 43 1167–1179. [DOI] [PubMed] [Google Scholar]
- 26.Di Carlo G., N. Mascolo, A. A. Izzo, and F. Capasso. 1999. Flavonoids: old and new aspects of a class of natural therapeutic drugs. Life Sci. 65 337–353. [DOI] [PubMed] [Google Scholar]
- 27.Hamberg M., and B. Samuelsson. 1973. Detection and isolation of an endoperoxide intermediate in prostaglandin biosynthesis. Proc. Natl. Acad. Sci. USA. 70 899–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Baumann J., F. von Bruchhausen, and G. Wurm. 1980. Flavonoids and related compounds as inhibition of Arachidonic acid peroxidation. Prostaglandins. 20 627–639. [DOI] [PubMed] [Google Scholar]
- 29.Kalkbrenner F., G. Wurm, and F. von Bruchhausen. 1992. In vitro inhibition and stimulation of purified prostaglandin endoperoxide synthase by flavonoids: structure-activity relationship. Pharmacology. 44 1–12. [DOI] [PubMed] [Google Scholar]
- 30.Mahmoud N. N., A. M. Carothers, D. Grunberger, R. T. Bilinski, M. R. Churchill, C. Martucci, H. L. Newmark, and M. M. Bertagnolli. 2000. Plant phenolics decrease intestinal tumors in an animal model of familial adenomatous polyposis. Carcinogenesis. 21 921–927. [DOI] [PubMed] [Google Scholar]
- 31.Welton, A. T., L. D. Tobias, C. Fiedler-Nagy, W. Anderson, W. Hope, K. Meyers, and J. W. Coffey. 1986. The effect of flavonoids on arachidonic acid metabolism. In Plant Flavonoids in Biology and Medicine. V. Cody, E. Middleton, and J. B. Harborne, editors. Alan R. Liss, New York. 231–242. [PubMed]
- 32.Chi Y. S., H. G. Jong, K. H. Son, H. W. Chang, S. S. Kang, and H. P. Kim. 2001. Effects of naturally occurring prenylated flavonoids on enzymes metabolizing arachidonic acid: cyclooxygenases and lipoxygenases. Biochem. Pharmacol. 62 1185–1191. [DOI] [PubMed] [Google Scholar]
- 33.Ju J., J. Hong, J. N. Zhou, Z. Pan, M. Bose, J. Liao, G. Y. Yang, Y. Y. Liu, Z. Hou, Y. Lin, et al. 2005. Inhibition of intestinal tumorigenesis in Apcmin/+ mice by (-)-epigallocatechin-3-gallate, the major catechin in green tea. Cancer Res. 65 10623–10631. [DOI] [PubMed] [Google Scholar]
- 34.Kulmacz R. J. 1986. Prostaglandin H synthase and hydroperoxides: peroxidase reaction and inactivation kinetics. Arch. Biochem. Biophys. 249 273–285. [DOI] [PubMed] [Google Scholar]
- 35.Hsuanyu Y., and H. B. Dunford. 1990. Reactions of prostaglandin H synthase in the presence of the stabilizing agents diethyldithiocarbamate and glycerol. Biochem. Cell Biol. 68 965–971. [DOI] [PubMed] [Google Scholar]
- 36.MacDonald I. D., G. Graff, L. A. Anderson, and H. B. Dunford. 1989. Optical spectra and kinetics of reactions of prostaglandin H synthase: effects of the substrates 13-hydroperoxyoctadeca-9,11-dienoic acid, arachidonic acid, N,N,N′,N′-tetramethyl-p-phenylenediamine, and phenol and of the nonsteroidal anti-inflammatory drugs aspirin, indomethacin, phenylbutazone, and bromfenac. Arch. Biochem. Biophys. 272 194–202. [DOI] [PubMed] [Google Scholar]
- 37.Hsuanyu Y., and H. B. Dunford. 1992. Reduction of prostaglandin H synthase compound II by phenol and hydroquinone, and the effect of indomethacin. Arch. Biochem. Biophys. 292 213–220. [DOI] [PubMed] [Google Scholar]
- 38.Pietta P. G. 2000. Flavonoids as antioxidants. J. Nat. Prod. 63 1035–1042. [DOI] [PubMed] [Google Scholar]
- 39.Cos P., L. Ying, M. Calomme, J. P. Hu, K. Cimanga, B. Van Poel, L. Pieters, A. J. Vlietinck, and D. Vanden Berghe. 1998. Structure-activity relationship and classification of flavonoids as inhibitors of xanthine oxidase and superoxide scavengers. J. Nat. Prod. 61 71–76. [DOI] [PubMed] [Google Scholar]
- 40.Bors W., and C. Michel. 2002. Chemistry of the antioxidant effect of polyphenols. Ann. N. Y. Acad. Sci. 957 57–69. [DOI] [PubMed] [Google Scholar]
- 41.Middleton, E., and C. Kandaswami. 1993. The impact of plant flavonoids on mammalian biology: Implications for immunity, inflammation and cancer. In The Flavonoids: Advances in Research Since 1986. J. Harborne, editor. Chapman & Hall, London. 619–652.
- 42.Flower R. J., and J. R. Vane. 1972. Inhibition of prostaglandin synthetase in brain explains the anti-pyretic activity of paracetamol (4-acetamidophenol). Nature. 240 410–411. [DOI] [PubMed] [Google Scholar]
- 43.Gottlieb S. 2001. COX 2 inhibitors may increase risk of heart attack. [news] BMJ. 323 471. [PMC free article] [PubMed] [Google Scholar]
- 44.Vane J. R., and R. M. Botting. 1998. Anti-inflammatory drugs and their mechanism of action. Inflamm. Res. 47 S78–S87. [DOI] [PubMed] [Google Scholar]
- 45.Schmedtje J. F., Y. S. Ji, W. L. Liu, R. N. DuBois, and M. S. Runge. 1997. Hypoxia induces cyclooxygenase-2 via the NF-kappaB p65 transcription factor in human vascular endothelial cells. J. Biol. Chem. 272 601–608. [DOI] [PubMed] [Google Scholar]
- 46.Belton O., D. Byrne, D. Kearney, A. Leahy, and D. J. Fitzgerald. 2000. Cyclooxygenase-1 and -2-dependent prostacyclin formation in patients with atherosclerosis. Circulation. 102 840–845. [DOI] [PubMed] [Google Scholar]
- 47.Bravo L. 1998. Polyphenols: chemistry, dietary sources, metabolism, and nutritional significance. Nutr. Rev. 56 317–333. [DOI] [PubMed] [Google Scholar]
- 48.Chow H. H., Y. Cai, I. A. Hakim, J. A. Crowell, F. Shahi, C. A. Brooks, R. T. Dorr, Y. Hara, and D. S. Alberts. 2003. Pharmacokinetics and safety of green tea polyphenols after multiple-dose administration of epigallocatechin gallate and polyphenon E in healthy individuals. Clin. Cancer Res. 9 3312–3319. [PubMed] [Google Scholar]
- 49.Ross J. A., and C. M. Kasum. 2002. Dietary flavonoids: bioavailability, metabolic effects, and safety. Annu. Rev. Nutr. 22 19–34. [DOI] [PubMed] [Google Scholar]
- 50.Manach C., G. Williamson, C. Morand, A. Scalbert, and C. Rémésy. 2005. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am. J. Clin. Nutr. 81 (1, Suppl): 230S–242S. [DOI] [PubMed] [Google Scholar]
- 51.Manach C., A. Scalbert, C. Morand, C. Rémésy, and L. Jiménez. 2004. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 79 727–747. [DOI] [PubMed] [Google Scholar]
- 52.Lee M. J., Z. Y. Wang, H. Li, L. Chen, Y. Sun, S. Gobbo, D. A. Balentine, and C. S. Yang. 1995. Analysis of plasma and urinary tea polyphones in human subjects. Cancer Epidemiol. Biomarkers Prev. 4 393–399. [PubMed] [Google Scholar]
- 53.Chow H. H., Y. Cai, D. S. Alberts, I. Hakim, R. Dorr, F. Shahi, J. A. Crowell, C. S. Yang, and Y. Hara. 2001. Phase I pharmacokinetic study of tea polyphenols following single-dose administration of epigallocatechin gallate and polyphenon E. Cancer Epidemiol. Biomarkers Prev. 10 53–58. [PubMed] [Google Scholar]
- 54.Bors W., and M. Saran. 1987. Radical scavenging by flavonoid antioxidants. Free Radic. Res. Commun. 2 289–294. [DOI] [PubMed] [Google Scholar]
- 55.Mira L., M. Silva, and C. F. Manso. 1994. Scavenging of reactive oxygen species by silibinin dihemisuccinate. Biochem. Pharmacol. 48 753–759. [DOI] [PubMed] [Google Scholar]
- 56.Cao G., E. Sofic, and R. L. Prior. 1997. Antioxidant and prooxidant behaviour of flavonoids: structure-activity relationships. Free Radic. Biol. Med. 22 749–760. [DOI] [PubMed] [Google Scholar]
- 57.Jovanovic, S. V., S. Steenken, M. G. Simic, and Y. Hara. 1998. Antioxidant properties of flavonoids: reduction potentials and electron transfer reactions of flavonoids radicals. In Flavonoids in Health and Disease. C. Rice-Evans and L. Packer, editors. Marcel Dekker, New York. 137–161.
- 58.Arora A., M. G. Nair, and G. M. Strasburg. 1998. Structure-activity relationships for antioxidant activities of a series of flavonoids in a liposomal system. Free Radic. Biol. Med. 24 1355–1363. [DOI] [PubMed] [Google Scholar]
- 59.Mira L., M. Silva, R. Rocha, and C. F. Manso. 1999. Measurement of relative antioxidant activity of compounds: a methodological note. Redox Rep. 4 69–74. [DOI] [PubMed] [Google Scholar]