Abstract
Phosphatidate phosphatase-1 (PAP1) enzymes have a key role in glycerolipid synthesis through the conversion of phosphatidate to diacylglycerol, the immediate precursor of triacylglycerol, phosphatidylcholine, and phosphatidylethanolamine. PAP1 activity in mammals is determined by the lipin family of proteins, lipin-1, lipin-2, and lipin-3, which each have distinct tissue expression patterns and appear to have unique physiological functions. In addition to its role in glycerolipid synthesis, lipin-1 also operates as a transcriptional coactivator, working in collaboration with known nuclear receptors and coactivators to modulate lipid metabolism gene expression. The requirement for different lipin activities in vivo is highlighted by the occurrence of lipodystrophy, insulin resistance, and neuropathy in a lipin-1-deficient mutant mouse strain. In humans, variations in lipin-1 expression levels and gene polymorphisms are associated with insulin sensitivity, metabolic rate, hypertension, and risk for the metabolic syndrome. Furthermore, critical mutations in lipin-2 result in the development of an inflammatory disorder in human patients. A key goal of future studies will be to further elucidate the specific roles and modes of regulation of each of the three lipin proteins in key metabolic processes, including triglyceride and phospholipid synthesis, fatty acid metabolism, and insulin sensitivity.
Keywords: triacylglycerol, lipodystrophy, obesity, insulin resistance, adipose tissue, muscle, liver, transcriptional coactivator
The synthesis and storage of glycerolipids has a central role in the maintenance of energy homeostasis. Triacylglycerols (TAG) are the most calorie-dense form of energy storage, and their accumulation in adipose tissue allows animals to withstand fasting during sleep or prolonged periods of food deprivation. The current prevalence of obesity and its association with pathologies including insulin resistance, diabetes, dyslipidemia, hypertension, coronary heart disease, and cancer has generated increased interest in understanding the processes that promote adipogenesis and modulate lipid storage in adipocytes. Furthermore, the study of rare genetic forms of lipodystrophy, and the rising incidence of acquired lipodystrophy associated with drugs used to treat human immunodeficiency virus infection, has highlighted the critical function of normal adipose tissue in maintaining metabolic homeostasis. As a result, recent years have been marked by the identification, cloning, and physiological characterization of many of the key enzymes in the glycerolipid biosynthesis pathway, some of which are featured in other articles in this Thematic Review series. This review focuses on the lipin proteins, their functions as phosphatidate phosphatase-1 enzymes in glycerolipid biosynthesis and as transcriptional coactivators with effects on fatty acid oxidation, and their physiological roles as determinants of adiposity and insulin sensitivity.
IDENTIFICATION OF THE LIPIN GENE FAMILY
In 1981, a spontaneous recessive mutation occurred in a mouse colony at the Jackson Laboratory (Bar Harbor, Maine). The mice appeared normal at birth, but when they began to suckle, they exhibited retarded growth rate and developed a profoundly fatty liver and hypertriglyceridemia (1). Interestingly, the fatty liver and hypertriglyceridemia resolved as the mice reached 2 weeks of age and were weaned from a high-milk-fat diet. However, the mutant animals maintained approximately 25% lower body weight and developed peripheral neuropathy characterized by tremor and hind limb motor defects throughout adulthood. Based on these phenotypes, the mutation was named fatty liver dystrophy (fld). It was later determined that fld mice have virtually no adipose tissue and develop insulin resistance (2), characteristics that these mice share with forms of severe lipodystrophy in humans (3).
In 2001, the gene affected by the fld mutation was identified using a positional cloning approach, revealing a null mutation in a novel gene (Lpin1) encoding a 98 kDa protein that was named lipin-1 (4). Commensurate with the lipodystrophy phenotype, Lpin1 is prominently expressed in white and brown adipose tissue, and it is induced during differentiation of adipocyte cell lines. Lpin1 expression also occurs at high levels in skeletal muscle and testis, and at lower levels in several tissues including liver, kidney, and brain. Lpin1 is also expressed in Schwann cells of peripheral nerves, and its deficit in these cells causes the peripheral neuropathy observed in the fld mouse (5, 6). Two lipin-1 protein isoforms of 891 amino acids (lipin-1A) and 924 amino acids (lipin-1B) are generated from the Lpin1 gene by alternative mRNA splicing. These have some distinct properties (7, 8), as discussed in a later section.
In mammals, there are two additional lipin protein family members, encoded by Lpin2 and Lpin3 (4). The three mammalian lipin genes differ in their tissue expression patterns (9), suggesting that they have unique physiological roles, although little is currently known about the functions of lipin-2 or -3. Lipins appear to play a fundamental role in cellular metabolism, as species ranging from yeast to invertebrates and plants have either one or two (in fish and plants) orthologous lipin genes. All lipin proteins share some common structural features. These include one or more nuclear localization signals, and large regions at the amino and carboxyl-terminal ends (N-LIP and C-LIP domains, respectively) that are highly conserved among all species. It was through the study of glycerolipid biosynthesis in yeast that the function of lipin-1 proteins as enzymes required for triglyceride and phospholipid synthesis was first elucidated, as described below.
LIPIN PROTEINS ARE PAP1 ENZYMES REQUIRED FOR GLYCEROLIPID SYNTHESIS
In mammals, the glycerol phosphate pathway is responsible for TAG synthesis in most tissues, including adipose tissue (10). TAG synthesis from glycerol phosphate occurs through the step-wise addition of acyl groups, catalyzed by distinct enzyme classes (Fig. 1). cDNA probes for members of each acyltransferase enzyme class have been isolated, but until recently, the molecular identity of the enzyme that converts phosphatidate to diacylglycerol remained elusive. The enzyme responsible, known as phosphatidate phosphatase-1 (PAP1), had been extensively studied since the 1950s and was shown to provide the diacylglycerol that is a necessary precursor for the synthesis of TAG as well as phosphatidylcholine and phosphatidylethanolamine (11–13). Unfortunately, the isolation of PAP1 from mammalian sources proved to be difficult, due in part to instability of the protein, the methods used at the time for determining PAP activity (14), and the existence of multiple isoforms in tissues such as liver.
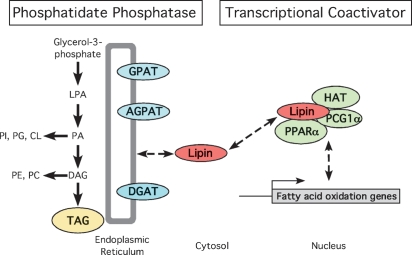
Fig. 1.
Dual molecular functions of lipin proteins as enzymes in glycerolipid biosynthesis and transcriptional coactivation. Left: Lipin proteins act as phosphatidate phosphatase-1 (PAP1) enzymes that catalyze the conversion of phosphatidate (PA) to diacylglycerol (DAG), a direct precursor of triacylglycerol (TAG) and of the phospholipids, phosphatidylethanolamine (PE), and phosphatidylcholine (PC). The acyltransferase enzymes that are required for TAG synthesis (GPAT, glycerol-3-phosphate acyltransferase; AGPAT, acylglycerol-3-phosphate acyltransferase; DGAT, diacylglycerol acyltransferase) are constitutive residents of the endoplasmic reticulum (ER) membrane. In contrast, lipin proteins reside in the cytosol and associate transiently with the ER membrane to perform the PAP1 reaction. Right: Lipin-1 also acts as a transcriptional coactivator that amplifies the effect of known coactivators on transcription of fatty acid oxidation genes. This occurs through lipin-1 interaction with peroxisome proliferator activated receptor γ coactivator-1α (PGC1α) and the nuclear receptor PPARα in a coactivation complex that likely includes other factors such as histone acetyltransferase (HAT). The coactivator activity has thus far been reported primarily for lipin-1, but lipin-2 and -3 proteins possess similar sequence motifs and could exhibit similar activity, although the physiological context for coactivator function of lipin-2 and -3 remains to be determined.
More recent studies in S. cerevisiae led to the successful purification and identification of yeast phosphatidate phosphohydrolase, Pah1p (15). Sequence analysis of this protein revealed that it is the yeast lipin ortholog. PAP1 enzyme activity is conferred by the DxDxT motif that is present in the C-LIP domain of Pah1p, and in all lipin family members in all species (see Fig. 2). Additional studies confirmed that each of the mammalian lipin proteins, lipin-1A, lipin-1B, lipin-2, and lipin-3, also exhibit PAP1 enzyme activity (9, 15). Despite the fact that all lipin proteins possess PAP1 enzyme activity, mouse and human mutations indicate that they have unique physiological roles. Studies of tissues from lipin-1-deficient fld mice revealed that lipin-1 accounts for all of the PAP1 activity in adipose tissue and skeletal muscle but that the other lipin protein family members likely contribute in other tissues (9, 16).
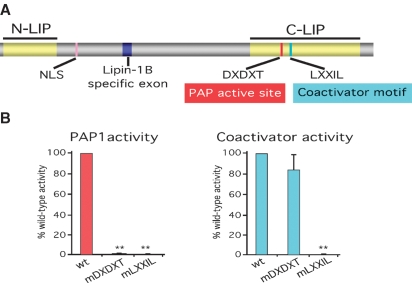
Fig. 2.
Lipin protein structure and functional motifs. A: Schematic view of lipin protein structure with known functional domains indicated. N-LIP and C-LIP regions represent domains that are highly conserved evolutionarily. The C-LIP domain contains critical functional motifs for PAP1 enzyme activity and coactivator interaction. A lysine and arginine-rich nuclear localization signal (NLS) is found in lipin proteins from most species. The alternatively spliced exon present in lipin-1B but not -1A is also indicated. B: Relative PAP1 and coactivator activities of lipin-1 proteins carrying mutation in the PAP1 motif (mDXDXT) or the coactivator interaction motif (mLXXIL). Lipin-1 protein carrying a mutation in the PAP1 active site motif (mDXDXT) lacks PAP1 activity, but retains coactivator function. In contrast, mutation of the coactiviator motif (mLXXIL) abolishes PAP1 and coactivator function. wt, wild-type lipin-1 protein. ** P < 0.001 vs. wt. (Data shown from the authors' laboratories; based on observations presented in Ref. 25.)
The identification of lipin proteins as PAP1 enzymes immediately made available a large body of literature concerning the biochemistry and regulation of these proteins. For example, previous biochemical studies demonstrated that glycerolipid synthesis occurs mainly on membranes of the endoplasmic reticulum (ER) of mammalian cells, although some contribution may come from mitochondria. However, when glycerolipid synthesis from fatty acids was measured with microsomal membranes, there was little production of diacylglycerol and TAG. The formation of these latter lipids was greatly enhanced by addition of the cytosolic fraction. Thus, it was postulated that the cytosol contributed unknown stimulating factors (13, 17). After several years of investigation, the heat-labile stimulating factor was identified as a soluble PAP (18, 19), which is now attributable to the activity of the lipins. Furthermore, from additional early studies of PAP1 activity, it is known that lipins translocate from the cytosol to the ER membrane in response to elevated cellular fatty acids levels (20) (Fig. 1).
It should be noted that in addition to the cytosolic PAP1 activity that corresponds to lipin proteins, there is also a very active membrane-bound PAP activity that does not normally contribute significantly to glycerolipid synthesis. This membrane-bound enzyme activity can be detected in microsomal membranes and in plasma membranes. This activity was originally called PAP2, but has recently been renamed as a family of lipid phosphate phosphatases (LPPs) (reviewed in Ref. 21). In determining lipin activity in cells or tissues, it is important to be aware of PAP1 and LPP activities and to take measures to distinguish these. Lipin PAP1 activity requires Mg2+ and is inhibited by N-ethylmaleimide, whereas LPP activity is Mg2+ independent and is insensitive to the inhibitor (22). Other differences also exist between the two activities, with LPPs having a broad substrate preference including several lipid phosphate species, while lipin proteins appear to be specific for phosphatidate (9, 21).
LIPIN-1 FUNCTIONS AS A TRANSCRIPTIONAL COACTIVATOR
Lipin-1 can localize to several cell compartments. As described earlier, lipin proteins occur in the cytosol and translocate to the ER membrane, a major site of phosphatidate production, to carry out their PAP1 function. Lipin proteins also possess a nuclear localization signal, and immunocytochemistry studies have shown that lipin-1 can reside in either the nucleus or cytoplasm in adipocytes, suggesting that it may have a specific nuclear function (8). There is evidence of a role for lipin in nuclear membrane metabolism in yeast. The lipin ortholog in S. pombe, known as Ned1p, interacts with three nuclear proteins that have roles in nuclear envelope formation and nuclear transport (23). In S. cerevisiae, Pah1p activity regulates nuclear membrane growth during the cell cycle (24).
Studies in mouse liver indicate that lipin-1 also has a role in the regulation of mammalian gene expression. This function of lipin-1 was uncovered after observing that mice lacking PPARγ coactivator 1α (PGC-1α) fail to activate expression of lipin-1 and fatty acid oxidation genes in the liver during fasting, leading to hepatic steatosis (25). Further exploration revealed that lipin-1 expression is required for the fasting-induced expression of the nuclear receptor PPARα and several of its target genes that promote fatty acid oxidation. The mechanism by which lipin-1 enhances gene expression is through its association with PGC-1α and PPARα in a physical complex. Lipin-1 does not contain a DNA binding domain but interacts with PPARα through an LxxIL motif located downstream of the PAP1 active site in the C-LIP domain (25). This motif is conserved in lipin-2 and -3 as well. Interestingly, mutation of the first aspartate residue in the PAP1 active site abolishes lipin-1 PAP1 enzyme activity, but does not diminish coactivator activity in an in vitro assay (Fig. 2) (25). This represents a difference between yeast and mammalian lipin protein function, since all activities of yeast Pah1p, including the effects on phospholipid biosynthesis gene expression, are dependent on PAP1 enzyme activity (26). In vitro assays indicate that, in addition to PPARα, lipin-1 can interact with nuclear receptors such as PPARδ, hepatocyte nuclear factor 4α, and the glucocorticoid receptor (25), raising the possibility that lipin-1 influences expression levels of genes regulated by these factors as well. It remains to be determined if lipin-1 has a physiological role as a coactivator in tissues other than liver, and whether lipin-2 and lipin-3 have analogous roles.
LIPIN-1 AND LIPID METABOLISM IN ADIPOSE TISSUE, MUSCLE, AND LIVER
Studies in mouse models and human subjects indicate that lipin-1 influences lipid homeostasis through its action in key metabolic tissues such as adipose tissue, skeletal muscle, and liver.
Adipose tissue
The lipodystrophic phenotype of lipin-1-deficient fld mice suggested a critical function of lipin-1 in adipose tissue, and some of the proposed functions of lipin-1 in adipocytes are outlined in Fig. 3. These will be discussed in following sections. Lipin-1 plays a critical role in adipocyte development and maturation. Studies of fld embryonic fibroblasts in culture revealed that lipin-1 is required for the expression of key adipogenic transcription factors, such as PPARγ and CAAT/enhancer binding protein α (C/EBPα), and for the attainment of mature adipocyte functions, including lipogenesis and lipid accumulation (27). The failure of lipin-1-deficient cells to accumulate triglyceride can now be attributed, at least partially, to the requirement of lipin-1 PAP1 activity for triglyceride synthesis. However, this is not the full story, because lipin-1 is also required for earlier events in adipogenesis, particularly PPARγ expression. One possibility is that the coactivator function of lipin-1 is important for induction of adipogenic genes during differentiation, but this remains to be determined.
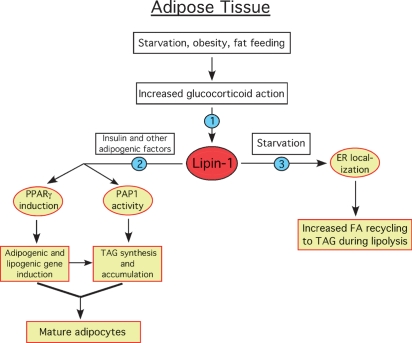
Fig. 3.
Proposed physiological regulation and role of lipin-1 in adipose tissue development and metabolism. Increased glucocorticoid action stimulates Lpin1 transcription and increases lipin-1 protein levels (1). Insulin and other adipogenic factors stimulate lipin-1 activity in an unknown manner, promoting expression of PPARγ and other transcription factors, which induce adipogenic and lipogenic gene expression. Increased lipin-1 PAP1 activity also stimulates TAG synthesis and accumulation to form mature adipocytes (2). During starvation, effects of adrenaline and fatty acids cause lipin-1 translocation to the ER to facilitate incorporation into TAG of fatty acids released during lipolysis (3). The model is based on literature described in the text.
Beyond the absolute requirement for lipin-1 in adipocyte development, the lipin-1A and -1B isoforms appear to have distinct roles in this process. During differentiation of 3T3-L1 preadipocytes, lipin-1A is most prominently expressed in early states of differentiation and gradually diminishes as differentiation progresses. By contrast, lipin-1B expression increases with differentiation and it becomes the predominant lipin isoform in mature adipocytes (8). Although both lipin-1 isoforms can localize to either the cytosol or the nucleus, the majority of lipin-1B in mature adipocytes is present in the cytosol and the remaining lipin-1A is prevalent in the nucleus. Most significantly, lipin-1A and -1B have distinct effects on adipocyte gene expression during differentiation. In studies in which lipin-1-deficient fibroblasts were reconstituted with each isoform independently, lipin-1A most effectively induced the expression of adipogenic transcription factors PPARγ and C/EBPα, whereas lipin-1B more effectively induced lipid synthesis genes such as fatty acid synthase and diacylglycerol acyltransferase (8). Thus, the two lipin-1 isoforms may have complementary roles in adipocyte differentiation.
An interesting new development is the implication of lipin-2 in adipocyte development. Lipin-2 is also expressed in 3T3-L1 preadipocytes and diminishes with differentiation, such that it is reciprocally regulated compared with total lipin-1 (28). A relationship between lipin-1 and -2 expression was further characterized by silencing expression of each separately. In cells treated with shRNA directed against lipin-1, lipin-2 expression increased and persisted longer during differentiation. However, the increased lipin-2 levels could not compensate for the function of lipin-1 in the development of mature adipocytes, confirming the absolute requirement for lipin-1 in adipogenesis. Silencing of lipin-2 expression resulted in increased lipin-1 levels and more rapid induction of the adipocyte fatty acid binding protein aP2, a marker of mature adipocytes. These results show an intriguing difference between lipin-1 and -2 function within adipocytes, despite the fact that both proteins function as effective PAP1 enzymes in biochemical assays (9).
In vivo, lipin-1 expression levels are robustly correlated with adiposity in mouse models. As described earlier, lipin-1 deficiency in the fld mouse causes lipodystrophy, affecting all of the white adipose tissue depots as well as interscapular brown adipose tissue (2). In contrast, enhanced lipin-1 expression targeted to adipose tissue in transgenic mice promotes obesity (29). This enhanced lipin-1 expression has no effect on food intake, yet adipose-specific transgenic mice gain weight more rapidly and accumulate twice the adipose tissue mass as wild-type littermates when fed a high-fat diet. The adipocytes of the transgenic mice are larger and contain 60% more triglyceride per cell than those in wild-type adipose tissue, but the number of cells per fat pad is unaltered (8, 29). This suggests that enhanced lipin-1 levels in adipose tissue leads to increased triglyceride storage due to increased PAP1 activity.
The relationship between lipin-1 expression levels and adiposity in humans is not as straightforward as that seen in the mouse. In fact, reduced lipin-1 expression levels have been detected in adipose tissue from obese compared with normal weight subjects (30, 31). These results appear at first glance to contradict those observed in the mouse. However, a key difference between the adipose tissue lipin-1 transgenic model and human subjects is that the transgene is under the control of a heterologous (adipocyte fatty acid binding protein, aP2) promoter that is not subject to signals that normally regulate lipin-1 gene expression. It appears that lipin-1 levels may increase during development of adipose tissue to promote triglyceride synthesis and adipocyte maturation, but may be subject to negative regulation in states in which triglyceride storage capacity has been reached, as in obesity. An eventual understanding of the factors that regulate lipin-1 gene expression may shed light on the determination of lipin-1 levels in lean and obese states.
A robust positive correlation exists between lipin-1 levels in adipose tissue and insulin sensitivity in mouse and man. Thus, lipin-1-deficient mice are severely insulin resistant, whereas adipose tissue lipin-1 transgenic mice are surprisingly more insulin sensitive than their wild-type littermates, despite their higher adiposity (2, 29). This correlation between lipin-1 expression in adipose tissue and insulin sensitivity has also been observed in several studies in human subjects, including obese subjects and healthy young subjects (30–33). As would be expected, lipin-1 expression levels in adipose tissue of individuals with human immunodeficiency virus-related lipodystrophy are reduced compared with normal subjects (30, 34). However, even in these patients, a positive relationship was observed between maintaining higher lipin-1 levels and better metabolic state, including improved insulin sensitivity and reduced inflammatory cytokine levels.
The mechanistic basis for the correlation between lipin-1 in adipose tissue and whole-body insulin sensitivity is not clear. One possibility is that lipin-1 promotes glucose transporter 4 expression and more efficient glucose uptake in adipocytes (35). Another possibility is that lipin-1 promotes the preferential storage of triglycerides in adipose tissue, thus protecting against ectopic lipid storage in tissues such as skeletal muscle, which may contribute to lipotoxicity and insulin resistance. Support for this mechanism comes from the demonstration that increased lipin-1 expression levels in human adipose tissue is associated with reduced intramyocellular lipid content in muscle of the same subjects (31). A third possibility is that lipin-1 in adipocytes has a role in promoting fatty acid oxidation under some conditions, as observed in liver (25). Evidence for this possibility comes from studies of human adipose tissue gene expression in which lipin-1 mRNA levels showed an extremely significant correlation with genes involved in fatty acid oxidation, PPARα and medium chain acyl-CoA dehydrogenase (32). Additional studies will be required to determine whether these mechanisms are responsible for the positive effect of lipin-1 expression in adipose tissue on insulin sensitivity.
Muscle
Lipin-1 expression levels in mouse skeletal muscle are similar to those in adipose tissue, suggesting an important role in muscle (4). This is supported by the observation that overexpression of a lipin-1 transgene in skeletal muscle dramatically alters energy balance. Thus, muscle lipin-1 transgenic mice eat a normal amount of food but become obese on a chow diet and extremely obese on a high-fat diet, with four times the fat mass of their wild-type littermates (29). The obesity is associated with reduced energy expenditure and decreased fatty acid oxidation in muscle. Lipin-1-deficient mice show the opposite phenotype, with increased energy expenditure and fatty acid oxidation. Interestingly, the energy expenditure of fld mice can be normalized by reconstituting lipin-1 expression specifically in muscle, demonstrating that the effect on energy metabolism is due to the direct function of lipin-1 in this tissue (29). A role for lipin-1 in exercise-induced changes in muscle has been proposed, because expression increases after acute exercise in rats and coincides with effects on oxidative gene expression (36). In contrast to the adipose tissue lipin-1 transgenics, the obese muscle transgenics become insulin resistant. This may be a secondary effect of the dramatically increased adipose tissue mass (without the beneficial increase in lipin-1 that is present in the adipose tissue transgenic mice) that accumulates due to reduced energy expenditure.
Liver
The lipin-1-deficient fld mouse exhibits a fatty liver during the neonatal period, suggesting that lipin-1 has an important physiological role in liver (1). However, lipin-1 is expressed at low levels in liver of adult mice in the fed state (4, 9), and it contributes little to the total hepatic PAP1 enzyme activity, raising the possibility that lipin-2 and/or lipin-3 fulfill a major part of this function in liver (9, 16). Nevertheless, lipin-1 expression in liver increases during fasting, as described earlier. This is instrumental for adaptation to fasting, which increases the potential for TAG synthesis in response to a high fatty acid influx (37). The increase also enhances the capacity for fatty acid oxidation through the action of lipin-1 as a transcriptional coactivator for fatty acid oxidation genes (25). In this sense, TAG synthesis should be viewed as a companion pathway, rather than an antagonistic pathway, for β-oxidation because the excess fatty acid stored in cytosolic TAG pools can be turned over later for oxidation. An emerging picture of the role of lipin-1 and its regulation in hepatocytes is presented in Fig. 4 and discussed in the following sections.
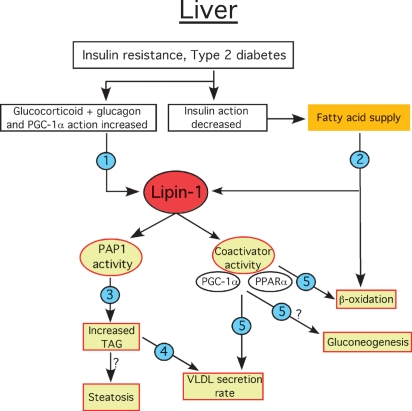
Fig. 4.
Proposed physiological regulation and role of lipin-1 in hepatic lipid metabolism and insulin resistance. Under conditions of insulin resistance, increased action of glucocorticoids relative to insulin stimulates Lpin1 transcription and increases lipin-1 protein levels and PAP1 activity (1). Lack of suppression of adipose tissue lipolysis by insulin increases the fatty acid supply to the liver, which provides substrate for TAG synthesis and β-oxidation. Fatty acids also activate lipin-1 PAP1 activity by promoting its translocation to the ER membrane (2). Translocation of lipin-1 to the ER stimulates TAG synthesis and accumulation, which could potentially lead to steatosis (3). Increased TAG synthesis also influences the rate of VLDL secretion (4). Lipin-1 coactivator activity in conjunction with PGC-1α and PPARα increases transcription of genes involved in fatty acid oxidation and modulates rate of VLDL synthesis and secretion. It may also influence gluconeogenesis (5). The model is based on literature described in the text.
Recent studies have revealed that lipin-1 has a role in hepatic very low density lipoprotein (VLDL) metabolism. Based on the role of lipin-1 as a PAP1 enzyme, one might expect that lipin-1 levels in liver promote synthesis of TAG and its incorporation into VLDL. However, observations that neonatal fld mice have increased plasma TAG levels (1) and that adenoviral overexpression of lipin-1 in liver leads to reduced circulating TAG levels (25) suggested that the effects of lipin-1 could be more complex. In fact, manipulation of lipin-1 expression levels in liver and hepatocytes isolated from fld mice indicate that lipin-1 levels do not determine rates of TAG synthesis, but rather influence secretion rates of TAG in the form of VLDL-sized particles that contain apoB48 (38). There was little secretion of apoB100-containing VLDL particles in these studies. Compared with wild-type, hepatocytes from fld mice had increased VLDL-TAG secretion and very high expression levels of stearoyl-CoA desaturase-1 (Scd1) (38), which is known to be a major regulator of VLDL secretion (39). Adenoviral overexpression of lipin-1B did not affect TAG synthesis rates, but it suppressed VLDL secretion and Scd1 expression without affecting the expression levels of microsomal triglyceride transfer protein and apoB (38). It was determined through the use of mutant lipin-1 molcules, that this effect requires lipin-1 transcriptional coactivator, but not PAP1, activity. However, it is unclear at present what transcriptional targets are important in mediating this effect. These results (38) also provide strong evidence for the participation of lipin-2 or -3 in promoting high levels of VLDL secretion.
Additional support for a role of lipin-1 in VLDL-TAG secretion comes from a study of hepatic gene expression performed in humans. Hepatic lipin-1 mRNA levels were measured in obese subjects before and after undergoing gastric bypass surgery (40). Analogous to relationships observed between lipin-1 levels in adipose tissue, lipin-1 mRNA levels were inversely related to body mass index and insulin resistance. But most interestingly, following the dramatic weight loss associated with the gastric bypass surgery, lipin-1 mRNA levels were increased and rates of VLDL-TAG secretion were decreased. Similarly, a reduction in VLDL secretion in a mouse model of the metabolic syndrome can be induced by overexpression of lipin-1 in the liver of these mice (38). Thus, lipin-1 levels in liver may be an important modulator of circulating TAG levels in both mouse and humans.
LIPINS AND SIGNAL TRANSDUCTION
In addition to the effects of the lipin proteins in controlling TAG synthesis and transcriptional regulation, PAP1 also appears to be involved in cell signaling. Both the substrate (phosphatidate, PA) and product (diacylglycerol, DAG) of the PAP1 reaction are bioactive lipids, which influence many aspects of cell activation (see Ref. 21 for review). Reported targets of PA action include activation of NADPH oxidase, protein kinase C-ζ, phosphatidylinositol 4-kinase, Raf, phospholipase C-γ, and Ras and inhibition of protein phosphatase-1. The effect of PA on Ras and Raf increases extracellular signal-related kinase activity and cell division. PA can increase cell division through mTOR and it stimulates stress fiber formation. The relative concentrations of lysophosphatidate and PA in membranes control their curvature and vesicle budding. DAG activates classical and novel isoforms of protein kinase C, Ras-GRP, and chimerins.
Direct evidence for PAP1 involvement in signal transduction has been reported. PAP1 appears to regulate epidermal growth factor signaling because it coimmunoprecipitates with epidermal growth factor receptors, which on activation appear to transfer the PAP1 activity to protein kinase-C-ɛ (PKC-ɛ), a DAG-dependent PKC (41). PAP1 activity is also involved in cyclo-oxygenase expression and eicosanoid formation when WISH cells are activated through PKC (42) and when macrophages are stimulated with lipopolysaccharide (43). The exact mechanisms for these actions are yet to be elucidated. However, this combined work, together with evidence that the regulation of PA concentration in yeast nuclei controls gene expression (44), point to a role for the lipins in controlling cell signaling through modulating PA and DAG levels.
Regulation of lipin-1 expression and activity
The accumulating evidence that lipin proteins are important determinants of lipid homeostasis has spurred interest in identifying mechanisms that regulate lipin expression and activity. Lipin activity appears to be regulated at several levels including transcription, posttranslational modification, and subcellular localization. As discovered many years before the lipin genes were identified, PAP1 activity is primarily cytosolic and translocates to the ER membrane in response to fatty acids. Treatment with okadaic acid displaces PAP1 from the ER membrane, suggesting that its translocation is regulated by phosphorylation (45). Direct evidence for this phosphorylation will be discussed later. In liver, PAP1 activity increases in starvation, diabetes, hypoxia, and ethanol-induced fatty liver, as well as in response to glucocorticoids (37). However, PAP1 activity in adipose tissue is regulated differently than in liver, with decreased activity in diabetes and starvation commensurate with decreased TAG accumulation and increased lipolysis (46). This decrease in PAP1 activity was reversed 2 h after administration of insulin, which indicates that it is probably not related to decreased lipin expression. Now that molecular probes and expression vectors are available for each of the three lipin genes, it is possible to examine the mechanisms underlying these changes in PAP1 activity versus concentration in different tissues and attribute them to specific lipin family members.
Lpin1 gene expression is modulated by several stimuli. Glucocorticoids are a key stimulus for lipin-1 expression during adipocyte differentiation and act through glucocorticoid receptor that is bound to sequences upstream of the Lpin1 gene (47). Lipin-1 expression in adipose tissue is also enhanced in vivo in circumstances characterized by increased glucocorticoid levels such as obesity and fasting (46, 47) (Fig. 3). The increase in lipin-1 expression in fasting probably provides the capacity for the increased synthesis of TAG, which is expressed by lipin-1 translocation to membranes in accelerated lipolysis as fatty acids accumulate (16). By contrast, the glucocorticoid-induced lipin-1 accumulation occurring in obesity is associated with increased TAG accumulation in adipose tissue.
In adipose tissue, lipin-1 is also induced by thiazolidinediones, insulin-sensitizing compounds that act as PPARγ agonists (31), and by the small molecule harmine, which also increases insulin sensitivity (48). These results raise the possibility that induction of lipin-1 expression contributes to the insulin-sensitizing effect of these compounds. In skeletal muscle, lipin-1 expression is induced by acute exercise as well as by treatment with activators of AMP-activated protein kinase or the β2-adrenergic receptor (36). The orphan nuclear receptor NOR-1 may mediate the effect of β-adrenergic signaling on lipin-1 expression, because NOR-1 is recruited to the Lpin1 promoter in response to β2-adrenergic receptor stimulation (49).
Expression of all three lipins, including both splice variants of the Lpin1 gene, can be detected in hepatocytes (9, 50), making it unclear which of these is responsible for the observed increase in hepatic PAP1 activity that occurs in various conditions whereby the activity of glucocorticoids is increased relative to insulin (45). Analysis of gene expression in mouse and rat hepatocytes has revealed distinct regulation of Lpin1, Lpin2, and Lpin3 genes. Glucocorticoids, which increase hepatic PAP1 activity, increase lipin-1A and -1B mRNA and protein levels (50). Glucocorticoid stimulation of Lpin1 expression is enhanced by glucagon or cAMP, but is antagonized by insulin (Fig. 4), responses that are similar to the regulation of key enzymes in gluconeogenesis. Neither lipin-2 nor -3 mRNA levels are affected by glucocorticoid and cAMP, indicating that the effects of these stimuli on PAP1 activity are attributable specifically to modulation of lipin-1 levels. These results provide a mechanism for the early observations of increased hepatic PAP1 activity in conditions characterized by increased glucocorticoid or cAMP effects, such as in the circadian cycle, severe stress, starvation, diabetes, and ethanol intoxication (45). Direct evidence that glucocorticoids increase lipin-1 levels in liver and adipose tissue during the circadian cycle has been provided (51), and this is confirmed by the abolishment of cycling in adrenalectomized mice (52). Dietary fatty acids, particularly saturated fatty acids, dramatically induce lipin-1 expression in liver, an effect that is mediated by PPARα (53). Lipin-1 expression levels in liver and uterus vary through the estrous cycle, with lowest levels when blood estrogen levels are at their peak (54). Thus, Lpin1 gene expression appears to be repressed by estrogen, which may contribute to a role for lipin-1 in fertility and in linking estrogens with lipid metabolism.
As described earlier, lipin-1 is responsible for the glucocorticoid-induced PAP1 activity in the liver during stress, insulin resistance, and diabetes. It was proposed that this provides the extra capacity for the liver to synthesize TAG and sequester fatty acids in cytosolic fat droplets when fatty acid build-up causes translocation of PAP1 (lipin-1) to the ER (37). This could provide protection against the lipotoxic effects of elevated unesterified fatty acid and acyl-CoA concentrations. Also, the glucocorticoid-induced increase in lipin-1 activity and fatty acid supply could help to maintain VLDL secretion in catabolic conditions to provide a source of energy together with glucose and ketones for other organs (37). Glucocorticoids are powerful inducers that increase VLDL secretion and this effect is antagonized by insulin (55), in parallel to the expression of lipin-1. In general, insulin insensitivity and increased glucocorticoid action in the metabolic syndrome is accompanied by hypertriglyceridemia (56).
Beyond transcription, phosphorylation of the lipin-1 protein appears to influence lipin-1 subcellular compartmentalization and activity. Lipin-1 is phosphorylated in response to insulin and amino acids, an effect that is inhibited by rapamycin and therefore dependent upon the mammalian target of rapamycin (mTOR) (7, 16). Although numerous phosphorylation sites have been identified in lipin-1 and in yeast Pah1p (16, 24), Ser106 of mouse lipin-1 appears to be a major site of insulin-stimulated phosphorylation (16). Lipin-1 is dephosphorylated in response to oleic acid or epinephrine. Phosphorylation does not appear to alter the intrinsic PAP1 activity of lipin-1 protein, but promotes cytosolic over microsomal localization (16), which may decrease the physiological expression of PAP1 activity. Reduced PAP1 activity during mitosis is associated with phosphorylation of lipin-1 and -2 (28). Thus, phosphorylation appears to be an important mechanism for modulation of PAP1 activity. Specific phosphatases have been identified for Pah1p in yeast (Nem1p) (24) and for lipin-1 (Dullard) (57). These phosphatases may have critical roles in modulating lipin PAP1 function, with important effects on processes such as nuclear membrane biogenesis and TAG synthesis. It remains to be determined whether phosphorylation also acts as a mechanism to regulate nuclear localization of lipin-1.
GENETIC VARIATION IN LIPIN GENES AND HUMAN DISEASE
As described earlier, Lpin1 mutations in the mouse cause lipodystrophy. Thus far, mutations causing human lipodystrophy have not been detected in the corresponding human gene, LPIN1 (58, 59). However, it must be noted that the subjects analyzed in these studies were not reported to recapitulate the additional aspects of the fld mouse phenotype, including transient fatty liver and peripheral neuropathy. More significantly perhaps, analyses of general human populations have identified genetic variations in LPIN1 that are associated with component traits of the metabolic syndrome.
The earliest LPIN1 genetic association study in humans showed a correlation between adipose tissue lipin-1 mRNA levels and fasting glucose and insulin values in a small group of individuals (33). To determine whether common LPIN1 genetic variants may influence these traits, single nucleotide polymorphisms (SNPs) throughout the gene were typed in a case-control sample of lean and obese subjects and in a large cohort of hyperlipidemic individuals. Significant associations were detected between individual SNPs as well as haplotypes comprised of multiple SNP markers and insulin levels and body mass index (BMI) (33). Associations between LPIN1 polymorphisms or haplotypes in several additional population samples have now been described for BMI (59–61), insulin levels (60, 61), resting metabolic rate (60), and systolic blood pressure (61, 62). One study that analyzed individuals from two different generations within families showed that some associations are age dependent. Thus, the association of LPIN1 genotype with insulin levels was stronger in the younger generation (offspring), whereas association with resting metabolic rate occurred in individuals from the older generation (parents) (62). It is significant that LPIN1 genotype is associated with several individual features of the metabolic syndrome, as well as with an overall “metabolic syndrome factor score” (61), and may reflect the roles that lipin-1 has in several tissues that influence metabolic homeostasis. Furthermore, a specific LPIN1 polymorphism is associated with the response of type 2 diabetics to thiazolidinedione treatment (63).
Recent work has revealed the occurrence of lipin-2 mutations in human disease. Mutation of LPIN2 causes Majeed syndrome, an inflammatory disorder characterized by recurrent bouts of osteomyelitis, dyserythropoietic anemia, and cutaneous inflammation, demonstrating that lipin-2 function cannot be substituted by other lipin family members (64, 65). Three LPIN2 mutations have been characterized, including a frame-shift mutation that produces a premature stop codon, a point mutation that results in a single amino acid substitution in the C-LIP domain, and a donor splice site mutation (64, 66). The mechanisms by which loss of lipin-2 causes this inflammatory disease are not known. It has been observed that lipin-2 expression is induced in liver and lung in response to experimentally induced oxidative stress, suggesting a potential role for lipin-2 in controlling inflammation resulting from oxidative stress (67, 68). Interestingly, using genome-wide genetic linkage analysis, a region on chromosome 18p11, which harbors the LPIN2 gene, was linked to BMI and insulin sensitivity in multiple populations (reviewed in Ref. 69). Subsequent high-resolution genotyping revealed an SNP within the 3′ untranslated region of the LPIN2 gene that is associated with increased diabetes risk in individuals with high BMI, and neutral or protective effects in lean individuals (70). At this point, no studies have been reported on LPIN3.
FUTURE PERSPECTIVES
The lipin proteins have now been established as PAP1 enzymes having critical roles in glycerolipid synthesis in key metabolic tissues such as adipose tissue, muscle, and liver. Compared with most other enzymes in the glycerolipid synthesis pathway, the lipin proteins are relatively unique in their multi-compartmental localization to cytosol, ER membranes, and nucleus. The only other enzymes known to have a similar profile are the CTP:phosphocholine cytidylyltransferases, CCT-α, and CCT-β, which regulate phosphatidylcholine synthesis. Signals that stimulate phosphatidylcholine synthesis recruit CCT-α from an inactive nuclear reservoir to a functional site on the ER (reviewed in Refs. 71, 72). Lipin-1A and -1B appear at present to be unique in facilitating glycerolipid synthesis and also having transcriptional coactivator function that is independent of PAP1 enzymatic activity.
Many questions remain. What regulates lipin-1 PAP1 versus coactivator function? Which effects of lipin protein action are attributable to PAP1 enzyme activity versus transcriptional regulator activity? What are the physiological roles of lipin-2 and -3, and do they also function as transcriptional coactivators? How do lipin-2 mutations cause the inflammatory symptoms observed in Majeed Syndrome? Are mutations or genetic variations in lipin-3 associated with any human diseases? Do the lipin proteins represent viable targets for the modulation of TAG storage in fat or other tissues, or for TAG secretion from the liver? Answers for these questions are within reach through the study of genetically modified mouse models, cell culture systems, and genetic analysis of human populations.
Note added in proof
Mutations in human LPIN1 have now been identified that cause recurrent acute myoglobinuria (Zeharia et al., 2008. Am J Hum Genet 83: 1–6).
Acknowledgments
D.N.B. is a recipient of a Medical Scientist Award from the Alberta Heritage Foundation for Medical Research.
Published, JLR Papers in Press, September 12, 2008.
Footnotes
The authors gratefully acknowledge support from the National Heart, Lung, and Blood Institute (HL-28481 and HL-90553 to K.R.) and the Heart and Stroke Foundation of Alberta, NWT & Nunavut (to D.N.B.).
References
- 1.Langner C. A., E. H. Birkenmeier, O. Ben-Zeev, M. C. Schotz, H. O. Sweet, M. T. Davisson, and J. I. Gordon. 1989. The fatty liver dystrophy (fld) mutation. A new mutant mouse with a developmental abnormality in triglyceride metabolism and associated tissue-specific defects in lipoprotein lipase and hepatic lipase activities. J. Biol. Chem. 264 7994–8003. [PubMed] [Google Scholar]
- 2.Reue K., P. Xu, X. P. Wang, and B. G. Slavin. 2000. Adipose tissue deficiency, glucose intolerance, and increased atherosclerosis result from mutation in the mouse fatty liver dystrophy (fld) gene. J. Lipid Res. 41 1067–1076. [PubMed] [Google Scholar]
- 3.Garg A. 2004. Acquired and inherited lipodystrophies. N. Engl. J. Med. 350 1220–1234. [DOI] [PubMed] [Google Scholar]
- 4.Péterfy M., J. Phan, P. Xu, and K. Reue. 2001. Lipodystrophy in the fld mouse results from mutation of a new gene encoding a nuclear protein, lipin. Nat. Genet. 27 121–124. [DOI] [PubMed] [Google Scholar]
- 5.Nadra K., A. S. de Preux Charles, J. J. Medard, W. T. Hendriks, G. S. Han, S. Gres, G. M. Carman, J. S. Saulnier-Blache, M. H. Verheijen, and R. Chrast. 2008. Phosphatidic acid mediates demyelination in Lpin1 mutant mice. Genes Dev. 22 1647–1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Verheijen M. H., R. Chrast, P. Burrola, and G. Lemke. 2003. Local regulation of fat metabolism in peripheral nerves. Genes Dev. 17 2450–2464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huffman T. A., I. Mothe-Satney, and J. C. Lawrence, Jr. 2002. Insulin-stimulated phosphorylation of lipin mediated by the mammalian target of rapamycin. Proc. Natl. Acad. Sci. USA. 99 1047–1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Péterfy M., J. Phan, and K. Reue. 2005. Alternatively spliced lipin isoforms exhibit distinct expression pattern, subcellular localization, and role in adipogenesis. J. Biol. Chem. 280 32883–32889. [DOI] [PubMed] [Google Scholar]
- 9.Donkor J., M. Sariahmetoglu, J. Dewald, D. N. Brindley, and K. Reue. 2007. Three mammalian lipins act as phosphatidate phosphatases with distinct tissue expression patterns. J. Biol. Chem. 282 3450–3457. [DOI] [PubMed] [Google Scholar]
- 10.Coleman R. A., and D. P. Lee. 2004. Enzymes of triacylglycerol synthesis and their regulation. Prog. Lipid Res. 43 134–176. [DOI] [PubMed] [Google Scholar]
- 11.Kates M. 1955. Hydrolysis of lecithin by plant plastid enzymes. Can. J. Biochem. Physiol. 33 575–589. [PubMed] [Google Scholar]
- 12.Smith S. W., S. B. Weiss, and E. P. Kennedy. 1957. The enzymatic dephosphorylation of phosphatidic acids. J. Biol. Chem. 228 915–922. [PubMed] [Google Scholar]
- 13.Stein Y., and B. Shapiro. 1957. The synthesis of neutral glycerides by fractions of rat liver homogenates. Biochim. Biophys. Acta. 24 197–198. [DOI] [PubMed] [Google Scholar]
- 14.Martin A., P. Hales, and D. N. Brindley. 1987. A rapid assay for measuring the activity and the Mg2+ and Ca2+ requirements of phosphatidate phosphohydrolase in cytosolic and microsomal fractions of rat liver. Biochem. J. 245 347–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Han G. S., W. I. Wu, and G. M. Carman. 2006. The Saccharomyces cerevisiae Lipin homolog is a Mg2+-dependent phosphatidate phosphatase enzyme. J. Biol. Chem. 281 9210–9218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harris T. E., T. A. Huffman, A. Chi, J. Shabanowitz, D. F. Hunt, A. Kumar, and J. C. Lawrence, Jr. 2007. Insulin controls subcellular localization and multisite phosphorylation of the phosphatidic acid phosphatase, lipin 1. J. Biol. Chem. 282 277–286. [DOI] [PubMed] [Google Scholar]
- 17.Hubscher G., D. N. Brindley, M. E. Smith, and B. Sudgwick. 1967. Stimulation of biosynthesis of glyceride. Nature. 216 449–453. [DOI] [PubMed] [Google Scholar]
- 18.Johnston J. M., G. A. Rao, P. A. Lowe, and B. E. Schawrz. 1967. The nature of the stimulatory role of the supernatant fraction on triglyceride synthesis by the alpha-glycerophosphate pathway. Lipids. 2 14–20. [DOI] [PubMed] [Google Scholar]
- 19.Smith M. E., B. Sedgwick, D. N. Brindley, and G. Hubscher. 1967. The role of phosphatidate phosphohydrolase in glyceride biosynthesis. Eur. J. Biochem. 3 70–77. [DOI] [PubMed] [Google Scholar]
- 20.Cascales C., E. H. Mangiapane, and D. N. Brindley. 1984. Oleic acid promotes the activation and translocation of phosphatidate phosphohydrolase from the cytosol to particulate fractions of isolated rat hepatocytes. Biochem. J. 219 911–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brindley D. N. 2004. Lipid phosphate phosphatases and related proteins: signaling functions in development, cell division, and cancer. J. Cell. Biochem. 92 900–912. [DOI] [PubMed] [Google Scholar]
- 22.Martin A., A. Gomez-Munoz, Z. Jamal, and D. N. Brindley. 1991. Characterization and assay of phosphatidate phosphatase. Methods Enzymol. 197 553–563. [DOI] [PubMed] [Google Scholar]
- 23.Tange Y., A. Hirata, and O. Niwa. 2002. An evolutionarily conserved fission yeast protein, Ned1, implicated in normal nuclear morphology and chromosome stability, interacts with Dis3, Pim1/RCC1 and an essential nucleoporin. J. Cell Sci. 115 4375–4385. [DOI] [PubMed] [Google Scholar]
- 24.Santos-Rosa H., J. Leung, N. Grimsey, S. Peak-Chew, and S. Siniossoglou. 2005. The yeast lipin Smp2 couples phospholipid biosynthesis to nuclear membrane growth. EMBO J. 24 1931–1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Finck B. N., M. C. Gropler, Z. Chen, T. C. Leone, M. A. Croce, T. E. Harris, J. C. Lawrence, Jr., and D. P. Kelly. 2006. Lipin 1 is an inducible amplifier of the hepatic PGC-1alpha/PPARalpha regulatory pathway. Cell Metab. 4 199–210. [DOI] [PubMed] [Google Scholar]
- 26.Han G. S., S. Siniossoglou, and G. M. Carman. 2007. The cellular functions of the yeast lipin homolog PAH1p are dependent on its phosphatidate phosphatase activity. J. Biol. Chem. 282 37026–37035. [DOI] [PubMed] [Google Scholar]
- 27.Phan J., M. Péterfy, and K. Reue. 2004. Lipin expression preceding peroxisome proliferator-activated receptor-gamma is critical for adipogenesis in vivo and in vitro. J. Biol. Chem. 279 29558–29564. [DOI] [PubMed] [Google Scholar]
- 28.Grimsey N., G. S. Han, L. O'Hara, J. J. Rochford, G. M. Carman, and S. Siniossoglou. 2008. Temporal and spatial regulation of the phosphatidate phosphatases lipin 1 and 2. J. Biol. Chem. 283 29166–29174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Phan J., and K. Reue. 2005. Lipin, a lipodystrophy and obesity gene. Cell Metab. 1 73–83. [DOI] [PubMed] [Google Scholar]
- 30.Miranda M., M. R. Chacon, J. Gomez, A. Megia, V. Ceperuelo-Mallafre, S. Veloso, M. Saumoy, L. Gallart, C. Richart, J. M. Fernandez-Real, et al. 2007. Human subcutaneous adipose tissue LPIN1 expression in obesity, type 2 diabetes mellitus, and human immunodeficiency virus–associated lipodystrophy syndrome. Metabolism. 56 1518–1526. [DOI] [PubMed] [Google Scholar]
- 31.Yao-Borengasser A., N. Rasouli, V. Varma, L. M. Miles, B. Phanavanh, T. N. Starks, J. Phan, H. J. Spencer 3rd, R. E. McGehee, Jr., K. Reue, et al. 2006. Lipin expression is attenuated in adipose tissue of insulin-resistant human subjects and increases with peroxisome proliferator-activated receptor gamma activation. Diabetes. 55 2811–2818. [DOI] [PubMed] [Google Scholar]
- 32.Donkor J., L. M. Sparks, H. Xie, S. R. Smith, and K. Reue. 2008. Adipose tissue lipin-1 expression is correlated with peroxisome proliferator-activated receptor alpha gene expression and insulin sensitivity in healthy young men. J. Clin. Endocrinol. Metab. 93 233–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Suviolahti E., K. Reue, R. M. Cantor, J. Phan, M. Gentile, J. Naukkarinen, A. Soro-Paavonen, L. Oksanen, J. Kaprio, A. Rissanen, et al. 2006. Cross-species analyses implicate Lipin 1 involvement in human glucose metabolism. Hum. Mol. Genet. 15 377–386. [DOI] [PubMed] [Google Scholar]
- 34.Lindegaard B., L. F. Larsen, A. B. Hansen, J. Gerstoft, B. K. Pedersen, and K. Reue. 2006. Adipose tissue lipin expression levels distinguish HIV patients with and without lipodystrophy. Int. J. Obes. 31 449–456. [DOI] [PubMed] [Google Scholar]
- 35.van Harmelen V., M. Ryden, E. Sjolin, and J. Hoffstedt. 2007. A role of lipin in human obesity and insulin resistance: relation to adipocyte glucose transport and GLUT4 expression. J. Lipid Res. 48 201–206. [DOI] [PubMed] [Google Scholar]
- 36.Higashida K., M. Higuchi, and S. Terada. 2008. Potential role of lipin-1 in exercise-induced mitochondrial biogenesis. Biochem. Biophys. Res. Commun. 374 587–591. [DOI] [PubMed] [Google Scholar]
- 37.Brindley, D. N. 1988. Phosphatidate Phosphohydrolase: Its Role in Glycerolipid Synthesis. CRC Press, Inc., Boca Raton, FL. 21–77.
- 38.Chen Z., M. C. Gropler, J. Norris, J. C. Lawrence, Jr., T. E. Harris, and B. N. Finck. 2008. Alterations in hepatic metabolism in fld mice reveal a role for lipin 1 in regulating VLDL-triacylglyceride secretion. Arterioscler. Thromb. Vasc. Biol. 28 1738–1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Attie A. D., R. M. Krauss, M. P. Gray-Keller, A. Brownlie, M. Miyazaki, J. J. Kastelein, A. J. Lusis, A. F. Stalenhoef, J. P. Stoehr, M. R. Hayden, et al. 2002. Relationship between stearoyl-CoA desaturase activity and plasma triglycerides in human and mouse hypertriglyceridemia. J. Lipid Res. 43 1899–1907. [DOI] [PubMed] [Google Scholar]
- 40.Croce M. A., J. C. Eagon, L. L. LaRiviere, K. M. Korenblat, S. Klein, and B. N. Finck. 2007. Hepatic lipin 1beta expression is diminished in insulin-resistant obese subjects and is reactivated by marked weight loss. Diabetes. 56 2395–2399. [DOI] [PubMed] [Google Scholar]
- 41.Jiang Y., Z. Lu, Q. Zang, and D. A. Foster. 1996. Regulation of phosphatidic acid phosphohydrolase by epidermal growth factor. Reduced association with the EGF receptor followed by increased association with protein kinase Cepsilon. J. Biol. Chem. 271 29529–29532. [DOI] [PubMed] [Google Scholar]
- 42.Johnson C. A., M. A. Balboa, J. Balsinde, and E. A. Dennis. 1999. Regulation of cyclooxygenase-2 expression by phosphatidate phosphohydrolase in human amnionic WISH cells. J. Biol. Chem. 274 27689–27693. [DOI] [PubMed] [Google Scholar]
- 43.Grkovich A., C. A. Johnson, M. W. Buczynski, and E. A. Dennis. 2006. Lipopolysaccharide-induced cyclooxygenase-2 expression in human U937 macrophages is phosphatidic acid phosphohydrolase-1-dependent. J. Biol. Chem. 281 32978–32987. [DOI] [PubMed] [Google Scholar]
- 44.O'Hara L., G. S. Han, S. Peak-Chew, N. Grimsey, G. M. Carman, and S. Siniossoglou. 2006. Control of phospholipid synthesis by phosphorylation of the yeast lipin Pah1p/Smp2p Mg2+-dependent phosphatidate phosphatase. J. Biol. Chem. 281 34537–34548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gomez-Munoz A., G. M. Hatch, A. Martin, Z. Jamal, D. E. Vance, and D. N. Brindley. 1992. Effects of okadaic acid on the activities of two distinct phosphatidate phosphohydrolases in rat hepatocytes. FEBS Lett. 301 103–106. [DOI] [PubMed] [Google Scholar]
- 46.Saggerson, E. D. 1988. Phosphatidate phosphohydrolase: its role in glycerolipid synthesis. In Phosphatidate phosphohydrolase: Its role in Glycerolipid Synthesis. CRC Press, Inc., Boca Raton. 79–129.
- 47.Zhang P., L. O'Loughlin, D. N. Brindley, and K. Reue. 2008. Regulation of lipin-1 gene expression by glucocorticoids during adipogenesis. J. Lipid Res. 49 1519–1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Waki, H., K. W. Park, N. Mitro, L. Pei, R. Damoiseaux, D. C. Wilpitz, K. Reue, E. Saez, and P. Tontonoz. 2007. The small molecule harmine is an anti-diabetic cell-type specific regulator of PPARg expression. Cell Metab. In press. [DOI] [PubMed]
- 49.Pearen M. A., S. A. Myers, S. Raichur, J. G. Ryall, G. S. Lynch, and G. E. Muscat. 2008. The orphan nuclear receptor, NOR-1, a target of beta-adrenergic signaling, regulates gene expression that controls oxidative metabolism in skeletal muscle. Endocrinology. 149 2853–2865. [DOI] [PubMed] [Google Scholar]
- 50.Manmontri B., M. Sariahmetoglu, J. Donkor, M. B. Khalil, M. Sundaram, Z. Yao, K. Reue, R. Lehner, and D. N. Brindley. 2008. Glucocorticoids and cyclic AMP selectively increase hepatic lipin-1 expression, and insulin acts antagonistically. J. Lipid Res. 49 1056–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Panda S., M. P. Antoch, B. H. Miller, A. I. Su, A. B. Schook, M. Straume, P. G. Schultz, S. A. Kay, J. S. Takahashi, and J. B. Hogenesch. 2002. Coordinated transcription of key pathways in the mouse by the circadian clock. Cell. 109 307–320. [DOI] [PubMed] [Google Scholar]
- 52.Oishi K., N. Amagai, H. Shirai, K. Kadota, N. Ohkura, and N. Ishida. 2005. Genome-wide expression analysis reveals 100 adrenal gland-dependent circadian genes in the mouse liver. DNA Res. 12 191–202. [DOI] [PubMed] [Google Scholar]
- 53.Martin P. G. P., H. Guillou, F. Lasserre, S. Dejean, A. Lan, J. M. Pascussi, M. Sancristobal, P. Legrand, P. Besse, and T. Pineau. 2007. Novel aspects of PPARa-mediated regulation of lipid and xenobiotic metabolism revealed through a nutrigenomic study. Hepatology. 45 767–777. [DOI] [PubMed] [Google Scholar]
- 54.Gowri P. M., S. Sengupta, S. Bertera, and B. S. Katzenellenbogen. 2007. Lipin1 regulation by estrogen in uterus and liver: implications for diabetes and fertility. Endocrinology. 148 3685–3693. [DOI] [PubMed] [Google Scholar]
- 55.Martin-Sanz P., J. E. Vance, and D. N. Brindley. 1990. Stimulation of apolipoprotein secretion in very-low-density and high-density lipoproteins from cultured rat hepatocytes by dexamethasone. Biochem. J. 271 575–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Brindley D. N., and Y. Rolland. 1989. Possible connections between stress, diabetes, obesity, hypertension and altered lipoprotein metabolism that may result in atherosclerosis. Clin. Sci. (Lond.). 77 453–461. [DOI] [PubMed] [Google Scholar]
- 57.Kim Y., M. S. Gentry, T. E. Harris, S. E. Wiley, J. C. Lawrence, Jr., and J. E. Dixon. 2007. A conserved phosphatase cascade that regulates nuclear membrane biogenesis. Proc. Natl. Acad. Sci. USA. 104 6596–6601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cao H., and R. A. Hegele. 2002. Identification of single-nucleotide polymorphisms in the human LPIN1 gene. J. Hum. Genet. 47 370–372. [DOI] [PubMed] [Google Scholar]
- 59.Fawcett K. A., N. Grimsey, R. J. Loos, E. Wheeler, A. Daly, M. Soos, R. Semple, H. Syddall, C. Cooper, S. Siniossoglou, et al. 2008. Evaluating the role of LPIN1 variation on insulin resistance, body weight and human lipodystrophy in UK populations. Diabetes 57 2527–2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Loos R. J., T. Rankinen, L. Perusse, A. Tremblay, J. P. Despres, and C. Bouchard. 2007. Association of lipin 1 gene polymorphisms with measures of energy and glucose metabolism. Obesity 15 2723–2732. [DOI] [PubMed] [Google Scholar]
- 61.Wiedmann S., M. Fischer, M. Koehler, K. Neureuther, G. Riegger, A. Doering, H. Schunkert, C. Hengstenberg, and A. Baessler. 2008. Genetic variants within the LPIN1 gene, encoding lipin, are influencing phenotypes of the metabolic syndrome in humans. Diabetes. 57 209–217. [DOI] [PubMed] [Google Scholar]
- 62.Ong K. L., R. Y. Leung, L. Y. Wong, S. S. Cherny, P. C. Sham, T. H. Lam, K. S. Lam, and B. M. Cheung. 2008. Association of a polymorphism in the lipin 1 gene with systolic blood pressure in men. Am. J. Hypertens. 21 539–545. [DOI] [PubMed] [Google Scholar]
- 63.Kang E. S., S. E. Park, S. J. Han, S. H. Kim, C. M. Nam, C. W. Ahn, B. S. Cha, K. S. Kim, and H. C. Lee. 2008. LPIN1 genetic variation is associated with rosiglitazone response in type 2 diabetic patients. Mol. Genet. Metab. 95 96–100. [DOI] [PubMed] [Google Scholar]
- 64.Ferguson P. J., S. Chen, M. K. Tayeh, L. Ochoa, S. M. Leal, A. Pelet, A. Munnich, S. Lyonnet, H. A. Majeed, and H. El-Shanti. 2005. Homozygous mutations in LPIN2 are responsible for the syndrome of chronic recurrent multifocal osteomyelitis and congenital dyserythropoietic anaemia (Majeed syndrome). J. Med. Genet. 42 551–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Majeed H. A., M. Al-Tarawna, H. El-Shanti, B. Kamel, and F. Al-Khalaileh. 2001. The syndrome of chronic recurrent multifocal osteomyelitis and congenital dyserythropoietic anaemia. Report of a new family and a review. Eur. J. Pediatr. 160 705–710. [DOI] [PubMed] [Google Scholar]
- 66.Al-Mosawi Z. S., K. K. Al-Saad, R. Ijadi-Maghsoodi, H. I. El-Shanti, and P. J. Ferguson. 2007. A splice site mutation confirms the role of LPIN2 in Majeed syndrome. Arthritis Rheum. 56 960–964. [DOI] [PubMed] [Google Scholar]
- 67.Boverhof D. R., L. D. Burgoon, C. Tashiro, B. Chittim, J. R. Harkema, D. B. Jump, and T. R. Zacharewski. 2005. Temporal and dose-dependent hepatic gene expression patterns in mice provide new insights into TCDD-Mediated hepatotoxicity. Toxicol. Sci. 85 1048–1063. [DOI] [PubMed] [Google Scholar]
- 68.Tomita M., T. Okuyama, H. Katsuyama, Y. Miura, Y. Nishimura, K. Hidaka, T. Otsuki, and T. Ishikawa. 2007. Mouse model of paraquat-poisoned lungs and its gene expression profile. Toxicology. 231 200–209. [DOI] [PubMed] [Google Scholar]
- 69.Reue K., and J. Donkor. 2007. Genetic factors in type 2 diabetes: all in the (lipin) family. Diabetes. 56 2842–2843. [DOI] [PubMed] [Google Scholar]
- 70.Aulchenko Y. S., J. Pullen, W. P. Kloosterman, M. Yazdanpanah, A. Hofman, N. Vaessen, P. J. Snijders, D. Zubakov, I. Mackay, M. Olavesen, et al. 2007. LPIN2 is associated with type 2 diabetes, glucose metabolism, and body composition. Diabetes. 56 3020–3026. [DOI] [PubMed] [Google Scholar]
- 71.Cornell R. B., and I. C. Northwood. 2000. Regulation of CTP:phosphocholine cytidylyltransferase by amphitropism and relocalization. Trends Biochem. Sci. 25 441–447. [DOI] [PubMed] [Google Scholar]
- 72.Li Z., and D. E. Vance. 2008. Phosphatidylcholine and choline homeostasis. J. Lipid Res. 49 1187–1194. [DOI] [PubMed] [Google Scholar]