Abstract
The prevalence of obesity and its associated metabolic diseases worldwide has focused attention on understanding the mechanisms underlying adipogenesis. The nuclear receptor PPARγ has emerged as a central regulator of adipose tissue function and formation. Despite the identification of numerous PPARγ targets involved in a range of processes, from lipid droplet formation to adipokine secretion, information is still lacking on targets downstream of PPARγ that directly affect fat cell differentiation. Here we identify HRASLS3 as a novel PPARγ regulated gene with a role in adipogenesis. HRASLS3 expression increases during the differentiation of preadipocyte cell lines and is highly expressed in white and brown adipose tissue in mice. HRASLS3 expression is induced by PPARγ ligands in preadipocyte cell lines as well in adipose tissue in vivo. We demonstrate that the HRASLS3 promoter contains a functional PPAR response element and is a direct target for regulation by PPARγ/RXR heterodimers. Finally, we show that overexpression of HRASLS3 augments PPARγ-driven lipid accumulation and adipogenesis, whereas siRNA-mediated knockdown of HRASLS3 expression decreases differentiation. Together, these results identify HRASLS3 as one of the downstream effectors of PPARγ action in adipogenesis.
Keywords: nuclear receptor, adipogenesis, lipid metabolism
Storing excess glucose and lipids as triglycerides is an important aspect of energy homeostasis. Adipose tissue, the primary site of this storage, has long been known to serve as a reserve fuel source, taking up energy in times of nutritional abundance and releasing it when energy and nutrients are scarce (1). In recent years, a broader role for adipose tissue, than simply a depot for triglycerides, has come to light. Adipose tissue is now recognized to be an endocrine tissue that releases various signaling molecules, or adipokines, including adiopnectin, leptin, and resistin (2). Despite the importance of adipose tissue in normal physiology, excessive accumulation of adipose tissue is one of the strongest risk factors for metabolic disease. In Western society, obesity has reached epidemic proportions and is becoming an important health concern worldwide (3), emphasizing the need to fully understand the pathways that regulate adipocyte differentiation and energy homeostasis.
To date, a number of key regulators of adipogenesis have been identified. These include negative modulators such as the GATA-binding and forkhead families of transcription factors and the Wnt signaling pathway (4, 5). Conversely, SREBP-1c and the C/EBP family of transcription factors (6, 7) are well characterized inducers of the adipogenic program. Many of these regulators of adipogenesis, both negative and positive, exert their effects through modulation of expression or activity of the nuclear receptor PPARγ (5). In fact, no factor has yet been discovered that promotes adipogenesis in the absence of PPARγ. Moreover, induced expression of PPARγ is sufficient to promote lipid accumulation in a number of cell types, including fibroblasts and myoblasts, whereas loss of PPARγ results in loss of the adipogenic capability of cells (8–12).
Like many other nuclear receptors, PPARγ is a ligand-activated transcription factor. Although its precise endogenous ligands remain unclear, PPARγ is believed to respond to fatty acids or their derivatives and to mediate an appropriate transcriptional response to these signals (13–15). In adipose tissue, this includes induction of pathways for lipid metabolism, adipokine signaling, and adipogenesis (16, 17). A number of PPARγ target genes that mediate such effects have now been identified. These include proteins involved in lipid uptake and droplet formation including lipoprotein lipase, perilipin, and adipophilin (18–21). PPARγ has also been shown to regulate the expression of a number of secreted adipokines that display systemic effects on glucose homeostasis, including leptin, adiponectin, and resistin (22). Despite the critical role of PPARγ in adipogenesis, however, transcriptional targets of PPARγ involved in fat cell differentiation are still being identified. Elucidating components of this pathway will be necessary for a complete understanding of adipogenesis.
Recent advances in gene profiling techniques, such as microarrays, have brought the ability to quickly identify large numbers of target genes. Results of such screens have identified many previously known PPAR targets, validating the approach (23–25). Among the novel targets identified in such screens, some have well-characterized functions that place them in specific biological pathways. Other targets identified by microarrays, however, have no known function and their role in PPAR biology is difficult to deduce. Furthermore, since PPARγ has other functions besides differentiation, such as roles in lipid metabolism and inflammation, it is difficult to separate targets specifically involved in differentiation from those involved in other functions. Nevertheless, it is likely that some of these uncharacterized PPARγ targets play a role in the differentiation process.
Previous experiments in NIH-3T3 fibroblasts have shown that of the PPAR isotypes, only PPARγ can promote adipocyte conversion (26). More recently, members of the PPAR family were shown to regulate overlapping but distinct sets of target genes when expressed at similar levels in a fibroblast cell line (24). Since the other PPAR family members, PPARα and PPARδ, regulate aspects of lipid metabolism but do not promote adipogenesis, isotype-selective PPARγ targets are good candidates for genes involved in fat cell differentiation. Here we show that HRas-like-supressor 3 (HRASLS3), a gene that we previously identified as selective PPARγ target (24), is a direct target of PPARγ with a role in adipogenesis.
MATERIALS AND METHODS
Retroviral expression and siRNA vectors
The full-length cDNA sequence for mouse HRASLS3 was generated by PCR and cloned into the pBabe retroviral vector between the BamHI and EcoRI sites. PCR primer sequences are available upon request. The retroviral RNAi vector pQCXIP gfp super forward was designed and provided by Caiyi Li and Stephen Smale (27). siRNA hairpins against HRASLS3 were designed using GenScript siRNA target finder and as detailed in Brummelkamp et al. (28). Control siRNA constructs were designed by mutating the HRASLS3 directed siRNA at position 2 and 9 of the stem as described (28). Hairpin sequences are available on request. Oligonucleotides of the HRASLS3 siRNA hairpin and corresponding mutant were annealed and cloned into the viral vector as described (27).
Stable cell lines
PPARγ expressing NIH-3T3 cell lines have been previously described (26). To generate HRASLS3 expressing cells, first phoenix A cells were grown in DMEM with 10% FBS and penicillin/streptomycin and plated in 10 cm dishes at 80% confluence. The pBabe HRASLS3 expression vector was transiently transfected into these cells for packaging into retrovirus using either calcium phosphate or lipofectamine 2000 (Invitrogen). Virus was collected 36 to 48 h after transfection and filtered through a 0.45 mm syringe filter (Nalgene). NIH-3T3 cells or hygromycin-resistent PPARγ expressing NIH-3T3 cell lines were infected with virus supernatant and 8 ug/ml polybrene at 50–80% confluence for 6 to 8 h. Cell lines stable expressing HRASLS3 directed siRNA, as well as nontargeting and mutated controls, were generated as described for the HRASLS3 overexpressing cell lines in 3T3-L1, 3T3-F442A, and PPARγ expressing NIH-3T3 cell lines. Stable cell lines were selected with 2 μg/ml (NIH-3T3) or 4 μg/ml (3T3-L1 and 3T3-F442A) of puromycin beginning 1 day after infection.
Cell culture and adipogenesis
All cells were maintained in high glucose DMEM containing 10% bovine calf serum and1% penicillin/streptomycin. For differentiation studies, cells were switched at confluence to high glucose DMEM supplemented with 10% fetal bovine serum, 1% penicillin/streptomycin and insulin (5 μg/ml). 3T3-L1 cell lines were further treated with dexamethasone (1 μM) and methylisobutylxanthine (0.5 μM) for 24 to 48 h postconfluence. NIH-3T3 cell lines were treated with dexamethasone (1 μM) for 24 to 48 h postconfluence. Subsequently cells were fixed with 37% formalin and stained with Oil Red O as previously described (11) or harvested for RNA analysis. PPARγ ligand (100 nM) or DMSO was initially added at confluence and replenished every 1–2 days. The PPARγ synthetic ligand GW7845 was provided by Jon Collins and Timothy Willson (GlaxoSmithKline). Prior to use in cell culture, the ligand was resuspended in DMSO. For in vivo gene expression studies, C57BL/6 mice were orally gavaged for 3 or 7 days with vehicle or 3 mg/kg GW7845.
RNA and protein analysis
Trizol reagent (Life Technologies, Inc.) was used to isolate RNA from cells and mouse tissue. One μg of total RNA was reverse transcribed with the iScript™ cDNA synthesis kit (BioRad). Sybrgreen real-time quantitative PCR assays were performed using an Applied Biosystems 7900HT sequence detector as described (29). Results are shown as the average of duplicate samples normalized to 36B4. Primer sequences are available on request. HA-tagged full-length mouse HRASLS3 was generated using gene-specific PCR primers with the HA epitope fused onto the N terminus and subcloned into BamHI/EcoRI restriction sites of the pCMX expression vector. The HA-HRASLS3 expression vector was cotransfected into HEK-293T cells with siRNA expression vectors (8 ug/10 cm dish of each construct) using calcium phosphate. Twenty-fhour h after transfection protein was isolated and Western blot analysis performed as described (30) using an antibody against the HA epitope (Covance) or actin (Sigma).
Electrophoretic mobility-shift assays
Radiolabeled oligonucleotides from the HRASLS3 promoter corresponding to the PPARγ binding site were used to analyze DNA binding as described (30). Competition assays were performed using 25 molar excess of cold competitor. RXRα and PPARγ proteins, for the electophoretic mobility, shift assays were in vitro translated using a TNT quick coupled transcription/translation system (Promega). The sequences for the oligonucleotide used are: HRASLS3, agataaGGGTCAcTGGTGAgccttc, nonbinding PPRE ctctccTCCGCTcTGACCTgagctt, LXRα atGGGCAaAGTTCAgca, perfect PPRE AGGTCAaAGGTCA. The PPRE half sites are in capitals.
Transfection assays
Transient transfection assays were performed in triplicate in 293T cells. Cells were plated at a density of 4 × 104 on poly-O-lysine coated 48-well plates 24 h prior to transfection. Lipofectamine 2000 transfection reagent (Invitrogen) was used according to manufacturer's directions to transfect cells with reporter plasmid (30 ng/well), empty or PPARγ pCMX receptor plasmid (60 ng/well), pCMX RXRα receptor plasmid (20 ng/well), and a renilla luciferase construct as an internal control for transfection efficiency. Subsequently cells were incubated in DMEM with .5% FBS and the indicated ligand or control. Luciferase activity was analyzed using a dual-luciferase assay system (Promega). pCMX expression vectors for PPARγ and RXRα have been previously described (31, 32). Reporter plasmids containing three copies of the HRASLS3 PPARγ response element and 6 bp of flanking sequence were subcloned into the 5′ BamHI and 3′ HindIII site of pTK-luciferase. The HRASLS3 reporter oligonucleotide used was agataaGGGTCAcTGGTGAgccttc. The PPRE half sites are in capitals and the basepairs altered in the HRALS3 mutant construct are underlined.
Cell cycle analysis
Fifty thousand cells/well were plated on gelatin-coated 6-well plates. Cells were trypsinized at time of analysis. Using the Z1 Couter Counter (Beckman), we took the mean of cell counts from two independent aliquots per well. Staining for DNA content was performed by resuspending PBS-washed cells in PBS containing 0.1% Na+citrate (w/v) (Sigma), 0.3% Triton-x-100 (v/v) (Sigma), 0.01% propidium iodide (w/v) (Calbiochem), and 0.002% RNase A (w/v) (Sigma), and incubating the cells for 30 min at 20–25°C. We acquired the cell cycle data on a FACS Calibur (BD Biosciences) and data were analyzed using FloJo Software (Treestar, Inc.).
RESULTS
Members of the PPAR nuclear receptor family (PPARγ, PPARδ, and PPARα) regulate distinct but overlapping sets of target genes in response to ligand when ectopically expressed in NIH-3T3 fibroblasts (24). Surprisingly many adipocyte-associated targets, such as the common adipogenic marker aP2, are regulated by all three PPARs, even though only PPARγ is able to drive the full adipogenic program. This suggests that as yet uncharacterized PPARγ-specific targets are involved in the differentiation of fat cells. To explore this hypothesis, we examined previously identified PPARγ-selective targets for roles in adipocyte biology and adipogenesis. We selected H-Ras like suppressor 3 (HRASLS3 or H-REV107) for further examination for several reasons. First, as stated above, it is regulated by PPARγ but not PPARα or PPARδ. Second, it has been shown to play a role in regulation of cell proliferation and there is a well-established inverse relationship between differentiation and proliferation (33). Finally, HRASLS3 has been linked to Ras signaling and prior studies have implicated Ras signaling in regulation of adipogenesis (34–37).
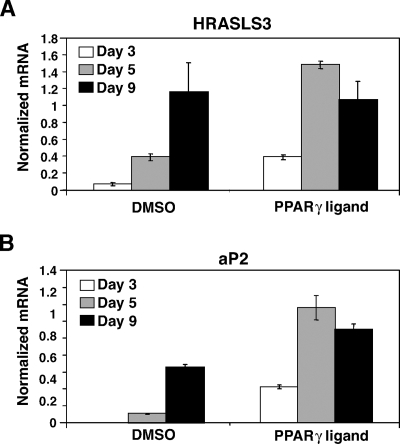
The expression of a number of adipogenic factors, including PPARγ itself, increases during the differentiation process. Therefore, we first examined whether HRASLS3 is also regulated during the differentiation of preadipocytes into lipid-laden adipocytes. 3T3-F442A preadipocytes were grown to confluence and cultured in 10% FBS in the presence of insulin to induce adipogenesis. RNA was isolated and gene expression analyzed on day 3, 5, and 9 postconfluence. Quantitative real-time PCR analysis showed increased HRASLS3 mRNA levels during the course of adipocyte differentiation (Fig. 1A). This increase in HRASLS3 expression corresponded with induction of aP2 (a marker for adipogenesis) as well as lipid droplet accumulation in these cells (Fig. 1B, data not shown), indicating that HRASLS3 expression increases in a differentiation-dependent manner. Treatment of cells with a synthetic PPARγ ligand (GW7845) under differentiation conditions increased both HRASLS3 expression and the rate of differentiation of the 3T3-F442A cells (Fig. 1). By day 5, ligand-treated cells were already fully differentiated and no additional increase in expression of either HRASLS3 or aP2 was observed. Similar results were seen with 3T3-L1 preadipocytes that were induced to differentiate using a dexamethasone, methylisobutyl xanthine, and insulin (DMI) differentiation cocktail or PPARγ ligand (data not shown).
Fig. 1.
HRASLS3 expression increases in response to both adipocyte differentiation and PPARγ ligand. A, B: HRASLS3 mRNA levels increase in preadipocytes as differentiation progresses similar to those of the differentiation marker PPARγ ligand treatment further increases HRASLS3 and aP2 expression. 3T3-F442A cells were induced to differentiate in the presence or absence of PPARγ ligand GW7845 (100 nM). RNA was isolated at days 3, 5, and 9 postconfluence and mRNA levels measured by real-time quantitative PCR. Error bars indicate ± SEM.
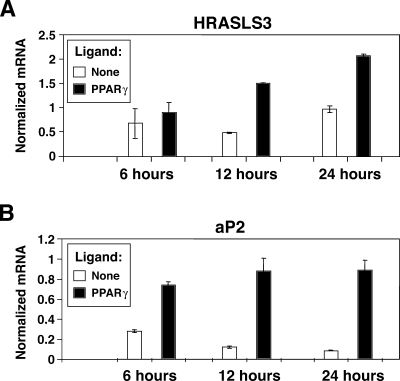
The above results clearly showed that HRASLS3 levels increase in response to both PPARγ ligand and adipocyte differentiation. It remained unclear, however, whether PPARγ directly regulates expression of HRASLS3 or whether HRASLS3 expression is induced by the differentiation process itself. We therefore examined whether changes in HRASLS3 expression in response to activation of PPARγ were observed before phenotypic adipogenic changes occurred. We treated 3T3-L1 cells for 6, 12, or 24 h with PPARγ ligand, in the absence of differentiation cocktail, and isolated RNA for gene expression analysis. Our results showed induction of HRASLS3 message beginning between 6 and 12 h after ligand treatment (Fig. 2A). For comparison, the cannonical PPARγ target aP2 is induced by PPARγ ligand as early as 6 h after ligand treatment (Fig. 2B). Although HRASLS3 is not regulated quite as robustly as aP2, the early induction of HRASLS3 levels is consistent with it being a primary target for PPARγ regulation.
Fig. 2.
HRASLS3 is an early transcriptional target of PPARγ. A: HRASLS3 expression levels are induced by PPARγ activation within 12 h of ligand treatment. B: Regulation of aP2 expression is shown as an example of regulation of a canonical PPARγ target upon ligand treatment. At confluence 3T3-L1cells were treated with PPARγ ligand GW7845 (100 nM) in the absence of additional pro-adipogenic stimulation. RNA was isolated 6, 12, and 24 h after ligand treatment and analyzed by real-time quantitative PCR. Error bars indicate ± SEM.
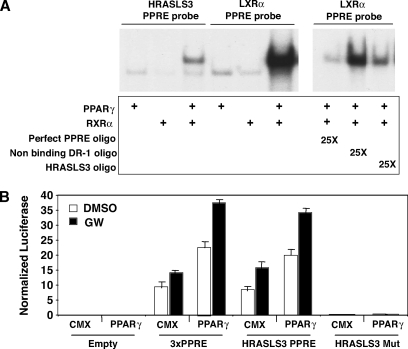
To confirm that PPARγ directly regulates HRASLS3 expression, we next employed sequence analysis to search for PPAR-binding elements (PPREs) in the HRASLS3 promoter region. We identified a site approximately 6 kb upstream of the HRASLS3 transcriptional start site for further analysis. Electophoretic mobility shift assays showed that in vitro translated PPARγ and RXRα bound as a heterodimer to a radiolabeled oligo containing this sequence (Fig. 3A). Furthermore, excess cold HRASLS3 site effectively competed away binding of PPAR/RXR heterodimers to a cannonical radiolabeled PPARγ binding site (Fig. 3A).
Fig. 3.
The HRASLS3 promoter contains a functional PPARγ response element. A: In vitro translated PPARγ and RXR bind to both the HRASLS3 and LXRα DR-1 sequences as a heterodimer. Twenty-five times molar excess of cold HRASLS3 DR-1 oligonucleotide effectively competes away binding of PPAR/RXR from the LXRα DR-1 in a similar manner as excess cold perfect PPRE, while a DR-1 sequence to which PPAR/RXR heterodimers do not bind is unable to displace PPAR/RXR heterodimers from the LXRα PPRE. Electrophoretic-mobility shift assays were performed using radiolabeled oligonucleotides corresponding to the direct repeat (DR-1) sequence in the promoter of HRASLS3 or LXRα, a characterized direct target of PPARγ. B: The HRASLS3 PPRE reporter construct, but not the mutant HRASL3 PPRE reporter, is activated by PPARγ in response to ligand in a manner similar to that seen for the aP2 PPRE. HEK-293T cells were transiently transfected with either empty reporter plasmid or reporter plasmids containing the aP2 PPRE, the HRASLS3 PPRE, or a mutated version of the HRASLS3 PPRE with or without a PPARγ expression plasmid. Cells were subsequently treated with ligand or vehicle for 24 h under low serum conditions and luciferase activity measured. Basal expression of the HRASLS3 reporter in the absence of transfected PPARγ is likely due to endogenous expression of PPARs. Error bars indicate ± SEM.
We next performed transfection assays to verify that PPARγ binding to the identified HRASLS3 PPRE was able to drive transcription. Two hundred and ninety-three T cells were transiently transfected with expression vectors for PPARγ and RXRα, along with a luciferase reporter construct containing three repeats of the putative HRASLS3 PPRE. Cells were then treated with DMSO or PPARγ ligand for 24 h under low serum conditions. Our results showed that PPARγ was able to induce activity of the HRASLS3 reporter in a manner similar to that seen for a reporter containing the aP2 PPRE (Fig. 3B). Moreover, stimulation of reporter activity by PPARγ was sequence specific, as no luciferase activity was seen with a reporter containing a mutated version of the HRASLS3 PPRE. These data, combined with the gel shift data, identify HRASLS3 as a direct transcriptional target of PPARγ.
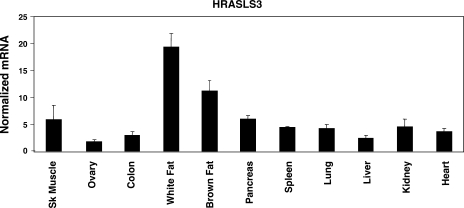
The in vitro results described above establish HRASLS3 as a direct target of PPARγ in various cell systems including preadipocyte cell lines. However, little is known about regulation of HRASLS3 in mice. We therefore sought to determine the expression and regulation of HRASLS3 in vivo. A panel of tissues was collected from wild-type mice and RNA was isolated. Quantitative real-time PCR analysis of these samples showed expression of HRASLS3 in all tissues examined (Fig. 4), consistent with what has been previously reported in rats (33). However, HRASLS3 expression was significantly higher in white and brown adipose tissue than in other tissues examined.
Fig. 4.
HRASLS3 is highly expressed in white and brown adipose tissue of mice. HRASLS3 mRNA levels are significantly higher in white and brown adipose tissue than in all other tissues examined. A panel of tissues was harvested from wild-type mice and total RNA isolated for analysis by real-time quantitative PCR. Error bars indicate ± SEM.
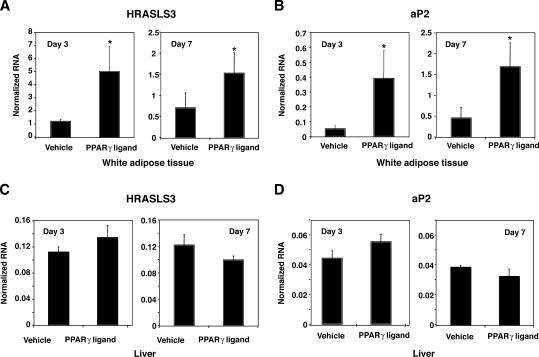
Having established that HRASLS3 is highly expressed in adipose tissue, we next endeavored to determine whether HRASLS3 is regulated by the PPARγ signaling pathway in vivo. Mice were gavaged with vehicle or PPARγ specific ligand (GW7845) for 3 or 7 days. Subsequently, white adipose tissue and liver were collected and RNA isolated for further examination. Quantitative real-time PCR analysis of these samples showed increased expression of HRASLS3 message in response to PPARγ activation in white adipose tissue (Fig. 5A). Moreover, PPARγ ligand treatment induced the expression of HRASLS3 to a similar degree as the well-characterized PPARγ target aP2 (Fig. 5B). Neither aP2 nor HRASLS3 expression was regulated by PPARγ in the liver (Fig. 5C, D). These results establish HRASLS3 as an adipose tissue-enriched and PPARγ-induced gene in mice, suggestive of a possible function for HRASLS3 as a downstream effector of PPARγ signaling in adipocytes.
Fig. 5.
HRASLS3 expression is regulated by PPARγ in fat but not liver in vivo. HRASLS3 and aP2 expression increases in response to PPARγ agonist treatment in white adipose tissue (A, B) but not in liver (C, D). Mice were gavaged daily with vehicle or PPARγ agonist (GW7845). After 3 or 7 days of ligand treatment, total RNA was isolated from white adipose tissue and liver and analyzed by real-time quantitative PCR. Error bars indicate ± SEM.
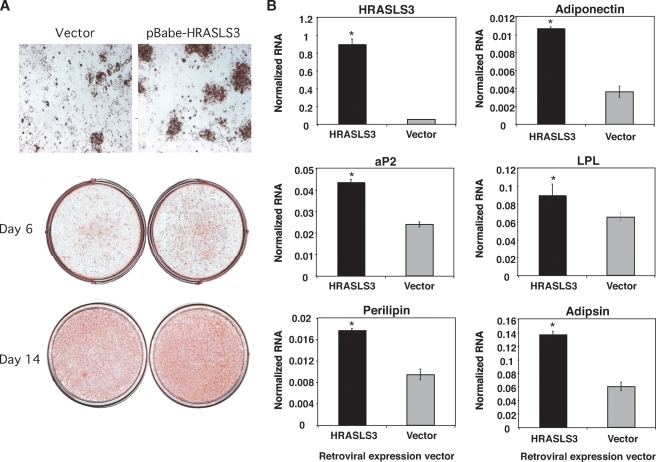
The results presented thus far establish HRASLS3 as a PPARγ target both in vitro and in vivo. Moreover, HRASLS3 likely plays a role in fat cell biology as it is induced during adipocyte differentiation in multiple preadipocyte cell lines and is highly expressed in adipose tissue in mice. These observations prompted us to examine whether HRASLS3 plays a direct role in the adipogenic program. To address this question, we ectopically expressed HRASLS3 in NIH-3T3 fibroblasts either alone, or together with PPARγ. Our pBabe retroviral expression system resulted in significantly higher levels of HRASLS3 mRNA levels than in control cells (Fig. 6). Cell lines overexpressing HRASLS3 were grown to confluence and switched to differentiation-inducing conditions including treatment with the synthetic PPARγ ligand GW7845 for 6 or 14 days. Subsequently, cells were fixed and lipid accumulation assessed by Oil Red O staining. Although the induced expression of HRASLS3 alone in NIH-3T3 fibroblasts did not promote lipid accumulation (data not shown), ectopic expression of HRASLS3 in combination with PPARγ resulted in increased lipid accumulation compared with cells only expressing PPARγ (Fig. 6A). In addition, examination of gene expression by quantitative real-time PCR showed increased levels of a number of adipogenic factors (e.g., aP2, perilipin) in the cells expressing both HRASLS3 and PPARγ 6 days after induction of differentiation and ligand treatment (Fig. 6B). Similar results were obtained with multiple independently derived stable pools of control and HRASLS3-expressing cells (data not shown).
Fig. 6.
HRASLS3 overexpression increases lipid accumulation and adipocyte-associated gene expression. Stable cell lines ectopically expressing HRASLS3 undergo enhanced adipogenesis as demonstrated by increased lipid accumulation (A) and higher expression of a number of adipogenic markers (B). NIH-3T3 cells stably expressing PPARγ were infected with retrovirus containing a pBabe HRASL3 expression vector to create cell lines stable expressing HRASLS3 (B). Cells were grown to confluence and induced to differentiate by treatment with dexamethasone (1 μM) for 48 h and continuous treatment with the synthetic PPARγ ligand GW7845 (100 nM). At the time points indicated, cells were fixed and stained with Oil Red O (A). In addition, 6 days after induction of differentiation mRNA was isolated and expression of adipogenic markers examined by real-time quantitative PCR (B). Similar results were obtained with multiple independent stable pools of control and HRASLS3-expressing cells. * P > 0.05. Error bars indicate ± SEM.
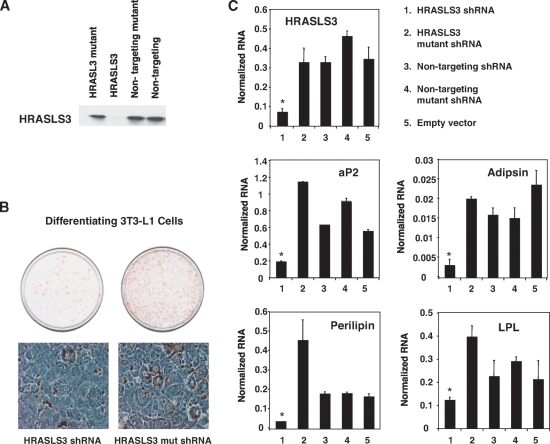
Next, we examined whether loss of HRASLS3 alters adipocyte-associated morphological changes and lipid accumulation in preadipocyte cell lines. First, we cloned siRNA oligonucleotides targeted against HRASLS3, as well as control siRNAs, into a retroviral vector to enable stable integration and expression of siRNA hairpins in cells. The ability of a number of siRNA constructs to efficiently knockdown HRASLS3 expression was initially tested in transiently transfected 293 T cells ectopically expressing HRASLS3. Fig. 7B shows that we were able to identify an siRNA construct that effectively depleted HRASLS3 mRNA and protein levels. The corresponding mutated siRNA sequence, which differed by only two nucleotides, did not lead to reduction in HRASLS3 protein levels. Once a suitable siRNA construct had been identified, we used this viral expression system to stably knockdown HRASLS3 expression in 3T3-L1 and 442A preadipocytes. Cells expressing either the HRASLS3 siRNA construct or mutant control were grown to confluence and induced to differentiate under standard differentiation conditions. Subsequently lipid accumulation was examined by Oil Red O staining. As shown in Fig. 7B, siRNA-mediated knockdown of HRASLS3 protein levels in 3T3-L1 preadipocytes led to reduced lipid accumulation compared with control cells in which HRALS3 expression was unaltered. Similar results were seen in 3T3-F442A cells (data not shown). Moreover reduction in HRASLS3 expression lead to decreased expression of multiple markers of adipogenesis compared with nontargeting siRNA constructs (Fig. 7C). These data combined, with the HRASLS3 overexpression data, identify HRASLS3 as a downstream mediator of the effects of PPARγ on fat cell differentiation.
Fig. 7.
Loss of HRASLS3 inhibits lipid accumulation and adipocyte-associated gene expression in preadipocyte cell lines. Reduction of HRALS3 protein (A) levels is achieved with an siRNA oligonucleotide targeted against HRASLS3, but not a mutated version of this siRNA or nontargeting siRNAs. The depletion of HRASLS3 in 3T3-L1 preadipocytes was associated with reduced lipid accumulation as seen by Oil Red O staining (B) and decreased expression of various adipocyte associated genes (C). Reduction in HRASLS3 protein was examined by Western blot analysis of whole cell extracts from HEK-293T cells that were cotransfected with an expression vector for HA-tagged HRASLS3 and siRNA viral expression vectors containing siRNA hairpins designed against HRASLS3, or mutant or nontargeting siRNAs. Lipid accumulation was examined by Oil Red O staining of 3T3-L1 preadipocytes induced to differentiate under standard conditions for 6 days. mRNA expression was examined using real-time quantitative PCR. Error bars indicate ± SEM.
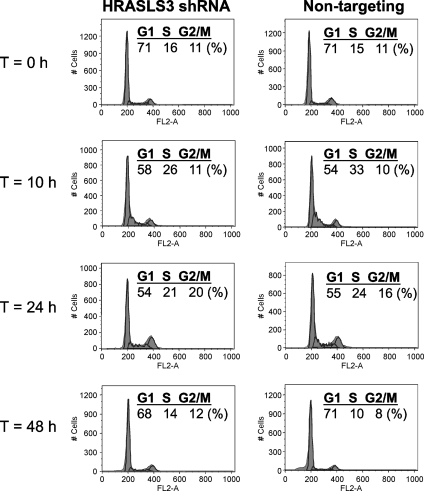
Finally, given the potential link between HRASLS3 and cell growth, we investigated whether HRASLS3 might affect mitotic clonal expansion in 3T3-L1 preadipocytes. Stable cell lines expressing HRASLS3 shRNA or nontargeting shRNA control were grown to confluence and then treated with DMI cocktail. DNA content analysis was performed at 12, 24, and 48 h postconfluence by flow cytometry. As shown in Fig. 8, no difference in cell cycle between cell lines was observed, suggesting that the effects of HRASL3 on differentiation may not be due to alterations in mitotic clonal expansion.
Fig. 8.
Loss of HRASLS3 does not affect mitotic clonal expansion of 3T3-L1 preadipocytes. Undifferentiated 3T3-L1 stable transformants were plated at a density of 50,000 cells per well. Dexamethasone, methylisobutyl xanthine, and insulin (DMI) was added at time of confluence. Cells were trypsinized and analyzed at the indicated time after the addition of DMI. DNA content was determined by propidium iodide staining using FACS.
DISCUSSION
PPARγ has been recognized as the major regulator of fat cell differentiation for many years (5, 11). However, a complete understanding of downstream factors in the PPARγ signaling pathway that contribute to adipocyte formation is still lacking. Moreover, PPARγ has been implicated in other biological processes such as inflammation and glucose metabolism, leading to difficulties in differentiating which PPARγ targets are directly involved in adipogenesis. Here we identify HRASLS3 as a novel factor involved in the adipogenic program that acts downstream of PPARγ signaling.
To date, little has been reported concerning the function of HRASLS3, and no studies have addressed its role in adipose tissue. Because PPARγ is a key regulator of both adipogenesis and mature fat cell function, the recent identification of HRASLS3 as a gene specifically regulated by PPARγ suggested that it also might be important in adipocyte biology (24). One caveat to the previous study is that HRASLS3 was identified as a PPARγ target in a fibroblast cell line ectopically expressing PPARγ. As a result, this work did not address whether HRASLS3 is a physiologically relevant target gene in fat cells. Here we show that HRALS3 expression increases during the course of differentiation in preadipocyte cells and is further increased by PPARγ activation, consistent with a role in fat cell differentiation and/or function.
A number of identified regulators of adipogenesis such as C/EBP β and δ are temporally regulated, displaying a transient spike in expression during the initiation of adipogenesis (5, 38). In contrast, HRASLS3 levels continue to increase throughout the differentiation process. It could thus be conjectured that HRASLS3 is involved in some later event in mature adipocytes and not the differentiation process itself. However, many of the early adipogenic factors act by regulating PPARγ expression and/or activity. Because HRASLS3 was initially characterized as being downstream of PPAR signaling, it would be expected to display an adipogenic expression profile similar to PPARγ itself (as we observe). Moreover, we observe induction of HRASLS3 as early as 12 h after PPARγ ligand treatment and before adipogenic changes are apparent, demonstrating that regulation of HRASLS3 by PPARγ is an early event in the PPARγ signaling pathway. Indeed, our results show that PPARγ binds to a PPRE in the HRASLS3 promoter and directly regulates activity at this site. Thus, HRASLS3 is not simply a marker of differentiation, but rather an early player in PPARγ signaling and thus appropriately timed to influence PPARγ-driven adipogenesis.
Experiments in cell lines both overexpressing HRASLS3 and cells with HRASLS3 level effectively reduced by siRNA confirm a role for HRASLS3 in the regulation of adipogenesis. Although HRASLS3 was unable to induce differentiation by itself in NIH-3T3 fibroblasts, expression of HRASLS3 in the presence of PPARγ resulted in enhanced lipid accumulation. The inability of HRASLS3 to promote differentiation in NIH-3T3 cells is not surprising. NIH-3T3 fibroblasts do not normally undergo adipocyte differentiation, even in the presence of adipogenic stimuli (11). In fact, only PPARγ and C/EBP α are capable of inducing adipogenesis in these cells (39). This can be largely attributed to the fact that NIH-3T3 cells do not express PPARγ. However, when HRASLS3 was expressed simultaneously with PPARγ, increased lipid accumulation compared with cells transfected with only PPARγ was observed. These data indicate that HRASLS3 is a contributing factor to PPARγ-mediated adipogenesis. In addition, we showed that siRNA-mediated knockdown of HRASLS3 expression led to reduced lipid accumulation confirming a role for HRALS3 in adipogenesis.
The mechanism by which HRASLS3 influences the adipogenic processes is an interesting avenue for future research. A review of previous work characterizing HRASLS3 function provides some clues as to how it might be working. First, HRASLS3 has been shown to inhibit cell proliferation and its expression is downregulated in a number of tumors and tumor cell lines (33). The antiproliferative properties of HRASLS3 suggest a possible role in terminal differentiation, as there is a well-established reciprocal relationship between proliferation and differentiation. Although we did not observe any noticeable difference in proliferation of cell lines in which HRASLS3 expression was reduced by shRNA (Fig. 8), more detailed cell cycle studies could potentially reveal differences. Alternatively, HRASLS3 has been linked to Ras signaling (33, 35). Several reports indicate an antagonistic role for Ras on adipocyte differentiation (34, 36). Specifically, Ras induced activation of MAPK signaling was shown to impair adipogenesis in 3T3-L1 cells (34). In addition, overexpression of HA-Ras in adipose tissue was shown to result in reduced fat mass in mice (36). Future studies on the influence of HRASLS3 on Ras signaling during adipogenesis would therefore be of interest.
Finally, our data examining HRASLS3 expression and regulation in mice suggest that HRASLS3 may also play a role in adipose tissue in vivo. Our analysis of a number of different tissues from mice showed HRASLS3 mRNA levels are significantly higher in white and brown fat compared with all other tissues examined. Moreover, ligand activation of PPARγ in white adipose tissue further increased HRASLS3 gene expression. The presence of HRASLS3 in a wide range of tissues in mice and rats likely indicates some general role for HRASLS3 in vivo. For example, HRASLS3 has been best characterized for its roles in cell proliferation and it is possible that this protein plays a role in cell cycle regulation, which is important in all tissues. Interestingly the known antiproliferative affect of HRASLS3 (33) could perhaps explain some of the antineoplastic effects that have been reported for PPARγ (40). However, the enhanced expression of HRASLS3 in adipose tissue suggests a prominent function in this tissue. Ultimately, the creation of mice with global or adipose-selective genetic deletion in HRASLS3 should aid in our understanding of the role of HRASLS3 in adipogenesis and adipocyte function in vivo.
Published, JLR Papers in Press, July 29, 2008.
Footnotes
The authors have no financial interest related to this work.
References
- 1.Spiegelman B. M., and J. S. Flier. 2001. Obesity and the regulation of energy balance. Cell. 104 531–543. [DOI] [PubMed] [Google Scholar]
- 2.Staiger H., and H. U. Haring. 2005. Adipocytokines: fat-derived humoral mediators of metabolic homeostasis. Exp. Clin. Endocrinol. Diabetes. 113 67–79. [DOI] [PubMed] [Google Scholar]
- 3.Flegal K. M., M. D. Carroll, C. L. Ogden, and C. L. Johnson. 2002. Prevalence and trends in obesity among US adults, 1999–2000. JAMA. 288 1723–1727. [DOI] [PubMed] [Google Scholar]
- 4.Prestwich T. C., and O. A. Macdougald. 2007. Wnt/beta-catenin signaling in adipogenesis and metabolism. Curr. Opin. Cell Biol. 19 612–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rosen E. D., O. A. MacDougald. 2006. Adipocyte differentiation from the inside out. Nature Reviews. 7 885–896. [DOI] [PubMed] [Google Scholar]
- 6.Rosen E. D., and B. M. Spiegelman. 2000. Molecular regulation of adipogenesis. Annu. Rev. Cell Dev. Biol. 16 145–171. [DOI] [PubMed] [Google Scholar]
- 7.MacDougald O. A., and M. D. Lane. 1995. Transcriptional regulation of gene expression during adipocyte differentiation. Annu. Rev. Biochem. 64 345–373. [DOI] [PubMed] [Google Scholar]
- 8.Hu E., P. Tontonoz, and B. M. Spiegelman. 1995. Transdifferentiation of myoblasts by the adipogenic transcription factors PPAR gamma and C/EBP alpha. Proc. Natl. Acad. Sci. USA. 92 9856–9860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mueller E., S. Drori, A. Aiyer, J. Yie, P. Sarraf, H. Chen, S. Hauser, E. D. Rosen, K. Ge, R. G. Roeder, et al. 2002. Genetic analysis of adipogenesis through peroxisome proliferator-activated receptor gamma isoforms. J. Biol. Chem. 277 41925–41930. [DOI] [PubMed] [Google Scholar]
- 10.Ren D., T. N. Collingwood, E. J. Rebar, A. P. Wolffe, and H. S. Camp. 2002. PPARgamma knockdown by engineered transcription factors: exogenous PPARgamma2 but not PPARgamma1 reactivates adipogenesis. Genes Dev. 16 27–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tontonoz P., E. Hu, and B. M. Spiegelman. 1994. Stimulation of adipogenesis in fibroblasts by PPAR gamma 2, a lipid-activated transcription factor. Cell. 79 1147–1156. [DOI] [PubMed] [Google Scholar]
- 12.Zhang J., M. Fu, T. Cui, C. Xiong, K. Xu, W. Zhong, Y. Xiao, D. Floyd, J. Liang, E. Li, et al. 2004. Selective disruption of PPARgamma 2 impairs the development of adipose tissue and insulin sensitivity. Proc. Natl. Acad. Sci. USA. 101 10703–10708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Forman B. M., P. Tontonoz, J. Chen, R. P. Brun, B. M. Spiegelman, and R. M. Evans. 1995. 15-Deoxy-delta 12, 14-prostaglandin J2 is a ligand for the adipocyte determination factor PPAR gamma. Cell. 83 803–812. [DOI] [PubMed] [Google Scholar]
- 14.Nolte R. T., G. B. Wisely, S. Westin, J. E. Cobb, M. H. Lambert, R. Kurokawa, M. G. Rosenfeld, T. M. Willson, C. K. Glass, and M. V. Milburn. 1998. Ligand binding and co-activator assembly of the peroxisome proliferator-activated receptor-gamma. Nature. 395 137–143. [DOI] [PubMed] [Google Scholar]
- 15.Xu H. E., M. H. Lambert, V. G. Montana, D. J. Parks, S. G. Blanchard, P. J. Brown, D. D. Sternbach, J. M. Lehmann, G. B. Wisely, T. M. Willson, et al. 1999. Molecular recognition of fatty acids by peroxisome proliferator-activated receptors. Mol. Cell. 3 397–403. [DOI] [PubMed] [Google Scholar]
- 16.Evans R. M., G. D. Barish, and Y. X. Wang. 2004. PPARs and the complex journey to obesity. Nat. Med. 10 355–361. [DOI] [PubMed] [Google Scholar]
- 17.Lehrke M., and M. A. Lazar. 2005. The many faces of PPARgamma. Cell. 123 993–999. [DOI] [PubMed] [Google Scholar]
- 18.Arimura N., T. Horiba, M. Imagawa, M. Shimizu, and R. Sato. 2004. The peroxisome proliferator-activated receptor gamma regulates expression of the perilipin gene in adipocytes. J. Biol. Chem. 279 10070–10076. [DOI] [PubMed] [Google Scholar]
- 19.Auwerx J., K. Schoonjans, J. C. Fruchart, and B. Staels. 1996. Transcriptional control of triglyceride metabolism: fibrates and fatty acids change the expression of the LPL and apo C–III genes by activating the nuclear receptor PPAR. Atherosclerosis. 124 (Suppl): S29–S37. [DOI] [PubMed] [Google Scholar]
- 20.Schoonjans K., J. Peinado-Onsurbe, A. M. Lefebvre, R. A. Heyman, M. Briggs, S. Deeb, B. Staels, and J. Auwerx. 1996. PPARalpha and PPARgamma activators direct a distinct tissue-specific transcriptional response via a PPRE in the lipoprotein lipase gene. EMBO J. 15 5336–5348. [PMC free article] [PubMed] [Google Scholar]
- 21.Targett-Adams P., M. J. McElwee, E. Ehrenborg, M. C. Gustafsson, C. N. Palmer, and J. McLauchlan. 2005. A PPAR response element regulates transcription of the gene for human adipose differentiation-related protein. Biochim. Biophys. Acta. 1728 95–104. [DOI] [PubMed] [Google Scholar]
- 22.Havel P. J. 2002. Control of energy homeostasis and insulin action by adipocyte hormones: leptin, acylation stimulating protein, and adiponectin. Curr. Opin. Lipidol. 13 51–59. [DOI] [PubMed] [Google Scholar]
- 23.Hodgkinson C. P., and S. Ye. 2003. Microarray analysis of peroxisome proliferator-activated receptor-gamma induced changes in gene expression in macrophages. Biochem. Biophys. Res. Commun. 308 505–510. [DOI] [PubMed] [Google Scholar]
- 24.Hummasti S., and P. Tontonoz. 2006. The peroxisome proliferator-activated receptor N-terminal domain controls isotype-selective gene expression and adipogenesis. Mol. Endocrinol. 20 1261–1275. [DOI] [PubMed] [Google Scholar]
- 25.Perera R. J., E. G. Marcusson, S. Koo, X. Kang, Y. Kim, N. White, and N. M. Dean. 2006. Identification of novel PPARgamma target genes in primary human adipocytes. Gene. 369 90–99. [DOI] [PubMed] [Google Scholar]
- 26.Brun R. P., P. Tontonoz, B. M. Forman, R. Ellis, J. Chen, R. M. Evans, and B. M. Spiegelman. 1996. Differential activation of adipogenesis by multiple PPAR isoforms. Genes Dev. 10 974–984. [DOI] [PubMed] [Google Scholar]
- 27.Ramirez-Carrozzi V. R., A. A. Nazarian, C. C. Li, S. L. Gore, R. Sridharan, A. N. Imbalzano, and S. T. Smale. 2006. Selective and antagonistic functions of SWI/SNF and Mi-2beta nucleosome remodeling complexes during an inflammatory response. Genes Dev. 20 282–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brummelkamp T. R., R. Bernards, R. Agami. 2002. A system for stable expression of short interfering RNAs in mammalian cells. Science. 296 550–553. [DOI] [PubMed] [Google Scholar]
- 29.Bradley M. N., C. Hong, M. Chen, S. B. Joseph, D. C. Wilpitz, X. Wang, A. J. Lusis, A. Collins, W. A. Hseuh, J. L. Collins, et al. 2007. Ligand activation of LXR beta reverses atherosclerosis and cellular cholesterol overload in mice lacking LXR alpha and apoE. J. Clin. Invest. 117 2337–2346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Castrillo A., S. B. Joseph, C. Marathe, D. J. Mangelsdorf, and P. Tontonoz. 2003. Liver X receptor-dependent repression of matrix metalloproteinase-9 expression in macrophages. J. Biol. Chem. 278 10443–10449. [DOI] [PubMed] [Google Scholar]
- 31.Kliewer S. A., B. M. Forman, B. Blumberg, E. S. Ong, U. Borgmeyer, D. J. Mangelsdorf, K. Umesono, and R. M. Evans. 1994. Differential expression and activation of a family of murine peroxisome proliferator-activated receptors. Proc. Natl. Acad. Sci. USA. 91 7355–7359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Laffitte B. A., J. J. Repa, S. B. Joseph, D. C. Wilpitz, H. R. Kast, D. J. Mangelsdorf, and P. Tontonoz. 2001. LXRs control lipid-inducible expression of the apolipoprotein E gene in macrophages and adipocytes. Proc. Natl. Acad. Sci. USA. 98 507–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sers C., U. Emmenegger, K. Husmann, K. Bucher, A. C. Andres, and R. Schafer. 1997. Growth-inhibitory activity and downregulation of the class II tumor-suppressor gene H-rev107 in tumor cell lines and experimental tumors. J. Cell Biol. 136 935–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Font de Mora J., A. Porras, N. Ahn, and E. Santos. 1997. Mitogen-activated protein kinase activation is not necessary for, but antagonizes, 3T3–L1 adipocytic differentiation. Mol. Cell. Biol. 17 6068–6075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hajnal A., R. Klemenz, and R. Schafer. 1994. Subtraction cloning of H-rev107, a gene specifically expressed in H-ras resistant fibroblasts. Oncogene. 9 479–490. [PubMed] [Google Scholar]
- 36.Houseknecht K. L., A. X. Zhu, L. Gnudi, A. Hamann, J. R. Zierath, E. Tozzo, J. S. Flier, and B. B. Kahn. 1996. Overexpression of Ha-ras selectively in adipose tissue of transgenic mice. Evidence for enhanced sensitivity to insulin. J. Biol. Chem. 271 11347–11355. [DOI] [PubMed] [Google Scholar]
- 37.Miyaoka Y., M. Tanaka, T. Naiki, and A. Miyajima. 2006. Oncostatin M inhibits adipogenesis through the RAS/ERK and STAT5 signaling pathways. J. Biol. Chem. 281 37913–37920. [DOI] [PubMed] [Google Scholar]
- 38.Rosen E. D., C. J. Walkey, P. Puigserver, and B. M. Spiegelman. 2000. Transcriptional regulation of adipogenesis. Genes Dev. 14 1293–1307. [PubMed] [Google Scholar]
- 39.Rosen E. D. 2005. The transcriptional basis of adipocyte development. Prostaglandins Leukot. Essent. Fatty Acids. 73 31–34. [DOI] [PubMed] [Google Scholar]
- 40.Gelman L., J. C. Fruchart, and J. Auwerx. 1999. An update on the mechanisms of action of the peroxisome proliferator-activated receptors (PPARs) and their roles in inflammation and cancer. Cell. Mol. Life Sci. 55 932–943. [DOI] [PMC free article] [PubMed] [Google Scholar]