Abstract
The origins of cholesterol utilized by intestinal ABCA1 were investigated in the human intestinal cell line Caco-2. Influx of apical membrane cholesterol increases ABCA1 mRNA and mass, resulting in enhanced efflux of HDL-cholesterol. Luminal (micellar) cholesterol and newly synthesized cholesterol are not transported directly to ABCA1 but reach the ABCA1 pool after incorporation into the apical membrane. Depleting the apical or the basolateral membrane of cholesterol by cyclodextrin attenuates the amount of cholesterol transported by ABCA1 without altering ABCA1 expression. Filipin added to the apical side but not the basal side attenuates ABCA1-mediated cholesterol efflux, suggesting that apical membrane “microdomains,” or rafts, supply cholesterol for HDL. Preventing cholesterol esterification increases the amount of cholesterol available for HDL. Ezetimibe, a Niemann-Pick C1-like 1 protein inhibitor, does not alter ABCA1-mediated cholesterol efflux. U18666A and imipramine, agents that mimic cholesterol trafficking defects of Neimann-Pick type C disease, attenuate cholesterol efflux without altering ABCA1 expression; thus, intestinal NPC1 may facilitate cholesterol movement to ABCA1. ABCA1-mediated cholesterol efflux is independent of cholesterol synthesis. The results suggest that following incorporation into plasma membrane and rafts of the apical membrane, dietary/biliary and newly synthesized cholesterol contribute to the ABCA1 pool and HDL-cholesterol. NPC1 may have a role in this process.
Keywords: cholesterol trafficking, Caco-2 cells, cholesterol, lipoproteins, high density lipoproteins, ABCA1
Reverse cholesterol transport is thought to be a key process through which peripheral cells unload their unwanted, excessive cholesterol by returning it to the liver for eventual elimination (1, 2). Through this mechanism, peripheral cells are protected from the potential harmful effects of cholesterol accumulation and subsequent foam cell formation. If these peripheral cells happen to be endothelial cells or macrophages that reside within a coronary arterial wall, reverse cholesterol transport, in theory, will prevent, or potentially reverse, atherosclerosis and ischemic heart disease. The lipoprotein particle responsible for transporting cholesterol from peripheral cells back to the liver for disposal is HDL. There is now good clinical evidence to suggest that elevated plasma HDL levels are beneficial, low plasma HDL levels are deleterious, and therapies that tend to raise plasma HDL levels are cardioprotective (3–8).
ABCA1 mediates the efflux of cellular cholesterol and phospholipids to apolipoprotein A-I (apoA-I) and is responsible for nascent HDL particle formation (1, 9–11). In the absence of ABCA1, HDL-cholesterol levels are extremely low, peripheral macrophages fill with cholesterol, and atherosclerosis is premature or accelerated (12–15). Although several tissues express ABCA1, liver and intestine express high levels of this transporter, suggesting that these two organs are the major sources for plasma HDL. Indeed, it is now clear that liver and intestine supply almost the entirety of plasma HDL (16, 17).
The ABCA1 gene is a target for the nuclear hormone transcription factor, liver X receptor (LXR) (18–20). LXR is believed to act as a sterol sensor, which, when activated, will result in the upregulation of genes that translate proteins which facilitate cholesterol elimination (19, 21–25). In a recent study in cultured intestinal cells, a nonsterol LXR agonist, T0901317, was used to enhance the expression of ABCA1 to address whether ABCA1 facilitates cholesterol transport at the apical or the basolateral membrane of the cell, at the time a very controversial question (26). The results were clear. ABCA1 facilitated the basolateral efflux of cholesterol to endogenously synthesized or exogenously supplied apoA-I. Thus, as in other cells, intestinal ABCA1 was responsible for nascent HDL particle formation and played no role in disposing of cholesterol back into the lumen. Other studies have since confirmed our observations (27–29). What was not clear from our earlier study, however, was the cellular origin of the HDL-cholesterol transported by ABCA1. Because intestinal ABCA1 might be an important target to enhance HDL-cholesterol production, the present study was performed in an attempt to elucidate the cellular pool of cholesterol that is used as substrate for ABCA1 and HDL production.
EXPERIMENTAL PROCEDURES
Materials
[3H]cholesterol (48.3 Ci/mmol) and [3H]mevalonolactone (33 Ci/mmol) were obtained from Perkin Elmer Life Sciences (Boston, MA). Protease inhibitor cocktail, sodium taurocholate, Tri Reagent, brefeldin A, monensin, U18666A, imipramine, Wortmannin, nigericin, nocodazole, monoclonal anti-β-actin antibody produced in mouse, and clone AC-15 were from Sigma Chemicals (St. Louis, MO). Rabbit polyclonal anti-human ABCA1 was from Cayman Chemicals (Ann Arbor, MI). Mouse monoclonal anti-human ABCA1 antibody was from Abcam Inc. (Cambridge, MA). The bicinchoninic protein assay kit, stabilized goat anti-mouse HRP-conjugated antibody, and superSignal west femto maximum sensitivity substrate chemiluminescent detection kit were from Pierce Biotechnology, Inc. (Rockford, IL). The ACAT inhibitor PD128042 was a generous gift from Brian Krause, Parke-Davis Pharmaceutical Division, Warner-Lambert Co. (Pfizer, Inc.). T0901317 was a gift from Tularik, Inc. (San Francisco, CA). Human apoA-I was purchased from Meridian Life Science, Inc. (Saco, ME). The Amplex Red Cholesterol Assay Kit was obtained from Invitrogen (Carlsbad, CA). Ezetimibe was purchased from Sequoia Research Products (Pangbourne, Berkshire, UK). The microsomal triglyceride transfer protein (MTP) inhibitor BMS-201038 was a gift from Bristol Myers Squibb (New Brunswick, NJ). Lovastatin was a gift from Merck (Rahway, NJ).
Cell culture
Caco-2 cells were cultured in T-75 flasks (Corning Glassworks, Corning, NY) in DMEM (GIBCO, Grand Island, NY) with 4.5 g/l glucose and supplemented with 10% FBS (Atlanta Biologicals, Norcross, GA), 2 mM glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin. Once the flasks reached 80% confluency, the cells were split and seeded at a density of 0.2 × 105 cells/well onto polyester membranes (0.4 μm pore size, 24 mm diameter) inserted into transwells (Costar, Cambridge, MA). Cells were fed every other day and were used 14 days after seeding.
Micelle preparation
Appropriate volumes of ethanol stock solutions containing taurocholate and individual lipids were evaporated under nitrogen, and the dried lipids were dissolved in DMEM. The resulting solution was stirred vigorously at 37°C until clear.
Estimation of efflux of [3H]cholesterol and cholesterol mass into basal medium
Caco-2 cells were incubated for 18 h with 1.5 ml 10% FCS-DMEM containing 2.5 μCi [3H]cholesterol to uniformly label cellular cholesterol. Radiolabeled cholesterol was added to the apical medium in ethanol (<0.5%, final concentration). To remove unincorporated labeled cholesterol, the filter insert was washed twice with DMEM and transferred to a new 6-well plate. The basal medium was replaced with 2.5 ml DMEM containing 3 μg/ml apoA-I. The cells were then incubated at 37°C in 1.5 ml DMEM, on the apical side, containing 0 or 2 μM T0901317, 0.05 μM MTP inhibitor, BMS-201038, and/or other treatments as described below. An aliquot (0.2 ml) of the basal medium was removed at the indicated time to measure basal efflux. An equal amount of the basal medium was replenished with fresh medium. The radioactivity recovered at each time point was used to calculate the percent of [3H]cholesterol effluxed into the basal medium. At the end of the incubation, the basal medium was collected and a sample was taken to estimate the percent of [3H]cholesterol effluxed into the medium. The remaining basal medium was extracted with 2.5 ml hexane-isopropyl alcohol-water (3:2:0.1; v/v/v). The hexane layer was removed, and the aqueous lower phase was extracted once again with 2 ml hexane. The combined hexane extracts were used for estimation of unesterified cholesterol mass by the Amplex Red method in the absence of cholesterol esterase according to the protocol described by the manufacturer. As a blank, cholesterol mass was estimated in an equal amount of basal medium from wells that were not exposed to cells, and this value was subtracted from the experimental values. The apical medium was removed, and the cells were washed two times with 1.5 ml cold DMEM. Cell lipids were extracted with 1.5 ml hexane-isopropyl alcohol-water (3:2:0.1; v/v/v). A portion of the lipid extract was taken and counted in a Packard liquid scintillation counter to determine total cholesterol label. Another portion was taken to estimate unesterified cholesterol mass by the Amplex Red method. In some experiments, the remaining lipid extract was separated by TLC on a silica gel G plate (250 μm; Uniplate, Analtech, Inc., Newark, DE), using a solvent system containing hexane-diethyl ether-acetic acid (90:10:1; v/v/v). Cholesterol and cholesteryl ester bands were localized by authentic standards, scraped from the plate, and counted.
Efflux of cholesterol taken up from micelles
Caco-2 cells were incubated at 37°C for 18 h in 1.5 ml of apical medium containing 0 or 2 μM T0901317 in 10% FCS-DMEM to upregulate the expression of ABCA1. At the end of this incubation, the apical medium was replaced with a micellar solution containing 5 mM taurocholate and 0.05 mM [3H]cholesterol (2.5 μCi cholesterol/dish). T0901317 was added back to the dishes that had it originally. The basal medium was replaced with 2.5 ml DMEM containing 3 μg/ml apoA-I. At specified times, the amount of labeled cholesterol taken up and the amount recovered in the basal medium were estimated. The amount of cholesterol mass was also estimated.
Efflux of newly synthesized cholesterol
Cells were incubated at 37°C for 18 h in 1.5 ml apical medium containing 0 or 2 μM T0901317 in DMEM to upregulate the expression of ABCA1. During the second incubation to measure efflux, the basal medium was replaced with 2.5 ml DMEM containing 3 μg/ml apoA-I. Radiolabeled mevalonate ± 2 μM T0901317 was added to the apical medium (10 μCi [3H]MVA/dish), and the incubation was continued for 3, 6, or 18 h. At the end of the indicated time period, basal medium and cells were analyzed for labeled cholesterol. The lipids were extracted twice with a chloroform-methanol mixture (water-methanol-chloroform; 0.9:1:1; v/v/v). The combined chloroform phase from two extractions was washed two times with a water-methanol mixture (1:1; v/v) to completely remove unincorporated [3H]MVA. A small aliquot was taken and counted to determine total sterol synthesis. The remaining lipid extract was used to isolate cholesterol on a silica gel TLC plate using hexane-diethylether-acetic acid (80:20:1, v/v/v). Radioactivity in the cholesterol fraction was determined using a Packard liquid scintillation counter.
RNA estimation by real-time quantitative RT-PCR
RNA was extracted from cells and subjected to DNase treatment followed by reverse transcription for 4 h at 50°C with SuperScript III (Invitrogen). The transcriptase was inactivated for 15 min at 70°C. The cDNA was mixed with the appropriate primers (Table 1) for the gene and 2× Sybr Green PCR master mix (Applied Biosystems), and the real-time RT-PCR was performed using a BIO-RAD Chromo4 real-time PCR detector system. The thermal cycler parameters were: hold for 2 min at 50°C and 10 min at 95°C for one cycle, followed by amplification of cDNA for 45 cycles with melting for 15 s at 95°C and annealing and extension for 1 min at 60°C. In this real-time PCR procedure, using Sybr Green dye, the mass of PCR product generated is estimated after each PCR cycle, and threshold cycle number is determined in the exponential phase of the curve. The values were normalized using 18S rRNA as endogenous internal standard. The relative expression of the gene was calculated using the comparative CT method (30).
TABLE 1.
Primers for real-time PCR
| Primer Name | Sequence | Accession Number |
|---|---|---|
| ABCA1- F | 5′-ATG-TCC-AGT-CCA-GTA-ATG-GTT-CTG-T-3′ | XM_032950.3 |
| ABCA1- R | 5′-CGA-GAT-ATG-GTC-CGG-ATT-GC-3′ | |
| ABCG1- F | 5′-TGC-ATC-CGG-CGA-GTA-CG-3′ | AY048757 |
| ABCG1- R | 5′-TCT-GAG-TCA-CAC-ATG-CCC-TCC-3′ | |
| HMG-CoA synthase- F | 5′-TTT-CCT-CTG-GTG-CCG-CTC-3′ | NM_001098272 |
| HMG-CoA synthase- R | 5′-GCA-GTC-TCC-AGG-TCT-GTC-ACT-G-3′ | |
| SREBP-1c- F | 5′-GGA-GGG-GTA-GGG-CCA-ACG-GCC-T-3′ | GI 409404 |
| SREBP-1c- R | 5′-CAT-GTC-TTC-GAA-AGT-GCA-ATC-C-3′ | |
| 18S- F | 5′-TAA-GTC-CCT-GCC-CTT-TGT-ACA-CA-3′ | M33069 |
| 18S- R | 5′-GAT-CCG-AGG-GCC-TCA-CTA-AAC-3′ |
Immunoblot analysis of ABCA1
Cells were incubated at 37°C for 18 h in 1.5 ml of apical medium containing 0 or 2 μM T0901317 and the indicated amounts of other compounds. Cells were washed and harvested in 1 ml DMEM buffer. Cells were sedimented at 16,000 g, 5 min, and the pellet was taken in 0.15 ml of RIPA buffer [50 mM Tris-HCl (pH 7.5), 150 mM NaCl, 1 mM Na2EDTA, 1 mM EGTA, 1% NP-40, 1% sodium deoxycholate, and 0.1% SDS] containing protease inhibitors. The cell suspension was sonicated for 10 s, followed by centrifugation at 16,000 g for 10 min. The supernatant was collected, and an equal amount of protein (40 μg) was applied to the SDS-PAGE and transferred to an Immobilon-P polyvinylidene difluoride (PVDF) membrane (Millipore, Bedford, MA). After rinsing in TBS (25 mM Tris-HCl, pH 7.5, 150 mM sodium chloride), the membrane was air-dried for 15 min, washed with water and methanol (1:1; v/v), followed by methanol alone. After drying for 10 min at room temperature, the blot was incubated at room temperature for 1 h with the mouse monoclonal anti-human ABCA1 and monoclonal anti-β-actin antibody diluted 2,000-fold in TBS containing 0.05% Tween-20 and 5% nonfat dry milk (Blotto). After washing in TBS containing Tween-20, the membrane was then incubated at room temperature for 1 h with goat anti-mouse antibody cross-linked to HRP (stock 10 μg/ml) diluted 2,500-fold in Blotto. The membrane was washed thoroughly in TBS containing Tween-20, and HRP was detected with superSignal west femto maximum sensitivity substrate chemiluminescent detection kit (Pierce). Equal loading of the samples was ensured by using an equal amount of protein per sample and by density of actin band on the blot.
ABCA1 mass was determined by immunoprecipitation with rabbit polyclonal anti-human ABCA1 antibody followed by Western blotting using anti-human ABCA1 mouse monoclonal antibody. Briefly, the cells were washed and harvested in 0.5 ml of RIPA buffer containing protease inhibitors. The cell suspension was sonicated for 10 s, followed by centrifugation at 16,000 g for 10 min. The supernatant was collected, and an equal amount of protein (200 μg) was taken for immunoprecipitation with 1 μg rabbit polyclonal anti-human ABCA1 antibody. This mixture was incubated overnight at 4°C with gentle mixing. Ten microliters of protein A/G slurry was added to the immunocomplexes and incubated at 37°C for 4 h. The mixture was centrifuged at 2,500 g for 10 min to sediment the complex. The supernatant was discarded, and 5 μl of 0.1 M glycine-HCl, pH 3.5, followed by 15 μl of 5× Laemmli sample buffer was added to dissociate the antigen-antibody complex. This mixture was applied quantitatively to the SDS-PAGE and transferred to an Immobilon-P PVDF membrane (Millipore). The Western blot analysis for ABCA1was performed as described above.
Other analyses
Protein content was estimated using the bicinchoninic acid assay kit (Pierce). One-way ANOVA with Tukey's post test or unpaired t-test was performed to compare the treatments using GraphPad Prism version 4 for Windows (GraphPad Software, San Diego CA).
RESULTS
Apical membrane cholesterol influx increases ABCA1-mediated HDL-cholesterol transport
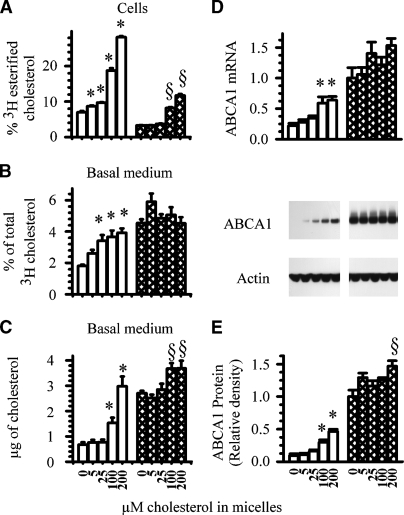
To address whether cholesterol influx from the apical membrane of intestinal cells contributes to ABCA1-mediated HDL formation, cells that were cultured on membranes separating an upper and lower well were labeled with cholesterol overnight to uniformly label all cholesterol pools. To drive apical membrane cholesterol into the cell, taurocholate micelles containing increasing concentrations of unlabeled cholesterol were added to the apical medium. As a cholesterol acceptor, all dishes contained apoA-I in the basolateral medium. In this experiment and all subsequent experiments, an MTP inhibitor, BMS-201038, was added to all dishes to prevent triglyceride-rich lipoprotein assembly and secretion. This MTP inhibitor, and one similar, prevents apoB and associated triglyceride and cholesterol secretion without altering ABCA1-mediated cholesterol efflux (26, 29). To enhance ABCA1 expression, another set of cells was incubated with micelles and the nonsterol LXR agonist T0901317. After an 18 h incubation, the amount of labeled apical membrane cholesterol esterified within cells (Fig. 1A), together with the amount of unesterified cholesterol label (Fig. 1B) and cholesterol mass (Fig. 1C) recovered in the basolateral medium, was estimated. In cells incubated in the absence of the LXR agonist (open bars), the amount of labeled cholesterol recovered in the basolateral medium increased in a concentration-dependent manner in response to the influx of micellar cholesterol (Fig. 1B). Analysis by TLC indicated that the labeled cholesterol recovered in the basolateral medium was essentially all unesterified. Uptake of micellar cholesterol resulted in the influx of apical membrane cholesterol to ACAT as cellular labeled cholesteryl esters increased in parallel fashion.
Fig. 1.
Effect of micellar cholesterol on ABCA1-mediated efflux of endogenous cholesterol. Caco-2 cells were labeled for 18 h with 2.5 μCi of [3H]cholesterol. After labeling, cells were washed and placed in new wells with the basal medium containing 3 μg/ml apolipoprotein A-I (apoA-I). Increasing amounts of cholesterol solubilized in 5 mM taurocholate micelles were added to the apical medium with 0 (open bars) or 2 μM (hatched bars) of the liver X receptor agonist T0901317. At the end of an 18 h incubation, the following were estimated: (A) cellular [3H]cholesteryl esters; (B) percent of [3H]cholesterol effluxed into the basal medium; (C) cholesterol mass effluxed into the basal medium; (D) ABCA1 mRNA; (E) ABCA1 mass. The data represent the mean ± SE of 6 dishes. * P < 0.05 versus cells incubated without cholesterol or T0901317. § P < 0.05 versus cells incubated without cholesterol but with T0901317.
The results differed, however, in the presence of an LXR agonist (Fig. 1A, hatched bars). As expected, in the absence of cholesterol, the LXR agonist significantly increased the basolateral efflux of labeled cholesterol (Fig. 1B). Adding increasing concentrations of micellar cholesterol to the apical medium, however, did not promote additional efflux. Thus, the differences observed in efflux between the two groups diminished; and in fact, at the highest concentration of micellar cholesterol (200 μM), the amount of labeled cholesterol recovered in the basolateral well was similar in cells incubated with or without the LXR agonist. Although fewer cholesteryl esters were formed in cells incubated with the LXR agonist compared with controls [an observation noted before (26)], cholesteryl esters did increase in cells incubated with micellar cholesterol, suggesting that apical membrane cholesterol was trafficking to ACAT (Fig. 1A).
When the amount of cholesterol mass recovered in the basolateral medium was estimated (Fig. 1C), the results were similar. In the absence of the LXR agonist, the amount of unesterified cholesterol recovered in the basolateral medium was significantly increased in cells incubated with 100 μM or 200 μM micellar cholesterol. In cells incubated with the LXR agonist but without micellar cholesterol, the basolateral efflux of cholesterol mass was increased approximately 5-fold. Adding 5 μM or 25 μM of micellar cholesterol did not significantly alter the amount of cholesterol mass recovered in the basolateral medium. At 100 μM or 200 μM of cholesterol, the amount of cholesterol mass recovered was modestly increased. As observed in the results using radiolabeled cholesterol, the differences in efflux observed between the two groups diminished with increasing concentrations of micellar cholesterol influx.
There are two possible but not mutually exclusive explanations for these results. Enhanced influx of apical membrane cholesterol (displaced by the uptake of micellar cholesterol) is transported to the ABCA1 cholesterol pool and is effluxed into the basolateral medium; and/or, enhanced influx of apical membrane cholesterol causes an increase in ABCA1 expression, which in turn, facilitates the efflux of more cholesterol.
To test the latter possibility, ABCA1 gene expression (Fig. 1D) and protein expression (Fig. 1E) were estimated in cells from the above experiment. In the absence of the LXR agonist, ABCA1 gene and protein expression increased in a step-wise fashion in cells incubated with increasing concentrations of micellar cholesterol. In the absence of micellar cholesterol, the LXR agonist significantly increased the expression of ABCA1. Adding increasing concentrations of micellar cholesterol, however, did not significantly further increase its gene or protein expression. These results essentially mirror those observed with cholesterol efflux, suggesting that changes in ABCA1 expression could explain the changes observed in cholesterol efflux. Whether apical membrane cholesterol contributes directly to the ABCA1 cholesterol pool remains in question and will be addressed below.
Cholesterol taken up from micelles is not a direct substrate for ABCA1-mediated HDL production
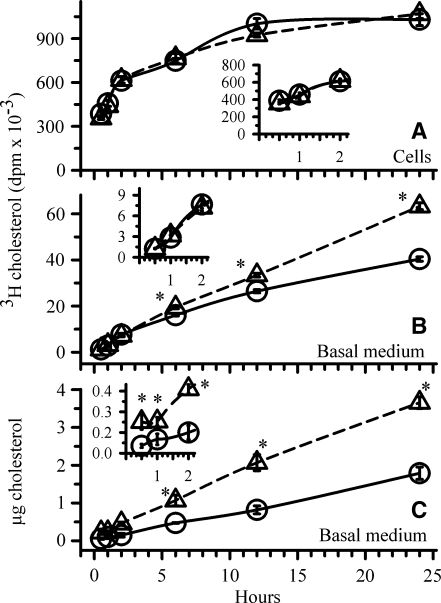
It has been suggested that the intestinal “HDL pathway” may serve as an alternative pathway for cholesterol absorption (17, 29, 31). To address whether luminal cholesterol taken up by the enterocyte enters the ABCA1 pathway and HDL formation, cells were first incubated for 18 h with the LXR agonist to enhance ABCA1 expression. Cells incubated without the LXR agonist served as controls. The cells were then incubated for 15, 30, 60, or 120 min with taurocholate micelles containing 50 μM labeled cholesterol. Over this time course, the amount of labeled cholesterol within cells and that recovered in the basolateral medium was estimated. The results are shown in Fig. 2. The amount of micellar cholesterol taken up was similar in cells incubated with or without the LXR agonist (Fig. 2A, inset). Thus, enhancing ABCA1 expression does not alter cholesterol uptake from the lumen. Moreover, over the 2 h time course, the amount of labeled micellar cholesterol recovered in the basal medium (Fig. 2B, inset) was similar in cells incubated in the presence or absence of the LXR agonist, suggesting that luminal cholesterol had not yet reached a pool of cholesterol that is exported by ABCA1. The incubation was then continued for 6, 12, and 22 h to determine at what time micellar cholesterol was entering the ABCA1 pool and was available for efflux. By 6 h, and certainly by 12 h and 22 h, the amount of labeled micellar cholesterol recovered in the basal medium (Fig. 2B) was significantly more in cells initially incubated with the LXR agonist, suggesting that cholesterol taken up from the lumen was reaching the ABCA1 pool, but only after a delay of several hours. The amount of cell-associated labeled cholesterol remained similar throughout the 22 h incubation (Fig. 2A). To verify that cholesterol efflux was indeed enhanced in cells first incubated with the LXR agonist, cholesterol mass recovered in the basal medium was estimated (Fig. 2C). At each of the time points, more cholesterol mass was recovered in the basal medium from cells in which ABCA1 was first upregulated. These results suggest that luminal micellar cholesterol does not directly traffick to the ABCA1 pool and to HDL. The delay would suggest that the cholesterol taken up is first incorporated into the apical membrane before it eventually becomes available to ABCA1 in the basolateral membrane.
Fig. 2.
Utilization of cholesterol taken up from micelles for ABCA1-mediated cholesterol efflux. ABCA1 expression was first enhanced by incubating cells for 18 h in the presence (open triangles) or absence (open circles) of 2 μM T0901317. Following this incubation, the basal medium was replaced with medium containing 3 μg/ml apoA-I. The apical medium was replaced with medium containing micelles consisting of 5 mM taurocholate and 50 μM [3H]cholesterol. T0901317 was added back to the cells that originally had it. At the end of each time point indicated on the X-axis, the amount of labeled cholesterol taken up (A) and that recovered in the basal medium (B) were estimated. Basal medium from two wells was combined to estimate cholesterol mass (C). The data represent the mean ± SE of 6 dishes. *P < 0.05 versus cells incubated without T0901317 at the corresponding time point by Student's t-test.
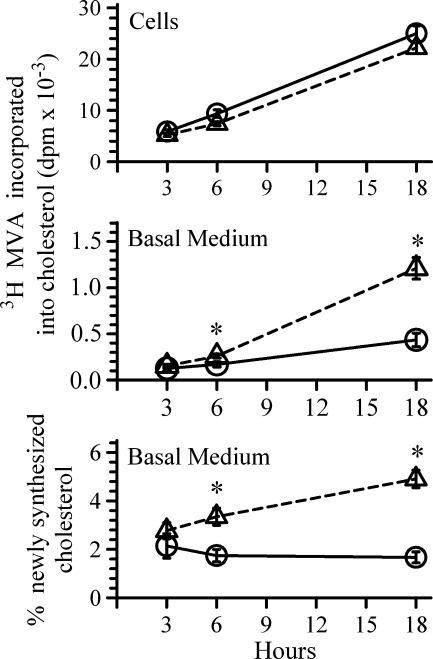
Newly synthesized cholesterol contributes to ABCA1-mediated HDL production
To address whether newly synthesized cholesterol contributes to intestinal HDL-cholesterol, ABCA1 expression was first enhanced by incubating cells with the LXR agonist for 18 h (Fig. 3, triangles). Cells incubated without the LXR agonist served as controls (Fig. 3, circles). Trace amounts of labeled mevalonate were then added, and the amount of labeled cholesterol within cells and that recovered in the basolateral medium were estimated over 18 h. In cells incubated with or without the LXR agonist, the rates of mevalonate incorporation into cellular cholesterol were similar. At 3 h, similar amounts of labeled cholesterol were recovered in the basal medium in both treatment groups. At 6 h and 18 h, however, the amount of newly synthesized cholesterol recovered in the basolateral medium was significantly more in cells incubated with the LXR agonist. At 18 h, approximately 5% of the newly synthesized cholesterol could be recovered in the basal medium (Fig. 3, bottom panel). Thus, newly synthesized cholesterol contributes to the ABCA1 cholesterol pool and is utilized for the production of HDL by the intestine. The several hours' delay in detecting ABCA1-mediated efflux of newly synthesized cholesterol, however, suggests initial incorporation of the sterol into the plasma membrane prior to its transport by ABCA1. This possibility will be addressed below.
Fig. 3.
Utilization of newly synthesized cholesterol for ABCA1-mediated cholesterol efflux. ABCA1 expression was first enhanced by incubating cells for 18 h in the presence (open triangles) or absence (open circles) of 2 μM T0901317. Following this incubation, the basal medium was replaced with medium containing 3 μg/ml apoA-I. The apical medium was replaced with medium containing 10 μCi [3H]mevalonate. T0901317 was added back to the cells that had it originally. At the end of each time point indicated on the X-axis, the amount of labeled cholesterol within cells and that recovered from the basal medium were estimated. The data represent the mean ± SE of 8 dishes. *P < 0.05 versus cells incubated without T0901317 at the corresponding time point by Student's t-test.
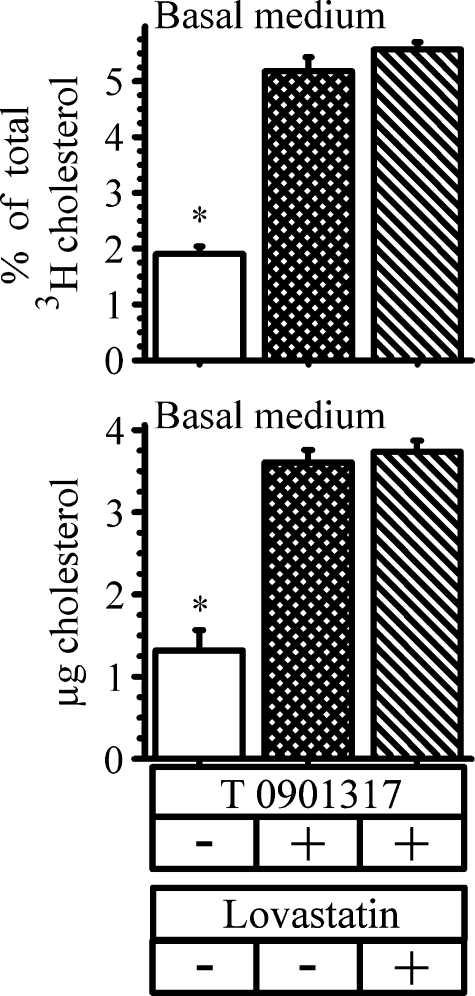
We also asked whether cholesterol synthesis was required for ABCA1-mediated cholesterol efflux. Cells were again labeled overnight with cholesterol to uniformly label all cholesterol pools. They were then incubated for 18 h with the LXR agonist in the presence or absence of 25 μM lovastatin, a concentration that essentially prevents cholesterol synthesis in Caco-2 cells (32, 33). Following the incubation, the amount of cholesterol label and cholesterol mass recovered in the basolateral medium was estimated (Fig. 4). Preventing cholesterol synthesis with lovastatin did not alter the amount of cholesterol label (Fig. 4, upper panel) or mass (Fig. 4, lower panel) found in the basolateral medium in response to LXR activation. These data, taken together, suggest that newly synthesized cholesterol contributes to ABCA1-mediated HDL-cholesterol (albeit, after its probable incorporation into the apical membrane) but that newly synthesized cholesterol is not required for normal ABCA1-mediated HDL-cholesterol efflux to occur.
Fig. 4.
Effect of inhibiting cholesterol synthesis on ABCA1-mediated cholesterol efflux. Cells were labeled for 18 h with 2.5 μCi of [3H]cholesterol. After labeling, cells were washed and placed in new wells with the basal medium containing 3 μg/ml apoA-I. The cells were then incubated with or without 2 μM T0901317 and in the presence or absence of 25 μM lovastatin. At the end of 18 h, the amount of cholesterol label and mass recovered in the basal medium was estimated. The data represent the mean ± SE of 6 dishes. *P < 0.05 versus cells incubated with T0901317.
Cholesterol from both apical and basolateral membranes contributes to HDL-cholesterol
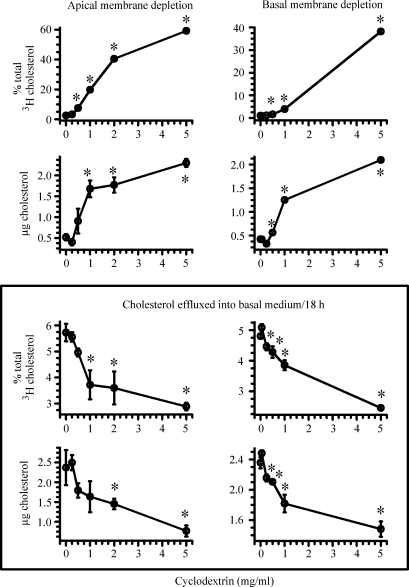
From the above results, there is a suggestion that cholesterol derived from the apical membrane may enter the ABCA1 cholesterol pool and contribute to the formation of HDL-cholesterol. To test this hypothesis, cells were labeled with cholesterol overnight to uniformly label all cholesterol pools. Cholesterol was then extracted from either the apical membrane or basolateral membrane by adding increasing concentrations of cyclodextrin to the apical or basolateral medium, respectively. After 2 h, the cells were extensively washed and placed in new wells. ABCA1 expression was then enhanced by adding the LXR agonist. Over the next 18 h, the efflux of labeled cholesterol and cholesterol mass into the basolateral medium was estimated. Figure 5 shows these results. The upper panel shows the amount of cholesterol label and mass extracted from the apical (left) and basal (right) membranes after 2 h of incubation with cyclodextrin. With increasing concentrations of cyclodextrin, the amount of cholesterol extracted from either the apical membrane or the basolateral membrane increased. There was no loss of apical cholesterol by cyclodextrin placed in the basal medium, and no loss of basal cholesterol by cyclodextrin placed in the apical medium (data not shown). The lower panel shows the amount of cholesterol recovered in the basal medium in response to the LXR agonist from cells in which cholesterol was extracted from the apical (left) or the basolateral (right) membrane. In both cases, the percent of labeled cholesterol or cholesterol mass that was recovered in the basal medium decreased in response to LXR activation. Thus, depleting cholesterol in either the apical membrane or the basolateral membrane resulted in less ABCA1-facilitated efflux of cellular cholesterol. These results also suggest two possible but not mutually exclusive explanations: cholesterol derived from either membrane contributes to intestinal HDL-cholesterol; and/or depleting either membrane of cholesterol suppresses ABCA1 expression and attenuates the efflux of cholesterol.
Fig. 5.
Effect of depleting cholesterol from either the apical or the basolateral membrane on ABCA1-mediated cholesterol efflux. Cells were labeled for 18 h with 2.5 μCi of [3H]cholesterol. After labeling, the indicated amounts of cyclodextrin were added for 2 h to either the apical (left side of figure) or basal (right side of figure) medium. Following this 2 h incubation, the amount of cholesterol label and mass recovered from the apical (left upper panel) or basal (right upper panel) medium was estimated. Following extensive washings, the basal medium was replaced with medium containing 3 μg/ml apoA-I. The apical medium was replaced with medium containing 2 μM T0901317. At the end of 18 h, the amount of cholesterol label and mass recovered in the basal medium was estimated (bottom panels). The data represent the mean ± SE of 12 dishes. *P < 0.05 versus cells incubated without cyclodextrin but with T0901317 by Student's t-test.
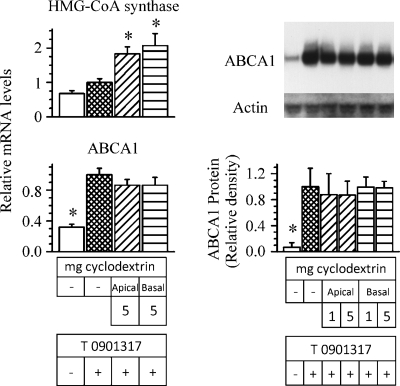
To address the latter possibility, the effect of cyclodextrin on ABCA1 gene expression and protein expression was determined. Except for including a set of cells incubated in the absence of both cyclodextrin and the LXR agonist (to validate enhanced expression of ABCA1), and using 1 mg/ml or 5 mg/ml of cyclodextrin, the protocol described in Fig. 5 was followed. The results are shown in Fig. 6. Extracting cholesterol from either the apical membrane or the basal membrane did not alter ABCA1 mRNA levels (Fig. 6, left lower data). Likewise, ABCA1 mass was not altered (Fig. 6, right data). In contrast, HMG-CoA synthase mRNA, a gene responsive to the removal of cholesterol, was significantly increased in cells incubated with cyclodextrin (Fig. 6, upper left data). These combined results would suggest, therefore, that cholesterol contained in both the apical membrane and the basal membrane of intestinal cells contributes to the pool utilized by ABCA1 for HDL-cholesterol production.
Fig. 6.
Effect of depleting cholesterol from either the apical or the basolateral membrane on expression of ABCA1 and HMG-CoA synthase. The experimental design was exactly as described for Fig. 5, except that either 1 mg/ml or 5 mg/ml of cyclodextrin was used and a set of cells were incubated in the absence of cyclodextrin but in the presence or absence of T0901317. Following the incubation, ABCA1 mRNA and protein and HMG-CoA synthase mRNA were estimated. The data represent the mean ± SE of 6 dishes for mRNA estimation and mean ± SE of 4 dishes for protein. *P < 0.05 versus cells incubated without cyclodextrin but with T0901317.
Cholesterol trafficking and ABCA1-mediated cholesterol efflux
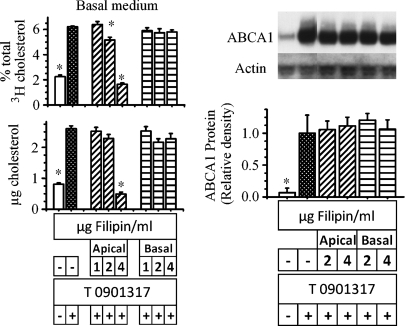
Filipin, a cholesterol-sequestering agent that binds to cholesterol-rich domains of cellular membranes causing disruption of raft structure and organization, was used to interfere with the normal movement of plasma membrane cholesterol (34–38). To explore whether disrupting raft structure would affect ABCA1-mediated cholesterol efflux, cells were incubated with labeled cholesterol overnight to uniformly label all cholesterol pools. The cells were then incubated with the LXR agonist and increasing concentrations of filipin added to either the apical medium or the basal medium. Following the incubation, the amount of labeled cholesterol and cholesterol mass recovered in the basolateral medium was estimated. Control incubations were without filipin. The results are shown in Fig. 7. In a concentration-dependent manner, the addition of filipin to the apical medium significantly decreased the amount of cholesterol mass and cholesterol label recovered in the basal medium in response to LXR activation (Fig. 7, diagonal bars). In contrast, filipin added to the basolateral side was without effect (Fig. 7, horizontal bars). Similar to what was observed with cyclodextrin, filipin did not alter ABCA1 protein mass. To prove that filipin was not interfering with the action of the LXR agonist, the observed increase in mRNA levels for two other LXR target genes, SREBP-1c and ABCG1, was not altered by the presence of filipin (data not shown). Thus, filipin prevents apical membrane cholesterol from reaching ABCA1 but not cholesterol contained in the basolateral membrane.
Fig. 7.
Effect of filipin on ABCA1-mediated cholesterol efflux. Cells were labeled for 18 h with [3H]cholesterol. After labeling, cells were washed and placed in new wells with the basal medium containing 3 μg/ml apoA-I. Filipin was then added to either the apical (diagonal bars) or the basal (horizontal bars) medium in the presence or absence of 2 μM T0901317 (added to the apical medium). At the end of 18 h, the amount of cholesterol mass (lower panel) and label (upper panel) recovered in the basal medium was estimated. The data represent the mean ± SE of 12 dishes. In another set of cells, following the incubation, ABCA1 mass was estimated (right side). The data represent the mean ± SE of 4 dishes. *P < 0.05 versus cells incubated without filipin but with T 0901317.
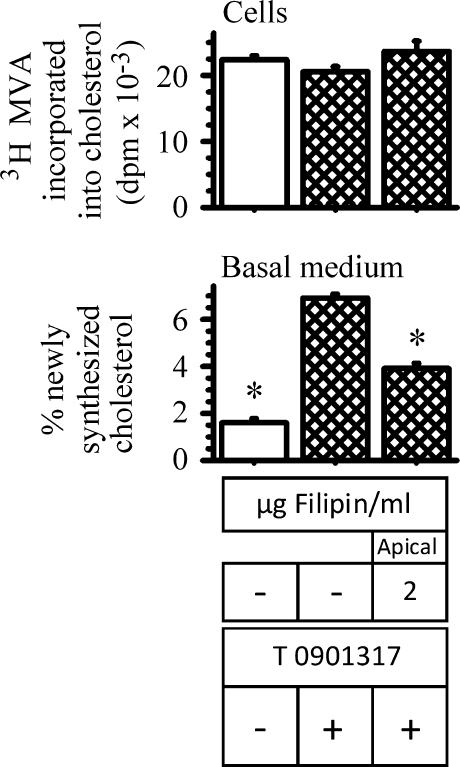
We then asked whether filipin would interfere with the basolateral efflux of newly synthesized cholesterol, because most newly synthesized cholesterol is believed to traffick to rafts of the apical membrane (as reviewed in Refs. 39, 40). Thus, the experiment described in Fig. 3 was repeated in the presence of 2 μg/ml filipin in the apical medium. These results are shown in Fig. 8. After 18 h, the incorporation of labeled mevalonate into cellular cholesterol was unaltered by the presence of filipin (Fig. 8, upper panel). The efflux of newly synthesized cholesterol by cells incubated with filipin, however, was decreased by approximately 40%, compared with controls (Fig. 8, lower panel). These results support the notion that newly synthesized cholesterol probably trafficks to the apical plasma membrane before becoming available for efflux via ABCA1, and they provide support that apical membrane rafts supply cholesterol for intestinal HDL production.
Fig. 8.
Effect of filipin on ABCA1-mediated newly synthesized cholesterol efflux. ABCA1 expression was first enhanced by incubating cells for 18 h in the presence or absence of 2 μM T0901317. Following this incubation, the basal medium was replaced with medium containing 3 μg/ml apoA-I. The apical medium was replaced with medium containing 10 μCi [3H]mevalonate with or without 2 μg/ml filipin. T0901317 was added back to cells that had it originally. At the end of 18 h, the amount of labeled cholesterol found within cells (upper bars) and that recovered in the basal medium (lower bars) were estimated. The data represent the mean ± SE of 4 dishes. *P < 0.05 versus cells incubated without filipin but with T0901317.
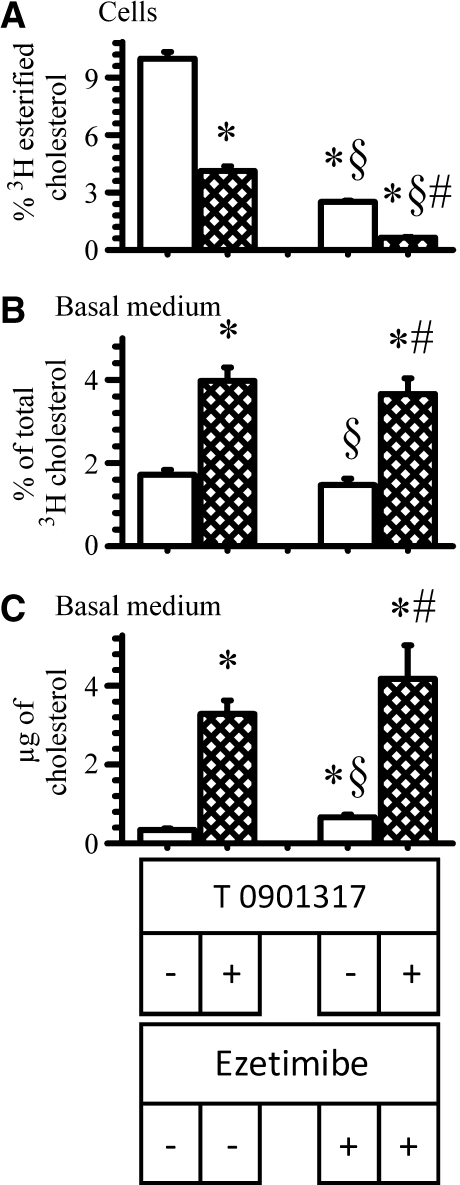
In Caco-2 cells, Niemann-Pick C1-like 1 protein (NPC1L1), the putative apical membrane cholesterol transporter, facilitates the trafficking of apical membrane cholesterol to the ACAT pool (41). Because the above results suggest that apical membrane cholesterol, and perhaps more specifically, apical membrane raft cholesterol contributes to HDL-cholesterol, it was questioned whether NPC1L1 had a role in delivering cholesterol to ABCA1. To address this, cells were incubated overnight with labeled cholesterol to uniformly label all cholesterol pools. The cells were then incubated with the LXR agonist and the NPC1L1 inhibitor, ezetimibe, at a concentration that significantly interferes with trafficking of cholesterol (41). Following the incubation, the amount of labeled cholesterol and cholesterol mass recovered in the basolateral medium was estimated (Fig. 9). In another set of cells, ABCA1 mass and gene expression were also estimated. Inhibiting the action of NPC1L1 by ezetimibe did not alter the amount of cholesterol label (Fig. 9B) or mass (Fig. 9C) in response to LXR activation, suggesting that NPC1L1 was not facilitating the delivery of apical membrane cholesterol to ABCA1. As expected, however, ezetimibe did interfere with the trafficking of cholesterol to ACAT (Fig. 9A). Ezetimibe did not alter ABCA1 mass or mRNA levels (data not shown).
Fig. 9.
Effect of ezetimibe on ABCA1-mediated cholesterol efflux. Cells were labeled for 18 h with [3H]cholesterol. After labeling, cells were washed and placed in new wells with the basal medium containing 3 μg/ml apoA-I. The cells were then incubated for 18 h in the presence (hatched bars) or absence (open bars) of 2 μM T0901317 and with or without 25 μM ezetimibe. At the end of the incubation, the percent of cholesterol esterified within cells (A) and the amount of cholesterol label (B) and mass (C) recovered in the basal medium were estimated. The data represent the mean ± SE of 6 dishes. *P < 0.05 versus cells incubated without ezetimibe or T0901317. §P < 0.05 versus cells incubated without ezetimibe but with T0901317. #P < 0.05 versus cells incubated with ezetimibe but without T0901317.
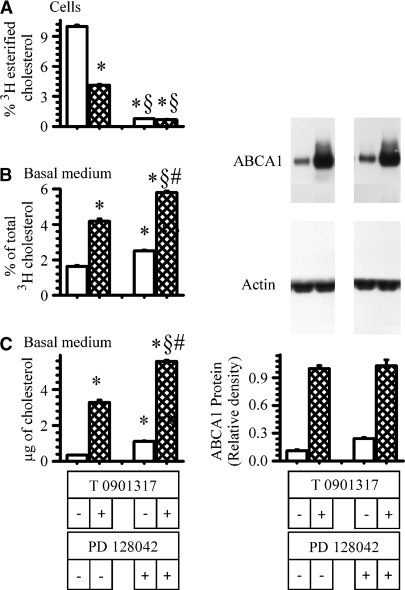
There is evidence to suggest that ABCA1 shares a pool of cholesterol with ACAT (39, 42). To address this, cells were incubated overnight with labeled cholesterol to uniformly label all cholesterol pools. The cells were then incubated with the LXR agonist T0901317 in the presence or absence of a potent ACAT inhibitor, PD128042. Following the incubation, the percent of cholesterol esterified within cells and the amount of labeled cholesterol and cholesterol mass recovered in the basolateral medium were estimated. In another set of cells, ABCA1 mass and gene expression were also estimated. The results are shown in Fig. 10. Preventing the esterification of cholesterol by almost 90% by PD128042 (Fig. 10A) caused a modest increase in the amount of cholesterol label (Fig. 10B) and mass (Fig. 10C) recovered in the basolateral medium from these cells. In the absence of the LXR agonist, ABCA1 mass was increased 2-fold in cells incubated with the ACAT inhibitor. In the presence of the LXR agonist, the ACAT inhibitor did not increase ABCA1 mass above that observed for the LXR agonist alone. ABCA1 mRNA levels were not significantly altered by the presence of the ACAT inhibitor (data not shown). These results would support the premise that ACAT and ABCA1 share a common cholesterol pool.
Fig. 10.
Effect of ACAT inhibition on ABCA1-mediated cholesterol efflux. Cells were labeled for 18 h with [3H]cholesterol. After labeling, cells were washed and placed in new wells with the basal medium containing 3 μg/ml apoA-I. The cells were then incubated for 18 h in the presence (hatched bars) or absence (open bars) of 2 μM T0901317 and with or without 2.6 μM of an ACAT inhibitor, PD128042. At the end of the incubation, the percent of cholesterol esterified within cells (A) and the amount of cholesterol label (B) and mass (C) recovered in the basal medium were estimated. The data represent the mean ± SE of 6 dishes. *P < 0.05 versus cells incubated without PD128042 or T0901317. §P < 0.05 versus cells incubated without PD128042 but with T0901317. #P < 0.05 versus cells incubated with PD128042 but without T0901317.
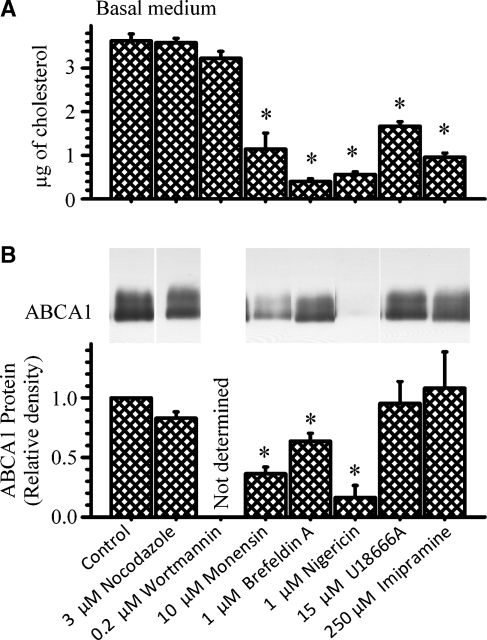
Finally, various agents that interfere with normal vesicular and/or cholesterol trafficking were used to investigate the importance of these pathways in ABCA1-mediated cholesterol efflux. Their effect on ABCA1 mass was also determined. Figure 11 shows these results. Neither nocodazole, which disrupts microtubule-mediated movement of vesicles (43), nor Wortmannin, which interferes with early endocytic trafficking/fusion by inhibiting PI-3 kinase (44), altered the amount of cholesterol mass recovered in the basal medium in response to LXR activation. In contrast, monensin and brefeldin A, agents that disrupt Golgi function (43, 45), nigericin, a potassium ionophore that interferes with the function of acidic vesicles (45), and U18666A and imipramine, hydrophobic amines that cause the accumulation of cholesterol in the late endosomal compartment, mimicking the phenotype of Niemann-Pick type C (NPC) cells (43, 46), all significantly decreased ABCA1-mediated cholesterol efflux. Monensin, brefeldin A, and nigericin, however, significantly decreased ABCA1 mass. In contrast, U18666A and imipramine did not. These results do not permit implicating a role for normal Golgi or acidic vesicle function in transporting cholesterol to ABCA1. The significant attenuation of ABCA1 expression by these agents could certainly explain their effect on cholesterol efflux. On the other hand, U18666A and imipramine did not alter ABCA1 mass. These results, therefore, could implicate a role for NPC1 in delivering cholesterol to ABCA1 in intestine.
Fig. 11.
Effect of various agents known to disrupt cellular cholesterol trafficking on ABCA1-mediated cholesterol efflux and ABCA1 protein. Cells were incubated for 18 h with 10% FBS-DMEM. The cells were washed and placed in new wells with the basal medium containing 3 μg/ml apoA-I. The cells were then incubated for 18 h with 2 μM T0901317 and the indicated amounts of the various compounds. At the end of the incubation, the amount of cholesterol mass recovered in the basal medium (A) was estimated. The amount of cellular ABCA1 protein (B) was estimated by immunoprecipitation and immunoblot analysis as described in the Experimental Procedures section. The data represent the mean ± SE of 6 dishes for cholesterol mass or 4 dishes for ABCA1 protein. *P < 0.05 versus control cells (with T0901317).
DISCUSSION
The results of this study show that in intestinal cells, the influx of endogenous apical membrane cholesterol enhances gene and protein expression of ABCA1, resulting in increased efflux of cellular cholesterol to apoA-I and HDL production. After uptake of cholesterol from micelles, however, micellar cholesterol itself is not directly transported to the ABCA1 pool but is instead first incorporated into the apical membrane before it becomes available for ABCA1-mediated efflux. Similarly, newly synthesized cholesterol contributes to the ABCA1 pool and HDL-cholesterol, but it, too, is first incorporated into the apical membrane before it enters the ABCA1 cholesterol pool. Thus, although intestinal HDL-cholesterol can come from newly synthesized cholesterol or cholesterol taken up from micelles, it is ultimately cholesterol contained within the apical and basolateral plasma membranes that is utilized by ABCA1. Moreover, it appears that it is cholesterol contained within specialized microdomains, or rafts, of the apical membrane that trafficks to ABCA1. ABCA1-mediated cholesterol efflux and intestinal HDL production are independent of normal NPC1L1 function, cholesterol synthesis, and microtubular function. NPC1 protein, however, may play a role in delivering cholesterol to ABCA1 in intestine.
With the discovery of ABCA1 and delineation of its role in facilitating cholesterol efflux and HDL formation (12, 13), it was initially proposed that intestinal ABCA1 was involved in cholesterol uptake and absorption (19). Over time, however, it became clear that intestinal ABCA1 functioned at the basolateral membrane, facilitating the efflux of cholesterol to apoA-I and producing nascent HDL particles. It played no role in facilitating the uptake of luminal cholesterol into the cell or its efflux back into the lumen, thought to be mediated by ABCG5/ABCG8 (47), and only a minor role in the absorption of cholesterol (28, 48, 49). In an elegant study performed in mice specifically lacking only intestinal ABCA1, it was found that the intestine contributed approximately 30% of the total plasma HDL-cholesterol (17). It was also observed that within 2 h of gavaging labeled cholesterol to these animals, the transport of cholesterol from the lumen to plasma, or more specifically to plasma HDL, was decreased by approximately 35% (similar to the overall decrease observed in HDL). Moreover, less labeled cholesterol was found in liver and more was retained within the intestine. In contrast, transport of dietary cholesterol into lymph was unaffected. It was concluded that cholesterol uptake from the lumen was not impaired in mice lacking intestinal ABCA1 but that transport of luminal cholesterol directly into plasma was attenuated (17). Our results would agree in part. ABCA1 does not facilitate the uptake of micellar cholesterol into the cell, inasmuch as markedly enhancing the expression of ABCA1 by an LXR agonist did not alter the amount of cholesterol taken up. In our cells, however, over a 2 h time period, cholesterol that was taken up from micelles did not find its way to the ABCA1 pool, suggesting that cholesterol taken up from the lumen was not being directly shuttled to the basolateral membrane, the site of ABCA1. Obviously, over time, the sterol taken up will eventually equilibrate with cholesterol contained within the plasma membrane, the location of 80–90% of total cellular cholesterol. This exogenous dietary/biliary cholesterol will then become substrate for either the chylomicron or the HDL pathway. From our data, however, there does not appear to be a direct cellular pathway by which luminal (dietary) cholesterol trafficks directly to ABCA1 and HDL production.
In mouse models deficient in either ACAT2, ABCA1, or a combined deficiency of ACAT2 and ABCA1, Temel et al. (31) concluded that ABCA1 contributed minimally to cholesterol absorption when ACAT2 was functional. In the absence of ACAT2 (which resulted in a 3-fold increase in intestinal ABCA1 expression), however, ABCA1 was able to partially compensate by facilitating the transport of more cholesterol into plasma, most likely through ABCA1-dependent efflux of free cholesterol. From these results, it is clear that compared with ABCA1, ACAT2 is more important in facilitating the transport of cholesterol from the intestinal lumen to plasma. Our results would agree. Potently inhibiting intestinal ACAT activity did enhance ABCA1 mass and ABCA1-mediated cholesterol efflux, but the effects were modest. A more dramatic effect might have been observed if the ACAT pathway had been “stressed” by increasing the amount of cholesterol influx. Indeed, in the study by Temel et al. (31), animals were fed a moderate cholesterol diet (0.17%, w/w) so that the unesterified cholesterol pool within the intestinal cells of animals deficient in ACAT2 was certain to be expanded. Despite this, the observed difference in cholesterol absorption between the ACAT2- and ACAT2/ABCA1-deficient animals was modest. In a previous study in Caco-2 cells (26), we observed that with cholesterol influx, ABCA1 gene expression could be enhanced if ACAT activity was inhibited, conditions that would favor accumulation of unesterified cholesterol and an increase in ABCA1-mediated cholesterol efflux. Nevertheless, it seems clear from our results and those of others (31, 48, 50–52) that the pathway of intestinal ABCA1-mediated cholesterol efflux and HDL production is a “low-capacity” system and is not designed for, nor meant for, transport of dietary cholesterol into the body, even if the chylomicron pathway were to be disrupted by interfering with NPC1L1, ACAT2, or MTP activity. Thus, attempting to lower plasma cholesterol by inhibiting cholesterol absorption through targeting intestinal ABCA1 would be futile and, by lowering plasma HDL, probably harmful.
Our results implicate cholesterol contained within the apical membrane as a major source for ABCA1-mediated cholesterol efflux. Moreover, the data suggest that it may be cholesterol contained within “specialized microdomains,” or rafts, of the apical membrane that supply cholesterol to ABCA1. Filipin, an agent recognized to disrupt raft function by binding to cholesterol-rich domains of cell membranes, markedly attenuated ABCA1-mediated cholesterol efflux when added to the apical medium. When added to the basolateral medium, filipin was without effect. This makes sense. In polarized intestinal cells, rafts are exclusively confined to the apical membrane (34). Because filipin did not alter the amount of ABCA1 protein, the results suggest that filipin was preventing cholesterol from reaching ABCA1. In an earlier study performed in fibroblasts, Mendez et al. (35), found that ABCA1 did not colocalize with membrane rafts and that disrupting raft structure did not alter cholesterol efflux to apoA-I. These investigators concluded that in fibroblasts, membrane rafts do not supply cholesterol to the ABCA1-mediated efflux pathway. The differences observed between the two studies may simply be related to the cell type, i.e., an enterocyte versus a fibroblast (certainly much different in function) or perhaps polarized cell versus nonpolarized cell. In the present study, it was possible to independently manipulate either the apical membrane or the basolateral membrane to investigate pathways involved in ABCA1-mediated cholesterol efflux. There is another potential explanation. In other cells studied, the activity of cholesterol efflux closely parallels the level of expression of ABCA1 (53–55). This suggests, and it is our impression as well, that the limiting factor in ABCA1-mediated cholesterol efflux is related to the amount of ABCA1 protein present in the basolateral membrane. To investigate the contributions of different pools of cellular cholesterol to the ABCA1-mediated pathway, we have eliminated the possibility of ABCA1 being the limiting factor by maximizing its expression with an LXR agonist. If ABCA1 were limiting in the studies by Mendez et al. (35), it may be difficult to demonstrate a decrease in cholesterol efflux following disruption of raft function.
Although we suspect, as do others (56), that membrane cholesterol in close proximity to ABCA1 is a substrate for ABCA1-mediated efflux, our results are not conclusive. In contrast to the results with filipin, cyclodextrin added to either the apical or the basolateral side caused a significant decrease in ABCA1-mediated cholesterol efflux. Because ABCA1 mass was not altered by cyclodextrin, this might suggest that cholesterol contained in both membranes contributes to the ABCA1 efflux pathway. Cyclodextrin added to the apical medium will disrupt raft structure and function (57). Thus, it would be expected that these results would parallel those of filipin, and they do. Adding cyclodextrin to the basal medium and removing cholesterol from the basolateral membrane should theoretically decrease the availability of cholesterol to ABCA1, and in fact, we did observe less efflux of ABCA1-mediated cholesterol. We cannot exclude, however, the possibility that with the extraction of cholesterol from the basolateral membrane (the location of ABCA1), the ABC transporter is rendered dysfunctional. To our knowledge, there is no information concerning the membrane microenvironment of ABCA1 and the role it might play in its function. Obviously, further studies would be required to address this issue. The observation that apical membrane cholesterol did not replenish cholesterol extracted from the basolateral membrane might suggest that ABCA1 was rendered nonfunctional or that trafficking of apical membrane cholesterol to the ABCA1 cholesterol pool occurs slowly.
There are several studies that imply that normal intracellular cholesterol trafficking is critical in ABCA1-mediated cholesterol efflux. Previous studies have suggested that the Golgi apparatus is important in cholesterol efflux (45, 58). Others have implicated NPC1 (59–61). Little is known about the role of cholesterol trafficking and intestinal HDL production. In the present study, there was little question that agents known to disrupt Golgi function, brefeldin A and monensin, caused a profound attenuation of ABCA1-mediated cholesterol efflux. Although this could imply that trafficking of cholesterol through this organelle is important for cholesterol efflux, both agents significantly decreased ABCA1 mass. Similarly, nigericin, a potassium ionophore that alkalinizes acidic vesicles, disrupting their function, profoundly decreased cholesterol efflux; but it, too, markedly decreased ABCA1 mass. The mechanism or mechanisms through which these agents decreased ABCA1 mass is not clear. These results, however, do not allow us to differentiate whether these agents are limiting the supply of substrate for ABCA1, i.e., disrupting cholesterol trafficking to ABCA1, or are just limiting ABCA1 mass.
It is clear from our results that the uptake of micellar cholesterol leads to the influx of apical membrane cholesterol and upregulation of ABCA1 gene and protein expression. These results agree with dietary studies in mice showing that intestinal ABCA1 gene expression is enhanced following the ingestion of cholesterol (62). Because cholesterol itself is not thought to be a ligand for LXR, the results imply that oxysterols generated within the cell act as the true ligand for LXR. Because dietary and/or biliary cholesterol enhances intestinal ABCA1 expression, it would seem to make sense that the apical cholesterol transporter NPC1L1 (63–65) would have a role in regulating ABCA1 expression. Indeed, in animals that lack NPC1L1, the expression of intestinal ABCA1 is attenuated (64), and feeding an LXR agonist to mice lacking NPC1L1 fails to normalize intestinal ABCA1 expression and raise plasma HDL-cholesterol levels (66). These results imply that when cholesterol uptake is attenuated by the absence of NPC1L1, ABCA1 is limiting and/or insufficient cholesterol is available for ABCA1-mediated HDL production. What seems clear from our results, however, is that NPC1L1 does not facilitate the transport of apical membrane cholesterol to ABCA1 in the basolateral membrane. Ezetimibe, an agent that interferes with NPC1L1 function (65), did not alter cholesterol efflux or ABCA1 expression. Because NPC1L1 facilitates the trafficking of apical membrane cholesterol to ACAT (41), our results would suggest that this specific pathway is not critical for normal HDL production by the intestine. Our findings would support clinical observations showing that administration of ezetimibe does not decrease HDL-cholesterol levels (67–70).
It has been observed in NPC disease fibroblasts that ABCA1-mediated cholesterol efflux is attenuated (59, 60). Although the exact function of the defective protein in this disease, NPC1, is unknown, the disorder is characterized by impaired cholesterol trafficking with the accumulation of unesterified cholesterol in late endosomes/lysosomes (71–75). It was suggested, therefore, that NPC1 plays a role in providing ABCA1 with cholesterol for efflux. ABCA1 mass, however, is decreased in NPC cells (60). Moreover, the defect in cholesterol efflux in these cells can be partially but not completely reversed by an LXR agonist, upregulating ABCA1 expression (61). It could be argued that ABCA1 mass is the limiting factor for cholesterol efflux in NPC cells, independent of NPC1 and defects in cholesterol trafficking. In our study, the hydrophobic amines U18666A and imipramine, agents that mimic the cholesterol trafficking defects found in NPC cells (as reviewed in Refs. 76–78), caused attenuation of ABCA1-mediated cholesterol efflux without altering ABCA1 mass. Thus, our results would implicate a role for intestinal NPC1 in supplying cholesterol to ABCA1. Moreover, implicating NPC1 would explain why an LXR agonist could not completely abrogate the attenuation of ABCA1-mediated cholesterol efflux observed in NPC cells (61). One must use caution, however, in interpreting results using inhibitors of cholesterol trafficking. Further experiments, perhaps using Caco-2 cells in which NPC1 expression has been attenuated, will be required to confirm the role of NPC1 in intestinal HDL-cholesterol transport.
Although not a direct substrate source for ABCA1, our results demonstrate that approximately 5–6% of newly synthesized cholesterol is eventually transported by ABCA1 to intestinal HDL-cholesterol. Hours elapsed, however, before newly synthesized cholesterol was found in the basolateral medium in response to LXR activation. If our postulate is correct, that it is membrane cholesterol that is the final substrate source for ABCA1 transport, then it would be expected that newly synthesized cholesterol is first transported to the plasma membrane before reaching the ABCA1 pool. Indeed, in the presence of filipin in the apical medium, the efflux of newly synthesized cholesterol was significantly attenuated, suggesting that it was initially trafficking to the apical membrane. This agrees with our previous findings in Caco-2 cells (38), and those of others in other cell types (73, 79–81), suggesting that most newly synthesized cholesterol trafficks to the cell surface. Obviously, following equilibration of the newly synthesized cholesterol with membrane cholesterol, the newly synthesized cholesterol will eventually enter the ABCA1 cholesterol pool. The present data also suggest that newly synthesized cholesterol is not required for intestinal HDL production. Inhibiting cholesterol synthesis with lovastatin did not alter ABCA1-mediated cholesterol efflux. This result makes sense and supports animal and human data showing that statins, potent inhibitors of cholesterol synthesis, either do not significantly alter plasma HDL-cholesterol levels or modestly increase them (82).
The real importance of intestinal ABCA1 is that it facilitates the basal efflux of cholesterol to apoA-I and contributes to approximately 30% of the circulating HDL-cholesterol (17). Targeting ways to enhance the production of intestinal HDL-cholesterol would obviously be beneficial. We postulated that if luminal (dietary or biliary) cholesterol could be channeled to the ABCA1 pathway and away from the chylomicron pathway, it would be possible to divert luminal cholesterol from a potentially atherogenic pathway to an anti-atherogenic pathway. Clearly, inhibiting ACAT activity is not a rational approach. Despite previous data (31, 52, 83, 84) and our present data, which show an increase in ABCA1-mediated cholesterol efflux in cells incubated with an ACAT inhibitor, this does not translate clinically into higher plasma HDL-cholesterol levels. In ACAT2-deficient animals, or in animals or humans treated with ACAT inhibitors, HDL-cholesterol levels are unaltered (52, 85–87). Likewise, ezetimibe, which disrupts cholesterol trafficking from the apical membrane to ACAT by interfering with NPC1L1, does not divert more cholesterol to the HDL pathway. A different approach was used in a recent study by Brunham et al. (88). These investigators fed mice an intestinal-specific LXR agonist that increased intestinal ABCA1 gene expression by 6-fold and plasma HDL-cholesterol levels by almost 50%. Because this agent only targeted intestinal LXR, it did not cause fatty liver or hypertriglyceridemia, serious side effects that limit the use of nonspecific LXR agonists. It is clear, therefore, that if the expression of intestinal ABCA1 can be upregulated (in our opinion, the limiting factor in intestinal HDL production), HDL-cholesterol levels will increase, having a favorable effect on atherosclerosis.
Abbreviations
apoA-I, apolipoprotein A-I
LXR, liver X receptor
MTP, microsomal triglyceride transfer protein
NPC, Niemann-Pick type C
NPC1L1, Niemann-Pick C1-like 1 protein
Published, JLR Papers in Press, August 18, 2008.
Footnotes
This work was supported by National Institutes of Health Grant DK-067132.
References
- 1.Wang N., and A. R. Tall. 2003. Regulation and mechanisms of ATP-binding cassette transporter A1-mediated cellular cholesterol efflux. Arterioscler. Thromb. Vasc. Biol. 23 1178–1184. [DOI] [PubMed] [Google Scholar]
- 2.Brewer H. B., Jr., A. T. Remaley, E. B. Neufeld, F. Basso, and C. Joyce. 2004. Regulation of plasma high-density lipoprotein levels by the ABCA1 transporter and the emerging role of high-density lipoprotein in the treatment of cardiovascular disease. Arterioscler. Thromb. Vasc. Biol. 24 1755–1760. [DOI] [PubMed] [Google Scholar]
- 3.Brewer H. B., Jr. 2004. High-density lipoproteins: a new potential therapeutic target for the prevention of cardiovascular disease. Arterioscler. Thromb. Vasc. Biol. 24 387–391. [DOI] [PubMed] [Google Scholar]
- 4.Assmann G., H. Schulte, A. von Eckardstein, and Y. Huang. 1996. High-density lipoprotein cholesterol as a predictor of coronary heart disease risk. The PROCAM experience and pathophysiological implications for reverse cholesterol transport. Atherosclerosis. 124 (Suppl.): 11–20. [DOI] [PubMed] [Google Scholar]
- 5.Curb J. D., R. D. Abbott, B. L. Rodriguez, K. Masaki, R. Chen, D. S. Sharp, and A. R. Tall. 2004. A prospective study of HDL-C and cholesteryl ester transfer protein gene mutations and the risk of coronary heart disease in the elderly. J. Lipid Res. 45 948–953. [DOI] [PubMed] [Google Scholar]
- 6.Sharrett A. R., C. M. Ballantyne, S. A. Coady, G. Heiss, P. D. Sorlie, D. Catellier, and W. Patsch. 2001. Coronary heart disease prediction from lipoprotein cholesterol levels, triglycerides, lipoprotein(a), apolipoproteins A-I and B, and HDL density subfractions: The Atherosclerosis Risk in Communities (ARIC) Study. Circulation. 104 1108–1113. [DOI] [PubMed] [Google Scholar]
- 7.Gordon T., W. P. Castelli, M. C. Hjortland, W. B. Kannel, and T. R. Dawber. 1977. High density lipoprotein as a protective factor against coronary heart disease. The Framingham Study. Am. J. Med. 62 707–714. [DOI] [PubMed] [Google Scholar]
- 8.Linsel-Nitschke P., and A. R. Tall. 2005. HDL as a target in the treatment of atherosclerotic cardiovascular disease. Nat. Rev. Drug Discov. 4 193–205. [DOI] [PubMed] [Google Scholar]
- 9.Francis G. A., R. H. Knopp, and J. F. Oram. 1995. Defective removal of cellular cholesterol and phospholipids by apolipoprotein A-I in Tangier Disease. J. Clin. Invest. 96 78–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Asztalos B. F. 2004. High-density lipoprotein metabolism and progression of atherosclerosis: new insights from the HDL Atherosclerosis Treatment Study. Curr. Opin. Cardiol. 19 385–391. [DOI] [PubMed] [Google Scholar]
- 11.Yokoyama S. 2006. Assembly of high-density lipoprotein. Arterioscler. Thromb. Vasc. Biol. 26 20–27. [DOI] [PubMed] [Google Scholar]
- 12.Bodzioch M., E. Orso, J. Klucken, T. Langmann, A. Bottcher, W. Diederich, W. Drobnik, S. Barlage, C. Buchler, M. Porsch-Ozcurumez, et al. 1999. The gene encoding ATP-binding cassette transporter 1 is mutated in Tangier disease. Nat. Genet. 22 347–351. [DOI] [PubMed] [Google Scholar]
- 13.Rust S., M. Rosier, H. Funke, J. Real, Z. Amoura, J. C. Piette, J. F. Deleuze, H. B. Brewer, N. Duverger, P. Denefle, et al. 1999. Tangier disease is caused by mutations in the gene encoding ATP-binding cassette transporter 1. Nat. Genet. 22 352–355. [DOI] [PubMed] [Google Scholar]
- 14.Schaefer E. J., L. A. Zech, D. E. Schwartz, and H. B. Brewer, Jr. 1980. Coronary heart disease prevalence and other clinical features in familial high-density lipoprotein deficiency (Tangier disease). Ann. Intern. Med. 93 261–266. [DOI] [PubMed] [Google Scholar]
- 15.Clee S. M., J. J. Kastelein, M. van Dam, M. Marcil, K. Roomp, K. Y. Zwarts, J. A. Collins, R. Roelants, N. Tamasawa, T. Stulc, et al. 2000. Age and residual cholesterol efflux affect HDL cholesterol levels and coronary artery disease in ABCA1 heterozygotes. J. Clin. Invest. 106 1263–1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Timmins J. M., J. Y. Lee, E. Boudyguina, K. D. Kluckman, L. R. Brunham, A. Mulya, A. K. Gebre, J. M. Coutinho, P. L. Colvin, T. L. Smith, et al. 2005. Targeted inactivation of hepatic Abca1 causes profound hypoalphalipoproteinemia and kidney hypercatabolism of apoA-I. J. Clin. Invest. 115 1333–1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brunham L. R., J. K. Kruit, J. Iqbal, C. Fievet, J. M. Timmins, T. D. Pape, B. A. Coburn, N. Bissada, B. Staels, A. K. Groen, et al. 2006. Intestinal ABCA1 directly contributes to HDL biogenesis in vivo. J. Clin. Invest. 116 1052–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Berge K. E., H. Tian, G. A. Graf, L. Yu, N. V. Grishin, J. Schultz, P. Kwiterovich, B. Shan, R. Barnes, and H. H. Hobbs. 2000. Accumulation of dietary cholesterol in sitosterolemia caused by mutations in adjacent ABC transporters. Science. 290 1771–1775. [DOI] [PubMed] [Google Scholar]
- 19.Repa J. J., S. D. Turley, J. A. Lobaccaro, J. Medina, L. Li, K. Lustig, B. Shan, R. A. Heyman, J. M. Dietschy, and D. J. Mangelsdorf. 2000. Regulation of absorption and ABC1-mediated efflux of cholesterol by RXR heterodimers. Science. 289 1524–1529. [DOI] [PubMed] [Google Scholar]
- 20.Venkateswaran A., B. A. Laffitte, S. B. Joseph, P. A. Mak, D. C. Wilpitz, P. A. Edwards, and P. Tontonoz. 2000. Control of cellular cholesterol efflux by the nuclear oxysterol receptor LXR alpha. Proc. Natl. Acad. Sci. USA. 97 12097–12102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Peet D. J., S. D. Turley, W. Ma, B. A. Janowski, J. M. Lobaccaro, R. E. Hammer, and D. J. Mangelsdorf. 1998. Cholesterol and bile acid metabolism are impaired in mice lacking the nuclear oxysterol receptor LXR alpha. Cell. 93 693–704. [DOI] [PubMed] [Google Scholar]
- 22.Lehmann J. M., S. A. Kliewer, L. B. Moore, T. A. Smith-Oliver, B. B. Oliver, J. L. Su, S. S. Sundseth, D. A. Winegar, D. E. Blanchard, T. A. Spencer, et al. 1997. Activation of the nuclear receptor LXR by oxysterols defines a new hormone response pathway. J. Biol. Chem. 272 3137–3140. [DOI] [PubMed] [Google Scholar]
- 23.Luo Y., and A. R. Tall. 2000. Sterol upregulation of human CETP expression in vitro and in transgenic mice by an LXR element. J. Clin. Invest. 105 513–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Venkateswaran A., J. J. Repa, J. M. Lobaccaro, A. Bronson, D. J. Mangelsdorf, and P. A. Edwards. 2000. Human white/murine ABC8 mRNA levels are highly induced in lipid-loaded macrophages. A transcriptional role for specific oxysterols. J. Biol. Chem. 275 14700–14707. [DOI] [PubMed] [Google Scholar]
- 25.Repa J. J., G. Liang, J. Ou, Y. Bashmakov, J. M. Lobaccaro, I. Shimomura, B. Shan, M. S. Brown, J. L. Goldstein, and D. J. Mangelsdorf. 2000. Regulation of mouse sterol regulatory element-binding protein-1c gene (SREBP-1c) by oxysterol receptors, LXRalpha and LXRbeta. Genes Dev. 14 2819–2830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Murthy S., E. Born, S. N. Mathur, and F. J. Field. 2002. LXR/RXR activation enhances basolateral efflux of cholesterol in CaCo-2 cells. J. Lipid Res. 43 1054–1064. [DOI] [PubMed] [Google Scholar]
- 27.Ohama T., K. Hirano, Z. Zhang, R. Aoki, K. Tsujii, Y. Nakagawa-Toyama, K. Tsukamoto, C. Ikegami, A. Matsuyama, M. Ishigami, et al. 2002. Dominant expression of ATP-binding cassette transporter-1 on basolateral surface of Caco-2 cells stimulated by LXR/RXR ligands. Biochem. Biophys. Res. Commun. 296 625–630. [DOI] [PubMed] [Google Scholar]
- 28.Mulligan J. D., M. T. Flowers, A. Tebon, J. J. Bitgood, C. Wellington, M. R. Hayden, and A. D. Attie. 2003. ABCA1 is essential for efficient basolateral cholesterol efflux during the absorption of dietary cholesterol in chickens. J. Biol. Chem. 278 13356–13366. [DOI] [PubMed] [Google Scholar]
- 29.Iqbal J., K. Anwar, and M. M. Hussain. 2003. Multiple, independently regulated pathways of cholesterol transport across the intestinal epithelial cells. J. Biol. Chem. 278 31610–31620. [DOI] [PubMed] [Google Scholar]
- 30.Wong M. L., and J. F. Medrano. 2005. Real-time PCR for mRNA quantitation. Biotechniques. 39 75–85. [DOI] [PubMed] [Google Scholar]
- 31.Temel R. E., R. G. Lee, K. L. Kelley, M. A. Davis, R. Shah, J. K. Sawyer, M. D. Wilson, and L. L. Rudel. 2005. Intestinal cholesterol absorption is substantially reduced in mice deficient in both ABCA1 and ACAT2. J. Lipid Res. 46 2423–2431. [DOI] [PubMed] [Google Scholar]
- 32.Field F. J., E. Born, S. Murthy, and S. N. Mathur. 2001. Regulation of sterol regulatory element-binding proteins by cholesterol flux in CaCo-2 cells. J. Lipid Res. 42 1687–1698. [PubMed] [Google Scholar]
- 33.Chen H., E. Born, S. N. Mathur, and F. J. Field. 1993. Cholesterol and sphingomyelin syntheses are regulated independently in cultured human intestinal cells, CaCo-2: role of membrane cholesterol and sphingomyelin content. J. Lipid Res. 34 2159–2167. [PubMed] [Google Scholar]
- 34.Field F. J., E. Born, S. Murthy, and S. N. Mathur. 1998. Caveolin is present in intestinal cells: role in cholesterol trafficking? J. Lipid Res. 39 1938–1950. [PubMed] [Google Scholar]
- 35.Mendez A. J., G. Lin, D. P. Wade, R. M. Lawn, and J. F. Oram. 2001. Membrane lipid domains distinct from cholesterol/sphingomyelin-rich rafts are involved in the ABCA1-mediated lipid secretory pathway. J. Biol. Chem. 276 3158–3166. [DOI] [PubMed] [Google Scholar]
- 36.Orlandi P. A., and P. H. Fishman. 1998. Filipin-dependent inhibition of cholera toxin: evidence for toxin internalization and activation through caveolae-like domains. J. Cell Biol. 141 905–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rothberg K. G., Y. S. Ying, B. A. Kamen, and R. G. Anderson. 1990. Cholesterol controls the clustering of the glycophospholipid-anchored membrane receptor for 5-methyltetrahydrofolate. J. Cell Biol. 111 2931–2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Field F. J., E. Born, S. Murthy, and S. N. Mathur. 1998. Transport of cholesterol from the endoplasmic reticulum to the plasma membrane is constitutive in CaCo-2 cells and differs from the transport of plasma membrane cholesterol to the endoplasmic reticulum. J. Lipid Res. 39 333–343. [PubMed] [Google Scholar]
- 39.Chang T. Y., C. C. Chang, N. Ohgami, and Y. Yamauchi. 2006. Cholesterol sensing, trafficking, and esterification. Annu. Rev. Cell Dev. Biol. 22 129–157. [DOI] [PubMed] [Google Scholar]
- 40.Matveev S., X. Li, W. Everson, and E. J. Smart. 2001. The role of caveolae and caveolin in vesicle-dependent and vesicle-independent trafficking. Adv. Drug Deliv. Rev. 49 237–250. [DOI] [PubMed] [Google Scholar]
- 41.Field F. J., K. Watt, and S. N. Mathur. 2007. Ezetimibe interferes with cholesterol trafficking from the plasma membrane to the endoplasmic reticulum in CaCo-2 cells. J. Lipid Res. 48 1735–1745. [DOI] [PubMed] [Google Scholar]
- 42.Field F. J., E. Born, S. Murthy, and S. N. Mathur. 2002. Polyunsaturated fatty acids decrease the expression of sterol regulatory element-binding protein-1 in CaCo-2 cells: effect on fatty acid synthesis and triacylglycerol transport. Biochem. J. 368 855–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Maxfield F. R., and D. Wustner. 2002. Intracellular cholesterol transport. J. Clin. Invest. 110 891–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shetty S., E. R. Eckhardt, S. R. Post, and D. R. van der Westhuyzen. 2006. Phosphatidylinositol-3-kinase regulates scavenger receptor class B type I subcellular localization and selective lipid uptake in hepatocytes. Arterioscler. Thromb. Vasc. Biol. 26 2125–2131. [DOI] [PubMed] [Google Scholar]
- 45.Mendez A. J. 1995. Monensin and brefeldin A inhibit high density lipoprotein-mediated cholesterol efflux from cholesterol-enriched cells. Implications for intracellular cholesterol transport. J. Biol. Chem. 270 5891–5900. [DOI] [PubMed] [Google Scholar]
- 46.Sobo K., I. Le Blanc, P. P. Luyet, M. Fivaz, C. Ferguson, R. G. Parton, J. Gruenberg, and F. G. van der Goot. 2007. Late endosomal cholesterol accumulation leads to impaired intra-endosomal trafficking. PLoS ONE. 2 e851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yu L., J. Li-Hawkins, R. E. Hammer, K. E. Berge, J. D. Horton, J. C. Cohen, and H. H. Hobbs. 2002. Overexpression of ABCG5 and ABCG8 promotes biliary cholesterol secretion and reduces fractional absorption of dietary cholesterol. J. Clin. Invest. 110 671–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McNeish J., R. J. Aiello, D. Guyot, T. Turi, C. Gabel, C. Aldinger, K. L. Hoppe, M. L. Roach, L. J. Royer, J. de Wet, et al. 2000. High density lipoprotein deficiency and foam cell accumulation in mice with targeted disruption of ATP-binding cassette transporter-1. Proc. Natl. Acad. Sci. USA. 97 4245–4250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Plosch T., T. Kok, V. W. Bloks, M. J. Smit, R. Havinga, G. Chimini, A. K. Groen, and F. Kuipers. 2002. Increased hepatobiliary and fecal cholesterol excretion upon activation of the liver X receptor is independent of ABCA1. J. Biol. Chem. 277 33870–33877. [DOI] [PubMed] [Google Scholar]
- 50.Drobnik W., B. Lindenthal, B. Lieser, M. Ritter, T. Christiansen Weber, G. Liebisch, U. Giesa, M. Igel, H. Borsukova, C. Buchler, et al. 2001. ATP-binding cassette transporter A1 (ABCA1) affects total body sterol metabolism. Gastroenterology. 120 1203–1211. [DOI] [PubMed] [Google Scholar]
- 51.Groen A. K., V. W. Bloks, R. H. Bandsma, R. Ottenhoff, G. Chimini, and F. Kuipers. 2001. Hepatobiliary cholesterol transport is not impaired in Abca1-null mice lacking HDL. J. Clin. Invest. 108 843–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Repa J. J., K. K. Buhman, R. V. Farese, Jr., J. M. Dietschy, and S. D. Turley. 2004. ACAT2 deficiency limits cholesterol absorption in the cholesterol-fed mouse: impact on hepatic cholesterol homeostasis. Hepatology. 40 1088–1097. [DOI] [PubMed] [Google Scholar]
- 53.Brooks-Wilson A., M. Marcil, S. M. Clee, L. H. Zhang, K. Roomp, M. van Dam, L. Yu, C. Brewer, J. A. Collins, H. O. Molhuizen, et al. 1999. Mutations in ABC1 in Tangier disease and familial high-density lipoprotein deficiency. Nat. Genet. 22 336–345. [DOI] [PubMed] [Google Scholar]
- 54.Lawn R. M., D. P. Wade, M. R. Garvin, X. Wang, K. Schwartz, J. G. Porter, J. J. Seilhamer, A. M. Vaughan, and J. F. Oram. 1999. The Tangier disease gene product ABC1 controls the cellular apolipoprotein-mediated lipid removal pathway. J. Clin. Invest. 104 R25–R31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bortnick A. E., G. H. Rothblat, G. Stoudt, K. L. Hoppe, L. J. Royer, J. McNeish, and O. L. Francone. 2000. The correlation of ATP-binding cassette 1 mRNA levels with cholesterol efflux from various cell lines. J. Biol. Chem. 275 28634–28640. [DOI] [PubMed] [Google Scholar]
- 56.Vedhachalam C., P. T. Duong, M. Nickel, D. Nguyen, P. Dhanasekaran, H. Saito, G. H. Rothblat, S. Lund-Katz, and M. C. Phillips. 2007. Mechanism of ATP-binding cassette transporter A1-mediated cellular lipid efflux to apolipoprotein A-I and formation of high density lipoprotein particles. J. Biol. Chem. 282 25123–25130. [DOI] [PubMed] [Google Scholar]
- 57.Hansen G. H., J. Pedersen, L. L. Niels-Christiansen, L. Immerdal, and E. M. Danielsen. 2003. Deep-apical tubules: dynamic lipid-raft microdomains in the brush-border region of enterocytes. Biochem. J. 373 125–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zha X., A. Gauthier, J. Genest, and R. McPherson. 2003. Secretory vesicular transport from the Golgi is altered during ATP-binding cassette protein A1 (ABCA1)-mediated cholesterol efflux. J. Biol. Chem. 278 10002–10005. [DOI] [PubMed] [Google Scholar]
- 59.Chen W., Y. Sun, C. Welch, A. Gorelik, A. R. Leventhal, I. Tabas, and A. R. Tall. 2001. Preferential ATP-binding cassette transporter A1-mediated cholesterol efflux from late endosomes/lysosomes. J. Biol. Chem. 276 43564–43569. [DOI] [PubMed] [Google Scholar]
- 60.Choi H. Y., B. Karten, T. Chan, J. E. Vance, W. L. Greer, R. A. Heidenreich, W. S. Garver, and G. A. Francis. 2003. Impaired ABCA1-dependent lipid efflux and hypoalphalipoproteinemia in human Niemann-Pick type C disease. J. Biol. Chem. 278 32569–32577. [DOI] [PubMed] [Google Scholar]
- 61.Boadu E., H. Y. Choi, D. W. Lee, E. I. Waddington, T. Chan, B. Asztalos, J. E. Vance, A. Chan, G. Castro, and G. A. Francis. 2006. Correction of apolipoprotein A-I-mediated lipid efflux and high density lipoprotein particle formation in human Niemann-Pick type C disease fibroblasts. J. Biol. Chem. 281 37081–37090. [DOI] [PubMed] [Google Scholar]
- 62.Repa J. J., J. M. Dietschy, and S. D. Turley. 2002. Inhibition of cholesterol absorption by SCH 58053 in the mouse is not mediated via changes in the expression of mRNA for ABCA1, ABCG5, or ABCG8 in the enterocyte. J. Lipid Res. 43 1864–1874. [DOI] [PubMed] [Google Scholar]
- 63.Altmann S. W., H. R. Davis, Jr., L. J. Zhu, X. Yao, L. M. Hoos, G. Tetzloff, S. P. Iyer, M. Maguire, A. Golovko, M. Zeng, et al. 2004. Niemann-Pick C1 Like 1 protein is critical for intestinal cholesterol absorption. Science. 303 1201–1204. [DOI] [PubMed] [Google Scholar]
- 64.Davis H. R., Jr., L. J. Zhu, L. M. Hoos, G. Tetzloff, M. Maguire, J. Liu, X. Yao, S. P. Iyer, M. H. Lam, E. G. Lund, et al. 2004. Niemann-Pick C1 Like 1 (NPC1L1) is the intestinal phytosterol and cholesterol transporter and a key modulator of whole-body cholesterol homeostasis. J. Biol. Chem. 279 33586–33592. [DOI] [PubMed] [Google Scholar]
- 65.Garcia-Calvo M., J. Lisnock, H. G. Bull, B. E. Hawes, D. A. Burnett, M. P. Braun, J. H. Crona, H. R. Davis, Jr., D. C. Dean, P. A. Detmers, et al. 2005. The target of ezetimibe is Niemann-Pick C1-Like 1 (NPC1L1). Proc. Natl. Acad. Sci. USA. 102 8132–8137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tang W., Y. Ma, L. Jia, Y. A. Ioannou, J. P. Davies, and L. Yu. 2008. Niemann-Pick C1-like 1 is required for an LXR agonist to raise plasma HDL cholesterol in mice. Arterioscler. Thromb. Vasc. Biol. 28 448–454. [DOI] [PubMed] [Google Scholar]
- 67.Bays H. E., P. B. Moore, M. A. Drehobl, S. Rosenblatt, P. D. Toth, C. A. Dujovne, R. H. Knopp, L. J. Lipka, A. P. Lebeaut, B. Yang, et al. 2001. Effectiveness and tolerability of ezetimibe in patients with primary hypercholesterolemia: pooled analysis of two phase II studies. Clin. Ther. 23 1209–1230. [DOI] [PubMed] [Google Scholar]
- 68.Dujovne C. A., M. P. Ettinger, J. F. McNeer, L. J. Lipka, A. P. LeBeaut, R. Suresh, B. Yang, and E. P. Veltri. 2002. Efficacy and safety of a potent new selective cholesterol absorption inhibitor, ezetimibe, in patients with primary hypercholesterolemia. Am. J. Cardiol. 90 1092–1097. [DOI] [PubMed] [Google Scholar]
- 69.Knopp R. H., H. Gitter, T. Truitt, H. Bays, C. V. Manion, L. J. Lipka, A. P. LeBeaut, R. Suresh, B. Yang, and E. P. Veltri. 2003. Effects of ezetimibe, a new cholesterol absorption inhibitor, on plasma lipids in patients with primary hypercholesterolemia. Eur. Heart J. 24 729–741. [DOI] [PubMed] [Google Scholar]
- 70.Tremblay A. J., B. Lamarche, J. S. Cohn, J. C. Hogue, and P. Couture. 2006. Effect of ezetimibe on the in vivo kinetics of apoB-48 and apoB-100 in men with primary hypercholesterolemia. Arterioscler. Thromb. Vasc. Biol. 26 1101–1106. [DOI] [PubMed] [Google Scholar]
- 71.Pentchev P. G., H. S. Kruth, M. E. Comly, J. D. Butler, M. T. Vanier, D. A. Wenger, and S. Patel. 1986. Type C Niemann-Pick disease. A parallel loss of regulatory responses in both the uptake and esterification of low density lipoprotein-derived cholesterol in cultured fibroblasts. J. Biol. Chem. 261 16775–16780. [PubMed] [Google Scholar]
- 72.Kruth H. S., M. E. Comly, J. D. Butler, M. T. Vanier, J. K. Fink, D. A. Wenger, S. Patel, and P. G. Pentchev. 1986. Type C Niemann-Pick disease. Abnormal metabolism of low density lipoprotein in homozygous and heterozygous fibroblasts. J. Biol. Chem. 261 16769–16774. [PubMed] [Google Scholar]
- 73.Liscum L., R. M. Ruggiero, and J. R. Faust. 1989. The intracellular transport of low density lipoprotein-derived cholesterol is defective in Niemann-Pick type C fibroblasts. J. Cell Biol. 108 1625–1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Garver W. S., K. Krishnan, J. R. Gallagos, M. Michikawa, G. A. Francis, and R. A. Heidenreich. 2002. Niemann-Pick C1 protein regulates cholesterol transport to the trans-Golgi network and plasma membrane caveolae. J. Lipid Res. 43 579–589. [PubMed] [Google Scholar]
- 75.Wojtanik K. M., and L. Liscum. 2003. The transport of low density lipoprotein-derived cholesterol to the plasma membrane is defective in NPC1 cells. J. Biol. Chem. 278 14850–14856. [DOI] [PubMed] [Google Scholar]
- 76.Underwood K. W., B. Andemariam, G. L. McWilliams, and L. Liscum. 1996. Quantitative analysis of hydrophobic amine inhibition of intracellular cholesterol transport. J. Lipid Res. 37 1556–1568. [PubMed] [Google Scholar]
- 77.Lange Y., J. Ye, M. Rigney, and T. Steck. 2000. Cholesterol movement in Niemann-Pick type C cells and in cells treated with amphiphiles. J. Biol. Chem. 275 17468–17475. [DOI] [PubMed] [Google Scholar]
- 78.Koh C. H., and N. S. Cheung. 2006. Cellular mechanism of U18666A-mediated apoptosis in cultured murine cortical neurons: bridging Niemann-Pick disease type C and Alzheimer's disease. Cell. Signal. 18 1844–1853. [DOI] [PubMed] [Google Scholar]
- 79.Neufeld E. B., A. M. Cooney, J. Pitha, E. A. Dawidowicz, N. K. Dwyer, P. G. Pentchev, and E. J. Blanchette-Mackie. 1996. Intracellular trafficking of cholesterol monitored with a cyclodextrin. J. Biol. Chem. 271 21604–21613. [DOI] [PubMed] [Google Scholar]
- 80.Lange Y., J. Ye, and T. L. Steck. 1998. Circulation of cholesterol between lysosomes and the plasma membrane. J. Biol. Chem. 273 18915–18922. [DOI] [PubMed] [Google Scholar]
- 81.Cruz J. C., and T. Y. Chang. 2000. Fate of endogenously synthesized cholesterol in Niemann-Pick type C1 cells. J. Biol. Chem. 275 41309–41316. [DOI] [PubMed] [Google Scholar]
- 82.Ballantyne C. M., M. A. Blazing, D. B. Hunninghake, M. H. Davidson, Z. Yuan, P. DeLucca, K. E. Ramsey, C. M. Hustad, and J. Palmisano. 2003. Effect on high-density lipoprotein cholesterol of maximum dose simvastatin and atorvastatin in patients with hypercholesterolemia: results of the Comparative HDL Efficacy and Safety Study (CHESS). Am. Heart J. 146 862–869. [DOI] [PubMed] [Google Scholar]
- 83.Chang T. Y., C. C. Chang, and D. Cheng. 1997. Acyl-coenzyme A:cholesterol acyltransferase. Annu. Rev. Biochem. 66 613–638. [DOI] [PubMed] [Google Scholar]
- 84.Yamauchi Y., C. C. Chang, M. Hayashi, S. Abe-Dohmae, P. C. Reid, T. Y. Chang, and S. Yokoyama. 2004. Intracellular cholesterol mobilization involved in the ABCA1/apolipoprotein-mediated assembly of high density lipoprotein in fibroblasts. J. Lipid Res. 45 1943–1951. [DOI] [PubMed] [Google Scholar]
- 85.Homan R., and B. R. Krause. 1997. Established and emerging strategies for inhibition of cholesterol absorption. Curr. Pharm. Des. 3 29–34. [Google Scholar]
- 86.Harris W. S., C. A. Dujovne, K. von Bergmann, J. Neal, J. Akester, S. L. Windsor, D. Greene, and Z. Look. 1990. Effects of the ACAT inhibitor CL 277,082 on cholesterol metabolism in humans. Clin. Pharmacol. Ther. 48 189–194. [DOI] [PubMed] [Google Scholar]
- 87.Insull W., Jr., M. Koren, J. Davignon, D. Sprecher, H. Schrott, L. M. Keilson, A. S. Brown, C. A. Dujovne, M. H. Davidson, R. McLain, et al. 2001. Efficacy and short-term safety of a new ACAT inhibitor, avasimibe, on lipids, lipoproteins, and apolipoproteins, in patients with combined hyperlipidemia. Atherosclerosis. 157 137–144. [DOI] [PubMed] [Google Scholar]
- 88.Brunham L. R., J. K. Kruit, T. D. Pape, J. S. Parks, F. Kuipers, and M. R. Hayden. 2006. Tissue-specific induction of intestinal ABCA1 expression with a liver X receptor agonist raises plasma HDL cholesterol levels. Circ. Res. 99 672–674. [DOI] [PubMed] [Google Scholar]