Abstract
Sound duration can play a pivotal role in the reproductive behavior of anuran amphibians. Here, we report the first whole-cell recordings from duration-selective neurons in the anuran torus semicircularis, in vivo. We show that most short-pass duration-selective cells exhibited short-latency inhibition and delayed excitation. The duration of the inhibition increased with tone burst duration. Hence, for long-duration tone bursts, inhibition overlapped with excitation, reducing or eliminating spikes; no postinhibitory rebound was present. Other short-pass cells, however, showed inhibition only for long-duration tone bursts. Bandpass duration selectivity also involved interplay between inhibition and excitation; inhibition negated excitation with tone bursts that exceeded the optimum duration. Additionally, however, bandpass selectivity arose from stimulus-dependent excitation; tone bursts of sufficiently long duration were required to elicit excitation. Similarly, long-pass neurons showed inhibition and duration-dependent enhancement of excitation; long-pass selectivity resulted from enhanced excitation outlasting the transient inhibition or, in some cases, excitation overriding concurrent inhibition. Last, we evaluated the stimulus specificity of duration-selective neurons to variations in pulse repetition rate. We show that (1) most neurons that exhibited long-pass selectivity for tone-burst duration nonetheless responded to short-duration pulses when repeated at particular rates, and (2) some neurons that showed selectivity for tone burst duration also showed selectivity for pulse train duration. These novel response profiles appear to result from interplay between inhibition and time- and activity-dependent changes in excitation strength. These findings are discussed in the context of prevailing models of duration selectivity and acoustic communication in anurans.
Keywords: acoustic, auditory, in vivo, inferior colliculus, intracellular, patch clamp, temporal coding
Introduction
Duration is an important temporal feature of many acoustic signals (Suga, 1988; Masterton, 1992). For example, stereotypy in syllable and/or phoneme duration is critical for the recognition of human speech (Lehiste et al., 1976; Shannon et al., 1995). Echolocation systems of bats (for review, see Casseday et al., 2002) and communication systems of anurans (Potter, 1965; Narins and Capranica, 1980; Gooler and Feng, 1992) provide the most thoroughly studied associations between behaviorally relevant acoustic signals that vary in duration and their neural representations.
In anurans, temporal call characteristics play a pivotal role in reproductive isolation, mate choice, and coordination of reproductive behavior (Gerhardt and Huber, 2002; Rose and Gooler, 2007; Wells and Schwartz, 2007). In particular, pulse repetition rate (PRR) and pulse duration (PD) constitute the primary temporal acoustic features that enable most sympatric frog and toad species to differentiate between conspecific and heterospecific calls (Gerhardt, 1982, 1988). In anurans, as in mammals, short-pass, bandpass, and long-pass duration-selective neurons occur in the auditory midbrain, and their selectivity corresponds well with characteristics of species-typical calls (Potter, 1965; Narins and Capranica, 1980; Gooler and Feng, 1992).
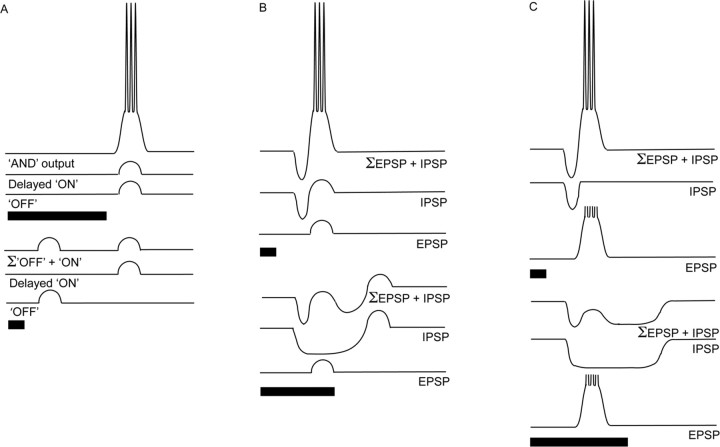
Several mechanistic models of duration tuning have been proposed (see Fig. 1). These models use either coincidence or anticoincidence mechanisms (for review, see Mora and Kössl, 2004). For example, Narins and Capranica (1980) proposed that duration tuning in the midbrain of the Puerto Rican treefrog, Eleutherodactylus coqui, could emerge from differences in the timing of phasic “ON” and “OFF” responses (see Fig. 1A). In this model, excitation of the OFF unit is triggered by the cessation of the stimulus, and excitation of the ON unit is delayed relative to the onset of the stimulus. Hence, for a particular tone burst duration, the two excitatory phases would coincide and trigger an excitatory “AND” response.
Figure 1.
Potential mechanisms for duration selectivity proposed by the following: Narins and Capranica, 1980 (A); Casseday et al., 1994, 2000; Covey et al., 1996; Ehrlich et al., 1997 (B); and Fuzessery and Hall, 1999 (C). For explanation, see Introduction.
Alternatively, Casseday et al. (1994) showed that duration tuning can arise from the interplay between excitation and inhibition (see Fig. 1B). In this model, inhibition persists for the duration of the stimulus, whereas the excitation is transient, subthreshold, and delayed relative to the onset of inhibition. Hence, for pulses of long-duration, delayed excitation overlaps with the inhibition. However, for pulses of shorter duration, excitation and rebound from inhibition coincide in time and elicit spikes. This model could also include coincidence of a second excitatory input tuned to the stimulus offset and, thus, may not require rebound from inhibition. Finally, Fuzessery and Hall (1999) proposed an “anticoincidence” variant of this model in which delayed excitatory inputs exceed threshold, and, hence, coincidence of excitation with inhibitory rebound is not required to elicit spikes (see Fig. 1C).
Less is known regarding the mechanisms that underlie long-pass selectivity. Recordings from long-pass inferior colliculus (IC) neurons in bats suggest that this selectivity can arise from transient inhibition and sustained excitation (Faure et al., 2003). Presumably, when the signal exceeds some duration, the decay in the transient inhibition allows the sustained excitatory input to bring the cell to threshold.
Currently, advances in understanding the mechanisms that underlie duration selectivity have come primarily from studies of mammals, although only a single intracellular study has been performed (Covey et al., 1996). A similar understanding has not been reached in anurans. For example, GABAergic inhibition is present in the anuran midbrain (Hall, 1999; Hollis and Boyd, 2004), but the role of inhibition, if any, in duration selectivity is unclear. Using whole-cell patch recordings, in vivo, we examined the response profiles of duration-selective units in the anuran IC and assessed how these response profiles relate to current mechanistic models.
Previous work on bats has shown that duration-selective units can also exhibit selectivity for PRR (Pinheiro et al., 1991; Jen and Wu, 2005), but the relationship between the specificity of these cells for duration and PRR is essentially unknown. In Pacific treefrogs, Hyla regilla, leopard frogs, Rana pipiens (the two species used in the current study), and many other anurans, advertising males produce pulse trains with stereotypical PD and PRR (Rose and Capranica, 1983; Rose and Brenowitz, 1997, 2002). Hence, a second objective of the present study was to determine whether duration-selective neurons are also selective for PRR.
Materials and Methods
Recording procedures.
H. regilla and R. pipiens were prepared for recording following the methods of Alder and Rose (2000). Briefly, frogs were immersed in 3% urethane, and a local anesthetic (lidocaine HCl) was applied topically to the dorsal surface of the skull in which a small opening was made to expose the optic tectum. Individuals were allowed to recover overnight from surgery and were subsequently immobilized with d-tubocurarine chloride for recording. Whole-cell patch intracellular recordings were made, in vivo, according to methods described in detail by Rose and Fortune, 1996) and Edwards et al., 2007). All procedures were approved by the University of Utah Institutional Animal Care and Use Committee.
Patch pipettes were constructed from borosilicate capillary glass (1 mm outer diameter, 0.58 mm inner diameter; catalog #5960; A-M Systems) using a Flaming-Brown type puller (model P-97; Sutter Instruments). These pipettes had outside tip diameters of ∼1–2 μm and had resistances between 15 and 25 MΩ. Electrode tips were backfilled with a solution (pH 7.4) consisting of (in mm) 100 potassium gluconate, 2 KCl, 1 MgCl2, 5 EGTA, 10 HEPES, 20 KOH, and biocytin at a concentration to bring the final osmolarity to ∼285 mOsm. Biocytin was replaced by mannitol in the solution used to fill pipette shanks.
Seal resistances were typically >2 GΩ with access resistances of 50 MΩ or less. Resting potentials of duration-selective neurons ranged from −94 to −48 mV (mean of −73 mV). Input resistances ranged from 104 MΩ to 1.2 GΩ (median of 571 MΩ).
The pipette was advanced into the brain using an “inchworm” microdrive (Burleigh Instruments) while applying positive pressure. After reaching the recording location, the pipette was advanced in 1.5 μm increments while maintaining positive pressure and passing −0.1 nA square-wave pulses (500 ms) to monitor resistance. Cell contact was indicated by a small increase (10%) in the voltage change. Negative pressure was then applied to the pipette to increase the seal resistance to gigaohm levels. Subsequent to seal formation, negative current (approximately −0.5 nA) was applied to rupture the patch and attain an intracellular recording.
Stimulus generation.
Search stimulus carrier frequencies were systematically varied from 300 to 2200 Hz with modulation frequencies (sinusoidal amplitude modulation) ranging from 20 to 100 Hz. Intracellular recordings were made in an audiometric chamber that was maintained at a constant 18°C. The advertisement calls of the R. pipiens and H. regilla have average PDs of ∼18 and 10 ms at this temperature, respectively. The average PRR at this temperature is ∼15 pulses/s for R. pipiens and 90 pulses/s in R. regilla. Acoustic stimuli were generated using Tucker Davis Technologies System II hardware and custom software (Alder and Rose, 2000). Stimuli were presented free field in an audiometric room (Alder and Rose, 2000). The speaker was situated 0.5 m from the animal and contralateral to the recording site. Unless stated otherwise, rise/fall times for tone burst stimuli were 1 ms.
Neurophysiological procedures and measurements.
To aid in examination of the relative contributions of inhibition and excitation to duration selectivity, recordings were made while the neuron was depolarized or hyperpolarized, respectively, to near the excitatory or inhibitory reversal potentials (current-clamp recording). Increased conductances in response to stimuli can shunt the excitation or inhibition, thereby obscuring their full amplitude. In some cases, we were able to isolate the relative contribution and time course of inhibition using stimulus carrier frequencies that were offset from the best excitatory frequency (BEF).
Results
To test the models shown in Figure 1, recordings were acquired from 22 neurons in the ICanuran of H. regilla and R. pipiens that exhibited duration-selective properties. Eleven of these units exhibited short-pass selectivity, 1 unit exhibited bandpass selectivity, and 10 units exhibited long-pass selectivity.
Short-pass neurons
Intracellular recordings from a representative short-pass neuron (Fig. 2) revealed an early hyperpolarization followed by a depolarization that, for short-duration tone bursts, elicited spike activity that persisted for at least 100 ms. The duration of the early hyperpolarization increased with tone burst duration and, for stimulus durations greater than ∼10–20 ms, was accompanied by reduced spike activity that was delayed in its onset. For tone durations of 80 and 160 ms, hyperpolarizations persisted for over 100 and 200 ms, respectively (Fig. 2). These observations suggest that the early hyperpolarization is inhibitory and tonic. There was no evidence of OFF excitation or postinhibitory rebound to long-duration tone bursts in this representative case, characteristic of the short-pass units sampled. For example, the longest duration tone burst (160 ms) did not elicit any obvious subthreshold depolarization after the inhibition (Fig. 2). This duration selectivity and pattern of inhibition and excitation was maintained over the entire (up to 27 dB above threshold) range of stimulus amplitude that was tested. The response strength was generally greater at low amplitudes (Fig. 2), suggesting that inhibition was recruited more strongly at the higher amplitudes.
Figure 2.
Averaged responses from a short-pass duration-selective unit to tone bursts that vary in duration. Responses were examined over three stimulus amplitudes: A, 72 dB SPL; B, 63 dB SPL; and C, 54 dB SPL. The number of spikes elicited over the number of stimulus presentations is shown above each trace. The carrier frequency was 1500 Hz, threshold was 43 dB SPL, and the resting potential was −60 mV. Spike amplitude is attenuated by the averaging process.
Recordings from another short-pass neuron (Fig. 3) further illustrate the interplay between tonic inhibition and delayed excitation in creating short-pass selectivity. As in the former case, the onset of inhibition appeared to precede the excitation. Also, the inhibition progressively overlapped with the excitation as the stimulus duration was increased (Fig. 3A). Strong responses were triggered for tone durations of 10 and 20 ms. However, for 30 ms tone bursts, spike rate was reduced to ∼25% of that for 10–20 ms bursts; the earliest phase of the excitatory response appeared to be attenuated by the inhibition. This attenuation was progressively extended with additional increases in tone burst duration, and depolarizations were subthreshold for durations greater than ∼50 ms. Inhibition completely overlapped with delayed excitation for tone bursts ≥100 ms (Fig. 3A).
Figure 3.
Averaged responses of a short-pass neuron to varying tone burst durations (A) and changes in carrier frequency (B). A, Inhibition was isolated by examining the responses to 20 ms, 300 Hz tone bursts (gray trace), a stimulus that evoked minimal excitation; stimulus carrier frequency was 750 Hz, the BEF, for all other traces. B, Responses to 20 ms (top) or 200 ms (bottom) tone bursts (frequencies shown), recorded under negative (−0.04 nA) or positive (+0.04 nA) current clamp, respectively. The time course of excitation with a 20 ms tone burst was similar to the time course of inhibition with a 200 ms tone burst. C, Time-expanded view of the initial responses to 800 Hz tone bursts (region denoted by the dashed box in B) to show latencies of excitation and inhibition. Depolarization peaks in negative current-clamp recordings are mirrored by corresponding peaks in positive current clamp (dashed lines). The stimulus amplitude was 65 dB SPL, threshold was 54 dB SPL, and the resting potential was −87 mV.
The relative timing of the excitation and inhibition for this neuron was most clearly seen in negative and positive current-clamp recordings, respectively (Fig. 3B,C). Presenting a 20 ms tone and recording with −0.04 nA current clamp emphasized the excitation (Fig. 3B, top row). The onset of the depolarization (latency of 31 ms) occurred ∼14 ms after the onset of the hyperpolarization (latency of 17 ms), which is most clearly seen in the positive current-clamp recordings of responses to 200 ms tone bursts (Fig. 3B,C, bottom row).
These findings suggest that short-pass duration selectivity results from the interplay between excitation and inhibition. Brief tone bursts elicit delayed excitation that persists well beyond the duration of the stimulus and the transient inhibition. The short time course of the inhibition is evident in recordings of responses to 20 ms, low-frequency (300 Hz) tone bursts (20 ms tone) (Fig. 3A, gray trace), which primarily recruited inhibition. This stimulus elicited a relatively brief hyperpolarization that reached peak amplitude ∼50 ms after stimulus onset. As with most short-pass units examined, the time course of inhibition only exceeded the time course of excitation when sufficiently long tone bursts were used, indicating that the time course of inhibition was dependent on stimulus duration. In the present example, the time course of inhibition to a 200 ms tone burst approximated the time course of excitation to a 20 ms tone burst (Fig. 3B). As in other short-pass cases examined, the frequency tuning of the inhibition generally spanned that of the excitation (Fig. 3B). There was no evidence of an OFF excitatory response or postinhibitory rebound in this case.
Although postinhibitory rebound excitation was not evident in the responses to long-duration tone bursts (Figs. 2, 3), we cannot exclude the possibility that it augments responses at short durations; strongest responses may result from the coincidence of subthreshold excitation and release from inhibition (Casseday et al., 1994; Covey et al., 1996). The neurons depicted in Figure 4, however, provided a partial test of this hypothesis. For example, for the neuron shown in Figure 4A, an inhibitory response was observed for tone bursts ≥10 ms and was only evident in positive current-clamp recordings (+0.04 nA, gray traces). As with the other short-pass units sampled, the duration of the inhibition appeared to be tonic and increased with the duration of the stimulus. The excitation, however, was long lasting regardless of stimulus duration (Fig. 4A). No inhibition could be seen for 5 ms tone bursts [the best duration (BD)] under positive current clamp, however, indicating that inhibition was only recruited by the longer tone bursts. This response property was characteristic of 3 of the 11 short-pass units sampled and suggests that postinhibitory rebound played little or no role in augmenting the excitation for this unit. One additional neuron provides further support for this hypothesis (Fig. 4B). In this case, the resting potential was approximately equal to the reversal potential for inhibition, thereby precluding postinhibitory rebound as a factor in amplifying excitation. The depolarization in response to a 10 ms tone burst was sufficient to elicit spikes (Fig. 4B). Inhibition, however, was clearly present under positive current clamp and, for tone burst durations <50 ms, ended before the prolonged phase of excitation (Fig. 4B, gray traces). As in the previous neuron, the time course of the excitation was long lasting across a broad range of tone burst durations (Fig. 4B). The interplay between excitation and inhibition accounted for the bimodal nature of the averaged response profile of this cell to a 100 ms tone burst (Fig. 4B, dark trace). For this stimulus, however, excitation was insufficient to overcome the inhibition. The lack of any evidence of postinhibitory rebound in this representative case and others (Figs. 2, 3), in combination with the aforementioned response profiles, suggest that the mechanisms underlying short-pass duration selectivity were most consistent with an anticoincidence model (Fuzessery and Hall, 1999).
Figure 4.
A, Averaged responses of a short-pass neuron to 1500 Hz tone bursts (the BEF) ranging from 5 ms (the best duration) to 160 ms. Recordings made under positive current clamp (+0.04 nA) are shown as gray traces. Inhibition was only evident for tone bursts ≥10 ms, and the time course of inhibition corresponded with stimulus duration. Stimulus amplitude was 56 dB SPL, threshold was 48 db SPL, and resting potential was −83 mV. B, Responses for a second short-pass neuron at rest and under positive current clamp (+0.01 nA, gray traces). Inhibition was primarily masked in this cell because the resting potential of the cell (−95 mV) approximated that of the reversal potential for inhibition. The time course of excitation exceeded the time course for tonic inhibition, even with a stimulus tone burst of 100 ms. The stimulus amplitude was 51 dB SPL, threshold was 42 dB SPL, and the carrier frequency was 550 Hz.
The response properties of the neurons shown in Figures 2 and 3 were generally characteristic of the short-pass neurons sampled. Short-pass duration-selective units typically responded best to tone bursts that were less than ∼20 ms (BD range of 4–15 ms) but responded with few or no spikes to bursts that exceeded ∼30 ms (Fig. 5A). This reduction in spike activity with tone bursts that exceeded the BD was paralleled by an increase in first-spike latency (Fig. 5B). The increase in first-spike latency stemmed from the extension of inhibition as tone burst duration was increased, as shown in Figures 2–4. These response characteristics were generally consistent across neurons, with the exception of a single case that exhibited short-pass selectivity but showed a relatively small increase in first-spike latency with progressively longer tone bursts (Fig. 5B, open diamonds). In contrast with other short-pass neurons, the time course of inhibition for this cell only weakly tracked the stimulus duration (Fig. 6) and, therefore, failed to sufficiently counter the excitation. Hence, this cell responded weakly to long-duration tone bursts. Collectively, these results suggest that inhibition plays a major role in creating short-pass selectivity.
Figure 5.

A, Pulse duration versus spikes per stimulus repetition for six short-pass units representing the range of variability found across the short-pass units sampled. B, Pulse duration versus first-spike latency for the same units shown in A. C, Last-spike latencies for the six neurons shown in A and B as well as other short-pass units (open circles). The range of last-spike latencies are shown for each neuron across several presentations of best-duration tone bursts. The best duration is indicated in parentheses above the range of last spike latencies for each cell. Stimulus parameters of representative cells shown in A–C are as follows: BEF of 350 Hz, 66 dB SPL (filled circles); BEF of 700 Hz, 57 dB SPL (open triangles); BEF of 700 Hz, 69 dB SPL (filled squares); BEF of 1500 Hz, 63 dB SPL (open diamonds); BEF of 1500 Hz, 54 dB SPL (filled triangles); BEF of 500 Hz, 51 dB (denoted with an “X”).
Figure 6.
Time course of inhibition to tone bursts of varying duration for a short-pass neuron shown in Figure 5 (open diamonds). Tone burst durations were 2 ms (black, shortest inhibitory time course), 10 ms (dark gray), 20 ms (light gray), and 100 ms (black, longest inhibitory time course). Responses are averaged over 4–11 stimulus repetitions. The carrier frequency was 1500 Hz (the BEF), 63 dB SPL.
Short-pass neurons also showed excitation that persisted well after the stimulus (Figs. 2–4). The persistence of the excitation was quantified across cells by measuring the latency of the last spike to each stimulus presentation, thereby indicating how long after the onset of a best-duration tone burst suprathreshold depolarization was present (Fig. 5C). Cells with short last-spike latencies were generally more sharply tuned to tone burst duration. For example, neurons 1 and 5 (Fig. 5C) had relatively short last-spike latencies, exhibited relatively strong tonic inhibitory responses that tracked tone burst duration, and, as expected, were sharply duration tuned (Fig. 5A, open squares and filled triangles). In contrast, neuron 6 had consistently long last-spike latencies (Fig. 5C) and was more broadly tuned (Fig. 5A). The prominent exception was the cell, described earlier, that showed little change in first-spike latency as tone burst duration was increased (Fig. 5B, open diamonds). Although this cell had short last-spike latencies (neuron 2) (Fig. 5C), it responded over a relatively wide range of tone burst durations (Fig. 5A, open diamonds), apparently because the inhibition only weakly tracked pulse duration (Fig. 6).
Our results indicate that tonic inhibition plays a central role in short-pass selectivity. Short-pass duration selectivity could also arise, however, if the excitation was weaker for long-duration pulses. Although there was no evidence to suggest this was the case based on the responses of the two neurons shown in Figure 4, we further addressed this issue by examining traces from an additional short-pass neuron at various levels of current clamp (Fig. 7). For this cell, there was particularly good correspondence between the time course of excitation to short and long tone bursts under negative current-clamp conditions (Fig. 7), suggesting that the excitation was not weaker for long-duration tone bursts. Because we do not have negative current-clamp data for most cells, we cannot entirely rule out the possibility that a decrease in excitation contributes to the duration tuning in all short-pass neurons sampled.
Figure 7.
Averaged responses of a short-pass neuron to 20 ms (black trace) and 100 ms (gray trace) tone bursts. Recordings were made at 0 nA (top) and −0.025 nA current clamp (bottom). The time course of excitation, which was most apparent in negative current-clamp recordings, is highly similar for the two tone burst durations. Carrier frequency was 650 Hz (the BEF), 57 dB SPL. Resting potential was −81 mV.
Responses of short-pass neurons to repeated pulses
As an initial step in examining the specificity of the short-pass unit shown in Figure 2 to repeated pulses, we examined response profiles of this cell to two sequential 10 ms pulses (rise time 1 ms, carrier of 1500 Hz) presented at various interpulse intervals (time between the onsets of successive pulses) (Fig. 8). Consistent with other short-pass units (Figs. 3, 4), the inhibition in this cell preceded the excitation. The excitation overcame the inhibition and elicited spiking ∼60 ms after the onset of a 10 ms tone burst (Fig. 8, gray trace). Accordingly, a second sound pulse that occurred less than ∼60 ms after the first pulse triggered additional inhibition and further delayed spiking. For interpulse intervals of 15 and 30 ms, the excitation triggered by the first pulse summated with that from the second pulse, resulting in a spike rate that exceeded the response to a single 10 ms tone burst (7.1, 7.0 vs 5 spikes/repetition) (Figs. 2, 8). For this cell, temporally discrete groups of spikes were elicited by each pulse only when the interpulse interval was greater than ∼60 ms.
Figure 8.
Responses of a short-pass unit (also shown in Fig. 2) to stimuli that consisted of two 10 ms pulses (1500 Hz carrier frequency) that were presented at rates ranging from 11 to 67 pulses/s (91–15 ms interpulse intervals). Response to a single 10 ms pulse is shown in gray (top left). The stimulus amplitude was 54 dB SPL.
We further examined how interactions between inhibition and excitation influenced the responses of this cell for pulse trains that varied in PRR and duration. Despite the fact that there was no response to tone bursts >80 ms (Fig. 2), this neuron responded strongly to 10 pulses (PD of 10 ms) when presented at 80 pulses/s (12.5 ms interpulse intervals; 2.3 spikes/repetition) but not at all for PRRs of 20 and 40 pulses/s (Fig. 9). Even at 10 pulses/s, for which spikes were expected to occur relatively consistently to each pulse (based on responses to two pulses at a similar rate) (Fig. 8), spiking was infrequent and predominately occurred to the first three pulses in the series (Fig. 9). Although these stimuli have the same number of pulses, they differ in train duration. The strong responses to 80 pulses/s could, therefore, be attributable to the shorter duration of this pulse train. In support of this hypothesis, no spikes were elicited when the duration of an 80 pulses/s stimulus was increased to 400 ms (Fig. 9, gray trace). That is, this short-pass duration-selective unit was also highly selective for pulse train duration; a reduction in the number of pulses in the series elicited a stronger response.
Figure 9.
Averaged responses of cell depicted in Figures 2 and 8 to pulse trains that vary in pulse repetition rate and pulse train duration (using a pulse duration of 10 ms). The stimulus amplitude was 54 dB SPL.
Properties that confer selectivity for pulse train duration appeared similar to those that underlie selectivity for tone burst duration. For example, the duration of the inhibition tracked the duration of the pulse train (Fig. 9, 80 pps), and the excitation to the short train persisted for well after stimulus offset. Also, the pulses at the beginning of the train appeared to elicit greater excitation than those at the end. Thus, for long-duration pulse trains, the inhibition overlapped with, and effectively countered, the delayed excitation. Even when pulses were presented at rates that were sufficient for achieving temporal summation (40–80 pps) and elicited spikes when two pulses were presented, inhibition appeared to counter the excitation and prevented spiking (Fig. 9). It is presently unclear whether an increase in the strength of inhibition also contributed to this selectivity for pulse train duration.
Bandpass neurons
A single unit exhibited bandpass duration selectivity. Over at least an 18 dB range in stimulus amplitude, this cell responded maximally to tone bursts that had durations between 30 and 50 ms, with a large reduction in spike rate for stimulus durations below and above this optimal range (Fig. 10A). As with short-pass units, selectivity for mid-duration tone bursts appeared to arise, in part, from interactions between tonic inhibition and delayed excitation. However, there were several critical features that distinguished this cell from short-pass neurons and accounted for its bandpass selectivity. First, excitatory input appeared to depend on stimulus duration. Tone bursts that were 10 ms in duration elicited hyperpolarizations, a characteristic that was primarily independent of stimulus amplitude (Fig. 10A). EPSPs could not be seen in response to these short tone bursts, even when the neuron was hyperpolarized to near the reversal potential of the inhibition [Fig. 10A, top gray trace, 10 ms tone burst, 65 dB sound pressure level (SPL)]. These data suggest that, in this neuron, unlike short-pass cells, brief tone bursts elicited little, if any, excitation. Instead, short tone bursts primarily elicited inhibition. When the stimulus was sufficiently long (∼30–50 ms), sustained depolarizations (EPSPs) were elicited that triggered spikes for much of their duration, similar to the responses of short-pass neurons. As in the former short-pass examples, a long-duration tone burst was required for the time course of tonic inhibition to exceed that of the excitation (∼160 ms) (Fig. 10A), thereby contributing to its bandpass selectivity. To better view the time course of the inhibition (IPSP), we presented a 40 ms tone burst that had a frequency (300 Hz) that elicited inhibition but little or no excitation (Fig. 10A, gray trace, 40 ms tone burst, 65 dB SPL). Comparing this recording with the response to a 40 ms burst at the BEF of the cell shows that the IPSP peaked (vertical, dashed line) ∼4 ms before the onset of spike activity, i.e., the hyperpolarization began to decline as spike activity ensued. We also presented a 10 ms tone burst at the BEF while recording with +0.045 nA current clamp (Fig. 10A, bottom gray trace, 10 ms tone burst, 65 db SPL), such that the cell was depolarized to near its excitatory reversal potential. The time course of the inhibition was similar to that in response to the 300 Hz tone bursts. The bandpass selectivity of this cell increased with stimulus amplitude (Fig. 10A, compare top and bottom rows), suggesting that inhibition was stronger at the higher amplitudes.
Figure 10.
Averaged responses of a bandpass duration-selective unit to tone bursts of varying duration with amplitudes of 59, 65, and 71 dB SPL (A). The time course of inhibition was isolated from excitation for 40 ms tone bursts (65 dB SPL) using a 300 Hz carrier that recruited little excitation (gray trace). The time of the IPSP peak is marked by a vertical, dashed line. Top and bottom gray traces for 10 ms tone burst (65 dB SPL) are recordings under negative (−0.025 nA) and positive (+0.045 nA) current-clamp conditions, respectively. Gray trace for 160 ms tone burst (65 dB SPL) shows averaged response recorded at +0.07 nA current clamp. B, Averaged responses of the same neuron shown in A to an 800 Hz carrier signal, amplitude modulated at various rates and broadcast at 65 dB SPL. Gray trace, Averaged response to two cycles (pulses) of 20 Hz amplitude modulation. The BEF was 800 Hz. Also shown for each stimulus condition is the number of spikes per the number of stimulus presentations. Threshold was 51 dB SPL. The resting potential was −72 mV.
Consistent with its selectivity for pulse duration, this neuron also exhibited bandpass selectivity for amplitude modulation (AM) rate (Fig. 10B). This cell responded best to ∼20 Hz AM, which corresponds to a pulse duration of 50 ms. At this AM rate, the response to two pulses (Fig. 10B, gray trace) was nearly as strong as that to a sequence of eight pulses. It is unclear why the additional six pulses failed to appreciably increase the response. One possibility is that these pulses elicited little additional excitation. Alternatively, the decreased depolarization with successive pulses, also evident at slower pulse rates, may have resulted from increased inhibition. For AM rates below 20 Hz, pulse duration and rise time increased (slope decreased). Differences in pulse rise time, along with duration, could have contributed to the decreased response of the cell at AM rates of 5 and 10 Hz. Although this possibility cannot be ruled out entirely, the similar response levels for long-duration tone bursts and initial pulses in 5 Hz AM suggest that the decline in response at low AM rates was primarily related to the increase in pulse duration.
Long-pass neurons
Long-pass duration-selective neurons responded when tone bursts exceeded a particular duration. Two physiologically distinct types were found. An exemplar of the first type (Fig. 11A) responded to 50 or 100 ms, but not 20 ms, tone bursts. Increasing the amplitude of the 20 ms tone burst by 6 dB failed to elicit depolarizations comparable with those seen for 50 ms bursts (Fig. 11A); the maximum depolarization for 20 ms tone bursts at 71 dB SPL was ∼5 mV, whereas 50 ms bursts at amplitudes of 65 and 53 dB SPL (data not shown) elicited depolarizations of 29 and 24 mV, respectively. These results suggest that this selectivity was not simply attributable to energy differences between stimuli of different durations. The ineffectiveness of short-duration (20 ms) tone bursts appeared to result from inhibition (Fig. 11A, gray trace, recorded with +0.07 nA current clamp) overlapping with relatively weak excitation. In contrast, the enhanced excitation for the 50 ms tone burst persisted beyond the inhibition and elicited spikes; the time course of the inhibition appeared to be comparable for 20 and 50 ms tone bursts (Fig. 11A, gray traces).
Figure 11.
From left to right, Averaged responses of a long-pass neuron to tone burst stimuli of 20, 50, and 100 ms (A) and presentation of one to four stimulus pulses, at a rate of 70 pulses/s (B), carrier frequency of 300 Hz. A, Responses to 20 ms tone bursts at 65 dB SPL (bottom dark trace) and 71 dB SPL (top dark trace) are shown; for all other cases shown with dark traces, stimulus amplitude was 65 dB SPL. Gray traces in A are the averaged responses to 20 ms tone bursts (300 Hz) at 59 dB SPL and +0.07 nA current clamp (left) and to 50 ms tone bursts (600 Hz) at 60 dB SPL (middle). Gray traces in B are recordings made with +0.1 or −0.03 nA current clamp to reveal the time courses of inhibition or excitation, respectively. The BEF was 300 Hz. The resting potential was −68 mV. C, Averaged responses of an additional long-pass duration-selective neuron to varying tone burst durations. Gray traces are responses to individual 40 ms tone bursts; EPSPs are denoted with filled circles. All traces were made at the BEF (1200 Hz), and the stimulus amplitude was 75 dB SPL. The resting potential was −64 mV. Ratios are number of spikes per the number of stimulus presentations.
We also examined the responses of this cell to pulsed stimuli. Individual brief (14 ms) naturalistic sound pulses elicited primarily inhibition (Fig. 11B). This cell responded, however, when at least four pulses, each having a duration of just 14 ms, were presented at a PRR of ∼70 pulses/s (Fig. 11B). Thus, short-duration pulses that were primarily inhibitory when presented singly elicited suprathreshold depolarizations when presented in a series of at least four pulses. This interplay between inhibition and enhanced excitation at fast pulse repetition rates is characteristic of interval-counting neurons (Edwards et al., 2007).
To better reveal the time courses of inhibition and excitation, recordings were also made under positive and negative current-clamp conditions (Fig. 11B, bottom and top gray traces, respectively). In response to a single pulse, the inhibition slightly preceded and spanned the main excitation to the cell and had a duration of 47 ms, measured at half-maximal amplitude. IPSP duration increased to 62 ms when two pulses were delivered. This increase (from 47 to 62 ms) approximated the 14.4 ms interpulse interval and indicated that a second inhibitory event had occurred. A nonlinear increase in EPSP amplitude was also evident. Additional pulses, presented with the same interpulse interval, resulted in pronounced enhancement of the excitation that overcame the concurrent inhibition; for three pulses, the depolarization reached 90% of its maximum value 68 ms after stimulus onset, at which time the inhibition, measured from the two-pulse stimulus (gray trace), was at its peak. Thus, in this neuron, the interplay between enhanced excitation and inhibition differed slightly for tone bursts versus pulsed stimuli; for long-duration tone bursts, the excitation extended beyond the relatively transient inhibition. This was characteristic of 5 of the 10 long-pass neurons sampled.
Recordings shown in Figure 11C demonstrate, however, that long-pass duration selectivity can arise even if excitation and inhibition are concurrent. For this neuron, representative of the remaining long-pass cells, the time course of the inhibition increased with tone burst duration. The enhanced excitation with tone burst duration, however, was sufficient for overcoming the concurrent inhibition. This interplay was particularly evident in the response to 40 ms tone bursts. Examination of responses to individual 40 ms tone bursts (Fig. 11C, gray traces) revealed EPSPs that progressively increased in amplitude, similar to that seen for interval-counting neurons to pulsed stimuli (Edwards et al., 2007). These results are consistent with a model proposed by Edwards and Rose, 2003) for AM band-suppression selectivity wherein long-duration pulses elicit a series of appropriately timed spikes in afferents to these neurons.
Cells of this type respond to slow and fast rates of sinusoidal AM but not to intermediate rates and are known to be a class of interval-counting neuron (Edwards and Rose, 2003). Accordingly, the recordings in Figure 12 show that, although stimulus energy is constant across modulation rates, the cell shown in Figure 11, A and B, responded only at very slow (5, 10 Hz) and fast (60, 80 Hz) AM rates.
Figure 12.
Responses to sinusoidally amplitude-modulated stimuli (5–80 Hz), carrier frequency 300 Hz. Successive traces are shifted to avoid overlap of spikes. Recordings are from the same neuron as shown in Figure 11A,B. The stimulus amplitude was 65 dB SPL.
The second type of long-pass duration-selective cell also responded to tone bursts only when they exceeded a particular duration but did not exhibit AM band-suppression characteristics; of the 10 long-pass neurons examined, one cell exhibited these characteristics (Fig. 13). As with the former long-pass and bandpass units, short-duration tone bursts elicited relatively weak excitation in this cell (Fig. 13A). Extensive recordings were made at stimulus amplitudes of 76 and 82 dB SPL. Over this limited range, this neuron showed level-tolerant long-pass, or weak bandpass, selectivity. Inhibition, although not easily discerned in recordings without current injection, was evident in recordings made under positive current clamp (Fig. 13A, gray traces) and contributed to the repolarization phase of the responses to the 10 and 20 ms tone bursts. Consistent with its long-pass selectivity, this cell responded best to slow AM rates, e.g., 5–10 Hz AM, and this selectivity was apparent when the pulse repetition rate was held constant but the AM duty cycle was reduced to 0.5, which halved pulse duration (Fig. 13B, gray traces). The effects of this reduction in pulse duration were particularly apparent in responses to 10 Hz AM, in which EPSP amplitude was markedly reduced (Fig. 13B) and only one spike was elicited in 21 stimulus presentations.
Figure 13.
A, Averaged responses of a representative long-pass duration-selective neuron to tone bursts (1800 Hz) that varied in duration. Stimulus amplitudes were 76 and 82 dB SPL for bottom and top recordings, respectively. Gray traces are averaged recordings made with +0.04 nA current clamp. B, Representative and averaged recordings of responses of this neuron to an 1800 Hz carrier signal that was amplitude modulated at rates of (left to right) 5, 10, 20, 50, or 100 Hz; responses for 1.0 (black) and 0.5 (gray) duty cycle modulation are shown. The stimulus amplitude was 76 dB SPL. Ratios are number of spikes per the number of stimulus presentations. Threshold was 67 dB SPL, and the BEF was 1800 Hz. The resting potential was −67 mV.
Discussion
Our results provide the first description of intracellular response profiles for duration-selective units in the anuran auditory midbrain and, thus, provide insights into the potential mechanisms underlying duration selectivity. We also have shown that some duration-selective neurons exhibit selectivity to PRR and pulse train duration, suggesting that these cells could contribute to temporal processing and selective filtering in ways that have not been recognized previously.
Current models for duration selectivity of auditory neurons use either coincidence or anticoincidence mechanisms (for review, see Mora and Kössl, 2004). Coincidence detection models predict that subthreshold excitatory inputs elicit spikes when they summate. For instance, Narins and Capranica (1980) proposed that duration tuning in the frog midbrain could be generated by integrating subthreshold, phasic ON and OFF responses that differ in latency; for a particular range of durations, these responses will overlap and, when combined in a logical AND manner, trigger spikes (Fig. 1A). Such a mechanism could create short-pass, bandpass, or long-pass duration selectivity through shifts in the delay of the ON response. Alternatively, coincidence detection mechanisms can involve the interplay between inhibition and excitation; subthreshold postinhibitory rebound coincides with subthreshold excitation that is delayed and transient to create duration selectivity (Fig. 1B). Such a model can account for short and/or bandpass duration selectivity (Casseday et al., 1994, 2000; Covey et al., 1996; Ehrlich et al., 1997). Yet, short-pass duration selectivity does not necessarily require coincidence detection mechanisms. Selective responses to short-duration tone bursts could arise from integrating tonic inhibition and suprathreshold excitation that is delayed and transient; for long-duration stimuli, inhibition would overlap with the delayed excitation and reduce or eliminate spiking (Fig. 1C) (Fuzessery and Hall, 1999).
Our results indicate that short-pass duration-selective units in the ICanuran exhibit sustained inhibition for the duration of tone bursts and delayed excitation, characteristics that are consistent with the latter two models. The depolarizations that result from excitation after short-duration tone bursts, e.g., 10–30 ms, exceed threshold levels for >100 ms. That is, the excitation persists beyond the inhibition. For long-duration tone bursts, however, inhibition overlaps with excitation, thereby reducing or eliminating spikes. This relationship causes the cell to track the offset of the stimulus. Hence, behavioral decisions based on analysis of the duration of the pulses would not be expected to be complete until well after the stimulus has occurred. Postinhibitory rebound might have contributed to the strength of responses to short-duration tone bursts, but it was not evident after long-duration tone bursts. Furthermore, strong short-pass selectivity was also seen in cells that showed no apparent inhibition to short-duration tone bursts; inhibition was strongest for long-duration tone bursts. In one other case, inhibition was elicited by short-duration tone bursts, but the inhibitory reversal potential was near the resting potential. Thus, the short-pass selectivity of these neurons does not appear to require coincidence of subthreshold excitation and postinhibitory rebound. Nevertheless, we cannot rule out the possibility that postinhibitory rebound for short-duration tone bursts contributed to short-pass selectivity in some neurons. Additional experimental procedures (e.g., blocking inhibition, determining the time course of inhibition from current-clamp experiments) will be required to further evaluate the extent to which short-pass selectivity in the anuran midbrain arises through coincidence or anticoincidence mechanisms.
Results from a single bandpass duration-selective ICanuran unit also suggested that the mechanisms underlying bandpass selectivity arises from the interplay between tonic inhibition and delayed transient excitation; with progressively longer tone bursts, inhibition appeared to negate excitation. In contrast with short-pass units, however, bandpass selectivity appeared to be attributable, in part, to the enhancement of excitation that occurred as tone-burst duration was increased to its optimal value. For instance, short-duration stimuli (≈10 ms) did not elicit any detectable depolarization of the cell (Fig. 10A), whereas longer tone bursts effectively recruited excitation. At the tone burst duration that best excited the cell, spiking occurred slightly after the inhibition had reached its peak and was decreasing. Thus, although no postinhibitory rebound was observed when only inhibition was present, the peak activity occurs during the release from inhibition.
Similar conclusions were reached for long-pass duration-selective units; a combination of local interplay between inhibition and excitation, and duration-dependent enhancement, appeared to contribute to long-pass selectivity in the IC anuran. In these cells, excitation and inhibition appeared to be highly temporally congruent for short-duration tone bursts. In some cells, longer-duration tone bursts elicited excitation that extended beyond the relatively transient inhibition and elicited spikes. Hence, long-pass selectivity in these neurons appears to result in part from integrating phasic inhibition with relatively tonic excitation, as has been suggested to be the basis for long-pass duration selectivity in bats (Faure et al., 2003). In other cases, temporal incongruencies between inhibition and excitation did not appear to be required to create long-pass duration selectivity. In these cells, the inhibition appeared to track stimulus duration (as seen for short-pass neurons), but enhancement of the excitation was sufficient to elicit spikes despite the overlapping inhibition. Under these conditions, temporal offset of excitation and inhibition is, therefore, not required to produce long-pass duration selectivity. Additional experiments are necessary to better evaluate the roles of phasic inhibition and tonic excitation (and, thus, temporal incongruencies in excitation and inhibition) in long-pass duration selectivity,
PRR specificity
Interplay between excitation and inhibition also shaped the response pattern of a cell to PRR. Two notable patterns were observed: (1) specificity for short pulse train duration in short-pass duration-selective units and (2) AM band-suppression characteristics in long-pass duration-selective units. The former types of units, to our knowledge, have not been described previously and may provide a neural substrate for detecting calls with few consecutive pulses, characteristic of the vocal repertoire of many anuran species. The latter units responded to slow and fast PRRs, but not to intermediate PRRs, and are a class of interval-counting neuron (Edwards and Rose, 2003). Hence, as mentioned above, these long-pass duration-selective cells nonetheless also respond to short-duration pulses if presented at high, but biologically relevant, repetition rates.
The response properties of many short-pass duration-selective units to a pulse series presented at varying repetition rates were, in many cases, unexpected based on the other response properties of the cell. For example, the interplay between short-latency inhibition and longer-latency transient excitation appeared to govern the response properties to a two-pulse series; spiking was observed for both pulses in the series only when there was sufficient decay of inhibition from the preceding pulse, i.e., when the interval between consecutive pulses was sufficiently long (≥60 ms) (Fig. 8). These results suggested that, for slow PRRs, spiking would occur to each pulse regardless of the duration of the train. This was not the case. In fact, spiking predominately occurred in response to the first, and occasionally last, few pulses in a train when pulses were presented at slow rates (Fig. 9). Consistent with these results, and perhaps more surprising, was that these cells exhibited high specificity for the duration of trains of pulses that were presented at fast PRRs. For instance, the exemplar cell (shown in Figs. 8, 9) responded optimally to 2–10 pulses presented in rapid succession (∼80 pps) but did not spike to much longer pulse trains that had the same PRR. Response profiles suggested that specificity for short pulse trains resulted from interplay between inhibition and excitation. As was seen for short-duration tone bursts, short-duration pulse trains elicited inhibition and delayed, suprathreshold excitation. Hence, when few consecutive pulses were presented, excitation extended beyond the inhibition. However, with longer pulse trains, the inhibition overlapped with and countered the excitation. This raises the question of why the last few pulses in the train did not elicit additional delayed depolarization and trigger spikes. One possibility is that excitation depressed over time for long pulse trains. Alternatively, an increase in the strength and duration of inhibition may have contributed to this selectivity.
Specificity for short pulse trains has important implications in the acoustic communication systems of anurans. Calls of varying pulse train duration are characteristic of the advertisement calls of both R. pipiens and H. regilla and many other anuran species. For example, in H. regilla, males produce diphasic advertisement vocalizations; the two “phases” are similar in PRR but differ in pulse number (for review, see Brenowitz et al., 2001). It is plausible that specificity of some short-pass duration-selective cells provide a neural substrate for the detection of short pulse trains of such calls.
Nine of the 10 long-pass neurons showed interval-counting properties and, therefore, responded to short-duration pulses provided that a sufficient number were presented at a fast rate. Cells of this type exhibited strong, facilitated excitation to short-duration pulses presented in rapid succession. Hence, these cells respond to single long tone bursts and rapid sequences of short-duration pulses but not to single short tone bursts. These cells are known to be interval-counting neurons (Edwards and Rose, 2003) and exhibit long-pass duration selectivity as well. These band-suppression, interval-counting neurons respond well to long-duration tone bursts apparently because these stimuli elicit sufficient optimal interspike intervals in the afferents (Edwards and Rose, 2003). To the best of our knowledge, this is the first evidence that long-pass neurons also show interval-counting properties (Edwards et al., 2002) and PRR selectivity. In these cells, enhancement of excitation overcomes inhibition at fast PRRs. Potential mechanisms of enhancement are discussed in a previous paper (Edwards et al., 2007). Only a single cell was recorded that did not respond to short-duration pulses, regardless of PRR, i.e., showed unconditional long-pass duration selectivity. Long-pass duration-selective neurons with AM band-suppression characteristics provide yet another example of how duration-selective cells potentially contribute to temporal processing and filtering of acoustic signals.
It is currently not known whether such cells also exist in the auditory midbrain of bats. Such cells could, potentially, perform dual functional roles in the bat echolocation system; long-duration calls are typically produced while searching for prey and are dramatically shortened and produced in rapid sequence during the pursuit of potential prey. Concordantly, such cells could provide the neural substrate for identification of various call types that often differ in duration and PRR in anurans.
Footnotes
This work was supported by grants from the National Institutes of Health. We thank Y. Hanabusa, J. Callaway, and Shushruth for technical assistance and E. Brenowitz and T. Bryenton for help collecting frogs.
References
- Alder TB, Rose GJ. Integration and recovery processes contribute to the temporal selectivity of neurons in the northern leopard frog, Rana pipiens. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2000;186:923–937. doi: 10.1007/s003590000144. [DOI] [PubMed] [Google Scholar]
- Brenowitz EA, Rose GJ, Alder T. The neuroethology of acoustic communication in Pacific treefrogs. In: Ryan MJ, editor. Anuran communication. Washington, DC: Smithsonian Institution; 2001. pp. 145–155. [Google Scholar]
- Casseday JH, Ehrlich D, Covey E. Neural tuning for sound duration: role of inhibitory mechanisms in the inferior colliculus. Science. 1994;264:847–850. doi: 10.1126/science.8171341. [DOI] [PubMed] [Google Scholar]
- Casseday JH, Erlich D, Covey E. Neural measurement of sound duration: control by excitatory-inhibitory interactions in the inferior colliculus. J Neurophysiol. 2000;84:1475–1487. doi: 10.1152/jn.2000.84.3.1475. [DOI] [PubMed] [Google Scholar]
- Casseday JH, Fremouw T, Covey E. The inferior colliculus: a hub for the central auditory system. In: Oertel D, Fay RR, Popper AN, editors. Integrative functions in the mammalian auditory pathway. New York: Springer; 2002. pp. 238–318. [Google Scholar]
- Covey E, Kauer JA, Casseday JH. Whole-cell patch-clamp recording reveals subthreshold sound-evoked postsynaptic current in the inferior colliculus of awake bats. J Neurosci. 1996;16:3009–3018. doi: 10.1523/JNEUROSCI.16-09-03009.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards CJ, Rose GJ. Interval-integration underlies amplitude modulation band-suppression selectivity in the anuran midbrain. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2003;189:907–914. doi: 10.1007/s00359-003-0467-2. [DOI] [PubMed] [Google Scholar]
- Edwards CJ, Alder TB, Rose GJ. Auditory midbrain neurons that count. Nat Neurosci. 2002;5:934–936. doi: 10.1038/nn916. [DOI] [PubMed] [Google Scholar]
- Edwards CJ, Leary CJ, Rose GJ. Counting on inhibition and rate-dependent excitation in the auditory system. J Neurosci. 2007;27:13384–13392. doi: 10.1523/JNEUROSCI.2816-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich D, Casseday JH, Covey E. Neural tuning to sound duration in the inferior colliculus of the big brown bat, Eptesicus fuscus. J Neurophysiol. 1997;77:2360–2372. doi: 10.1152/jn.1997.77.5.2360. [DOI] [PubMed] [Google Scholar]
- Faure PA, Fremouw T, Casseday JH, Covey E. Temporal masking reveals properties of sound-evoked inhibition in duration-tuned neurons of the inferior colliculus. J Neurosci. 2003;23:3052–3065. doi: 10.1523/JNEUROSCI.23-07-03052.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuzessery ZM, Hall JC. Sound duration selectivity in the pallid bat inferior colliculus. Hear Res. 1999;137:137–154. doi: 10.1016/s0378-5955(99)00133-1. [DOI] [PubMed] [Google Scholar]
- Gerhardt HC. Sound pattern recognition in some North American treefrogs (Anura: Hylidae): implications for mate choice. Am Zool. 1982;22:581–595. [Google Scholar]
- Gerhardt HC. Acoustic properties used in call recognition by frogs and toads. In: Fritszch B, Wilczynski W, Ryan MJ, Hetherington TE, Walkowiak W, editors. The evolution of the amphibian auditory system. New York: Wiley; 1988. pp. 275–294. [Google Scholar]
- Gerhardt HC, Huber F. Acoustic communication in insects and anurans. Chicago: University of Chicago; 2002. [Google Scholar]
- Gooler DM, Feng AS. Temporal coding in the frog midbrain: the influence of duration and rise-fall time on the processing of complex amplitude-modulated stimuli. J Neurophysiol. 1992;67:1–22. doi: 10.1152/jn.1992.67.1.1. [DOI] [PubMed] [Google Scholar]
- Hall JC. GABAergic inhibition shapes frequency tuning and modifies response properties in the midbrain of the leopard frog. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 1999;185:479–491. doi: 10.1007/s003590050409. [DOI] [PubMed] [Google Scholar]
- Hollis DM, Boyd SK. Distribution of GABA-like immunoreactive cell bodies in the brains of two amphibians, Rana catesbeiana and Xenopus laevis. Brain Behav Evol. 2004;65:127–142. doi: 10.1159/000082981. [DOI] [PubMed] [Google Scholar]
- Jen P H-S, Wu CH. The role of GABAergic inhibition in shaping the response size and duration selectivity of bat inferior collicular neurons to sound pulses in rapid sequences. Hear Res. 2005;202:222–234. doi: 10.1016/j.heares.2004.11.008. [DOI] [PubMed] [Google Scholar]
- Lehiste I, Olive JP, Streeter LA. Role of duration in disambiguating syntactically ambiguous sentences. J Acoust Soc Am. 1976;60:1199–1202. [Google Scholar]
- Masterton RB. Role of the central auditory system in hearing: the new direction. Trends Neurosci. 1992;15:280–285. doi: 10.1016/0166-2236(92)90077-l. [DOI] [PubMed] [Google Scholar]
- Mora EC, Kössl M. Ambiguities in sound-duration selectivity by neurons in the inferior colliculus of the bat Molossus molossus from Cuba. J Neurophysiol. 2004;91:2215–2226. doi: 10.1152/jn.01127.2003. [DOI] [PubMed] [Google Scholar]
- Narins PM, Capranica RR. Neural adaptations for processing the two-note call of the Puerto Rican treefrog, Eleutherodactylus coqui. Brain Behav Evol. 1980;17:48–66. doi: 10.1159/000121790. [DOI] [PubMed] [Google Scholar]
- Pinheiro AD, Wu M, Jen PH. Encoding repetition rate and duration in the inferior colliculus of the big brown bat Eptesicus fuscus. J Comp Physiol. 1991;169:69–85. doi: 10.1007/BF00198174. [DOI] [PubMed] [Google Scholar]
- Potter HD. Patterns of acoustically-evoked dischanges of neurons in the mesencephalon of the bullfrog. J Neurophysiol. 1965;28:1155–1184. doi: 10.1152/jn.1965.28.6.1155. [DOI] [PubMed] [Google Scholar]
- Rose G, Capranica RR. Temporal selectivity in the central auditory system of the leopard frog Rana pipiens. Science. 1983;219:1087–1089. doi: 10.1126/science.6600522. [DOI] [PubMed] [Google Scholar]
- Rose GJ, Brenowitz EA. Plasticity of aggressive thresholds in Hyla regilla: discrete accommodation to encounter calls. Anim Behav. 1997;53:353–361. [Google Scholar]
- Rose GJ, Brenowitz EA. Pacific treefrogs use temporal integration to differentiate advertisement from encounter calls. Anim Behav. 2002;63:1183–1190. [Google Scholar]
- Rose GJ, Fortune ES. New techniques for making whole-cell recordings from CNS neurons in vivo. Neurosci Res. 1996;26:89–94. doi: 10.1016/0168-0102(96)01074-7. [DOI] [PubMed] [Google Scholar]
- Rose GJ, Gooler DM. Function of the anuran central auditory system. In: Feng AS, Narins PM, Fay RH, Popper AH, editors. Hearing and sound communication in amphibians. Springer handbook of auditory research. New York: Springer; 2007. pp. 250–290. [Google Scholar]
- Shannon RV, Zeng FG, Kamath V, Wygonski J, Ekelid M. Speech recognition with primarily temporal cues. Science. 1995;270:303–304. doi: 10.1126/science.270.5234.303. [DOI] [PubMed] [Google Scholar]
- Suga N. Auditory neuroethology and speech processing: complex-sound processing by combination-sensitive neurons. In: Edelman GM, Gall WE, Cowan WM, editors. Auditory function: neurobiological bases of hearing. New York: Wiley; 1988. pp. 679–720. [Google Scholar]
- Wells KD, Schwartz JJ. The behavioral ecology of anuran communication. In: Feng AS, Narins PM, Fay RH, Popper AH, editors. Hearing and sound communication in amphibians. Springer handbook of auditory ressearch. New York: Springer; 2007. pp. 44–86. [Google Scholar]