Abstract
Exendin 4 (Ex4) is a glucagon-like peptide-1 receptor (GLP- 1R) agonist which is available as a short-acting injectable treatment for type 2 diabetes. Our aim was to characterize the long-term effects of elevated steady-state levels of Ex4 provided by in vivo gene therapy. We constructed a helper-dependent adenoviral (HDAd) vector for long-term expression of Ex4 in vivo. A high-fat diet (HFD)-induced obesity (DIO) mouse model was chosen to approximate the metabolic derangements seen in obese patients. Mice were treated with a single injection of HDAd-Ex4 and were monitored for 15 weeks. Both hepatic Ex4 RNA and plasma Ex4 were detectable at the end of the study. HDAd-Ex4 treatment improved glucose homeostasis without increasing insulin levels. However, there was evidence of enhanced insulin action and decreased gluconeogenic enzyme expression. HDAd-Ex4 caused decreased weight gain without detectable changes in food intake, in part, due to increases in energy expenditure (EE). HDAd-Ex4 DIO mice also had reduced hepatic fat and an improved adipokine profile. In the liver, there was decreased expression of genes that were involved in de novo fatty acid synthesis. These observations are important in considering the development of longer acting GLP-1R agonists for the treatment of type 2 diabetes.
INTRODUCTION
The “incretin effect” refers to the historical observation that intestinal factors, released in response to oral glucose or nutrients, cause lower plasma glucose levels and increased insulin levels compared to parenteral glucose (reviewed in ref. 1). Glucagon-like peptide-1 (GLP-1) is a major incretin hormone and is a cleavage product of the pro-glucagon peptide. It is secreted from intestinal L cells, but is degraded within minutes by proteases, mostly dipeptidyl peptidase 4.
GLP-1 receptor (GLP-1R) agonists augment glucose-stimulated insulin secretion from beta cells, while inhibiting glucagon secretion.1 They also increase satiety and promote weight loss, mediated in part by effects on gastric emptying and central nervous system appetite centers.2,3. These properties have stimulated investigations to extend the actions of GLP-1 mimetics for treatment of type 2 diabetes, either by producing a more stable peptide or nonpeptide agonist for parenteral administration, or by inhibiting dipeptidyl peptidase 4 in vivo, enhancing endogenous action of GLP-1.4–6 The gila monster (Heloderma suspectum) salivary peptide, exendin-4 (Ex4), shares homology with GLP-1 (ref. 7), enabling it to activate the receptor8 while resisting degradation by dipeptidyl peptidase 4. Ex4 is the first GLP-1R agonist to be marketed for treatment of type 2 diabetes, requiring twice daily injections (Exenatide).
Studies of GLP-1R agonists in animal models have mainly focused on glucose homeostasis, weight and food intake.9 There may be additional metabolic effects with long-term treatment1,10–12 but these are less well described. Gene therapy provides an approach to maintain an effective serum level of an expressed peptide for a prolonged period as opposed to repeated injections, with peaks and troughs. Recently, plasmid-based or first-generation adenoviral (FGAd) systems have been used to express GLP-1R agonists.13–16 However, these systems are relatively short term, with expression usually decreasing after the first week and dropping off significantly thereafter.13,15,16 In this study, we have chosen a helper-dependent (gutless) adenoviral (HDAd) vector because, unlike first-generation adenovirus, which contains a full complement of viral protein genes, HDAd encodes no viral proteins and has negligible toxicity.17–19 It is an ideal vector for studying the effects of long-term steady-state expression of a peptide. In mice, systemic administration of HDAd efficiently transduces the liver and can lead to lifetime transgene expression.19 Recent studies using GLP-1 gene therapy have used extreme genetic rodent models of obesity such as ob/ob, db/db, and Zucker diabetic fatty rats.13–16 These models have leptin or leptin receptor mutations which could confound the metabolic observations made in long-term studies, because leptin and GLP-1 may cooperate for effects on appetite.20,21 On the other hand, we have used a diet-induced obesity (DIO) model to emulate the metabolic derangements seen with obesity and type 2 diabetes. Combining HDAd and the DIO mouse model has allowed us to observe interesting metabolic changes that occur with long-term, steady-state levels of a GLP-1R agonist. This is relevant considering that very long-acting versions of GLP-1R agonists are under development for clinical treatment of type 2 diabetes.
RESULTS
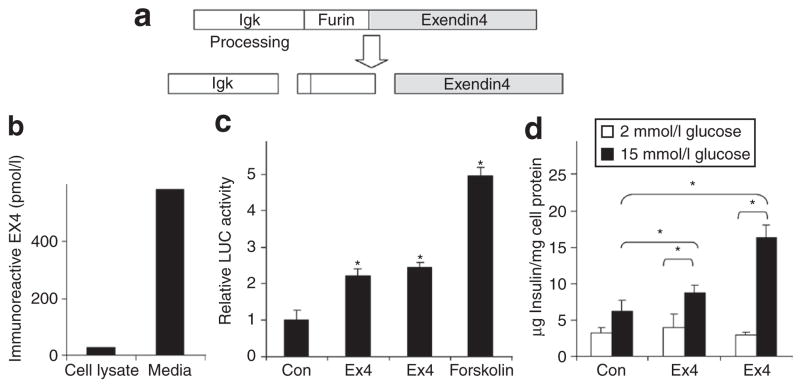
The Ex4 cassette produces immunoreactive and biologically active Ex4 in vitro and in vivo
The expression of Ex4 peptide from the constructed cassette (Figure 1a) was confirmed by transduction of Hep3B cells with HDAd-Ex4. Ex4 could be detected by enzyme immunoassay in the cell lysate with higher levels in the media (Figure 1b), confirming that immunoreactive Ex4 was expressed and secreted. The biological activity of the expressed peptide was confirmed by transfection of 832/13 insulinoma cells with CMV-Ex4. This resulted in (i) enhanced cyclic adenosine monophosphate–responsive element–driven luciferase (CRE-LUC) activity (Figure 1c) and (ii) augmented glucose-stimulated insulin secretion (Figure 1d), both expected of an active GLP-1R agonist. Mice injected with HDAd-Ex4 had immunoreactive Ex4 in plasma with a mean of 335 ± 152 pmol/l at 4 weeks (n = 10) and 159 ± 26 at 15 weeks. These levels are several fold higher than reported peak levels of endogenous human GLP-1 after a meal (~40 pmol/l)13,22 and steady-state Ex4 levels attained in human clinical studies with 10 μg injected exenatide (50 pmol/l) or 2 mg exenatide LAR (55 pmol/l).23 Ex4 transcripts also were detected in the liver using reverse transcription PCR at 15 weeks at the end of the study (data not shown).
Figure 1. The exendin 4 (Ex4) cassette expresses immunoreactive and biologically active secreted Ex4.
(a) Schematic diagram of the Ex4 expression cassette. (b) Transduction of Hep3B cells with HDAd-Ex4 (100 viral particles/cell) leads to detection of Ex4 in the cell lysate and the media after 48 hours as measured by enzyme immunoassay. (c) Rat 832/13 insulinoma cells were plated in 48-well dishes and cotransfected with 10 ng of pcDNA3.1 or pcDNA3.1-Ex4 (CMV-Ex4) and 400 ng of cyclic adenosine monophosphate–responsive element–driven luciferase reporter plasmid. After 48 hours, Ex4 peptide (10 nmol/l) or forskolin (10 μmol/l) was added in the indicated wells for an additional 6 hours before harvesting (*P < 0.05 compared to control). (d) 832/13 cells were transfected in quadruplicate with 10 ng of pcDNA3.1 or pcDNA3.1-Ex4. After 48 hours of expression, the media was removed and cells were treated with Hanks’ Balanced Salt Solution (HBSS) containing low (2 mmol/l) or high (15 mmol/l) glucose for 4 hours, with or without Ex4 peptide (10 nmol/l) as indicated. Immunoreactive insulin secreted into the HBSS was measured by enzyme-linked immunosorbent assay and normalized to total cell protein for each well (*P < 0.05 indicated by brackets). LUC, luciferase.
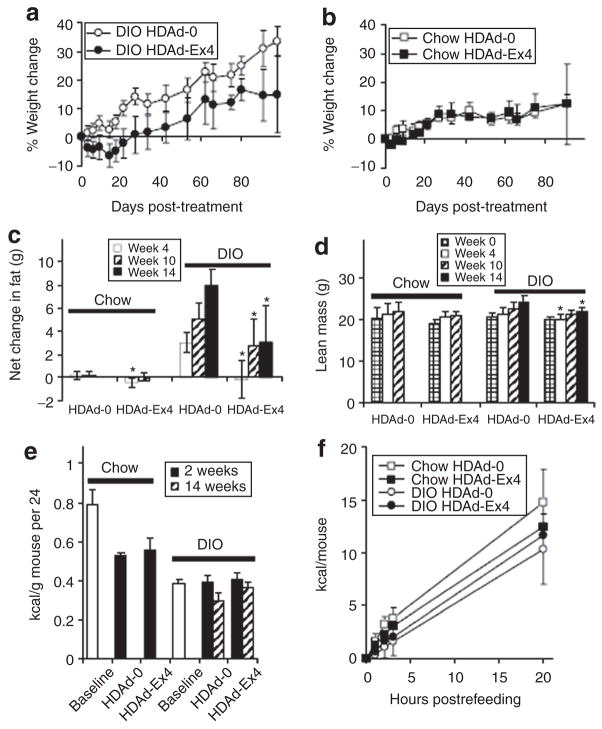
Improvement in weight without changes in food intake
Age-matched mice were started on regular chow or high-fat diet (HFD) at 6 weeks of age. After 8 weeks on these diets, the HFD-fed mice weighed an average of 31.78 g ± 2.71 while their chow-fed counterparts weighed 24.8 ± 2.07 g. At this point (14 weeks of age), mice were treated with HDAd. Control DIO HDAd-0 (empty vector) mice continued to gain weight throughout the study (Figure 2a), which was terminated 15 weeks after HDAd injection (29 weeks of age). In contrast, DIO HDAd-Ex4 mice had initial weight loss and then significantly reduced weight gain (Figure 2a). HDAd-Ex4 treatment of lean chow-fed mice did not cause differences in weight (Figure 2b). The increase in body fat in DIO mice also was slowed by HDAd-Ex4 treatment, as measured by magnetic resonance imaging (Figure 2c), while lean mass was mildly decreased at 4 and 14 weeks compared to DIO HDAd-0 mice (Figure 2d).
Figure 2. HDAd-Ex4 decreases weight gain and fat mass in diet-induced obesity (DIO) mice without detectable changes in food intake.
The % weight change from baseline is shown with time from helper-dependent adenovirus (HDAd) injection (14 weeks of age) is shown for (a) DIO (n = 10 in each group) and (b) chow-fed mice (n = 5 in each group). The change was significant for the DIO mice (P < 0.05) at all time points. The net change in (c) fat mass and the (d) lean mass was measured by magnetic resonance imaging (*P < 0.05 HDAd-Ex4 compared to HDAd-0). (e) Twenty-four-hour food intakes were measured for 5 days at baseline (DIO, n = 20; chow, n = 10) and at 2 and 14 weeks after HDAd injection (DIO groups, n = 10; chow groups, n = 5) and are shown as kilocalories per gram of mouse per 24 hours. (f) Acute food intake was also measured after a 24-hours fast (n = 5 each group). Values are mean ± SD. Ex4, exendin 4.
Baseline food intake was compared to that at 2 and 14 weeks after treatment (Figure 2e). There was no difference between the HDAd groups for either mouse model, whether calculated as kilocalories (kcal) per mouse or kcal/g of mouse (Figure 2e). Although the DIO mice gained more weight than chow-fed mice, they consumed similar kcal/day and fewer kcal/g of mouse, as was reported in another study using the DIO mouse model.24 Refeeding after a 24-hour fast at 4 weeks after HDAd injection also did not reveal any significant differences among the groups (Figure 2f). Interestingly, a parallel study in hyperphagic db/db mice showed that HDAd-Ex4 significantly decreased weight gain as well as food intake at 2 weeks after HDAd-Ex4 treatment (Supplementary Figure S1b and c).
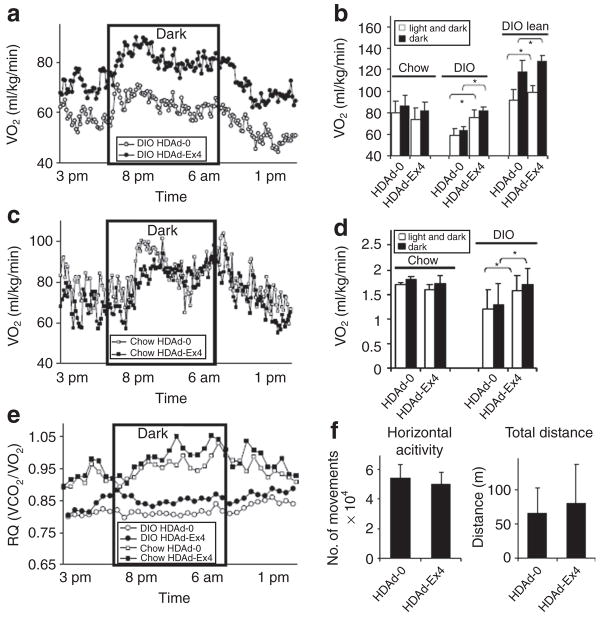
Alterations in metabolism measured by indirect calorimetry
We performed indirect calorimetry to investigate why HDAd-Ex4-treated DIO mice lost weight without detectable changes in food intake. DIO HDAd-Ex4 mice had higher oxygen consumption [VO2 (ml/kg/min)] than DIO HDAd-0 mice, even when calculated using lean mass (Figure 3a and b), and this was further reflected by their energy expenditure (EE) (Figure 3d). Treatment of db/db mice with HDAd-Ex4 also caused increased VO2 (Supplementary Figure S1d). The chow HDAd-Ex4 mice, which did not have sustained weight changes, did not have significant alterations in VO2 or EE (Figure 3b–d), indicating that the most significant effects of Ex4 are seen in the context of obesity and/or a HFD. The average respiratory quotient [Respiratory quotient (RQ) = VCO2/VO2)] was also higher in the DIO HDAd-Ex4 group compared to HDAd-0 (Figure 3e), with a lesser effect in the chow-fed HDAd-Ex4 mice, suggesting that there is more carbohydrate utilization in HDAd-Ex4-treated mice. In spite of the higher EE, we did not detect changes in activity in DIO HDAd-Ex4 mice measured as horizontal activity, total distance (Figure 3f), or total movement time (data not shown). These results were in contrast to a study reporting decreased VO2 and RQ with acute Ex4 injection of chow-fed mice,25 which we replicated using an intraperitoneal injection of Ex4 peptide at the beginning of the dark cycle. Consistent with the previous report,25 acute Ex4 injection led to decreases in VO2 and RQ (Supplementary Figure S2a and b). Therefore, dietary differences and/or long-term steady-state Ex4 levels affect metabolism differently compared to acute injections.
Figure 3. HDAd-Ex4 treatment alters metabolic rate.
Indirect calorimetry was measured for singly housed mice 4 weeks post-treatment for 22 hours from 3 PM to 1 PM the next day. The dark cycle was from 8 PM to 6 AM. (a) VO2 (ml/kg/min) of diet-induced obesity (DIO) mice over the light and dark cycle (n = 7 each group). (b) Average VO2 (ml/kg/min) of chow and DIO mice (c) VO 2 (ml/kg/min) of chow-fed mice over the light and dark cycle (n = 4 each group). (d) Calculated energy expenditure (EE). (e) The respiratory quotient (RQ) was calculated from the VCO 2-to-VO2 ratio. The average VO2 and calculated energy expenditure (kJ/kg/min) is compared among the different treatment groups for the total 22-hours light and dark cycle as well as the 10-hour dark cycle alone (*P < 0.05 HDAd-Ex4 compared to HDAd-0). (f) Activity measurements. Values indicate mean ± SD. Ex4, exendin 4; HDAd, helper-dependent adenovirus.
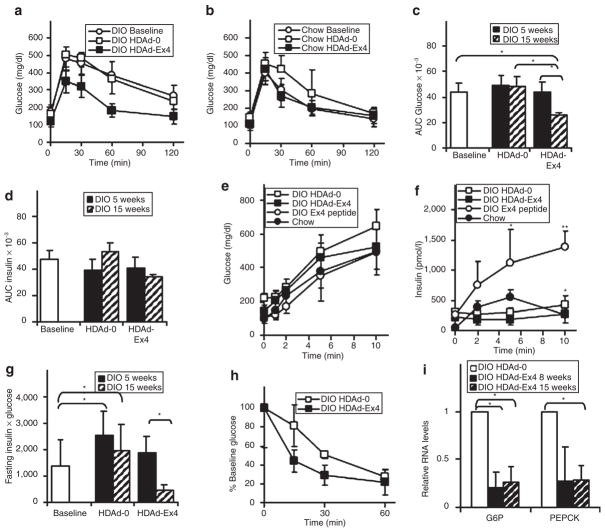
Improvements in glucose homeostasis
GLP-1R agonists are thought to improve glucose levels mainly by enhancing insulin secretion in the presence of high glucose. The DIO mice that received a single injection of HDAd-Ex4 15 weeks earlier showed marked improvement in glucose tolerance compared to baseline and DIO HDAd-0 mice (Figure 4a), with a significant decrease in the area under the curve (AUC) for glucose (Figure 4c). HDAd-Ex4 treatment of chow-fed mice led to mild improvement in age-related glucose intolerance (Figure 4b) but the decrease in the AUC glucose was not statistically significant (data not shown) suggesting that the major effects of Ex4 are seen in the DIO model. Unexpectedly, the AUC for insulin during glucose tolerance testing (Figure 4d) was no different among HDAd-treated DIO mice, suggesting that the improved glucose clearance in the HDAd-Ex4 mice was not due to increased insulin levels.
Figure 4. Glucose homeostasis is improved by HDAd-Ex4.
Intraperitoneal (IP) glucose tolerance tests were performed (1.5 mg glucose/g mouse IP) for (a) diet-induced obesity (DIO) mice (n = 10, each group) and (b) chow-fed mice (n = 5, each group) at 15 weeks post-treatment. The area under the curve (AUC) for (c) blood glucose and (d) plasma insulin was calculated (*P < 0.05 HDAd-Ex4 compared to helper-dependent adenovirus (HDAd)-0 at the same time point). Acute insulin secretion (3.0 mg glucose/g mouse IP) was performed for at 15 weeks post-HDAd injection for DIO HDAd-treated mice (n = 10, each group), chow-fed mice (n = 5) and DIO mice injected with 2.4 nmol/kg mouse of commercial exendin 4 (Ex4) (n = 5) for measurement of (e) blood glucose and (f) plasma insulin levels (*P < 0.05 HDAd-0 compared to HDAd-Ex4; **P <0.05 for Ex4 peptide injection compared to both HDAd-Ex4 and HDAd-0). (g) The product of the fasting insulin × fasting glucose was calculated (*P < 0.05). (h) An insulin tolerance test was performed with 1.0 unit/kg mouse insulin IP. (i) Relative liver RNA levels of glucose-6-phosphatase (G6P) and PEPCK were measured by quantitative PCR and normalized to cyclophilin. The HDAd-0 values were set at 1.0 for comparison with HDAd-Ex4 (*P < 0.05). Values are mean ± SD.
We measured acute insulin secretion to ascertain whether enhanced insulin release had occurred within the first 15 minutes after glucose injection and was overlooked by the standard glucose tolerance testing protocol. Glucose levels were not significantly different among the different groups (Figure 4e) and DIO mice injected with Ex4 at 2 hours before the test had the highest insulin levels (Figure 4f), revealing the expected enhancement of insulin secretion with acute Ex4 treatment. Surprisingly, insulin levels were lower for DIO HDAd-Ex4 mice compared to HDAd-0 (Figure 4f). This demonstrates that hepatic expression of Ex4, which achieves long-term and/or steady-state levels of GLP-1R agonists, may not enhance insulin secretion as with acute Ex4 injections, and is not the basis for the improved glucose homeostasis. We were unable to detect differences in insulin content or islet area in the pancreata of DIO HDAd-Ex4 and HDAd-0 mice (data not shown), possibly because both groups already had islet hyperplasia, compared to chow-fed mice, which is expected as a result of HFD and obesity.36
The significantly lower product of fasting insulin and glucose (Figure 4g) suggests improved insulin sensitivity, which is consistent with the results of an insulin tolerance test (Figure 4h). Interestingly, the most profound effects were observed at 15 weeks, compared to 5 weeks (Figure 4g), suggesting that chronic exposure to Ex4 is needed to see this improvement in glucose homeostasis.
The expression levels of key gluconeogenic genes, glucose-6-phosphatase and PEPCK, were reduced in the liver of HDAd-Ex4 mice (Figure 4i), suggesting that suppressed gluconeogenesis may also have a role in the improved glucose homeostasis observed in the DIO model. Because these genes are regulated by insulin, the decreased levels of gluconeogenic gene expression could be secondary to improved hepatic insulin sensitivity. GLP-1R agonists are also known to decrease the secretion of glucagon, which affects gluconeogenic gene expression.4,27 However, fasting glucagon levels were low and highly variable among the DIO mice (data not shown), so no conclusions can be drawn about possible contributions of decreased circulating glucagon in this model.
Adipokine profile improves with HDAd-Ex4 treatment
Multiple adipokines modulate insulin resistance,28 which appears to be altered in the HDAd-Ex4 DIO mice. We measured fasting levels of adiponectin, leptin, and resistin at 10 weeks after HDAd injection (Figure 5a). Although leptin did not differ between the DIO groups, adiponectin was increased and resistin was decreased in the DIO HDAd-Ex4 group. Adiponectin was also increased in the chow-fed HDAd-Ex4 mice (P = 0.05) (data not shown).
Figure 5. Adipokine levels are altered by HDAd-Ex4 treatment.
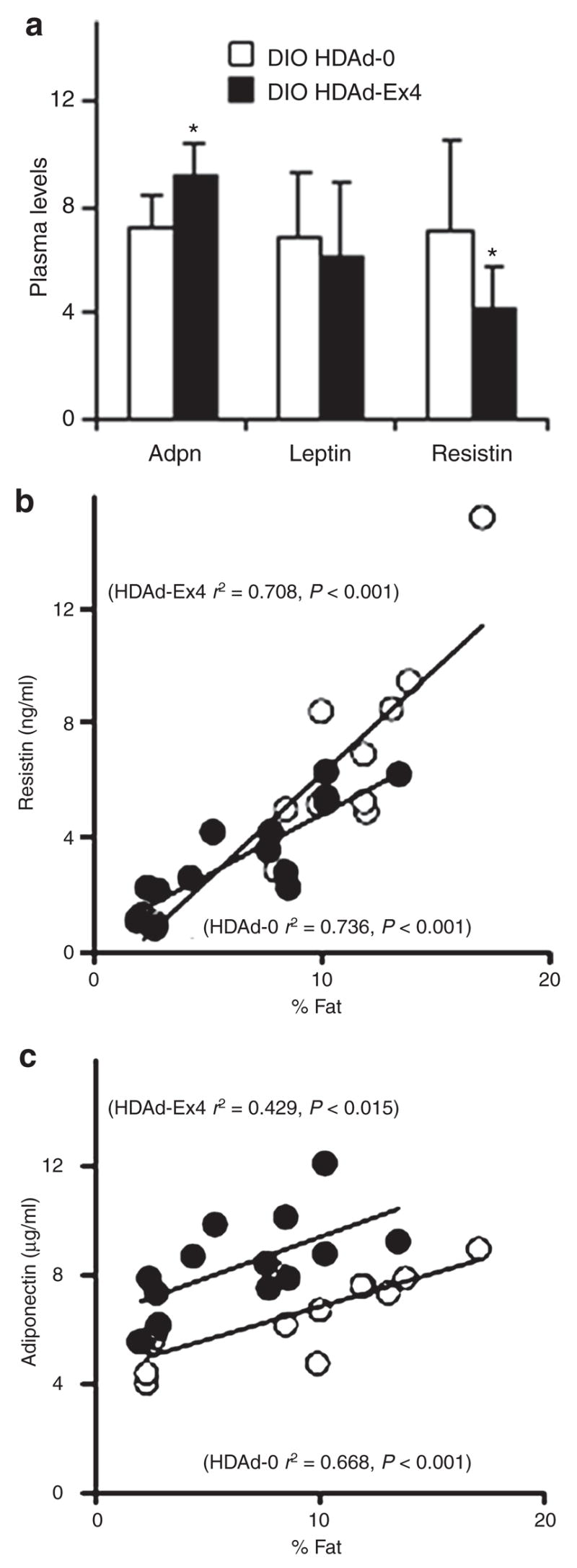
(a) Fasting (24 hours) plasma levels of adiponectin (Adpn; μg/ml), leptin (ng/ml), and resistin (ng/ml) are shown at 10 weeks after helper-dependent adenovirus (HDAd) injection for diet-induced obesity (DIO) mice (n = 10 each group; *P < 0.05 for HDAd-Ex4 compared to HDAd-0). Values are mean ±SD. Scatter plots of (b) resistin versus % fat and (c) adiponectin versus % fat are shown (HDAd-0, white circles; HDAd-Ex4, black circles). Ex4, exendin 4.
Scatter plots are shown for resistin (Figure 5b) and adiponectin (Figure 5c). Multiple linear regression revealed that resistin levels correlated with weight (r2 = 0.738, P < 0.001), fat (r2 = 0.784, P < 0.001), or % fat (r2 = 0.704, P < 0.001) with no contribution by HDAd-Ex4 in DIO and chow-fed mice (Figure 5b). For adiponectin, inclusion of both chow-fed and DIO adiponectin levels in the analysis revealed that Ex4 contributed to the regression model (P < 0.001, Figure 5b) if the independent variable was weight, fat, or % fat. Using only the DIO data, adiponectin levels of HDAd-0 correlated with % fat (r2 = 0.465, P < 0.05) but for HDAd-Ex4-treated mice, weight (P = 0.323), fat (P = 0.839), and % fat (P = 0.118) did not play a role, and the only contributor to adiponectin levels was HDAd-Ex4 (P < 0.01 for all three variables). This suggests that the effects of HDAd-Ex4 on resistin levels are indirect and dependant on body weight and/or composition, while Ex4 may directly contribute to increasing adiponectin levels in vivo and most significantly in an obesity model. We did not find that the level of adiponectin RNA was increased in the adipose tissue of HDAd-Ex4 mice (data not shown). Moreover, injections of Ex4 peptide did not increase adiponectin levels acutely (Supplementary Figure S3). At this juncture, the mechanism by which adiponectin is elevated remains unclear but appears to be related to long-term exposure to Ex4.
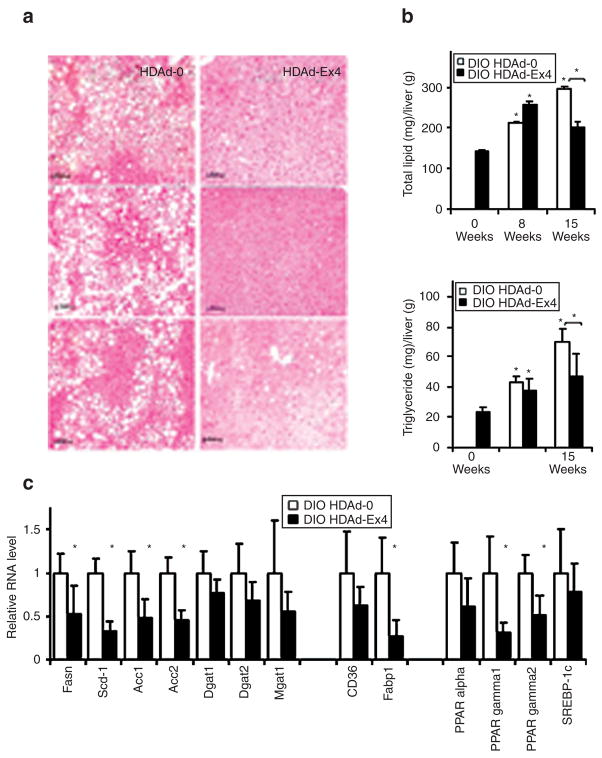
Liver lipid content and lipid metabolism gene expression
Fasting (4 hours) triglycerides were similar among DIO HDAd treatment groups at 15 weeks (HDAd-0, 0.44 ± 0.12 versus HDAd-Ex4, 0.47 ± 0.22). However, free-fatty acids were significantly decreased by 15 weeks (HDAd-0, 1.53 ± 0.37 versus HDAd-Ex4, 0.53 ± 0.07, P < 0.05). The DIO HDAd-0 mice accumulated increasing total lipid and triglyceride in the liver throughout the study, by histology (Figure 6a) and direct measurement (Figure 6b). However, the HDAd-Ex4 mice had significantly less liver total lipid and triglyceride levels than their HDAd-0-treated counterparts and this effect was maximal by 15 weeks.
Figure 6. Hepatic fat is decreased in HDAd-Ex4-treated mice.
(a) Liver tissue from mice treated for 15 weeks with helper-dependent adenovirus (HDAd)-0 or HDAd-Ex4 were fixed in phosphate-buffered formalin, paraffin sectioned, and hematoxylin and eosin staining was performed. Mice were fasted for 4 hours before killing. Representative sections from three separate mice for each HDAd treatment group are shown. Bars = 50 μm. (b) Lipid and triglyceride content of the liver was measured (n = 5/group; *P < 0.05 compared to baseline and brackets indicate P < 0.05 HDAd-Ex4 compared to HDAd-0). (c) The relative liver RNA expression of levels of key lipogenic gene products were measured by quantitative PCR normalized to cyclophilin (*P < 0.05 for HDAd-Ex4 compared to HDAd-0). Mice were fasted for 4 hours before killing. Values are mean SD. Ex4, exendin 4. Fasn, fatty acid synthase; Scd1, stearoyl coA desaturase 1; Acc, acetyl CoA carboxylase; Dgat, diacylglycerol acetyltransferase; Mgat, monoacylglycerol acetyltransferase; Fabp1, Fatty acid–binding protein 1; PPAR, peroxisome proliferator–activated receptor; SREBP-1c, sterol regulatory-element-binding protein-1c.
The relative hepatic transcript levels of key lipogenic and lipid transport genes were analyzed by quantitative PCR (Figure 6c). Transcripts of acetyl CoA carboxylase (Acc) 1 and 2, stearoyl coA desaturase 1 (Scd1), and fatty acid synthase (Fasn) were down-regulated in HDAd-Ex4-treated mice.29 Triglyceride synthesis enzymes, diacylglycerol acetyltransferase (Dgat) 1 and 2, and monoacylglycerol acetyltransferase (Mgat) 1, were not significantly affected. Fatty acid–binding protein 1(Fabp1)30 mRNA was decreased but there was no significant effect on CD36, another transport protein which may contribute to steatosis.31 The peroxisome proliferator–activated receptors (PPARs) have also been implicated in the process of hepatic steatosis, with elevated and inappropriate PPAR-γexpression.32,33 PPAR-γand PPAR-γ2 transcripts were downregulated in HDAd-Ex4-treated liver, while PPAR-α was not significantly affected. The transcript level of the sterol regulatory-element-binding protein-1c (SREBP-1c), a regulator of lipogenic gene expression, was unchanged but this factor is also regulated by multiple post-translational mechanisms.34
Discussion
GLP-1R agonists are coming to the forefront of therapy for diabetes and obesity and a complete understanding of their effects on glucose homeostasis, adipokines, and metabolism is essential. Long-acting formulations (i.e., weekly injections) are in testing for clinical use, as opposed to short-acting injections, and hence the metabolic consequences of steady-state elevations of GLP-1R agonists are of great interest. To this end, we have used HDAd-mediated hepatic expression of Ex4 to characterize the effects of long-term and steady-state levels of a GLP-1R agonist. We have avoided db/db or ob/ob mouse models because the absence of leptin action could confound observations of the true impact of a GLP-1R agonist.20,21 The proexendin-4 transgenic mouse has potential for similar studies of long-term and steady-state levels, except that this model has been hampered by autoimmunity induced by the native Ex4 signal peptide.35,36 With a single injection of HDAd-Ex4, we achieved systemic Ex4 levels approximately five- to tenfold higher than endogenous levels of GLP-1 (refs. 13,22) and expression was sustained for at least 15 weeks.
There was improved glucose homeostasis in DIO HDAd-Ex4 mice which was expected of a GLP-1R agonist. Surprisingly, this improvement could not be attributed to enhanced insulin secretion. Also, the pancreatic insulin content was similar between the DIO groups and there were no detectable changes in islet area on histology, possibly due to the already increased beta cell mass caused by obesity.26 Rather, there was improved insulin sensitivity as evidenced by the lowered [fasting glucose × insulin] product, improved insulin tolerance test data, and increased glucose clearance during glucose tolerance testing in the absence of elevated insulin levels. Although it is difficult to separate the direct effects of HDAd-Ex4 from those caused by decreased adiposity, it is notable that the most profound effects on glucose homeostasis occurred at the longest time point (15 weeks of treatment) compared to baseline, even though the HDAd-Ex4 mice had increased in weight and fat mass. Also, this corroborates other in vivo and in vitro data which support direct effects of GLP-1 on insulin sensitivity.9,16,37,38
Decreased gluconeogenesis in HDAd-Ex4 mice may also contribute to improved glucose levels through decreases in rate-limiting enzyme expression, specifically PEPCK and glucose-6-phosphatase. This has also been noted by others using a gene therapy approach for GLP-1R agonist delivery.16 GLP-1R agonists could cause this through improvements in insulin action or lowered glucagon secretion.1,4 but whether the latter hormone contributed to the lowered enzyme expression is not clear because of the wide variation in systemic glucagon levels in our study.
There was an improved adipokine profile with decreased resistin and increased adiponectin, changes which could enhance insulin sensitivity. Two recent studies have reported similar adipokine changes using GLP-1R agonist injections.39,40 In our study, adiponectin correlated with HDAd-Ex4 treatment in DIO mice rather than decreased weight or fat mass, and the possibility of direct effects by Ex4 and other GLP-1R agonists requires further investigation. The decrease in hepatic steatosis observed with HDAd-Ex4 treatment is concordant with another reports,10 and could also contribute to decreased insulin resistance.41 The changes in lipogenic gene expression could be a cause of these observations, due to direct effects of Ex4 (ref. 10) or an indirect effect secondary to improved insulin sensitivity and/or decreased steatosis.32 The presence of the known GLP-1R on hepatocytes is controversial8,10 but there is evidence of specific binding of GLP-1 and activation of signal transduction cascades in hepatocytes42 and further work is needed to determine the mechanism by which Ex4 can affect hepatocyte gene expression. The considerable effects that we observed using the HDAd-Ex4 system could be due to systemic levels of expressed Ex4, but there also may be local effects due to hepatic-based expression.
Pharmacologic levels of GLP-1R agonists are known to promote weight loss, attributed to delayed gastric emptying and satiety, and direct effects on central nervous system appetite centers.2,3 However, food intake appeared unaffected in mice exposed to sustained high levels of Ex4 in this study. Baggio et al.36 also did not observe changes in food intake in transgenic proexendin-4 mice on a chow diet. One explanation is that effects on food intake may require spikes in systemic peptide levels, such as with acute injections, rather than steady-state levels of peptide, as with gene therapy or transgenic expression. However, a long-acting form of exenatide is able to achieve sustained decreases in food intake for weeks.43 We cannot rule out the possibility that the appetite effects were transient, and missed by the intermittent measurements done here. However, we believe that this is unlikely, because we made the measurements more than once, at 2 weeks post-treatment during weight loss in the DIO HDAd-Ex4 mice and, again at 14 weeks, when the mice still showed decreased weight gain. We cannot rule out that the intestinal absorption of fat and other nutrients was affected by HDAd-Ex4 treatment as we did not measure fecal fat, but this possibility should be explored in the future. It is interesting that HDAd-Ex4 treatment did decrease food intake early on in a parallel db/db study (Supplementary Figure S1), in agreement with reports by others.14,16 Perhaps, the major effects are seen in mouse models of extreme hyperphagia.
Increased VO2 and EE could also contribute to the weight effects in DIO HDAd-Ex4 mice in the absence of altered food intake or detectable changes in physical activity. Our observations differ from a report showing systemic or intracerebroventricular injections of Ex4 acutely reduce RQ and VO2 in chow-fed mice.25 We have replicated this finding here (Supplementary Figure S2). One explanation is that acute injections of GLP-1R agonists cause acute reductions in food intake and delayed absorption of nutrients,25,44 similar to a fasting state. Thus, the physiological response is to increase fat oxidation and lower the RQ and VO2, as is observed with acute leptin injections.24 Further, Baggio et al.25 used chow-fed mice, and we have had differential findings depending on the diet (chow or high fat) and/or body weight (lean or obese). A recent report described increased EE and resistance to HFD in GLP-1/GIP double incretin receptor knockout mice, and the authors conclude that incretins are negative regulators of energy balance.45,46 Although our findings are somewhat different from their results, they are consistent with the ability of GLP-1R agonists to promote sustained weight loss. Perhaps other compensatory changes, such as the decreased leptin levels in their study, could explain the different observations. In lean humans, fasting GLP-1 levels are positively correlated with resting EE47 and another GLP-1R agonist, liraglutide, has been reported to increase resting EE mildly (~10%) in candy-fed DIO rats with a relative increase in feeding-associated EE when adjusted for weight.26 Because there were no detectable changes in activity of the HDAd-Ex4 mice, the mechanism by which EE is affected requires further investigation.
Given that we predicted that the decreased fat mass in DIO HDAd-Ex4 mice would be secondary to increased fat oxidation with a lowered RQ, the increased RQ also is unexpected. We speculate that the higher RQ in HDAd-Ex4-treated mice is a reflection of increased carbohydrate utilization due to improved whole-body insulin sensitivity and is consistent with the observed changes in glucose homeostasis.48
In conclusion, our HDAd-mediated expression system allowed for sustained high plasma levels of a GLP-1R agonist, which revealed new information on the chronic metabolic effects of Ex4 beyond incretin-mediated enhancement of insulin secretion. This included changes in insulin sensitivity, adipokine levels, hepatic steatosis, and EE. Many of our observations pertained only to a diet-induced obesity model and were not seen in lean chow-fed mice, suggesting that the most important contributions of long-term steady-state Ex4 are under conditions of metabolic derangement. Our results are especially pertinent in light of the ongoing development of depot or longer-acting GLP-1 analogs for clinical use and are the impetus for further investigation into the mechanisms by which GLP-1R agonists can have such widespread effects on metabolism beyond glucose homeostasis.
MATERIALS AND METHODS
Reagents and assays
Lyophilized Ex4 (Sigma-Aldrich, St Louis, MO) was resuspended in water/0.1% bovine serum albumin at 100 μg/ml. Immunoassays were as follows: Ex4 enzyme immunoassay kit (Phoenix Pharmaceuticals, Burlingame, CA); adiponectin enzyme-linked immunosorbent assay (Millipore, St Charles, MO); human and mouse insulin Ultrasensitive enzyme-linked immunosorbent assays (Mercodia, Uppsala, Sweden) Resistin, leptin, and glucagon were measured using a Lincoplex multiplex assay (Millipore) with the Bio-Plex system (Bio-Rad Laboratories, Hercules, CA). Plasma nonesterified free-fatty acids (Wako Chemicals USA, Richmond, VA) and triglycerides (Infinity assay by Thermo Electron, Melbourne, Australia) were measured by colorimetric assay. Oligonucleotides were purchased from Sigma-Aldrich.
DNA constructs
An Ex4 expression cassette was synthesized from a series of complementary oligonucleotides which were annealed and ligated. The cassette (Figure 1a) contains the mouse immunoglobulin κ light chain leader from pSecTag2a (Invitrogen, Carlsbad, CA), a furin cleavage site, and the Ex4 sequence7 modified for mammalian codon usage. The cassette was inserted into pcDNA 3.1(+) (Invitrogen) downstream of the cytomegalovirus promoter (CMV) to create CMV-Ex4 for transfection experiments.
For HDAd construction, the Ex4 cassette was ligated with pLPBL1 downstream of the elongation factor 1α (BOS) promoter and upstream of the rabbit β-globin polyadenylation signal.18 The fragment containing the promoter, Ex4 cassette, and polyadenylation signal was inserted into pΔ28 (ref. 18).
Cell culture and transfection
Cell culture reagents were purchased from GIBCO-BRL (Grand Island, NY). Hep3B cells were maintained in Dulbecco’s modified Eagle’s medium/10% fetal bovine serum/antibiotic–antimycotic. Rat 832/13 insulinoma49 cells were a gift from Dr. C. Newgard (Duke University) and were maintained in Rosewell Park Memorial Institute medium/10% fetal bovine serum/antibiotic–antimycotic. For glucose-stimulated insulin release, 832/13 cells were plated at 1 × 105/well in 48-well plates. At 90% confluence, cells were transfected in quadruplicate with pcDNA3.1 (400 ng) or CMV-Ex4 (400 ng). After 48 hours, cells were incubated overnight in media with 2 mmol/l glucose. The next day, insulin secretion assays were performed as described.49
HDAd production
HDAd was produced in the 293Cre66 cell line in the Adenoviral Core Laboratory at Baylor College of Medicine as described.18 After seven passages, final HDAd-Ex4 and the control HDAd-0 preparations were purified from empty capsid by cesium chloride gradient, and HDAd DNA was analyzed by Southern blotting to confirm <1% helper virus contamination.
Animals
Animal experiments were approved by the Institutional Animal Care and Use Committee at Baylor College of Medicine. C57/BL6 male mice were from The Jackson Laboratory (Bar Harbor, ME) and housed in the Taub Animal Facility. At 6 weeks of age, DIO mice were switched from chow to HFD (42% of calories from fat; Harlan Teklad, Houston, TX) and control mice were fed chow (12.1% fat; Purina Rodent Laboratory Diet) for the duration of the study. Animals were injected via tail vein with 1 × 1011 viral particles of HDAd-0 or HDAd-Ex4 virus at 14 weeks of age.
Weights and 4-hours fasting glucose were monitored on a weekly basis. Echo magnetic Resonance Imaging (Echo Medical System, Houston TX) was used for body composition analysis. Mice were killed under anesthesia. Tissues were fixed in formalin or frozen in liquid nitrogen and stored at −80 °C for further analysis.
Indirect calorimetry
RQ, VO2, and activity were determined for singly housed mice using Physioscan and VersaMax equipment and software (AccuScan Instruments, Columbus, OH). Mice were equilibrated for 4 hours and data were collected for 1 minute every 8 minutes over 22 hours from 3 PM to 1 PM the next day. The dark cycle of the room was from 8 PM to 6 AM.
Food intake
Mice were equilibrated in separate housing for 2 days and then the weights of each mouse and food were measured every 24 hours for 5 days. To calculate caloric intake, HFD has 4.5 kcal/g while chow diet has 3.35 kcal/g of physiologic fuel value.
Glucose and insulin tolerance tests
Glucose tolerance and acute insulin secretion testing were performed by intraperitoneal injection after a 16-hours fast, and glucose levels were measured by glucometer (Lifescan, Milpitas, CA) from tail blood. Insulin tolerance tests were performed after a 4-hour fast with 1.0 units/kg humulin R insulin intraperitoneally (Eli Lilly and Company, Indianapolis, IN).
Hepatic fat content
Total lipid was extracted from 100 mg of liver by the Bligh–Dyer method, dried under N2 gas, and weighed.50 Lipid components were separated by one-dimensional thin-layer chromatography (Silica Gel 60), using hexane–diethyl ether–glacial acetic acid (75:35:1) and developed with iodine. Triglyceride was quantified by computer analysis (SigmaScan Pro 5.0) by comparison to a marker containing a known amount of triglyceride.
Reverse transcription PCR
Total RNA was extracted from liver using Trizol reagent (Invitrogen) as per the manufacturer’s instructions. RNA was treated with DNase I (Invitrogen) and reverse transcribed using Superscript III First Strand Synthesis System for reverse transcription PCR (Invitrogen). Quantitative PCR was performed using iQ™ SYBR Green Supermix (Bio-Rad) with Rox reference dye and the Mx3000P quantitative PCR machine (Stratagene, La Jolla, CA). Cyclophilin was used as the housekeeping gene. Primers for quantitative PCR were designed to cross an intron, to have a Tm of 58 to 60 °C, and produce a 200–base pair product (Supplementary Table S1).
Statistical analysis
Comparisons between groups were made using the Student’s t-test. Effects of HDAd-0 and HDAd-Ex4 on adipokine and insulin levels in relation to weight, fat mass, and % fat were performed using multiple linear regression calculations in SigmaStat 3.1 (SyStat Software, San Jose, CA). Error bars in figures indicate ± SD.
Supplementary Material
Treatment of diabetic db/db mice with HDAd-Ex4 or HDAd-0 (n = 5 each group) at 8 weeks of age.
Indirect calorimetry results are shown for Ex4 injected mice.
Acute injection of Ex4 did not raise adiponectin levels in chow fed or DIO mice (n = 4 in each treatment group).
Primer sequences used for quantitative PCR.
Acknowledgments
This research was supported by the US National Institutes of Health (NIH) grants HL-51586 and DK-68037 (to L.C.), the Diabetes and Endocrinology Research Center DERC (P30DK079638), and the Adenovirus Core of the DERC. L.C. is also supported by the Rutherford Chair for Diabetes Research from St. Luke’s Episcopal Hospital and by the T.T. and W.F. Chao Foundation. S.L.S. is supported by a Canadian Institutes of Health Research fellowship. V.Y. is supported by an NIH K08 award K08-DK068391. We appreciate the technical assistance of Vonda Avery for animal care and Shelley Cormier for adenoviral work.
References
- 1.Holst JJ. The physiology of glucagon-like peptide 1. Physiol Rev. 2007;87:1409–1439. doi: 10.1152/physrev.00034.2006. [DOI] [PubMed] [Google Scholar]
- 2.Aja S, Ewing C, Lin J, Hyun J, Moran TH. Blockade of central GLP–1 receptors prevents CART–induced hypophagia and brain c–Fos expression. Peptides. 2006;27:157–164. doi: 10.1016/j.peptides.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 3.Schick RR, Zimmermann JP, Walde TV, Schusdziarra V. Peptides that regulate food intake – Glucagon–like peptide 1–(7–36) amide acts at lateral and medial hypothalamic sites to suppress feeding in rats. Am J Physiol Reg Integr and Comp Physiol. 2003;284:R1427–R1435. doi: 10.1152/ajpregu.00479.2002. [DOI] [PubMed] [Google Scholar]
- 4.Brubaker PL. Incretin–based therapies: mimetics versus protease inhibitors. Trends Endocrinol Metab. 2007;18:240–245. doi: 10.1016/j.tem.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 5.Knudsen LB, Kiel D, Teng M, Behrens C, Bhumralkar D, Kodra JT, et al. Small–molecule agonists for the glucagon–like peptide 1 receptor. Proc Natl Acad Sci USA. 2007;104:937–942. doi: 10.1073/pnas.0605701104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen DS, Liao JY, Li N, Zhou CH, Liu Q, Wang GX, et al. A nonpeptidic agonist of glucagon–like peptide 1 receptors with efficacy in diabetic db/db mice. Proc Natl Acad Sci USA. 2007;104:943–948. doi: 10.1073/pnas.0610173104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eng J, Kleinman WA, Singh L, Singh G, Raufman JP. Isolation and characterization of exendin–4, an exendin–3 analog, from heloderma–suspectum venom – further evidence for an exendin receptor on dispersed acini from guinea–pig pancreas. J Biol Chem. 1992;267:7402–7405. [PubMed] [Google Scholar]
- 8.Mayo KE, Miller LJ, Bataille D, Dalle S, Goke B, Thorens B, et al. International union of pharmacology. XXXV. The glucagon receptor family. Pharmacol Rev. 2003;55:167–194. doi: 10.1124/pr.55.1.6. [DOI] [PubMed] [Google Scholar]
- 9.Young AA, Gedulin BR, Bhavsar S, Bodkin N, Jodka C, Hansen B, et al. Glucose–lowering and insulin–sensitizing actions of exendin–4—studies in obese diabetic (ob/ob, db/db) mice, diabetic fatty Zucker rats, and diabetic rhesus monkeys (Macaca mulatta) Diabetes. 1999;48:1026–1034. doi: 10.2337/diabetes.48.5.1026. [DOI] [PubMed] [Google Scholar]
- 10.Ding XK, Saxena NK, Lin SB, Gupta N, Anania FA. Exendin–4, a glucagon–like protein–1 (GLP–1) receptor agonist, reverses hepatic steatosis in ob/ob mice. Hepatology. 2006;43:173–181. doi: 10.1002/hep.21006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Park CW, Kim HW, Ko SH, Lim JH, Ryu GR, Chung HW, et al. Long–term treatment of glucagon–like peptide–1 analog exendin–4 ameliorates diabetic nephropathy through improving metabolic anomalies in db/db mice. J Am Soc Nephrol. 2007;18:1227–1238. doi: 10.1681/ASN.2006070778. [DOI] [PubMed] [Google Scholar]
- 12.Vella A, Rizza RA. Extrapancreatic effects of GIP and GLP–1. Horm Metab Res. 2004;36:830–836. doi: 10.1055/s-2004-82617. [DOI] [PubMed] [Google Scholar]
- 13.Kumar M, Hunag Y, Glinka Y, Prud’homme GJ, Wang Q. Gene therapy of diabetes using a novel GLP–1/IgG1–Fc fusion construct normalizes glucose levels in db/db mice. Gene Ther. 2007;14:162–172. doi: 10.1038/sj.gt.3302836. [DOI] [PubMed] [Google Scholar]
- 14.Parsons GB, Souza DW, Wu H, Yu D, Wadsworth SG, Gregory RJ, et al. Ectopic expression of glucagon–like peptide 1 for gene therapy of type II diabetes. Gene Ther. 2007;14:38–48. doi: 10.1038/sj.gt.3302842. [DOI] [PubMed] [Google Scholar]
- 15.Oh S, Lee M, Ko KS, Choi S, Kim SW. GLP–1 gene delivery for the treatment of type 2 diabetes. Mol Ther. 2003;7:478–483. doi: 10.1016/s1525-0016(03)00036-4. [DOI] [PubMed] [Google Scholar]
- 16.Lee YS, Shin S, Shigihara T, Hahm E, Liu MJ, Han J, et al. Glucagon-like peptide-1 gene therapy in obese diabetic mice results in long-term cure of diabetes by improving insulin sensitivity and reducing hepatic gluconeogenesis. Diabetes. 2007;56:1671–1679. doi: 10.2337/db06-1182. [DOI] [PubMed] [Google Scholar]
- 17.Ng P, Parks RJ, Cummings DT, Evelegh CM, Sankar U, Graham FL. A high-efficiency Cre/loxP-based system for construction of adenoviral vectors. Hum Gene Ther. 1999;10:2667–2672. doi: 10.1089/10430349950016708. [DOI] [PubMed] [Google Scholar]
- 18.Oka K, Chan L. Helper-dependent adenoviral vectors. Curr Protoc Mol Biol. 2005;16:16.2.1–16.2.24. doi: 10.1002/0471142727.mb1624s69. [DOI] [PubMed] [Google Scholar]
- 19.Oka K, Belalcazar LM, Dieker C, Nour EA, Nuno-Gonzalez P, Paul A, et al. Sustained phenotypic correction in a mouse model of hypoalphalipoproteinemia with a helper-dependent adenovirus vector. Gene Ther. 2007;14:191–202. doi: 10.1038/sj.gt.3302819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goldstone AP, Mercer JG, Gunn I, Moar KM, Edwards CMB, Rossi M, et al. Leptin interacts with glucagon-like peptide-1 neurons to reduce food intake and body weight in rodents. FEBS Lett. 1997;415:134–138. doi: 10.1016/s0014-5793(97)01103-4. [DOI] [PubMed] [Google Scholar]
- 21.Williams DL, Baskin DG, Schwartz MW. Leptin regulation of the anorexic response to glucagon-like peptide-1 receptor stimulation. Diabetes. 2006;55:3387–3393. doi: 10.2337/db06-0558. [DOI] [PubMed] [Google Scholar]
- 22.Orskov C, Rabenhoj L, Wettergren A, Kofod H, Holst JJ. Tissue and plasma-concentrations of amidated and glycine-extended glucagon-like peptide-i in humans. Diabetes. 1994;43:535–539. doi: 10.2337/diab.43.4.535. [DOI] [PubMed] [Google Scholar]
- 23.Kim D, MacConell L, Zhuang DL, Kothare PA, Trautmann M, Fineman M, et al. Effects of once-weekly dosing of a long-acting release formulation of exenatide on glucose control and body weight in subjects with type 2 diabetes. Diabetes Care. 2007;30:1487–1493. doi: 10.2337/dc06-2375. [DOI] [PubMed] [Google Scholar]
- 24.Martin TL, Alquier T, Asakura K, Furukawa N, Preitner F, Kahn BB. Diet-induced obesity alters AMP kinase activity in hypothalamus and skeletal muscle. J Biol Chem. 2006;281:18933–18941. doi: 10.1074/jbc.M512831200. [DOI] [PubMed] [Google Scholar]
- 25.Baggio LL, Huang QL, Brown TJ, Drucker DJ. Oxyntomodulin and glucagon-like peptide-1 differentially regulate murine food intake and energy expenditure. Gastroenterology. 2004;127:546–558. doi: 10.1053/j.gastro.2004.04.063. [DOI] [PubMed] [Google Scholar]
- 26.Raun K, von Voss P, Gotfredsen CF, Golozoubova V, Rolin B, Knudsen LB. Liraglutide, a long-acting glucagon-like peptide-1 analog, reduces body weight and food intake in obese candy-fed rats, whereas a dipeptidyl peptidase-IV inhibitor, vildagliptin, does not. Diabetes. 2007;56:8–15. doi: 10.2337/db06-0565. [DOI] [PubMed] [Google Scholar]
- 27.Jiang G, Zhang BB. Glucagon and regulation of glucose metabolism. Am J Physiol Endocrinol Metab. 2003;284:E671–E678. doi: 10.1152/ajpendo.00492.2002. [DOI] [PubMed] [Google Scholar]
- 28.Kershaw EE, Flier JS. Adipose tissue as an endocrine organ. J Clin Endocrinol Metab. 2004;89:2548–2556. doi: 10.1210/jc.2004-0395. [DOI] [PubMed] [Google Scholar]
- 29.Miyazaki M, Flowers MT, Sampath H, Chu K, Otzelberger C, Liu X, et al. Hepatic stearoyl-CoA desaturase-1 deficiency protects mice from carbohydrate-induced adiposity and hepatic steatosis. Cell Metab. 2007;6:484–496. doi: 10.1016/j.cmet.2007.10.014. [DOI] [PubMed] [Google Scholar]
- 30.Newberry EP, Xie Y, Kennedy S, Han XL, Buhman KK, Luo JY, et al. Decreased hepatic triglyceride accumulation and altered fatty acid uptake in mice with deletion of the liver fatty acid-binding protein gene. J Biol Chem. 2003;278:51664–51672. doi: 10.1074/jbc.M309377200. [DOI] [PubMed] [Google Scholar]
- 31.Zhou J, Febbraio M, Wada T, Zhai Y, Kuruba R, He J, et al. Hepatic fatty acid transporter Cd36 is a common target of LXR, PXR, and PPAR gamma in promoting steatosis. Gastroenterology. 2008;134:556–567. doi: 10.1053/j.gastro.2007.11.037. [DOI] [PubMed] [Google Scholar]
- 32.Inoue M, Ohtake T, Motomura W, Takahashi N, Hosoki Y, Miyoshi S, et al. Increased expression of PPAR gamma in high fat diet-induced liver steatosis in mice. Biochem Biophys Res Commun. 2005;336:215–222. doi: 10.1016/j.bbrc.2005.08.070. [DOI] [PubMed] [Google Scholar]
- 33.Rao MS, Reddy JK. PPAR alpha in the pathogenesis of fatty liver disease. Hepatology. 2004;40:783–786. doi: 10.1002/hep.20453. [DOI] [PubMed] [Google Scholar]
- 34.Raghow R, Yellaturu C, Deng X, Park EA, Elam MB. SREBPs: the crossroads of physiological and pathological lipid homeostasis. Trends Endocrinol Metab. 2008;19:65–73. doi: 10.1016/j.tem.2007.10.009. [DOI] [PubMed] [Google Scholar]
- 35.Baggio LL, Holland D, Wither J, Drucker DJ. Lymphocytic infiltration and immune activation in metallothionein promoter-exendin-4 (MT-Exendin) transgenic mice. Diabetes. 2006;55:1562–1570. doi: 10.2337/db05-1502. [DOI] [PubMed] [Google Scholar]
- 36.Baggio L, Adatia F, Bock T, Brubaker PL, Drucker DJ. Sustained expression of exendin-4 does not perturb glucose homeostasis, beta-cell mass, or food intake in metallothionein-preproexendin transgenic mice. J Biol Chem. 2000;275:34471–34477. doi: 10.1074/jbc.M005119200. [DOI] [PubMed] [Google Scholar]
- 37.Idris I, Patiag D, Gray S, Donnelly R. Exendin-4 increases insulin sensitivity via a PI-3-kinase-dependent mechanism: contrasting effects of GLP-1. Biochem Pharmacol. 2002;63:993–996. doi: 10.1016/s0006-2952(01)00924-8. [DOI] [PubMed] [Google Scholar]
- 38.Gedulin BR, Nikoulina SE, Smith PA, Gedulin G, Nielsen LL, Baron AD, et al. Exenatide (Exendin-4) improves insulin sensitivity and beta-cell mass in insulin-resistant obese fa/fa Zucker rats independent of glycemia and body weight. Endocrinology. 2005;146:2069–2076. doi: 10.1210/en.2004-1349. [DOI] [PubMed] [Google Scholar]
- 39.Li L, Yang YTX, Liu H, Tang Y, Boden G. Exenatide prevents fat-induced insulin resistance and raises adiponectin expression and plasma levels. Diabetes Obes Metab. 2007 doi: 10.1111/j.1463-1326.2007.00832.x. epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 40.Baggio LL, Huang QL, Cao XM, Drucker DJ. An albumin-exendin-4 conjugate engages central and peripheral circuits regulating murine energy and glucose Homeostasis. Gastroenterology. 2008;134:1137–1147. doi: 10.1053/j.gastro.2008.01.017. [DOI] [PubMed] [Google Scholar]
- 41.Qureshi K, Abrams GA. Metabolic liver disease of obesity and role of adipose tissue in the pathogenesis of nonalcoholic fatty liver disease. World J Gastroenterol. 2007;13:3540–3553. doi: 10.3748/wjg.v13.i26.3540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Redondo A, Trigo MV, Acitores A, Valverde I, Villanueva-Penacarrillo ML. Cell signalling of the GLP-1 action in rat liver. Mol Cell Endocrinol. 2003;204:43–50. doi: 10.1016/s0303-7207(03)00146-1. [DOI] [PubMed] [Google Scholar]
- 43.Gedulin BR, Smith P, Prickett KS, Tryon M, Barnhill S, Reynolds J, et al. Dose-response for glycaemic and metabolic changes 28 days after single injection of long-acting release exenatide in diabetic fatty Zucker rats. Diabetologia. 2005;48:1380–1385. doi: 10.1007/s00125-005-1795-2. [DOI] [PubMed] [Google Scholar]
- 44.Flint A, Raben A, Rehfeld JF, Holst JJ, Astrup A. The effect of glucagon-like peptide-1 on energy expenditure and substrate metabolism in humans. Int J Obesity Relat Metab Disord. 2000;24:288–298. doi: 10.1038/sj.ijo.0801126. [DOI] [PubMed] [Google Scholar]
- 45.Hansotia T, Baggio LL, Delmeire D, Hinke SA, Yamada Y, Tsukiyama K, et al. Double incretin receptor knockout (DIRKO) mice reveal an essential role for the enteroinsular axis in transducing the glucoregulatory actions of DPP-IV inhibitors. Diabetes. 2004;53:1326–1335. doi: 10.2337/diabetes.53.5.1326. [DOI] [PubMed] [Google Scholar]
- 46.Ayala JE, Bracy DP, Hansotia T, Flock G, Seino Y, Wasserman DH, et al. Insulin action in the double incretin receptor knockout mouse. Diabetes. 2008;57:288–297. doi: 10.2337/db07-0704. [DOI] [PubMed] [Google Scholar]
- 47.Pannacciulli N, Bunt JC, Koska J, Bogardus C, Krakoff J. Higher fasting plasma concentrations of glucagon-like peptide 1 are associated with higher resting energy expenditure and fat oxidation rates in humans. Am J Clin Nutr. 2006;84:556–560. doi: 10.1093/ajcn/84.3.556. [DOI] [PubMed] [Google Scholar]
- 48.Braun B, Sharoff C, Chipkin SR, Beaudoin F. Effects of insulin resistance on substrate utilization during exercise in overweight women. J Appl Physiol. 2004;97:991–997. doi: 10.1152/japplphysiol.00231.2004. [DOI] [PubMed] [Google Scholar]
- 49.Hohmeier HE, Mulder H, Chen GX, Henkel-Rieger R, Prentki M, Newgard CB. Isolation of INS-1-derived cell lines with robust ATP-sensitive K+ channel-dependent and -independent glucose-stimulated insulin secretion. Diabetes. 2000;49:424–430. doi: 10.2337/diabetes.49.3.424. [DOI] [PubMed] [Google Scholar]
- 50.Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Treatment of diabetic db/db mice with HDAd-Ex4 or HDAd-0 (n = 5 each group) at 8 weeks of age.
Indirect calorimetry results are shown for Ex4 injected mice.
Acute injection of Ex4 did not raise adiponectin levels in chow fed or DIO mice (n = 4 in each treatment group).
Primer sequences used for quantitative PCR.