Abstract
Light information reaches the suprachiasmatic nucleus (SCN) through a subpopulation of retinal ganglion cells. Previous work raises the possibility that brain-derived neurotrophic factor (BDNF) and its high-affinity receptor TrkB may be important as modulators of this excitatory input into the SCN. To test this possibility, we used whole-cell patch-clamp methods to measure excitatory currents in rat SCN neurons. These currents were evoked by electrical stimulation of the optic nerve. We found that the amplitude of the N-methyl-D-aspartate (NMDA) component of the evoked excitatory postsynaptic currents (NMDA-EPSC) was increased by application of BDNF. The neurotrophin also increased the magnitude of NMDA-evoked currents in SCN neurons. The BDNF enhancement of the NMDA-EPSC was blocked by treatment with the neurotrophin receptor antagonist K252a as well as treatment with the soluble form of the TrkB receptor engineered as an immunoadhesin (TrkB IgG). Finally, the BDNF enhancement was lost in brain slices treated with the NR2B antagonist ifenprodil. The results demonstrate that BDNF and TrkB receptors are important regulators of retinal glutamatergic synaptic transmission within the SCN.
Keywords: circadian rhythms, NMDA, rat, TrkB, suprachiasmatic nucleus, SCN
During development, neurotrophic factors promote neuronal survival and differentiation (Lewin and Barde, 1996). Neurotrophins also modify the growth of developing axons (Alsina et al., 2001; Frost, 2001) and through this mechanism are likely to be involved in the establishment of neural circuits. Once the circuits are established in adult tissue, neurotrophins appear to be utilized for other functions, including the regulation of cellular communication and plasticity. In the adult nervous system, brain-derived neurotrophic factor (BDNF) and its high-affinity TrkB receptor are highly expressed in select regions, including the hippocampus and cortex (McAllister et al., 1999). In these regions, BDNF expression and release can be rapidly regulated by neural activity. Mechanistically, BDNF can regulate transcription through CREB activation (Finkbeiner et al., 1997). On a cellular level, BDNF can produce a wide range of effects including regulation of presynaptic release (Lessmann, 1998; Xu et al., 2000; Schinder et al., 2000), modulation of postsynaptic receptors (Levine et al., 1998; Lin et al., 1998; Levine and Kolb, 2000), and direct activation of membrane channels (Blum and Konnerth, 2005).
Relatively less is known about the role of BDNF in the context of behaviorally relevant circuits. In mammals, one of the best-understood behavioral control circuits is found in the suprachiasmatic nucleus (SCN). Neurons in this hypothalamic region are critical for the generation of circadian behaviors and their synchronization to light. SCN neurons gain access to information about external lighting conditions, at least in part, through a specialized subpopulation of light-sensitive retinal ganglion cells that contain the photopigment melanopsin (Van Gelder, 2005). These ganglion cells utilize glutamate and the neuropeptide pituitary adenylyl cyclase activating peptide (PACAP) as cotransmitters to communicate with the SCN (Hannibal et al., 2000). A variety of evidence indicates that, within the SCN, the N-methyl-D-aspartate (NMDA) class of glutamate receptors plays a critical role in mediating the behavioral effects of light (Colwell and Menaker, 1996).
Recent work raises the possibility that BDNF is an important modulator of photic regulation in the circadian system. Both BDNF and its high-affinity TrkB receptor are expressed in the SCN (Liang et al., 1998; Allen and Earnest, 2005). Levels of BDNF fluctuate rhythmically in this region, with peak protein levels found during the night, when exposure to light can alter the phase of circadian oscillations (Liang et al., 1998). Functionally, both BDNF- and TrkB-deficient mice exhibit a reduction in the phase shifting effects of light on the circadian system (Liang et al., 2000; Allen et al, 2005). Thus, we became interested in exploring the possible role of BDNF as a regulator of synaptic-evoked NMDA currents in SCN neurons.
MATERIALS AND METHODS
Animals and Housing
Male Sprague-Dawley rats (n = 36; 100–250 g) purchased from Charles River Laboratories International (Wilmington, MA) were used in this study. The experimental procedures conformed to the U.S. National Institutes of Health guidelines. Before being used for experiments, the animals were housed in a temperature-controlled chamber (23–24°C) with a 12:12 hr light/dark schedule for at least 1 week. By definition, zeitgeber time (ZT) 0 is the time of lights-on and ZT 12 is the time of lights-off in the colony. All experiments were conducted during the animal’s night based on the prior light/dark (LD) cycle.
Hypothalamic Slice Preparation
Rats were anesthetized with sodium pentobarbitone (100 mg/kg, i.p.) between ZT 10:30 and 11:30, and the brains were quickly excised and submerged in ice-cold physiological saline (composition in mM: 127 NaCl, 2 MgCl2, 3 KCl, 1.25 NaH2PO4, 26 NaHCO3, 2 CaCl2, 10 glucose; osmolality 300 mOsm/kg). After ∼1 min of chilling, the brains were trimmed to a block containing the hypothalamus and optic nerves. With the use of a vibroslicer (model NVSLM1; World Precision Instruments, Sarasota, FL), two parasagittal slices (400–550 μm thickness), each containing one optic nerve (2–4 mm) and an SCN, were cut from the tissue block. In some cases, two coronal slices containing the SCN (450 μm thickness) were cut. During the slicing, the tissue was maintained in ice-cold physiological saline. One of the slices was transferred to a modified Hass-type gas interface recording chamber (Haas et al., 1979) and superfused continuously, at a rate of ∼0.7 ml/min, with physiological saline (pH 7.4) aerated with 95% O2/5% CO2. Air humidified by 95% O2/5% CO2 was also continuously blown over the slice to ensure further adequate oxygenation of the cells in the tissue. The remaining slice was kept, for later use, in a storage chamber filled with physiological saline aerated by 95% O2/5% CO2.
Whole Cell Voltage-Clamp Recording
After ∼1 hr of equilibration of the slice in the recording chamber, electrophysiological experiments were begun. Micropipettes (tip diameter: 1.5–2.0 μm; 3–7 MΩ) pulled from borosilicate tubing (od: 1.5 mm, id: 0.84 mm; World Precision Instruments) and filled with an internal solution (composition in mM: 130 Cs-methanesulfate, 4 NaCl, 1 MgCl2, 3KCl, 3 QX-314, 5 Mg ATP, 0.2 EGTA, 8 Na-HEPES, 1 GTP, 0.1 leupeptin, 10 phosphocreatine; pH 7.2; osmolality 285 mOsm/kg) were used as recording electrodes. Whole-cell voltage-clamp recordings from SCN neurons were obtained, at room temperature, in continuous voltage-clamp mode with the use of Axoclamp-2A amplifier (Axon Instruments, Foster City, CA). The recorded signals were filtered at 1 kHz and sampled at 50-μsec intervals with Digidata 1320A and pClamp8.0 (Axon Instruments).
Optic Nerve Stimulation
The optic nerve was stimulated with the use of a custom-made suction electrode; constant current pulses (biphasic square wave) of 0.1–1.0 mA intensity and 0.5–2.0 msec duration were applied in 30-sec intervals.
DRUGS
Bicuculline methiodide (BIC; a GABAA receptor antagonist), 2-amino-5-phosphonopentanoic acid (APV; an NMDA receptor antagonist), 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX; a non-NMDA receptor antagonist), BDNF, NMDA, K252a (a neurotrophin receptor antagonist), anti-mouse TrkB receptor antibody, and ifenprodil (an NR2B NMDA receptor subunit antagonist) were used in this study. All the drugs were purchased from Sigma (St. Louis, MO), except the antibody TrkB receptor IgG (R&D Systems, Inc., Minneapolis, MN). BIC (25 μM), APV (50 μM), CNQX (20 μM), and ifenprodil (3 μM) were bath applied, and BDNF (200 ng/ml) and NMDA (0.5 mM) were applied directly to the SCN with the “Y-tube” method (Murase et al., 1989). K252a was either bath applied (100 nM) or focally applied (200 nM) using the “Y-tube” method. TrkB receptor IgG (1 μg/μl) was applied directly to the SCN in the form of droplets (a total of 10 μl). Approximately 1 min prior to the TrkB receptor IgG application, bath perfusion was stopped, and it was resumed 2 min following the application.
Statistical Analyses
Numerical data are shown as mean ± SEM. Drug effects on the synaptic responses from optic nerve stimulation were estimated with the use of paired t-test. P < 0.05 was considered to be significant.
RESULTS
Characterization of the NMDA Excitatory Postsynaptic Currents Evoked by Optic Nerve Stimulation
Experiments were conducted on >50 neurons located in the ventral region of the SCN in rat brain slices recorded during the night. All of the neurons included in this study exhibited stable, short-latency excitatory currents in response to optic nerve stimulation. By this criterion, we considered these cells to be retinorecipient SCN neurons. For these experiments, NMDA receptor-mediated currents in SCN neurons were isolated by recording responses evoked by optic nerve stimulation in the presence of the non-NMDA receptor antagonist CNQX (20 μM) and the GABAA receptor antagonist BIC (25 μM). Because the NMDA currents are strongly voltage dependent and exhibit a reversal potential of about 0 mV, the neurons were held at positive holding potentials (+30 to +80 mV). The NMDA excitatory postsynaptic currents (EPSCs) exhibited a 10–90% rise time of 20.3 ± 1.8 msec with a duration at half-maximal amplitude of 304 ± 16 msec (n = 9). As expected, the peak NMDA synaptic current in SCN neurons displayed a nonlinear current-voltage relationship with marked rectification at membrane potentials more negative than -10 mV (Fig. 1). The NMDA-EPSCs were blocked by the NMDA antagonist APV (50 μM; Fig. 1).
Fig. 1.
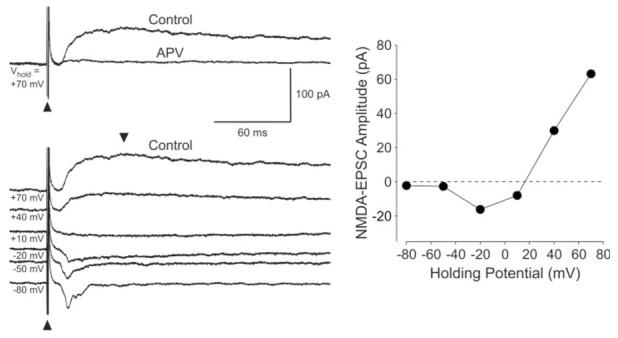
Isolation of NMDA receptor-mediated synaptic currents in SCN neurons. Left: Example of the time courses of the EPSCs in an SCN neuron evoked by optic nerve stimulation. The holding potential (Vh) is indicated to the right of each current trace. The stimulus artifacts are indicated with arrowheads. Each of the current traces in this and the following figures is an average of three to eight individual traces. The top panel illustrates that NMDA-EPSCs were blocked by the bath application of the NMDA receptor antagonist APV (50 μM, 5 min). The bottom panel shows the EPSCs measured at six different holding potentials. Right: Relationship between holding potential and the peak NMDA-EPSC current in the neuron from the left panel. The time of peak current measurement occurred between 30 and 80 msec poststimulation. As is characteristic of NMDA currents, the current-voltage relationship is not linear, with significant rectification seen at the hyperpolarized voltages. Depending on the cell, the peak inward current occurred with Vh between -20 and -40 mV.
Enhancement of NMDA Receptor-Mediated Response by BDNF
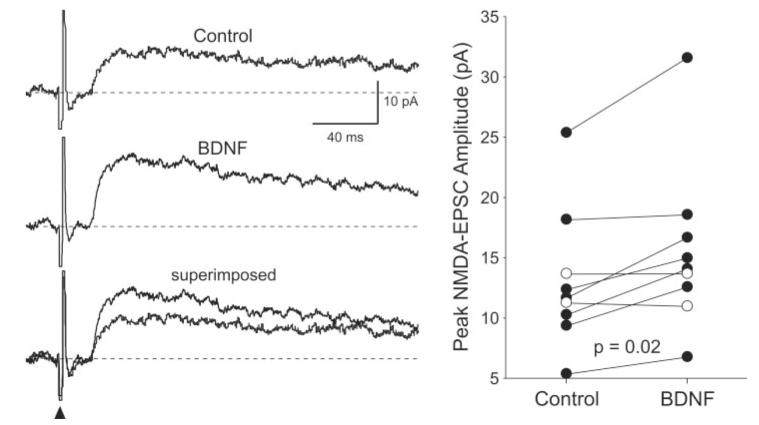
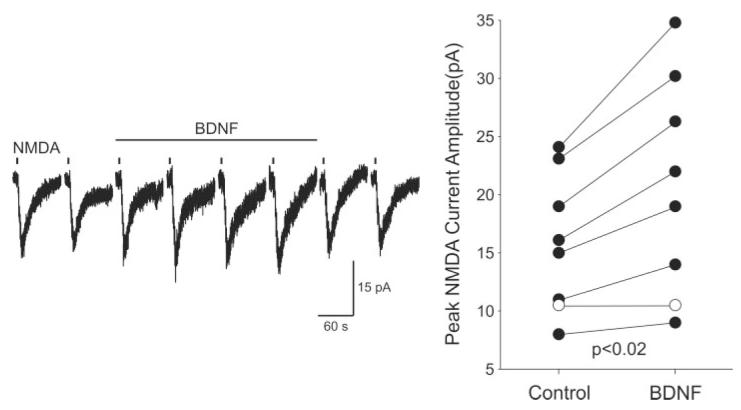
The effects of BDNF on the NMDA-EPSCs were examined in nine retinorecipient SCN neurons (Fig. 2). Overall, BDNF (200 ng/ml; 5 min) caused a rapid increase in the peak magnitude of the NMDA-EPSC (20% ± 6% change; P = 0.02; n = 9). Enhancement of at least 10% was found in six of nine neurons and fully reversed in three of these six cases. We next examined the effect of BDNF (200 ng/ml; 5 min) on the non-NMDA-EPSC. In these experiments, APV (50 μM) and BIC (25 μM) were added to the bath solution, and the neuron was held at -60 mV. These synaptic evoked currents were completely blocked by CNQX. BDNF (200 ng/ml; 5 min) had no effect of the magnitude of these AMPA-EPSCs (0% ± 6% change; P > 0.48; n = 6). We then examined whether BDNF enhanced the inward currents induced by exogenously applied NMDA (0.5 mM; 3- or 5-sec pulse; delivered once every 120 sec; Fig. 3). These NMDA currents were elicited in SCN neurons recorded in coronal slices, at holding potentials between -25 and -35 mV in the presence of BIC (25 μM) and CNQX (20 μM). BDNF (200 ng/ml; 5 min) enhanced the NMDA currents significantly (24% ± 6% change; n = 8; P < 0.02; Fig. 3). Enhancement of at least 10% was found in six of eight neurons and fully reversed in four of these six cases.
Fig. 2.
BDNF enhanced the magnitude of NMDA-EPSC. Left: Example of the time courses of the NMDA-EPSCs evoked by optic nerve stimulation before and during treatment with BDNF (200 ng/ml; 5 min). The two traces are superimposed at the bottom to make the comparison easier. These recordings were made with Vh at +50 mV. Right: The peak amplitudes of the NMDA-EPSCs measured in each SCN neuron before and during BDNF treatment are plotted. In this and the other figures, lines connect data from a single neuron before and during BDNF treatment. Solid circles indicate data from those cells whose response increased with BDNF treatment.
Fig. 3.
BDNF enhanced the magnitude of NMDA currents in SCN neurons. The whole-cell patch-clamp recording technique was used to measure directly currents evoked by NMDA in ventral SCN neurons during the night. To isolate the postsynaptic response better, BIC (25 μM) and CNQX (20 μM) were added to the bath. In these experiments, the inward currents evoked by the focal application of NMDA (0.5 mM; 3-sec or 5-sec pulse; applied once every 120 sec) were measured with Vh = -30 mV. The magnitudes of the NMDA-evoked currents were measured before, during, and after treatment with BDNF (200 ng/ml, 5 min). Left: An example of the NMDA-evoked currents recorded using this protocol before, during and after treatment with BDNF. Right: The peak amplitudes of the NMDA-evoked currents measured in each SCN neuron before and during the BDNF treatment are plotted. Application of BDNF increased the magnitude of the NMDA currents. Solid circles indicate data from those cells whose response increased with BDNF treatment.
TrkB Receptors Mediate the Effects of BDNF
To determine whether the effect of BDNF on NMDA currents was mediated by activation of neurotrophin receptors, we utilized the tyrosine kinase inhibitor K252a (100–200 nM). We found that, by itself, K252a (200 nM; focal application) attenuated the NMDA components significantly (-27% ± 5% change; n = 8; P < 0.001). In the presence of K252a (100 nM), application of BDNF (200 ng/ml; 5 min) did not enhance the NMDA-EPSCs (-18% ± 12% change; n = 7; P > 0.23; Fig. 4A,B). Bath application of K252a also blocked the BDNF-induced enhancement of exogenous NMDA-induced currents (3% ± 5% change; n = 7; P > 0.8; Fig. 4C). Finally, we used a soluble form of the TrkB receptor engineered as an immunoadhesin (TrkB-IgG) to block TrkB ligands. We found that pretreatment with the TrkB receptor IgG (1 μg/μl; 10 μl) prevented BDNF enhancement of the NMDA-EPSC (-5% ± 5% change; n = 6; P > 0.25; Fig. 5).
Fig. 4.
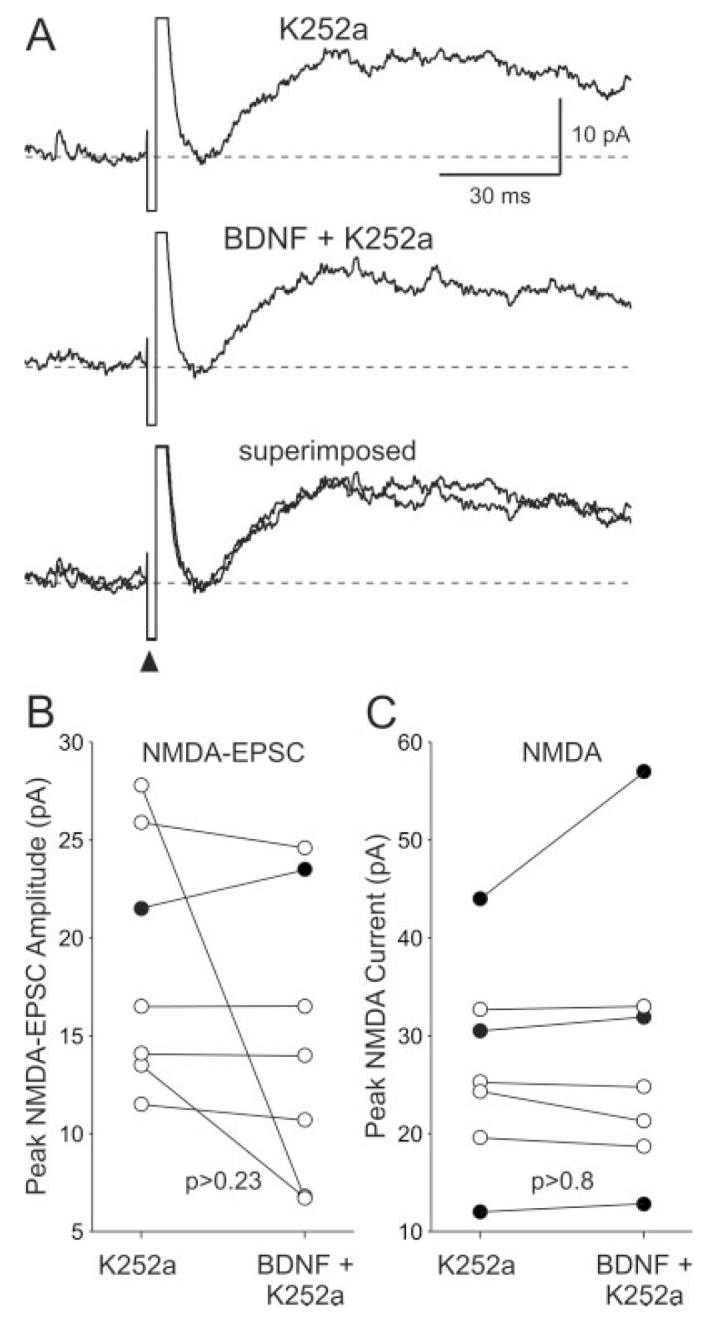
The neurotrophin receptor antagonist K252a inhibited the effects of BDNF on SCN neurons. A: Example showing the time courses of the NMDA-EPSCs evoked before and during BDNF treatment (focal application; 200 ng/ml, 5 min) in the presence of K252a in the bath (100 nM). B: The peak amplitudes of the NMDA-EPSCs measured in each SCN neuron before and during BDNF treatment are plotted. C: The peak amplitudes of the NMDA-evoked currents measured in each SCN neuron before and during BDNF treatment are plotted. For both B and C, solid circles indicate data from those cells whose response increased with BDNF treatment in the presence of K252a.
Fig. 5.
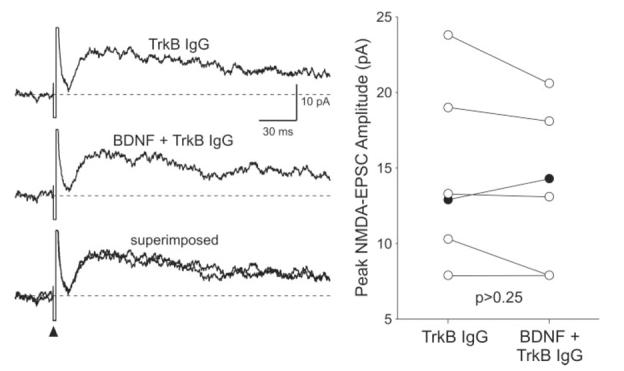
TrkB receptor IgG prevented the BDNF enhancement of the NMDA-EPSC evoked by optic nerve stimulation. Left: Example showing the time courses of the NMDA-EPSCs evoked before and during BDNF treatment (200 ng/ml, 5 min) in a slice pretreated with TrkB IgG (1 μg/μl; 10 μl). Right: The peak amplitudes of the NMDA-EPSCs measured in each SCN neuron before and during BDNF treatment are plotted. Solid circles indicate data from those cells whose response increased with BDNF treatment in the presence of TrkB IgG.
Treatment With the NR2B Receptor Blocker Ifenprodil Prevented BDNF Enhancement
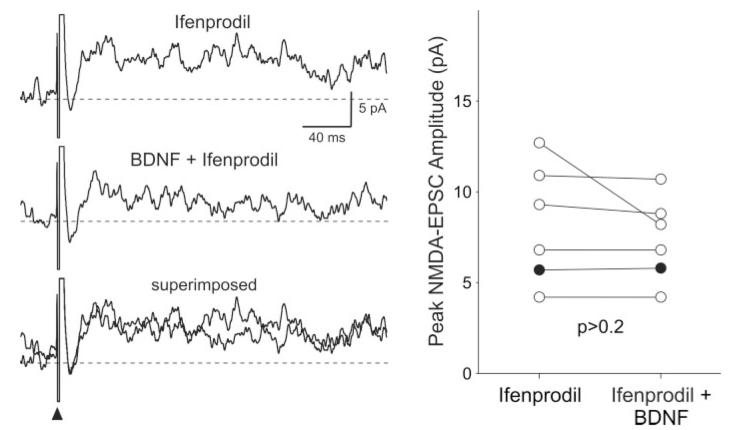
Finally, we examined the role of the NR2B subunit of the NMDA receptor in mediating the effects of BDNF on NMDA-EPSC in SCN neurons. By itself, bath application of the noncompetitive NR2B antagonist ifenprodil (3 μM, 5 min) significantly reduced the magnitude of the NMDA-EPSC (-40% ± 7% change; n = 7; P < 0.05). With the conditions under which these experiments were performed, the inhibition by ifenprodil did not wash out within 30 min. For comparison, similar treatment with the competitive NMDA antagonist APV (50 μM, 5 min) completely suppressed the NMDA-EPSC (Fig. 1). In the presence of ifenprodil (3 μM), the application of BDNF (200 ng/ml, 5 min) was no longer able to enhance the NMDA-EPSC (-7% ± 6% change; n = 6; P > 0.2; Fig. 6).
Fig. 6.
BDNF failed to enhance the magnitude of the NMDA-EPSC when the NR2B subunit of the NMDA receptor was inhibited. Left: Example showing the time courses of the NMDA-EPSCs evoked before and during treatment with BDNF (200 ng/ml, 5 min) in the presence of ifenprodil (3 μM). Right: The peak amplitudes of the NMDA-EPSCs measured in each SCN neuron before and during BDNF treatment are plotted. Solid circles indicate data from those cells whose response increased with BDNF treatment in the presence of ifenprodil.
DISCUSSION
The circadian system is largely responsible for the temporal organization of an organism’s physiology and behavior. The cells within the SCN are responsible for the generation of the daily rhythms as well as their regulation by light. The light information is detected by specialized light-sensitive retinal ganglion cells that utilize the photo-pigment melanopsin (Peirson and Foster, 2006). The axons of these retinal ganglion cells form a fiber tract (retinohypothalamic tract or RHT) that terminates in the SCN. Previous work has established that these axon terminals utilize glutamate and the neuropeptide PACAP as transmitters (Hannibal et al., 2000). Postsynaptically, the NMDA receptor plays a critical role in transducing the light information signaled by the release of glutamate to a behavioral response of the circadian system. Among other findings, blocking NMDA receptors prevents the effects of light on the circadian system (Colwell et al., 1990), whereas activation of these receptors can mimic the effect of light (Mintz et al., 1999). The SCN is made up of functionally distinct cell populations (Antle and Silver, 2005) with only a subset of SCN neurons in the ventral region receiving these direct innervations from the RHT (Morin et al., 2006). Previous data raise the possibility that BDNF may play a role in regulation of this light-input pathway. For example, a recent anatomical study found fluorescent immunostaining for the TrkB receptor throughout the SCN with a substantial overlap in distribution of TrkB immunoreactivity and RHT innervation (Allen and Earnest, 2005). The present study uses physiological tools to examine the hypothesis that BDNF modulates excitatory communication at the RHT/SCN synaptic connection in rats.
BDNF Regulation of NMDA-EPSCs
We focused our analysis on SCN neurons that receive monosynaptic excitatory input from the RHT based on two criteria. First, stimulation of the optic nerves evoked EPSCs in the SCN with low stimulus intensity, and the amplitude of the EPSCs was relatively fixed. Second, the latency of the response to stimulation was consistent and short (<25 msec). These types of criteria are widely used to identify monosynaptic connections (Berry and Pentreath, 1976). For the retinorecipient SCN neurons, we found that BDNF increased, whereas the neurotrophin receptor antagonist K252a decreased, the amplitude of synaptic-evoked NMDA currents. These changes in the amplitude of the NMDA-EPSC could reflect a modulation in presynaptic release of glutamate or postsynaptic changes in receptor sensitivity or a change in ionic driving force. However, our finding that the AMPA-EPSC was not similarly enhanced by BDNF argues against BDNF working presynaptically to increase the release of glutamate in the present preparation. This selectivity to NMDA receptors was a surprise to us, insofar as we have previously found evidence for BDNF enhancement of both AMPA and NMDA currents in the mouse (Michel et al., 2006b). It is not clear whether the difference lies in the species used, the SCN cell populations measured, or some other variable. Regardless, in the present study, we directly demonstrate that application of BDNF enhances the magnitude of the currents evoked by application of NMDA in rat SCN neurons. Although these results do not rule out a presynaptic modulation of glutamate release, they do demonstrate that BDNF can regulate the postsynaptic NMDA receptor in the SCN.
Underlying Mechanisms
To clarify the mechanisms underlying BDNF modulation of NMDA-EPSCs in the SCN, we undertook three sets of experiments. First, we found that the Trk-signaling pathway inhibitor K252a prevented the effects of BDNF. Although the inhibitor K252a is the standard pharmacological agent used to assess the role of neurotrophin receptors, K252a is not selective for TrkB receptor (Knusel and Hefti, 1992). It is also possible that some of the effects of K252a alone on the NMDA-EPSC amplitude are due to an inhibition of the endogenous actions of PACAP. This neuropeptide can activate Trk neurotrophin receptors (Lee et al., 2002; Rajagopal et al., 2004), and we have recently found physiological evidence that PACAP regulates NMDA currents in SCN neurons (Michel et al., 2006a). Next, to explore specifically the role of the TrkB receptors, we made use of a TrkB receptor antibody (TrkB IgG). This antibody competes with native TrkB receptors to sequester BDNF and prevent TrkB receptor activation (Davis et al., 1994; Shelton et al., 1995). Because of the expense of applying this antibody through the bath, we applied small drops of the treatment directly onto the SCN. Our finding that the microdrops of TrkB-IgG blocked the effect of BDNF gives us confidence that the TrkB receptors specifically mediate the effects of BDNF.
The NMDA receptor is composed of different subunits (Cull-Candy et al., 2001), and previous work indicates that blocking the NR2B subunit prevents BDNF action on NMDA currents (Crozier et al., 1999; Levine and Kolb, 2000). To explore the role of the NR2B subunit in the NMDA-EPSCs recorded in the SCN, we used the non-competitive antagonist ifenprodil (Williams, 2001; Neyton and Paoletti, 2006). At 3 μM, ifenprodil almost completely blocks NR2B-containing NMDARs but does not affect those containing NR2A. We found that, by itself, ifenprodil significantly reduced the magnitude of the NMDA-EPSCs, demonstrating a role for the NR2B receptor sub-type in the SCN. In the presence of ifenprodil, BDNF no longer enhanced the NMDA current, suggesting that the BDNF regulation of NMDA-EPSCs might work through the NR2B subunit. Interestingly, TrkB is an intrinsic component of the postsynaptic density in neurons, as are NMDA subunits, including NR2B (Kennedy, 1997; Aoki et al., 2000). This physical proximity raises the possibility that a localized TrkB-mediated phosphorylation cascade modulates NMDA receptors in SCN neurons. This model is consistent with previous findings in the hippocampus, where BDNF enhances the phosphorylation of the NR2B subunit (Lin et al., 1998) and increases the open probability of NMDA channels (Levine and Kolb, 2000). An alternative explanation is that BDNF could drive changes in the surface expression of NMDA receptors in the SCN. There is growing evidence that NMDA receptor subunits can undergo rapid cycling in and out of the membrane (Barria and Malinow, 2002; Nong et al., 2004; Lavezzari et al., 2004). Regulation of synaptic strength by phosphorylation of NMDA receptors and changes in subcellular localization are not mutually exclusive, and both mechanisms could be at work in the SCN.
As with other neurotrophins and neuropeptides, BDNF is synthesized as a precursor (pro-BDNF), which is proteolytically cleaved to form BDNF. Recent work suggests that the pro-BDNF interacts preferentially with the low-affinity receptor p75NTR, which in turn triggers intracellular signaling pathways (Lee et al., 2001; Huang and Reichardt, 2003). Endogenous pro-BDNF is thought to be secreted from neurons and may influence synaptic physiology (see, e.g., Woo et al., 2005) in addition to its better-described role promoting apoptosis. The p75NTR receptors are expressed at the afferent pathways innervating the SCN, and targeted lesions of the p75NTR containing cells with the immunotoxin 192 IgG-saporin impacts circadian rhythms in the rat (Beaule and Amir, 2002; Erhardt et al., 2004). Although the physiological effects of pro-BDNF and p75NTR activation at the RHT/SCN synapse are not known, it is quite possible that pro-BDNF as well as BDNF will play a role in the regulation of the SCN and circadian rhythms.
Functional Significance
Our results indicate that BDNF alters the ratio of the NMDA/AMPA currents evoked in the rat SCN in response to optic nerve stimulation. The magnitude of this effect on amplitude of the NMDA-EPSC was modest (∼20%). However, the long time course of these NMDA currents (∼300 msec duration at half-maximal amplitude) indicates the potential for BDNF to have a larger impact on the integration of synaptic input in the SCN neurons than would be predicted by amplitude alone. Interestingly, computational models suggest that changing the NMDA/AMPA current ratio can have a profound effect on the processing of afferent input (Wolf et al., 2005). Thus, BDNF’s modulation of dendritic integration may be particularly important in SCN neurons in which synaptic inputs have to be translated into intracellular processes to cause phase shifts of the circadian system. Calcium levels are critical for mediating the transduction of membrane events into transcriptional regulation (see, e.g., Finkbeiner et al., 1997), and a calcium influx has been shown to be critical for phase shifting circadian oscillators in nonmammalian species (Colwell et al., 1994). Because the activation of NMDA receptors containing the NR2B subunit results in slower and larger EPSCs (Monyer et al., 1994; Barria and Malinow, 2002), BDNF enhancement of the NR2B subunit would result in more current carried by the NMDA receptor and more calcium entry. Previous studies have shown that blocking the NR2B subunit causes a major reduction in NMDA-induced calcium changes in neurons (Sobczyk et al., 2005). There is also evidence that the NR2B subunit is physically associated with Ras (Krapivinsky et al., 2003) and can play an important role in the regulation of the Ras-ERK pathway (Kim et al., 2005). This signaling pathway is thought to be a critical component of the mechanisms underlying light-induced phase shifts of the circadian system (Butcher et al., 2005). Therefore, we propose that BDNF regulates the effects of light on the circadian system through the modulation of the NR2B subunit.
Previous work of Earnest and colleagues found that BDNF- or TrkB-deficient mice exhibit reduced magnitude of light-induced phase shifts (Liang et al., 2000; Allen et al., 2005). In both cases, the mice homozygous for these mutations do not survive to adulthood, so the effects of the complete loss of BNDF/TrkB signaling on circadian behavior could not be evaluated. In addition, with a systemic knockout of the gene, the site of action of the loss of BDNF or TrkB receptors on circadian behavior cannot be established, although it can be presumed to be in the SCN or the light-input pathway to the SCN. Nevertheless, this work strongly suggests a role for BDNF signaling in light- and glutamate-induced phase shifts of the circadian system. Our data provide support for the behavioral studies and indicate that the site of action of BDNF is on NMDA receptors within the SCN. Together, the data indicate that neurotrophins are important modulators of light’s actions on the circadian system.
ACKNOWLEDGMENTS
Y.I. Kim and H.-J. Choi were supported by the Brain Korea 21 Project in 2005.
REFERENCES
- Allen GC, Earnest DJ. Overlap in the distribution of TrkB immunoreactivity and retinohypothalamic tract innvervation of the rat suprachiasmatic nucleus. Neurosci Lett. 2005;376:200–204. doi: 10.1016/j.neulet.2004.11.076. [DOI] [PubMed] [Google Scholar]
- Allen GC, Qu X, Earnest DJ. TrkB-deficient mice show diminished phase shifts of the circadian activity rhythm in response to light. Neurosci Lett. 2005;378:150–155. doi: 10.1016/j.neulet.2004.12.023. [DOI] [PubMed] [Google Scholar]
- Alsina B, Vu T, Cohen-Cory S. Visualizing synapse formation in arborizing optic axons in vivo: dynamics and modulation by BDNF. Nat Neurosci. 2001;4:1093–1101. doi: 10.1038/nn735. [DOI] [PubMed] [Google Scholar]
- Antle MC, Silver R. Orchestrating time: arrangements of the brain circadian clock. Trends Neurosci. 2005;28:145–151. doi: 10.1016/j.tins.2005.01.003. [DOI] [PubMed] [Google Scholar]
- Aoki C, Wu K, Elste A, Len G, Lin S, McAuliffe G, Black IB. Localization of brain-derived neurotrophic factor and TrkB receptors to postsynaptic densities of adult rat cerebral cortex. J Neurosci Res. 2000;59:454–463. doi: 10.1002/(SICI)1097-4547(20000201)59:3<454::AID-JNR21>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Barria A, Malinow R. Subunit-specific NMDA receptor trafficking to synapses. Neuron. 2002;35:345–353. doi: 10.1016/s0896-6273(02)00776-6. [DOI] [PubMed] [Google Scholar]
- Beaule C, Amir S. Effect of 192 IgG-saporin on circadian activity rhythms, expression of P75 neurotrophin receptors, calbindin-D28K, and light-induced Fos in the suprachiasmatic nucleus in rats. Exp Neurol. 2002;176:377–389. doi: 10.1006/exnr.2002.7969. [DOI] [PubMed] [Google Scholar]
- Berry MS, Pentreath VW. Criteria for distinguishing between monosynaptic and polysynaptic transmission. Brain Res. 1976;105:1–20. doi: 10.1016/0006-8993(76)90919-7. [DOI] [PubMed] [Google Scholar]
- Blum R, Konnerth A. Neurotrophin-mediated rapid signaling in the central nervous system: mechanisms and functions. Physiology. 2005;20:70–78. doi: 10.1152/physiol.00042.2004. [DOI] [PubMed] [Google Scholar]
- Butcher GQ, Lee B, Cheng HY, Obrietan K. Light stimulates MSK1 activation in the suprachiasmatic nucleus via a PACAP-ERK/MAP kinase-dependent mechanism. J Neurosci. 2005;25:5305–5313. doi: 10.1523/JNEUROSCI.4361-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colwell CS, Menaker M. Regulation of circadian rhythms by excitatory amino acids. In: Brann DW, Mahesh VB, editors. Excitatory amino acids: their role in neuroendocrine function. CRC Press; New York: 1996. pp. 223–252. [Google Scholar]
- Colwell CS, Ralph MR, Menaker M. Do NMDA receptors mediate the effects of light on circadian behavior? Brain Res. 1990;523:117–120. doi: 10.1016/0006-8993(90)91643-u. [DOI] [PubMed] [Google Scholar]
- Colwell CS, Whitmore D, Michel S, Block GD. Calcium plays a central role in phase shifting the ocular circadian pacemaker of Aplysia. J Comp Physiol. 1994;A175:415–423. doi: 10.1007/BF00199249. [DOI] [PubMed] [Google Scholar]
- Crozier RA, Black IB, Plummer MR. Blockade of NR2B-containing NMDA receptors prevents BDNF enhancement of glutamatergic transmission in hippocampal neurons. Learn Mem. 1999;6:257–266. [PMC free article] [PubMed] [Google Scholar]
- Cull-Candy S, Brickley S, Farrant M. NMDA receptor subunits: diversity, development and disease. Curr Opin Neurobiol. 2001;11:327–35. doi: 10.1016/s0959-4388(00)00215-4. [DOI] [PubMed] [Google Scholar]
- Davis S, Gale NW, Aldrich TH, Maisonpierre PC, Lhotak V, Pawson T, Goldfarb M, Yancopoulos GD. Ligands for EPH-related receptor tyrosine kinases that require membrane attachment or clustering for activity. Science. 1994;266:816–819. doi: 10.1126/science.7973638. [DOI] [PubMed] [Google Scholar]
- Erhardt C, Galani R, Jeltsch H, Cassel JC, Klosen P, Menet JS, Pevet P, Challet E. Modulation of photic resetting in rats by lesions of projections to the suprachiasmatic nuclei expressing p75 neurotrophin receptor. Eur J Neurosci. 2004;19:1773–1788. doi: 10.1111/j.1460-9568.2004.03281.x. [DOI] [PubMed] [Google Scholar]
- Finkbeiner S, Tavazoie SF, Maloratsky A, Jacobs KM, Harris KM, Greenberg ME. CREB: a major mediator of neuronal neurotrophin responses. Neuron. 1997;19:1031–1047. doi: 10.1016/s0896-6273(00)80395-5. [DOI] [PubMed] [Google Scholar]
- Frost DO. BDNF/trkB signaling in the developmental sculpting of visual connections. Prog Brain Res. 2001;134:35–49. doi: 10.1016/s0079-6123(01)34004-9. [DOI] [PubMed] [Google Scholar]
- Haas HL, Schaerer B, Vosmansky M. A simple perfusion chamber for the study of nervous tissue slices in vitro. J Neurosci Methods. 1979;1:323–325. doi: 10.1016/0165-0270(79)90021-9. [DOI] [PubMed] [Google Scholar]
- Hannibal J, Moller M, Ottersen OP, Fahrenkrug J. PACAP and glutamate are co-stored in the retinohypothalamic tract. J Comp Neurol. 2000;418:147–155. [PubMed] [Google Scholar]
- Huang EJ, Reichardt LF. Trk receptors: roles in neuronal signal transduction. Annu Rev Biochem. 2003;72:609–642. doi: 10.1146/annurev.biochem.72.121801.161629. [DOI] [PubMed] [Google Scholar]
- Kennedy MB. The postsynaptic density at glutamatergic synapses. Trends Neurosci. 1997;20:264–268. doi: 10.1016/s0166-2236(96)01033-8. [DOI] [PubMed] [Google Scholar]
- Kim MJ, Dunah AW, Wang YT, Sheng M. Differential roles of NR2A- and NR2B-containing NMDA receptors in Ras-ERK signaling and AMPA receptor trafficking. Neuron. 2005;46:745–760. doi: 10.1016/j.neuron.2005.04.031. [DOI] [PubMed] [Google Scholar]
- Knusel B, Hefti F. K-252 compounds: modulators of neurotrophin signal transduction. J Neurochem. 1992;59:1987–1996. doi: 10.1111/j.1471-4159.1992.tb10085.x. [DOI] [PubMed] [Google Scholar]
- Krapivinsky G, Krapivinsky L, Manasian Y, Ivanov A, Tyzio R, Pellegrino C, Ben-Ari Y, Clapham DE, Medina I. The NMDA receptor is coupled to the ERK pathway by a direct interaction between NR2B and RasGRF1. Neuron. 2003;40:775–784. doi: 10.1016/s0896-6273(03)00645-7. [DOI] [PubMed] [Google Scholar]
- Lavezzari G, McCallum J, Dewey CM, Roche KW. Subunit-specific regulation of NMDA receptor endocytosis. J Neurosci. 2004;24:6383–6391. doi: 10.1523/JNEUROSCI.1890-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee FS, Rajagopal R, Kim AH, Chang PC, Chao MV. Activation of Trk neurotrophin receptor signaling by pituitary adenylate cyclase-activating polypeptides. J Biol Chem. 2002;277:9096–9102. doi: 10.1074/jbc.M107421200. [DOI] [PubMed] [Google Scholar]
- Lee R, Kermani P, Teng KK, Hempstead BL. Regulation of cell survival by secreted proneurotrophins. Science. 2001;294:1945–1948. doi: 10.1126/science.1065057. [DOI] [PubMed] [Google Scholar]
- Lessmann V. Neurotrophin-dependent modulation of glutamatergic synaptic transmission in the mammalian CNS. Gen Pharmacol. 1998;31:667–674. doi: 10.1016/s0306-3623(98)00190-6. [DOI] [PubMed] [Google Scholar]
- Levine ES, Kolb JE. Brain-derived neurotrophic factor increases activity of NR2B-containing N-methyl-D-aspartate receptors in excised patches from hippocampal neurons. J Neurosci Res. 2000;62:357–362. doi: 10.1002/1097-4547(20001101)62:3<357::AID-JNR5>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Levine ES, Crozier RA, Black IB, Plummer MR. Brain-derived neurotrophic factor modulates hippocampal synaptic transmission by increasing N-methyl-D-aspartic acid receptor activity. Proc Natl Acad Sci U S A. 1998;95:10235–10239. doi: 10.1073/pnas.95.17.10235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewin GR, Barde YA. Physiology of the neurotrophins. Annu Rev Neurosci. 1996;19:289–317. doi: 10.1146/annurev.ne.19.030196.001445. [DOI] [PubMed] [Google Scholar]
- Liang FQ, Walline R, Earnest DJ. Circadian rhythm of brain-derived neurotrophic factor in the rat suprachiasmatic nucleus. Neurosci Lett. 1998;242:89–92. doi: 10.1016/s0304-3940(98)00062-7. [DOI] [PubMed] [Google Scholar]
- Liang FQ, Allen G, Earnest D. Role of brain-derived neurotrophic factor in the circadian regulation of the suprachiasmatic pacemaker by light. J Neurosci. 2000;20:2978–2987. doi: 10.1523/JNEUROSCI.20-08-02978.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin SY, Wu K, Levine ES, Mount HT, Suen PC, Black IB. BDNF acutely increases tyrosine phosphorylation of the NMDA receptor subunit 2B in cortical and hippocampal postsynaptic densities. Brain Res Mol Brain Res. 1998;55:20–27. doi: 10.1016/s0169-328x(97)00349-5. [DOI] [PubMed] [Google Scholar]
- McAllister AK, Katz LC, Lo DC. Neurotrophins and synaptic plasticity. Annu Rev Neurosci. 1999;22:295–318. doi: 10.1146/annurev.neuro.22.1.295. [DOI] [PubMed] [Google Scholar]
- Michel S, Itri J, Han JH, Gniotczynski K, Colwell CS. Regulation of glutamatergic signalling by PACAP in the mammalian suprachiasmatic nucleus. BMC Neurosci. 2006a;7:15. doi: 10.1186/1471-2202-7-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel S, Clark JP, Ding JM, Colwell CS.BDNF and neurotrophin receptors modulate glutamate-induced phase shifts of the suprachiasmatic nucleus Eur J Neurosci (in press).2006b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mintz EM, Marvel CL, Gillespie CF, Price KM, Albers HE. Activation of NMDA receptors in the suprachiasmatic nucleus produces light-like phase shifts of the circadian clock in vivo. J Neurosci. 1999;19:5124–5130. doi: 10.1523/JNEUROSCI.19-12-05124.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monyer H, Burnashev N, Laurie DJ, Sakmann B, Seeburg PH. Developmental and regional expression in the rat brain and functional properties of four NMDA receptors. Neuron. 1994;12:529–540. doi: 10.1016/0896-6273(94)90210-0. [DOI] [PubMed] [Google Scholar]
- Morin LP, Shivers KY, Blanchard JH, Muscat L. Complex organization of mouse and rat suprachiasmatic nucleus. Neuroscience. 2006;137:1285–1297. doi: 10.1016/j.neuroscience.2005.10.030. [DOI] [PubMed] [Google Scholar]
- Murase K, Ryu PD, Randic M. Excitatory and inhibitory amino acids and peptide-induced responses in acutely isolated rat spinal dorsal horn neurons. Neurosci Lett. 1989;103:56–63. doi: 10.1016/0304-3940(89)90485-0. [DOI] [PubMed] [Google Scholar]
- Neyton J, Paoletti P. Relating NMDA receptor function to receptor subunit composition: limitations of the pharmacological approach. J Neurosci. 2006;26:1331–1333. doi: 10.1523/JNEUROSCI.5242-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nong Y, Huang YQ, Salter MW. NMDA receptors are movin’ in. Curr Opin Neurobiol. 2004;14:353–361. doi: 10.1016/j.conb.2004.05.001. [DOI] [PubMed] [Google Scholar]
- Peirson S, Foster RG. Melanopsin: another way of signaling light. Neuron. 2006;49:331–339. doi: 10.1016/j.neuron.2006.01.006. [DOI] [PubMed] [Google Scholar]
- Rajagopal R, Chen ZY, Lee FS, Chao MV. Transactivation of Trk neurotrophin receptors by G-protein-coupled receptor ligands occurs on intracellular membranes. J Neurosci. 2004;24:6650–6658. doi: 10.1523/JNEUROSCI.0010-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schinder AF, Berninger B, Poo M. Postsynaptic target specificity of neurotrophin-induced presynaptic potentiation. Neuron. 2000;25:151–163. doi: 10.1016/s0896-6273(00)80879-x. [DOI] [PubMed] [Google Scholar]
- Shelton DL, Sutherland J, Gripp J, Camerato T, Armanini MP, Phillips HS, Carroll K, Spencer SD, Levinson AD. Human trks: molecular cloning, tissue distribution, and expression of extracellular domain immunoadhesins. J Neurosci. 1995;15:477–491. doi: 10.1523/JNEUROSCI.15-01-00477.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobczyk A, Scheuss V, Svoboda K. NMDA receptor subunit-dependent [Ca2+] signaling in individual hippocampal dendritic spines. J Neurosci. 2005;25:6037–6046. doi: 10.1523/JNEUROSCI.1221-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Gelder RN. Nonvisual ocular photoreception in the mammal. Methods Enzymol. 2005;393:746–755. doi: 10.1016/S0076-6879(05)93039-5. [DOI] [PubMed] [Google Scholar]
- Williams K. Ifenprodil, a novel NMDA receptor antagonist: site and mechanism of action. Curr Drug Targets. 2001;2:285–298. doi: 10.2174/1389450013348489. [DOI] [PubMed] [Google Scholar]
- Wolf JA, Moyer JT, Lazarewicz MT, Contreras D, Benoit-Marand M, O’Donnell P, Finkel LH. NMDA/AMPA ratio impacts state transitions and entrainment to oscillations in a computational model of the nucleus accumbens medium spiny projection neuron. J Neurosci. 2005;25:9080–9095. doi: 10.1523/JNEUROSCI.2220-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo NH, Teng HK, Siao CJ, Chiaruttini C, Pang PT, Milner TA, Hempstead BL, Lu B. Activation of p75NTR by pro-BDNF facilitates hippocampal long-term depression. Nat Neurosci. 2005;8:1069–1077. doi: 10.1038/nn1510. [DOI] [PubMed] [Google Scholar]
- Xu B, Gottschalk W, Chow A, Wilson RI, Schnell E, Zang K, Wang D, Nicoll RA, Lu B, Reichardt LF. The role of brain-derived neurotrophic factor receptors in the mature hippocampus: modulation of long-term potentiation through a presynaptic mechanism involving TrkB. J Neurosci. 2000;20:6888–6897. doi: 10.1523/JNEUROSCI.20-18-06888.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]